Abstract
Background:
Statins were initially used to improve cardiovascular outcomes in people with established coronary artery disease, but recently their use has become more common in people at low cardiovascular risk. We did a systematic review of randomized trials to assess the efficacy and harms of statins in these individuals.
Methods:
We searched MEDLINE and EMBASE (to Jan. 28, 2011), registries of health technology assessments and clinical trials, and reference lists of relevant reviews. We included trials that randomly assigned participants at low cardiovascular risk to receive a statin versus a placebo or no statin. We defined low risk as an observed 10-year risk of less than 20% for cardiovascular-related death or nonfatal myocardial infarction, but we explored other definitions in sensitivity analyses.
Results:
We identified 29 eligible trials involving a total of 80 711 participants. All-cause mortality was significantly lower among patients receiving a statin than among controls (relative risk [RR] 0.90, 95% confidence interval [CI] 0.84–0.97) for trials with a 10-year risk of cardiovascular disease < 20% [primary analysis] and 0.83, 95% CI 0.73–0.94, for trials with 10-year risk < 10% [sensitivity analysis]). Patients in the statin group were also significantly less likely than controls to have nonfatal myocardial infarction (RR 0.64, 95% CI 0.49–0.84) and nonfatal stroke (RR 0.81, 95% CI 0.68–0.96). Neither metaregression nor stratified analyses suggested statistically significant differences in efficacy between high-and low-potency statins, or larger reductions in cholesterol.
Interpretation:
Statins were found to be efficacious in preventing death and cardiovascular morbidity in people at low cardiovascular risk. Reductions in relative risk were similar to those seen in patients with a history of coronary artery disease.
Although statins are known to improve survival and relevant clinical outcomes in high-risk populations,1 evidence of their clinical benefit in lower risk populations is more equivocal. Initially, low-risk populations were defined by the absence of known coronary artery disease (and their treatment was termed “primary prevention”). However, it was subsequently recognized that these populations included both patients at very high risk of coronary artery disease (e.g., those with severe peripheral vascular disease) and those at very low risk (e.g., those aged < 40 years who have no diabetes or hypertension and have low-density lipoprotein cholesterol level of less than 1.8 mmol/L). Accordingly, current guidelines for the use of statins are based on the projected risk of an atherosclerotic event rather than solely on the presence or absence of known coronary artery disease.2,3
Results of the recent JUPITER study (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin)4 have renewed enthusiasm for the use of statins in people without a history of coronary artery disease and have generated further controversy as to whether high-potency statins such as rosuvastatin and atorvastatin lead to better clinical outcomes than low-potency statins such as pravastatin, simvastatin, fluvastatin and lovastatin. We did a systematic review of randomized trials to assess the efficacy and harms of statins in people at low cardiovascular risk, including indirect comparisons of high-potency and low-potency statins.
Methods
We performed a systematic review of published and unpublished randomized controlled trials that compared statins with no statin or placebo. We used accepted methods for literature searches, article selection, data extraction and risk-of-bias assessment. The review was reported according to accepted guidelines.5,6
Literature search
We searched MEDLINE (1950 to Jan. 28, 2011) and EMBASE (1950 to Jan. 28, 2011). Details of the search strategy appear in Appendix 1 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.101280/-/DC1). Because of high search yields, an update of a modified version of the National Institute for Health and Clinical Excellence search7 was performed, with elimination of studies published before 2003. Searches were not restricted by language (when a translator could be found).
We also searched registries of health technology assessments and clinical trials, conducted manual searches of reference lists of relevant reviews, and contacted Canadian manufacturers of statins (Astra Zeneca, Merck Frosst, Pfizer, Bristol Myers Squibb, Novartis) for additional studies and unpublished reports of trials.
Study selection and validity assessment
We included parallel-group randomized controlled trials if they included people 16 years or older who were at low cardiovascular risk (as defined in the next paragraph), the follow-up period was at least six months, and an eligible statin (atorvastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin or simvastatin) was compared with no statin (placebo or standard care). We categorized statins according to their pharmacologic effect on lowering cholesterol as either low potency (fluvastatin, lovastatin, pravastatin and simvastatin) or high potency (atorvastatin and rosuvastatin).8 To be eligible, studies also had to report one or more of the following outcomes: all-cause mortality, unstable angina, acute myocardial infarction (fatal or nonfatal), stroke or transient ischemic attack (fatal or nonfatal), surgical or percutaneous revascularization, length of stay, quality of life, persistence on statin therapy, and adverse events. Trials with fewer than 30 participants per study arm were excluded to improve the efficiency of the work without an appreciable loss of power.9
Trials were considered to have enrolled participants at low cardiovascular risk if the 10-year risk of cardiovascular-related death or nonfatal myocardial infarction among participants was less than 20%,10 as assessed by extrapolation of observed risk in the control group of each trial. In general, this corresponded to participants who were free from cardiovascular disease (i.e., no prior acute coronary syndrome or coronary revascularization, no prior ischemic stroke and no prior revascularization or loss of limb owing to peripheral arterial disease) and diabetes. Data from trials in which some, but not all, participants had known cardiovascular disease were included if the control group had a low cardiovascular risk (as defined above).
