Abstract
Activation of the aryl hydrocarbon receptor (AhR) in immune cells, such as dendritic cells (DCs), can lead to suppressed immune responses. Although AhR activation is most recognized for mediating the effects of its prototypical ligand 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), many compounds existing in dietary sources can also bind the AhR. Because the immunomodulatory effects of indole-3-carbinol (I3C) and indirubin-3′-oxime (IO) have yet to be investigated in DCs, we evaluated the potential immunomodulatory effects of these compounds on murine DCs. We hypothesized that I3C and IO suppress immune and inflammatory responses in DCs. We found that both I3C and IO decreased the expression of CD11c, CD40, and CD54 while they increased expression of MHC2 and CD80. Following lipopolysaccharide (LPS)-activation, I3C and IO suppressed the production of pro-inflammatory mediators including tumor necrosis factor-α, interleukin (IL)-1β, IL-6, IL-12, and nitric oxide but increased IL-10 levels. These effects of I3C and IO were partially mediated by the AhR. Additionally, immunoregulatory genes, such as ALDH1A, IDO and TGFB, were upregulated following treatment with I3C or IO. Both I3C and IO decreased basal levels of nuclear factor-kappa B p65, but only I3C suppressed the LPS-induced activity of RelB. Finally, when cultured with naïve T cells, bone marrow-derived dendritic cells treated with the dietary AhR ligands increased the frequency of Foxp3+ Tregs in an antigen-specific manner. Taken together, these results indicate that I3C and IO exhibit immunosuppressive and anti-inflammatory effects on DCs. Because I3C and IO are significantly less toxic than TCDD, these natural products may ultimately become useful therapeutics for the treatment of autoimmune and inflammatory diseases.
Keywords: aryl hydrocarbon receptor (AhR), dendritic cells (DCs), indole-3-carbinol (I3C), indirubin, immune modulation
Dendritic cells (DCs) are professional antigen presenting cells (APCs) that bridge the innate and adaptive branches of the immune system. As such, these cells are critical to mounting successful immune and inflammatory responses against antigens as well as promoting tolerance. Following antigen uptake in the periphery, DCs mature and migrate to lymph nodes where they present processed antigen to naïve T cells. Many factors contribute to the ensuing adaptive immune response. One of these involves activation of the aryl hydrocarbon receptor (AhR), the cytosolic ligand-activated transcription factor responsible for the toxic and immunomodulatory effects of its prototypical ligand, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). The AhR is expressed in immune cells and is involved in many important physiological processes, because AhR-deficient mice have numerous defects including slowed early growth, anal prolapses, and hepatic abnormalities (Schmidt et al., 1996). Importantly, AhR activation by TCDD in DCs results in immune suppression via induction of indoleamine-2,3-dioxygenase (IDO) and subsequent generation of regulatory T cells (Tregs) (Mezrich et al., 2010; Nguyen et al., 2010; Simones and Shepherd, 2011; Vogel et al., 2008).
Besides environmental contaminants, additional compounds present in the diet have the potential to bind and activate the AhR. One of these dietary constituents is indole-3-carbinol (I3C), which is found in cruciferous vegetables, and another is indirubin, which is a component in the traditional Chinese medicine Danggui Longhui Wan. However, neither of these compounds bind the AhR as potently as TCDD. Both I3C and indirubin have well-documented anticancer properties, as they both work in part by inhibiting cyclin dependent kinases that leads to cell cycle arrest in various cell lines. More specifically, I3C has been evaluated for the treatment of both breast and prostate cancer (Weng et al., 2008), whereas indirubin has been used traditionally for the treatment of chronic myelocytic leukemia (Eisenbrand et al., 2004).
Despite these known effects on cancerous cells, the effects of I3C and indirubin on immune cells have not yet been investigated in depth. I3C suppresses the production of pro-inflammatory cytokines in macrophages (Chen et al., 2003; Chang et al., 2011; Tsai et al., 2010), whereas indirubin has been demonstrated to suppress these mediators in splenocytes and microglial cells (Jung et al., 2011; Kunikata et al., 2000). Therefore, studies evaluating the effects of these dietary AhR ligands on additional immune cell populations, such as DCs that constitutively express the AhR, are warranted because these compounds are present in the diet or readily consumed supplements and have great potential as complementary therapies in chronic inflammatory diseases.
In this study, we aimed to define the specific immunomodulatory effects of I3C and indirubin on murine bone marrow-derived DCs (BMDCs). We hypothesized that these natural AhR ligands alter DC maturation such that they suppress immune and inflammatory responses in DCs. To test this hypothesis, we conducted an array of in vitro experiments to assess changes in BMDC fate and function following exposure to I3C and indirubin. The results obtained in this study suggest that both I3C and indirubin generate immunosuppressive effects on DCs that may promote a regulatory environment, which may be useful to suppress chronic inflammatory diseases and/or autoimmunity in vivo.
MATERIALS AND METHODS
Chemicals and reagents.
Indole-3-carbinol (I3C) and indirubin-3′-oxime (IO), a commercially available indirubin derivative that is also an AhR agonist (Guengerich et al., 2004), were obtained from Sigma-Aldrich (St Louis, MO) and dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich). Both compounds were >99% pure when purchased, and the purities of I3C and IO were also confirmed in-house prior to use via nuclear magnetic resonance and high-performance liquid chromatography, respectively (data not shown). For initial concentration-response studies, I3C was used at concentrations of 25, 50, 100, and 200μM and IO was used at 0.01, 0.1, 1.0, and 5.0μM. For all other experiments, I3C and IO were used at final concentrations of 50 and 1μM, respectively. The final concentration of solvent used in cell culture was below 0.1% and did not induce DC cytotoxicity.
Mice.
Six- to 8-week-old male AhR+/+, AhR−/−, and OTII Foxp3egfp mice (all on a C57Bl/6 background) were bred and maintained in the animal research facilities at the University of Montana. C57Bl/6 AhR+/+ were originally obtained from The Jackson Laboratories (Bar Harbor, ME) and bred in-house. C57Bl/6 AhR−/− (B6.AhRtm1Bra) mice were generously provided by Dr B.P. Lawrence (University of Rochester Medical College, Rochester, NY) and bred as previously described (Schmidt and Bradfield, 1996). OTII Foxp3egfp mice were kindly provided by Dr Randolph J. Noelle (Dartmouth Medical School, Lebanon, NH), who originally obtained these mice from Dr A. Rudensky (University of Washington School of Medicine, Seattle, WA), and bred as previously described (Wang et al., 2008). To verify identity of mutant mice prior to use in experiments, AhR−/− mice and OT II Foxp3egfp mice were genotyped via PCR and phenotyped via flow cytometry, respectively. The genotype of AhR+/+ and AhR−/− mice is based on the presence of AhRb or AhRd alleles. The AhR+/+ mice possess the AhRb allele (AhRb/b), which is 300 bp, whereas AhR−/− mice possess the d allele (AhRd/d), which is 260 bp. AhR+/− mice possess both the b and d alleles thus resulting in both the 300 and 260 bp products. Mice were housed under specific pathogen-free conditions and maintained on 12-h dark/light cycles. Standard laboratory food and water were provided ad libitum. All protocols for the use of animals were approved by the University of Montana Institutional Animal Care and Use Committee and adhered to the current National Institutes of Health guidelines for animal usage.
Bone marrow-derived dendritic cells.
BMDCs were prepared as previously described (Bankoti et al., 2010). Briefly, bone marrow cells were collected by flushing murine femurs and tibias with complete media (cRPMI) comprised RPMI (Gibco-BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), 50μM mercaptoethanol, 20mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 10mM sodium pyruvate, and 50 μg/ml gentamicin (Gibco-BRL). Subsequent gradient centrifugation using the Lympholyte-M reagent (Cedarlane Laboratories Limited, Ontario, Canada) removed red blood cells. The hematopoietic cells were cultured at a density of 1 × 106 cells/ml in tissue culture flasks or six-well plates in cRPMI supplemented with 30 ng/ml murine granulocyte macrophage-colony stimulating factor (PeproTech, Rocky Hill, NJ) and treated with AhR ligands or vehicle control. Cells were grown for 7 days at 37°C and 5% CO2. Media, growth factor, and AhR ligand treatment were refreshed on days 3 and 5. On day 7, nonadherent cells representing immature DCs were harvested and purified using anti-CD11c (N418) beads (Miltenyi Biotec, Auburn, CA) and the Miltenyi AutoMACS per the manufacturer’s instructions. BMDC purity was verified via flow cytometry, and cells subsequently cultured were >90% CD11c+. Cells were ≥95% viable as determined by Trypan blue exclusion.
Flow cytometry.
Accessory molecule expression on isolated cells was determined by flow cytometry, as previously described (Shepherd et al., 2001). Briefly, cells were washed with PAB (1% bovine serum albumin and 0.1% sodium azide in PBS). Fc block (BioLegend, San Diego, CA) was used to eliminate nonspecific staining. Optimal concentrations of fluorochrome-conjugated monoclonal antibodies were used to stain cells for an additional 10 min on ice. Antibodies used in these experiments included CD11c-APC (HL3), MHC2-PECy7 (M5/114.15.2), CD86-AlexaFluor700 (GL-1), CD80-PE (16-10A1), CD54-Pacific Blue (YN1/1.7.4), CD40-FITC (3/23), CD4-APC (RM4-5), CD25-PerCPCy5.5 (PC61), and their corresponding isotype controls, all of which were purchased from BioLegend or BD Biosciences (San Jose, CA). One to 500,000 events were collected using a BD FACSAria flow cytometer and analyzed using FACSDiva (Version 6.1.2; BD Biosciences) and FlowJo (Version 8.7.1; TreeStar, Inc., Ashland, OR) software programs.
Cell activation and cytokine assays.
Purified vehicle- or AhR ligand-treated BMDCs were cultured in six-well plates at a density of 1 × 106 cells/ml and stimulated with 1 μg/ml lipopolysaccharide (LPS, Escherichia coli [055:B5], Sigma-Aldrich) for 24 h. Cells were harvested for immunophenotypic and quantitative real-time (qRT)-PCR analyses while supernatants were collected and evaluated for cytokine production using ELISAs. Levels of interleukin (IL)-1β, IL-2, IL-6, IL-10, IL-12, interferon (IFN)-γ and tumor necrosis factor (TNF)-α were measured using BD ELISA kits (BD Biosciences) per the manufacturer’s instructions. Levels of nitric oxide (NO) were measured using the Griess Reagent System (Promega, Madison, WI) per the manufacturer’s instructions.
Quantitative real-time PCR.
RNA was isolated from BMDCs with TRIzol reagent (Invitrogen, Carlsbad, CA) followed by RNA clean up using the Total RNA Kit with optional DNase treatment (Omega Bio-Tek, Norcross, GA) according to the manufacturers’ instructions. First strand complementary DNA (cDNA) synthesis was performed using qScript cDNA Supermix (Quanta Biosciences, Gaithersburg, MD). Resulting cDNA was subjected to qRT-PCR using commercially obtained primers (SABiosciences, Frederick, MD) including aldehyde dehydrogenase 1 family, member A1 (ALDH1A1), aldehyde dehydrogenase 1 family, member A2 (ALDH1A2), cytochrome P450 A1 (CYP1A1), indoleamine-2,3-dioxygenase 1 (IDO1), indoleamine-2,3-dioxygenase 2 (IDO2), IL6, IL10, IL27, latent-binding protein 3 (LTBP), transforming growth factor, beta 1 (TGFB1), transforming growth factor, beta 2 (TGFB2), and transforming growth factor, beta 3 (TGFB3). Reactions were performed with PerfeCTa SYBR Green Supermix (Quanta Biosciences) on an Agilent Technologies Stratagene Mx3005P QPCR System (Santa Clara, CA). Resulting data were normalized to β-actin, and fold changes were calculated using the ΔΔCt method, which compares threshold values of the samples of interest for a particular gene relative to a housekeeping gene.
Nuclear factor-kappa B activity.
DMSO-, I3C-, and IO-treated BMDCs were prepared and purified, as described above. Purified BMDCs (2 × 106 cells per well) were stimulated with LPS (1 μg/ml) for 45 min in six-well plates. Cells were harvested, and nuclear protein extracts were prepared using the Active Motif Nuclear Lysis kit (Active Motif, Carlsbad, CA). Protein concentrations were measured using the Bradford reagent (Bio-Rad Laboratories, Hercules, CA), and nuclear protein (3 μg/well) was subsequently used in the Active Motif TransAM colorimetric assay to evaluate the DNA binding activity of nuclear factor-kappa B (NF-κB) p65 and RelB per the manufacturer’s instructions.
DC:T cell co-cultures.
BMDCs were grown in the presence of the DMSO vehicle, I3C, or IO for 7 days and subsequently purified, as described above. BMDCs were cultured with 1 μg/ml OVA323–339 peptide (Mimotopes, Clayton, Victoria, Australia) for 2 h and washed twice prior to culturing with T cells. Spleens as well as the popliteal and brachial lymph nodes of OTII Foxp3egfp mice were harvested, and CD4+ T cells were purified to >75% using a naïve CD4 T cell isolation kit (Miltenyi Biotec) and the Miltenyi AutoMACS per the manufacturer’s instructions. Cells were ≥95% viable as determined by Trypan blue exclusion. DCs and T cells were co-cultured for 3 days at a ratio of 1:5 (DC:T cells) in 96-well plates. On day 3, cells were harvested, evaluated via flow cytometry for the frequency of CD4+Foxp3+ T cells, and supernatants were collected for subsequent evaluation of cytokine production, as described above.
Statistical analyses.
Data sets with multiple comparisons were evaluated by one-way ANOVA with Dunnett’s post hoc test, whereas data sets with two groups were analyzed by Student’s t-test. Values of p < 0.05 were considered significant.
RESULTS
Concentration-Dependent Effects of I3C and Indirubin-3′Oxime (IO) on the Viability and Proliferation of BMDCs
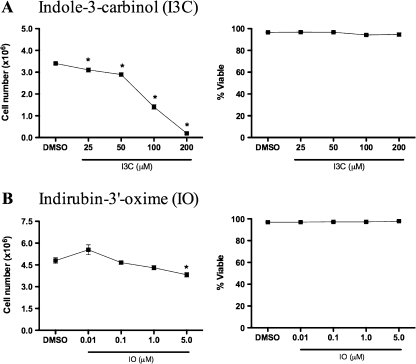
Because the effects of I3C and IO on DCs have not yet been evaluated and both compounds can trigger cell cycle arrest, the concentration-dependent effects on proliferation and viability of these dietary AhR ligands was initially examined in GM-CSF-derived BMDCs. Concentrations of I3C were based on physiologically relevant concentrations (reviewed in Howells et al., 2007), whereas IO concentrations were based on other published in vitro studies (reviewed in Eisenbrand et al., 2004), because the bioavailability of IO has not yet been determined to our knowledge. Neither compound affected the viability of the nonadherent immature DCs at any concentration tested; however, all concentrations of I3C examined decreased DC cell numbers, the highest concentrations of I3C (100 and 200μM) decreased cell proliferation by as much as 60 and 97%, respectively, as indicated by decreased cell numbers (Fig. 1). The highest concentration of IO (5μM) also significantly decreased DC cell numbers. Therefore, based on the results obtained in this initial experiment demonstrating no cytotoxicity and slight antiproliferative effects, concentrations of 50μM I3C and 1μM IO were selected for subsequent experiments.
FIG. 1.
Concentration-dependent effects of I3C and IO on BMDC proliferation and viability. BMDCs were grown in the presence of the 0.1% DMSO vehicle control or various concentrations of I3C and IO. After 7 days, nonadherent cells were stained with Trypan blue to determine cell numbers and viability. Results are representative of three separate experiments with n = 3. *Indicates significance of p ≤ 0.05.
Immunophenotypic Changes Induced by I3C and IO
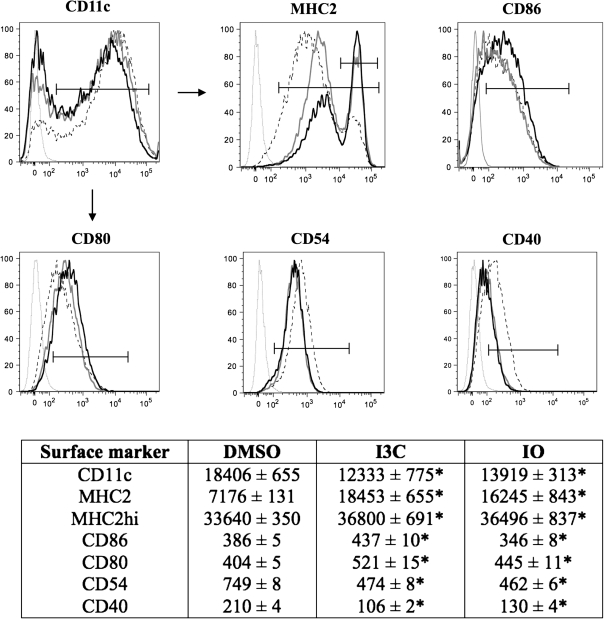
After determining noncytotoxic concentrations of I3C and IO, the effects of these compounds on BMDC immunophenotype were evaluated via flow cytometry (Fig. 2). Both compounds significantly decreased the relative expression of CD11c, CD40, and CD54 on BMDCs, whereas the expression of MHC class 2 (MHC2), MHC2hi, and CD80 was increased. Differential effects were observed on CD86 expression, as I3C increased the expression of this co-stimulatory molecule, whereas IO decreased its expression.
FIG. 2.
Alterations in BMDC phenotype following AhR ligand treatment. BMDCs were grown in the presence of the DMSO vehicle control, 50μM I3C, or 1μM IO for 7 days and subsequently evaluated for their relative expression (mean fluorescence intensity) of surface markers via flow cytometry (numerical values are listed in the corresponding table below the histograms). Thin gray lines indicate isotype control, dotted black lines indicate DMSO-treated cells, thick black lines indicate I3C-treated cells, and thick gray lines indicate IO-treated cells. Arrows indicate MHC2/MHC2hi, CD86, CD80, CD54, and CD40 expression on CD11c+ cells. Results are representative of three separate experiments with n = 3. *Indicates significance of p ≤ 0.05.
I3C and IO Alter the LPS-Induced Changes in Surface Marker Expression and Cytokine Production
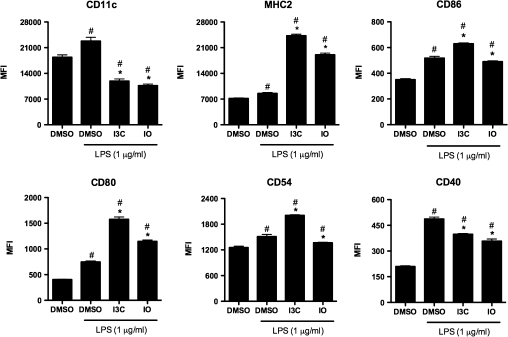
DCs play critical roles in mounting appropriate immune responses to various pathogens and inflammatory insults; therefore, the effects of LPS stimulation on BMDCs treated with I3C or IO were examined. Alterations in cell surface molecule expression were assessed initially (Fig. 3). Both compounds inhibited the LPS-induced upregulation of CD11c and CD40, whereas enhancing the LPS-induced increase in the expression of MHC2 and CD80. I3C upregulated CD86 and CD54; however, IO slightly decreased the LPS-induced expression of both CD86 and CD54.
FIG. 3.
Dietary AhR ligands alter LPS-induced changes in surface phenotype of BMDCs. BMDCs were grown in the presence of the DMSO vehicle control, 50μM I3C, or 1μM IO for 7 days and purified. The immature BMDCs were subsequently treated with 1 μg/ml LPS for 24 h, and the immunophenotype was evaluated by flow cytometry. Results are representative of three separate experiments with n = 3. #Indicates significance of p ≤ 0.05 compared with unstimulated DMSO control, *Indicates significance of p ≤ 0.05 compared with LPS-stimulated DMSO control.
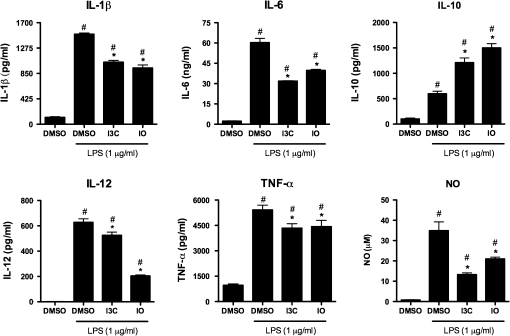
The effects of I3C and IO on pro-inflammatory cytokine production following LPS activation in BMDCs were also assessed. As anticipated, 1 μg/ml LPS induced the production of several pro-inflammatory cytokines including IL-1β, IL-6, IL-12, and TNF-α. BMDCs exposed to I3C or IO and subsequently stimulated with LPS generally produced lower levels of pro-inflammatory cytokines (Fig. 4). I3C and IO decreased the LPS-induced production of IL-1β from 1506 to 1040 and 941 pg/ml, respectively. This effect was also observed with the LPS-induced IL-6 production, in which I3C and IO decreased IL-6 levels from 60 to 32 and 40 ng/ml, respectively. IL-12 production decreased from 628 to 525 pg/ml with I3C and 204 pg/ml with IO. This effect was also observed with TNF-α, as this cytokine was decreased from 5415 to 4338 pg/ml with I3C and to 4427 pg/ml with IO. Lastly, the LPS-induced nitric oxide (NO) production was suppressed from 35 to 13μM with I3C and to 21μM with IO. Conversely, I3C and IO increased the production of the anti-inflammatory cytokine IL-10 after LPS stimulation from 598 to 1211 pg/ml and 1501 pg/ml, respectively. Taken together, these results suggest that I3C and IO significantly suppress the inflammatory responsiveness of LPS-stimulated BMDCs by inhibiting inflammatory mediator production and promoting anti-inflammatory mediator production.
FIG. 4.
Suppression of LPS-induced pro-inflammatory cytokine production by I3C- and IO-treated BMDCs. BMDCs were grown in the presence of the DMSO vehicle control, 50μM I3C, or 1 μm IO for 7 days and purified. The immature BMDCs were subsequently treated with 1 μg/ml LPS for 24 h, and protein levels of cytokines present in the supernatants were measured by ELISA. Results are representative of three separate experiments with n = 3. #Indicates significance of p ≤ 0.05 compared with unstimulated DMSO control, *Indicates significance of p ≤ 0.05 compared with LPS-stimulated DMSO control.
I3C and IO Induce Immunoregulatory Genes in BMDCs
AhR activation in DCs results in the induction of regulatory genes, an effect that correlates with a DC-induced expansion of CD4+Foxp3+ Tregs (Bankoti et al., 2010; Quintana et al., 2010; Simones and Shepherd 2011). Thus, the expression of several key immunoregulatory genes was assessed in BMDCs treated with I3C or IO to further evaluate the immunosuppressive potential of these dietary AhR ligands (Table 1). Without stimulation, BMDCs grown in the presence of I3C upregulated ALDH1A1 by 3.6-fold, CYP1A1 by 5.2-fold, IDO1 by 6.7-fold, IDO2 by 5.7-fold, TGFB2 by 2.4-fold, and TGFB3 by 5.8-fold (Table 1). Conversely, ALDH1A2 and IL27 decreased by 2.3- and 4.1-fold, respectively. Following LPS stimulation, IDO1, IDO2, IL6, TGFB2, and TGFB3 was upregulated, whereas IL10, IL27, and TGFB1 decreased in I3C-treated BMDCs. Similar trends were observed in IO-treated BMDCs with and without LPS stimulation (Table 2). In unstimulated cells, IO upregulated ALDH1A1 by 2.6-fold, CYP1A1 by 4.7-fold, IDO1 by 3.1-fold, IDO2 by 3.0-fold, TGFB2 by 3.5-fold, and TGFB3 by 4.0-fold. In LPS-activated cells, IDO1, IDO2, TGFB2, and TGFB3 were upregulated and ALDH1A2, IL6, and IL27 were downregulated. Overall, in an inflammatory environment, I3C- and IO-treated BMDCs increase their expression of key immunoregulatory genes and thus may possess the potential to suppress immune responses.
TABLE 1.
Dietary AhR Ligands Induce Regulatory Gene Expression in Unstimulated and LPS-Stimulated BMDCsa—Indole-3-carbinol (I3C)
| Gene | Unstimulated |
LPS |
||
| Fold change | p-value | Fold change | p-value | |
| ALDH1A1 | 3.6* | 0.008 | −1.1 | 0.420 |
| ALDH1A2 | −2.3* | <0.001 | −3.4* | <0.001 |
| CYP1A1 | 5.2* | 0.008 | 1.0 | 0.475 |
| IDO1 | 6.7* | 0.001 | 13.5* | <0.001 |
| IDO2 | 5.7* | <0.001 | 5.1* | <0.001 |
| IL6 | 1.3 | 0.328 | 1.5* | 0.008 |
| IL10 | −1.6 | 0.144 | −1.6* | 0.040 |
| IL27 | −4.1* | <0.001 | −2.6* | <0.001 |
| LTBP | −1.2 | 0.324 | 1.2 | 0.139 |
| TGFB1 | 1.1 | 0.373 | −1.3* | 0.032 |
| TGFB2 | 2.4* | <0.001 | 2.5* | 0.001 |
| TGFB3 | 5.8* | 0.005 | 1.8* | 0.034 |
Purified BMDCs grown in the presence of vehicle or 50μM I3C for 7 days were activated with 1 μg/ml LPS for 24 h to evaluate changes in gene transcription by qRT-PCR. Fold change is relevant to vehicle-treated cells and normalized to β-actin. Results are representative of three separate experiments.
*Significance of p ≤ 0.05 compared with the vehicle control.
TABLE 2.
Dietary AhR Ligands Induce Regulatory Gene Expression in Unstimulated and LPS-Stimulated BMDCsa—Indirubin-3′-oxime (IO)
| Gene | Unstimulated |
LPS |
||
| Fold change | p-value | Fold change | p-value | |
| ALDH1A1 | 2.6* | 0.040 | 1.4 | 0.140 |
| ALDH1A2 | −1.2* | 0.019 | −1.4* | 0.016 |
| CYP1A1 | 4.7* | 0.009 | −1.4 | 0.262 |
| IDO1 | 3.1* | 0.004 | 6.6* | 0.001 |
| IDO2 | 3.0* | <0.001 | 4.2* | <0.001 |
| IL6 | 1.2 | 0.367 | −1.3* | 0.016 |
| IL10 | −1.4 | 0.051 | −1.2 | 0.262 |
| IL27 | −3.4* | <0.001 | −3.1* | 0.004 |
| LTBP | 1.4 | 0.407 | 1.6* | 0.011 |
| TGFB1 | 1.1 | 0.244 | 1.1 | 0.362 |
| TGFB2 | 3.5* | 0.001 | 3.2* | 0.001 |
| TGFB3 | 4.0* | 0.027 | 1.7* | 0.037 |
Purified BMDCs grown in the presence of vehicle or 1μM IO for 7 days were activated with 1 μg/ml LPS for 24 h to evaluate changes in gene transcription by qRT-PCR. Fold change is relevant to vehicle-treated cells and normalized to β-actin. Results are representative of three separate experiments.
*Significance of p ≤ 0.05 compared with the vehicle control.
AhR-Dependence of I3C and IO on BMDCs
To investigate whether the observed effects of I3C were dependent on the AhR, BMDCs from AhR−/− mice were generated in the presence of I3C or IO. Surface marker expression of unstimulated and LPS-stimulated cells (Tables 3 and 4) as well as cytokine production (Table 5) was assessed. When AhR-deficient BMDCs were grown in the presence of I3C, changes in the expression of CD11c and CD54 were not observed relative to the vehicle control thereby indicating that the effects on these molecules were dependent on the AhR. The immunomodulatory effects of IO appeared to be more dependent on the AhR, as changes in CD11c, MHC2, CD80, and CD54 were not observed in unstimulated AhR−/− BMDCs. LPS-activated cells revealed that the expected alterations in the expression of CD11c on I3C-treated BMDCs, and CD11c, MHC2, CD80 and CD54 on IO-treated BMDCs were not observed in cells lacking the AhR. Additionally, suppression of many of the LPS-induced pro-inflammatory cytokines examined was observed in AhR-deficient BMDCs treated with I3C (IL-1β, IL-6, TNF-α, and NO) and IO (IL-6, IL-12, and TNF-α). Conversely, the effects of I3C on IL-12 and the effects of IO on IL-1β, IL-10, and NO were demonstrated to be dependent on the AhR. Interestingly, IL-10 production was decreased in LPS-stimulated, AhR−/− BMDCs treated with I3C in contrast to the effects observed in AhR+/+ BMDCs. Finally, the induction of several immunoregulatory genes also appeared to be independent of the AhR, as significant upregulation of ALDH1A1, ALDH1A2, IDO1, and TGFB2 was observed in I3C- and IO-treated AhR-deficient BMDCs (data not shown). Thus, both I3C and IO exert effects on BMDCs by a mechanism that is partially, but not entirely, dependent on the AhR.
TABLE 3.
Immune Modulation by I3C and IO Is Not Entirely AhR-Mediated in BMDCsa—Immunophenotype of Unstimulated Cells
| Surface marker | AhR+/+ |
AhR−/− |
||||
| DMSO | I3C | IO | DMSO | I3C | IO | |
| CD11c | 3391 ± 142 | 2603 ± 326* | 2219 ± 312* | 4611 ± 548 | 4822 ± 107 | 3992 ± 280 |
| MHC2 | 2833 ± 81 | 8056 ± 400* | 6432 ± 255* | 1970 ± 100 | 2912 ± 125* | 2067 ± 100 |
| CD86 | 1299 ± 15 | 1493 ± 3* | 1270 ± 10 | 1279 ± 5 | 950 ± 27* | 1180 ± 31* |
| CD80 | 1176 ± 23 | 1901 ± 8* | 1412 ± 8* | 839 ± 14 | 1129 ± 14* | 822 ± 13 |
| CD54 | 548 ± 7 | 507 ± 11* | 435 ± 6* | 649 ± 6 | 630 ± 12 | 633 ± 10 |
| CD40 | 334 ± 72 | 290 ± 11* | 291 ± 5* | 409 ± 18 | 308 ± 16* | 358 ± 6* |
AhR+/+ and AhR−/− BMDCs were grown in the presence of vehicle, 50μM I3C or 1μM IO and subsequently immunophenotyped prior to LPS stimulation. Mean fluorescence intensity values are shown as mean ± standard error. Data are representative of two independent experiments with n = 3.
*Significance of p ≤ 0.05 compared with DMSO control of respective cell type.
TABLE 4.
Immune Modulation by I3C and IO Is Not Entirely AhR-Mediated in BMDCsa—Immunophenotype of LPS-Stimulated Cells
| Surface marker | AhR+/+ |
AhR−/− |
||||
| DMSO | I3C | IO | DMSO | I3C | IO | |
| CD11c | 1950 ± 74 | 1702 ± 55* | 1770 ± 96 | 2665 ± 74 | 2645 ± 98 | 2198 ± 174 |
| MHC2 | 3182 ± 37 | 8189 ± 61* | 6514 ± 170* | 1817 ± 11 | 3386 ± 143* | 1901 ± 71 |
| CD86 | 1433 ± 9 | 1787 ± 21* | 1370 ± 8* | 1665 ± 5 | 1304 ± 8* | 1523 ± 25* |
| CD80 | 1219 ± 29 | 3257 ± 92* | 1874 ± 30* | 981 ± 15 | 1544 ± 52* | 998 ± 39 |
| CD54 | 510 ± 6 | 545 ± 3* | 439 ± 2* | 583 ± 5 | 650 ± 12* | 560 ± 17 |
| CD40 | 452 ± 11 | 417 ± 12* | 373 ± 21* | 589 ± 12 | 497 ± 9* | 499 ± 18* |
AhR+/+ and AhR−/− BMDCs were grown in the presence of vehicle, 50μM I3C or 1μM IO and subsequently immunophenotyped following activation with LPS (1 μg/ml) for 24 h. Mean fluorescence intensity values are shown as mean ± standard error. Data are representative of two independent experiments with n = 3.
*Significance of p ≤ 0.05 compared with DMSO control of respective cell type.
TABLE 5.
Immune Modulation by I3C and IO Is Not Entirely AhR-Mediated in BMDCsa—Cytokine Production Following LPS Stimulation
| Cytokine | AhR+/+ |
AhR−/− |
||||
| DMSO | I3C | IO | DMSO | I3C | IO | |
| IL-1β (pg/ml) | 1056 ± 53 | 813 ± 31* | 992 ± 42* | 1212 ± 56 | 862 ± 69* | 1053 ± 52 |
| IL-6 (ng/ml) | 47 ± 2 | 21 ± 1* | 24 ± 1* | 47 ± 0.3 | 31 ± 1.6* | 36 ± 2.6* |
| IL-10 (pg/ml) | 598 ± 49 | 1211 ± 92* | 1501 ± 83* | 417 ± 67 | 127 ± 10* | 543 ± 67 |
| IL-12 (pg/ml) | 1463 ± 159 | 984 ± 25* | 427 ± 30* | 1921 ± 140 | 2018 ± 93 | 1323 ± 88* |
| TNF-α (pg/ml) | 5347 ± 181 | 5121 ± 135* | 3962 ± 283* | 7471 ± 603 | 5972 ± 280* | 5040 ± 194* |
| NO (μM) | 9.3 ± 0.3 | 2.4 ± 0.5* | 7.5 ± 0.8* | 28 ± 1 | 16 ± 2* | 30 ± 2 |
AhR+/+ and AhR−/− BMDCs were grown in the presence of vehicle, 50μM I3C or 1μM IO and subsequently stimulated with LPS (1 μg/ml) for 24 h. Cytokine production was assessed in the supernatants. Protein concentrations are shown as mean ± standard error. Data are representative of two independent experiments with n = 3.
*Significance of p ≤ 0.05 compared with DMSO control of respective cell type.
I3C and IO Differentially Alter NF-kB Signaling in BMDCs
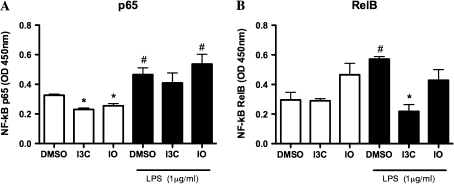
Because the immunomodulatory effects of I3C and IO on BMDCs were not entirely dependent on the AhR, NF-kB binding activity was evaluated because both I3C and IO can disrupt NF-kB signaling (Fig. 5). In unstimulated cells, both I3C and IO reduced the activity of nuclear NF-kB p65; however, similar effects were not observed in LPS-activated DCs. Conversely, there was a trend for increased basal levels of RelB following IO but not I3C treatment, whereas I3C, but not IO, significantly decreased the levels of RelB following LPS activation.
FIG. 5.
I3C and IO differentially alter NF-κB signaling. Purified I3C- and IO-treated BMDCs were stimulated with 1 μg/ml LPS for 45 min, and nuclear protein extracts were subsequently prepared for evaluation of the binding activity of NF-κB p65 (A) and RelB (B). Results are representative of one experiment with n = 3. #Indicates significance of p ≤ 0.05 compared with unstimulated vehicle control and *indicates significance of p ≤ 0.05 compared with vehicle control of similarly activated samples.
I3C- and IO-Treated BMDCs Promote the Generation of Antigen-Specific Tregs
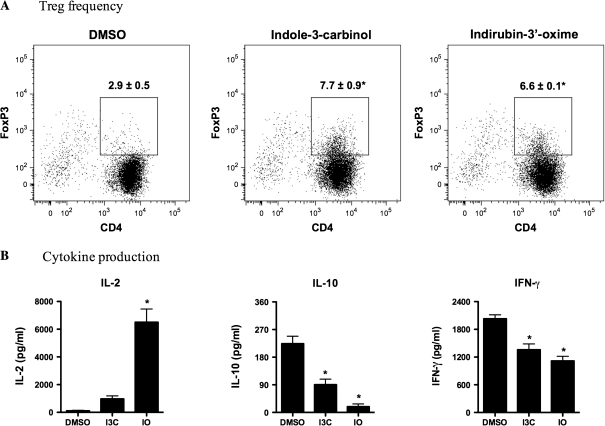
AhR-activated immunoregulatory DCs can induce the generation of CD4+Foxp3+ Tregs (Quintana et al., 2010; Simones and Shepherd, 2011); therefore, the potential for dietary AhR ligand-treated BMDCs to induce OVA323–339-specific Tregs was investigated. Culturing naïve CD4+ OTII T cells for 3 days in the presence of I3C- or IO-treated, OVA323–339-loaded BMDCs significantly increased the frequency of CD4+Foxp3+ T cells (Fig. 6A). When compared with vehicle-treated BMDCs, I3C treatment increased the frequency of OTII Tregs by ∼2.4-fold, whereas IO treatment increased the frequency by almost 2-fold. Cytokine production was also measured in the supernatants from these cultures (Fig. 6B). OTII T cells cultured with IO-treated BMDCs significantly increased IL-2 production from 112 to 6505 pg/ml. I3C- and IO-treated BMDCs decreased the production of IFN-γ in the co-cultures by ∼33 and 45%, respectively. IL-10 production was also decreased by both the I3C- and IO-treated BMDCs when cultured with naïve OTII T cells.
FIG. 6.
DCs treated with dietary AhR ligands increase the frequency of CD4+Foxp3+ Tregs and alter cytokine production. AhR ligand-treated BMDCs and naïve CD4+ OTII T cells were prepared as described in the “Materials and Methods.” (A) The percent of CD4+Foxp3+ T cells is shown in the histogram. (B) Cytokine production of IL-2, IL-10, and IFN-γ was also measured from the culture supernatants as described in the “Materials and Methods.” Results are representative of two separate experiments with n = 3. *Indicates significance of p ≤ 0.05.
DISCUSSION
Modulating the fate and function of DCs has important therapeutic implications in the treatment of a large variety of immune-mediated diseases. The AhR signaling pathway can play an important role in shaping the fate and function of DCs in immune and inflammatory responses. Given that activation of the AhR generates immunoregulatory cells, including DCs and Tregs, it is essential to evaluate the effects of nontoxic AhR ligands, including agonists and antagonists, on critical cell populations in the immune system. I3C and indirubin are particularly interesting dietary AhR ligands because they both possess potent anticancer properties, but currently our knowledge of their effects on immune cells is limited. Therefore, in this study, we investigated the potential of I3C and indirubin to promote immunosuppressive and anti-inflammatory effects via the AhR.
Following AhR activation in DCs, several changes in cell surface molecule expression, cytokine production, and regulatory gene induction contribute to suppressive and tolerogenic immune responses. The exogenous, prototypical ligand TCDD and the endogenous ligand 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE) both decrease the expression of CD11c and upregulation of MHC2 and CD86 in murine BMDCs (Bankoti et al., 2010). Alternatively, the small molecule VAF347 decreases CD86 and HLA-DR in human DCs (Ettmayer et al., 2006), whereas the environmental contaminant benzo(a)pyrene (BaP) induces few changes in the expression of CD11c, MHC2, and CD86 on murine DCs (Hwang et al., 2007). Consistent with the results from TCDD- and ITE-treated DCs, we demonstrated that I3C and IO decreased the expression of CD11c, CD40, and CD54 while increasing the expression of MHC2 and CD80. Decreased CD11c expression on DCs following exposure to I3C and IO suggests altered differentiation of these professional APCs. Furthermore, because CD40 and CD54 are critical immune molecules involved in the interactions of T cells with DCs, decreased expression of these markers on DCs suggests that their ability to successfully activate effector T cells may be defective. Although both I3C and IO are AhR agonists (Guengerich et al., 2004; Miller, 1997), changes in the immunophenotype of BMDCs were not entirely dependent on the AhR, as observed when using BMDCs from mice lacking the AhR. Similarly, it has been noted that the effects of BaP are not entirely AhR-dependent (Hwang et al., 2007). This is distinctly different from the effects of TCDD, which are entirely dependent on the AhR (Bankoti et al., 2010). Overall, the changes in surface marker expression on DCs treated with I3C and IO could be contributing to the generation of immune suppression, in part via AhR activation.
Cytokines are soluble mediators that play a critical role in influencing immune responses. Following activation by bacterial stimuli, such as LPS, DCs secrete many pro-inflammatory cytokines that can promote inflammatory responses. Consequently, we determined the effects I3C and IO on the LPS-induced production of inflammatory mediators. In general, we found that I3C and IO suppressed the secretion of many pro-inflammatory cytokines, thereby indicating anti-inflammatory activity. Interestingly, TCDD was recently shown to increase the LPS-induced production of IL-6 and TNF-α (Bankoti et al., 2010), whereas other AhR ligands including BaP, ITE, and VAF347 decrease the production of pro-inflammatory cytokines (Ettmayer et al., 2006; Lawrence et al., 2008; Quintana et al., 2010). Thus, I3C and IO elicit cytokine patterns much more similar to readily metabolized AhR ligands than TCDD, an extremely stable and persistent AhR agonist.
To further explore the immunosuppressive and anti-inflammatory properties of dietary AhR ligands, gene transcription was evaluated in unstimulated and LPS-activated BMDCs. Both I3C and IO upregulated IDO and TGFB genes, which have important implications in the generation of regulatory immune cells. Several investigators have demonstrated that AhR activation by TCDD in DCs induces regulatory genes that promote the expansion of Tregs (Bankoti et al., 2010; Mellor et al., 2004; Tas et al., 2007; Simones and Shepherd, 2011). Our laboratory previously reported that TCDD induced IDO1, IDO2, and TGFB3 in both unstimulated and LPS-stimulated BMDCs. Prior to activation, TCDD upregulated TGFB3 while TGFB1 and TGFB2 were upregulated following LPS activation (Bankoti et al., 2010). Importantly, the induction of IDO expression by DCs has been associated with the noncanonical NF-κB pathway (Tas et al., 2007; Vogel et al., 2008). Aldehyde dehydrogenase is associated with retinoic acid metabolism, which has been linked to regulatory DCs that drive Treg expansion. In our studies, ALDH1A1 was upregulated by both compounds while ALDH1A2 did not seem to have an important role in producing DCs of a regulatory phenotype. This effect was also observed with the endogenous ligand ITE, as ALDH1A1 was induced in DCs possessing regulatory functions (Quintana et al., 2010). Overall, upregulation of these immunoregulatory genes by I3C and IO suggest that these compounds have the potential to generate a regulatory environment that promotes the induction of regulatory T cells.
Because DCs acquired an immunoregulatory phenotype following treatment with I3C or IO, we evaluated their ability to generate Tregs from naïve CD4+ T cells in an antigen-specific manner. In this study, I3C- and IO-treated BMDCs significantly increased the frequency of CD4+Foxp3+ T cells in vitro. These findings are consistent with a study that demonstrated that indirubin increased CD4+CD25+Foxp3+ T cells in vivo (Zhang et al., 2007). However, in this study, it was not determined if indirubin acted directly on the T cells or indirectly via other immune cells such as the DCs. To date, no studies have reported direct and/or indirect effects of I3C on Foxp3+ Treg induction. TCDD, VAF347, and ITE have all been shown to increase CD4+CD25+Foxp3+ Tregs (Hauben et al., 2008; Quintana et al., 2010; Simones and Shepherd, 2011). ITE was recently demonstrated to induce Tregs both directly via effects on T cells and indirectly by altering DC function. Moreover, these effects in the DCs were found to be dependent on the generation of retinoic acid, as Foxp3+ Treg generation was blocked following the addition of a retinoic acid inhibitor (LE135) to the DCs (Hauben et al., 2008; Quintana et al., 2010; Simones and Shepherd, 2011). On the other hand, a recent study from our laboratory demonstrated that DCs treated with TCDD increased the frequency of OVA-specific, CD4+Foxp3+ Tregs in an IDO-dependent manner (Simones and Shepherd, 2011). Thus, based on the imunoregulatory profiles of DCs exposed to different AhR ligands, it is possible that Treg induction can occur through several mechanisms including retinoic acid, kynurenines, and/or TGF-β.
Both I3C and indirubin have traditionally been used in anticancer and anti-inflammatory applications. To date, neither I3C nor IO has been specifically evaluated for their potential anti-inflammatory effects on DCs, and information regarding the effects of these compounds on other immune cells is also limited. I3C has been assessed in vitro using the murine macrophage cell line RAW264.7 and primary murine macrophages. These studies revealed that I3C decreased the LPS-induced production of several inflammatory mediators, such as nitric oxide (NO) and TNF-α, which has been associated with decreased translocation of NF-κB into the nucleus (Chang et al., 2011; Chen et al., 2003; Tsai et al., 2010). Our laboratory has recently demonstrated that I3C ameliorates disease severity and inflammation associated with a murine model of colitis (manuscript in preparation), which is consistent with our previously published work regarding the anti-inflammatory and immunosuppressive effects of TCDD on colitis (Benson and Shepherd, 2011). The anti-inflammatory effects of indirubin have been considerably less studied. Kunikata and colleagues reported that indirubin inhibited IFN-γ production from human myelomonocytic HBL-38 cells, IL-6 production by murine splenocytes, and ear swelling in a murine model of delayed-type hypersensitivity (Kunikata et al., 2000). Most recently, IO inhibited the LPS-induced production of NO, TNF-α, IL-1β, and PGE-2 by rat brain microglia cells via inhibition of NF-κB activation (Jung et al., 2011).
Because suppression of NF-κB signaling has been associated with decreased inflammatory mediator production by both I3C and IO, it is likely that these compounds also interfere with NF-κB signaling to contribute to the anti-inflammatory responses we observed in BMDCs. The AhR can interact with NF-kB signaling components (Ruby et al., 2005; Vogel et al., 2007a,b). Therefore, it is conceivable that I3C and IO may interact with the AhR and/or NF-κB in DCs to reduce their inflammatory responsiveness. In this study, we demonstrated that I3C and IO decrease the binding activity of NF-κB p65 found in the nucleus of the BMDCs, but only I3C significantly decreases RelB activity following LPS activation. Therefore, it is possible that altered NF-κB signaling contributes, at least in part, to the suppression of LPS-induced inflammatory cytokine production in DCs. Overall, our data suggest that both the AhR and NF-κB signaling pathways may contribute toward the immunoregulatory and anti-inflammatory effects of I3C and IO in DCs. Additional studies utilizing DRE and NF-κB luciferase reporter assays conducted in DCs may help delineate the contribution of these pathways, as most studies on these compounds to date have been conducted in cancer cells. For example, it was shown that 60μM I3C decreased relative luciferase activity in breast cancer cells with regards to NF-κB activity (Rahman et al., 2004), whereas the DRE-driven luciferase of indigoids has been documented in a derivative of human hepatoma HepG2 cells (Guengerich et al., 2004).
Given that the effects observed in this study were not entirely dependent on either the AhR or NF-κB, it is possible that other factors contributed to the observed effects, as IO has been reported to inhibit Src kinase activity and subsequently the phosphorylation of STAT3, which has been implicated as an important factor in Th17 cell differentiation (Kimura et al., 2007; Nam et al., 2005). AhR activation leads to the generation of Tregs and IO may decrease Th17 differentiation so these factors together may contribute to the observed immunosuppressive environment.
It should be noted that in the acidic environment of the stomach, I3C forms the acid condensation product diindolylmethane (DIM). Because I3C is rapidly metabolized into DIM following oral consumption, the use of I3C in cell culture experiments has been criticized, as it has been shown that DIM is primarily responsible for the anticancer and anti-proliferative effects in vivo. However, a recent study demonstrated that following the addition of I3C to cultured cells, DIM spontaneously forms and accounts for much of the documented effects in vitro (Bradlow and Zeligs, 2010). Accordingly, we expect that DIM also formed in our cell cultures and thereby is at least partially responsible for the observed effects on murine BMDCs.
Collectively, we have demonstrated that the dietary AhR ligands I3C and IO exert anti-inflammatory and immunoregulatory effects on DCs in vitro. Although both of these compounds bind the AhR, the effects we observed on cell surface marker expression, cytokine production, and gene transcription were not entirely dependent on the AhR. Moreover, altered NF-kB signaling pathway may be contributing to the anti-inflammatory effects of both I3C and IO. Because no severe adverse reactions have been reported in humans consuming I3C or indirubin (clinical trials reviewed in Eisenbrand et al., 2004; Minich and Bland, 2007), these compounds may ultimately be useful complementary therapeutics for treating inflammatory disorders by altering DC fate and function, consequently creating an immunosuppressive environment. Importantly, our results also suggest that the generation of regulatory DCs and T cells following exposure to I3C and indirubin may significantly reduce the utility of these natural products as anticancer reagents. It is plausible that different metabolism occurs in vivo versus in vitro leading to a differential effect in animals compared with cell culture. It is also possible that the antiproliferative effects of I3C/DIM on tumor cells combined with additional anti-inflammatory and regulatory effects on the immune system may limit damage generated by the tumor cells, permitting a more robust immune response. Therefore, focused in vivo studies to investigate these possibilities are warranted based on previously published studies and our results presented herein.
FUNDING
This project was supported by the National Institutes of Health grant ES013784 (D.M.S) and by award F31AT005557 (J.M.B.) from the National Center for Complementary and Alternative Medicine (NCCAM).
Acknowledgments
The authors wish to thank the CEHS Fluorescence Cytometry Core and Molecular Biology Core at the University of Montana (supported by the National Institutes of Health grant RR017670) for their support. The authors also thank Drs Celine Beamer, Jerry Smith, and Scott Wetzel for critical review of this manuscript. Drs Earle Adams and Fernando Cardozo-Pelaez are also acknowledged, as they assisted with compound purity and stability analyses. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, NIEHS, or NCCAM.
References
- Bankoti J, Rase B, Simones T, Shepherd DM. Functional and phenotypic effects of AhR activation in inflammatory dendritic cells. Toxicol. Appl. Pharmacol. 2010;246:18–28. doi: 10.1016/j.taap.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson JM, Shepherd DM. Aryl hydrocarbon receptor activation by TCDD reduces inflammation associated with Crohn's disease. Toxicol. Sci. 2011;120:68–78. doi: 10.1093/toxsci/kfq360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradlow HL, Zeligs MA. Diindolylmethane (DIM) spontaneously forms from indole-3-carbinol (I3C) during cell culture experiments. In Vivo. 2010;24:387–391. [PubMed] [Google Scholar]
- Chang HP, Wang ML, Hsu CY, Liu ME, Chan MH, Chen YH. Suppression of inflammation-associated factors by indole-3-carbinol in mice fed high-fat diets and in isolated, co-cultured macrophages and adipocytes. Int. J. Obes. (Lond). 2011 doi: 10.1038/ijo.2011.12. doi:10.1038/ijo.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Dai HJ, Chang HP. Suppression of inducible nitric oxide production by indole and isothiocyanate derivatives from Brassica plants in stimulated macrophages. Planta Med. 2003;69:696–700. doi: 10.1055/s-2003-42790. [DOI] [PubMed] [Google Scholar]
- Eisenbrand G, Hippe F, Jakobs S, Muehlbeyer S. Molecular mechanisms of indirubin and its derivatives: Novel anticancer molecules with their origin in traditional Chinese phytomedicine. J. Cancer Res. Clin. Oncol. 2004;130:627–635. doi: 10.1007/s00432-004-0579-2. [DOI] [PubMed] [Google Scholar]
- Ettmayer P, Mayer P, Kalthoff F, Neruda W, Harrer N, Hartmann G, Epstein MM, Brinkmann V, Heusser C, Woisetschläger M. A novel low molecular weight inhibitor of dendritic cells and B cells blocks allergic inflammation. Am. J. Respir. Crit. Care Med. 2006;173:599–606. doi: 10.1164/rccm.200503-468OC. [DOI] [PubMed] [Google Scholar]
- Guengerich PF, Martin MV, McCormick WA, Nguyen LP, Glover E, Bradfield CA. Aryl hydrocarbon receptor response to indigoids in vitro and in vivo. Arch. Biochem. Biophys. 2004;423:309–316. doi: 10.1016/j.abb.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Hauben E, Gregori S, Draghici E, Migliavacca B, Olivieri S, Woisetschläger M, Roncarolo MG. Activation of the aryl hydrocarbon receptor promotes allograft-specific tolerance through direct and dendritic cell-mediated effects on regulatory T cells. Blood. 2008;112:1214–1222. doi: 10.1182/blood-2007-08-109843. [DOI] [PubMed] [Google Scholar]
- Howells LM, Moiseeva EP, Neal CP, Foreman BE, Andreadi CK, Sun YY, Hudson EA, Manson MM. Predicting the physiological relevance of in vitro cancer preventive activities of phytochemicals. Acta Pharmacol. Sin. 2007;28:1274–1304. doi: 10.1111/j.1745-7254.2007.00690.x. [DOI] [PubMed] [Google Scholar]
- Hwang JA, Lee JA, Cheong SW, Youn HJ, Park JH. Benzo(a)pyrene inhibits growth and functional differentiation of mouse bone marrow-derived dendritic cells. Downregulation of RelB and eIF3 p170 by benzo(a)pyrene. Toxicol. Lett. 2007;169:82–90. doi: 10.1016/j.toxlet.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Jung HJ, Nam KN, Son MS, Kang H, Hong JW, Kim JW, Lee EH. Indirubin-3′-oxime inhibits inflammatory activation of rat brain microglia. Neurosci. Lett. 2011;487:139–143. doi: 10.1016/j.neulet.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Kimura A, Naka T, Kishimoto T. IL-6-dependent and -independent pathways in the development of interleukin 17-producing T helper cells. Proc Natl. Acad. Sci. U.S.A. 2007;104:12099–12104. doi: 10.1073/pnas.0705268104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunikata T, Tatefuji T, Aga H, Iwaki K, Ikeda M, Kurimoto M. Indirubin inhibits inflammatory reactions in delayed-type hypersensitivity. Eur. J. Pharmacol. 2000;410:93–100. doi: 10.1016/s0014-2999(00)00879-7. [DOI] [PubMed] [Google Scholar]
- Lawrence BP, Denison MS, Novak H, Vorderstrasse BA, Harrer N, Neruda W, Reichel C, Woisetschläger M. Activation of the aryl hydrocarbon receptor is essential for mediating the anti-inflammatory effects of a novel low-molecular-weight compound. Blood. 2008;112:1158–1165. doi: 10.1182/blood-2007-08-109645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor AL, Chandler P, Baban B, Hansen AM, Marshall B, Pihkala J, Waldmann H, Cobbold S, Adams E, Munn DH. Specific subsets of murine dendritic cells acquire potent T cell regulatory functions following CTLA4-mediated induction of indoleamine 2,3 dioxygenase. Int. Immunol. 2004;16:1391–1401. doi: 10.1093/intimm/dxh140. [DOI] [PubMed] [Google Scholar]
- Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 2010;185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA., III Expression of the human aryl hydrocarbon receptor complex in yeast. Activation of transcription by indole compounds. J. Biol. Chem. 1997;272:32824–32829. doi: 10.1074/jbc.272.52.32824. [DOI] [PubMed] [Google Scholar]
- Minich DM, Bland JS. A review of the clinical efficacy and safety of cruciferous vegetable phytochemicals. Nutr. Rev. 2007;65:259–267. doi: 10.1301/nr.2007.jun.259-267. [DOI] [PubMed] [Google Scholar]
- Nam S, Buettner R, Turkson J, Kim D, Cheng JQ, Muehlbeyer S, Hippe F, Vatter S, Merz KH, Eisenbrand G, Jove R. Indirubin derivatives inhibit Stat3 signaling and induce apoptosis in human cancer cells. Proc. Natl. Acad. Sci. U.S.A. 2005;102:5998–6003. doi: 10.1073/pnas.0409467102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen NT, Kimura A, Nakahama T, Chinen I, Masuda K, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc. Natl. Acad. Sci. U.S.A. 2010;107:19961–19966. doi: 10.1073/pnas.1014465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana FJ, Murugaiyan G, Farez MF, Mitsdoerffer M, Tukpah AM, Burns EJ, Weiner HL. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. U.S.A. 2010;107:20768–20773. doi: 10.1073/pnas.1009201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman KM, Li Y, Sarkar FH. Inactivation of akt and NF-kappaB play important roles during indole-3-carbinol-induced apoptosis in breast cancer cells. Nutr. Cancer. 2004;48:84–94. doi: 10.1207/s15327914nc4801_12. [DOI] [PubMed] [Google Scholar]
- Ruby CE, Funatake CJ, Kerkvliet NI. 2,3,7,8 tetrachlorodibenzo-p-dioxin (TCDD) directly enhances the maturation and apoptosis of dendritic cells in vitro. J. Immunotoxicol. 2005;1:159–166. doi: 10.1080/15476910490920968. [DOI] [PubMed] [Google Scholar]
- Schmidt JV, Bradfield CA. Ah receptor signaling pathways. Annu. Rev. Cell Dev. Biol. 1996;12:55–89. doi: 10.1146/annurev.cellbio.12.1.55. [DOI] [PubMed] [Google Scholar]
- Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: Involvement of the Ah receptor in hepatic growth and development. Proc. Natl. Acad. Sci. U.S.A. 1996;93:6731–6736. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd DM, Steppan LB, Hedstrom OR, Kerkvliet NI. Anti-CD40 Treatment of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-exposed C57Bl/6 mice induces activation of antigen presenting cells yet fails to overcome TCDD-induced suppression of allograft immunity. Toxicol. Appl. Pharmacol. 2001;170:10–22. doi: 10.1006/taap.2000.9080. [DOI] [PubMed] [Google Scholar]
- Simones T, Shepherd DM. Consequences of AhR activation in steady-state dendritic cells. Toxicol. Sci. 2011;119:293–307. doi: 10.1093/toxsci/kfq354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tas SW, Vervoordeldonk MJ, Hajji N, Schuitemaker JH, van der Sluijs KF, May MJ, Ghosh S, Kapsenberg ML, Tak PP, de Jong EC. Noncanonical NF-kappaB signaling in dendritic cells is required for indoleamine 2,3-dioxygenase (IDO) induction and immune regulation. Blood. 2007;110:1540–1549. doi: 10.1182/blood-2006-11-056010. [DOI] [PubMed] [Google Scholar]
- Tsai JT, Liu HC, Chen YH. Suppression of inflammatory mediators by cruciferous vegetable-derived indole-3-carbinol and phenylethyl isothiocyanate in lipopolysaccharide-activated macrophages. Mediators Inflamm. 2010;2010:293642. doi: 10.1155/2010/293642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CF, Goth SR, Dong B, Pessah IN, Matsumura F. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem. Biophys. Res. Commun. 2008;375:331–335. doi: 10.1016/j.bbrc.2008.07.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CF, Sciullo E, Li W, Wong P, Lazennec G, Matsumura F. RelB, a new partner of aryl hydrocarbon receptor-mediated transcription. Mol. Endocrinol. 2007a;21:2941–2955. doi: 10.1210/me.2007-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CF, Sciullo E, Matsumura F. Involvement of RelB in aryl hydrocarbon receptor-mediated induction of chemokines. Biochem. Biophys. Res. Commun. 2007b;363:722–726. doi: 10.1016/j.bbrc.2007.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Pino-Lagos K, de Vries VC, Guleria I, Sayegh MH, Noelle RJ. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc. Natl. Acad. Sci. U.S.A. 2008;105:9331–9336. doi: 10.1073/pnas.0710441105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng JR, Tsai CH, Kulp SK, Chen CS. Indole-3-carbinol as a chemopreventive and anti-cancer agent. Cancer Lett. 2008;262:153–163. doi: 10.1016/j.canlet.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Qu Y, Zhang B, Zhang L, Zeng C, Peng J, Ji X, Hou M, Zhao Y. The different effects of indirubin on effector and CD4+CD25+ regulatory T cells in mice: Potential implication for the treatment of autoimmune diseases. J. Mol. Med. 2007;85:1263–1270. doi: 10.1007/s00109-007-0235-9. [DOI] [PubMed] [Google Scholar]