In sensitivity analyses, we calculated the estimated 10-year risk of cardiovascular-related death or nonfatal myocardial infarction for the average participant in each trial, using mean baseline characteristics from the control group and two commonly used formulas from D’Agostino and colleagues11 and the Third Adult Treatment Panel.12 We also used a number of other definitions for low risk in sensitivity analyses.
Two reviewers screened each citation. Trials considered to be relevant by one or both reviewers were retrieved, and the full text was independently assessed by two reviewers for inclusion. Disagreements were resolved by a third party through consensus.
Two reviewers independently assessed each study’s risk of bias using a condensed version of the Chalmers Index13 as well as other characteristics that influence the risk of biased estimates of effectiveness in meta-analyses.14–16 Disagreements were resolved by a third party through consensus.
Data extraction
One reviewer extracted data from selected trials, and a second reviewer checked the data for accuracy. Results of intention-to-treat analyses were collected if reported.
We classified adverse events as serious if they were defined as such by the primary authors or if their severity was unspecified but they led to withdrawal from therapy or study. We also collected data on the incidence of new diabetes, cancer and rhabdomyolysis. Myocardial infarctions were classified as fatal, nonfatal or fatal/nonfatal; those in the fatal/nonfatal category were from studies that did not specify the type of myocardial infarction or separate totals for fatal and nonfatal myocardial infarctions. Strokes were classified in a similar manner. Angina was classified as unstable if defined as such by the primary authors, if it required admission to hospital or if it necessitated revascularization. Angina that did not meet these criteria was classified as “unspecified” angina. Types of revascularization included coronary artery bypass graft surgery, percutaneous coronary intervention, percutaneous transluminal coronary angioplasty, interventional vascular procedure, coronary revascularization, arterial revascularization and coronary angioplasty. For studies in which the number of cardiovascular-related deaths or nonfatal myocardial infarctions was unclear, we contacted the original authors for information so that we could calculate the 10-year risk.
Data synthesis and analysis
We pooled the results using the random-effects model described by DerSimonian and Laird.17 We used relative risks (RRs) to summarize dichotomous results. We quantified statistical heterogeneity using the I2 statistic and used “small,” “moderate” and “large” to describe values of 25%, 50% and 75%.18,19 A weighted regression test20 was used to test for publication bias.
We used univariable and bivariable meta-regression analyses18 to examine whether the following variables influenced the association between statin use and all-cause mortality: duration of study, year of study, baseline cholesterol levels, observed annual cardiovascular risk in the control arm, the absolute and percent change in low-density lipoprotein cholesterol from baseline in the statin arm, the point at which low-density lipoprotein cholesterol was measured, daily initial dose of statin, potency of statin (high v. low), mean age of participants, proportion of participants who were male, proportion who had diabetes, proportion who had hypertension, and risk-of-bias items.
The number needed to treat and the absolute risk reduction were calculated for outcomes with statistically significant relative risks. The number needed to treat indicates the number of patients who need to be given statin treatment to prevent one event and is the reciprocal of the absolute risk reduction (difference in probabilities of an event between treatment and control groups).21
In subgroup analyses, we examined all-cause mortality and other clinical outcomes for trials stratified by statin potency (high v. low). We also explored the relative effects of statins using a method of indirect comparison of treatments described by Bucher and colleagues,22 a technique that facilitates the comparison of any two statins not directly compared in any one study. Here, the magnitude of treatment effects (e.g., relative risk) for the direct evidence of treatment A versus B and treatment B versus C were compared with acquired indirect evidence of treatment A versus C.
Analyses were conducted using RevMan Version 4.2 (The Cochrane Collaboration, 2002) and Stata/MP 11.2 software (Stata Corp, 2011).
Results
Literature search
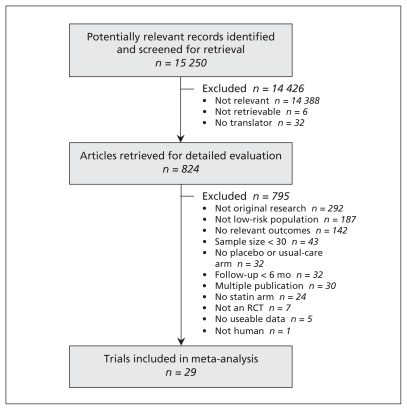
Of the 15 250 articles identified, 29 (n = 80 711) met our inclusion criteria (Figure 1).4,23–50 Atorvastatin was the study drug in six trials (n = 11 894), fluvastatin in four trials (n = 2107), lovastatin in three (n = 15 769), pravastatin in nine (n = 30 974), rosuvastatin in four (n = 19 129) and simvastatin in three (n = 838). Twenty-two of the trials were placebo-controlled, and the remaining seven reported usual care, conventional treatment, standard treatment, no treatment or diet as the comparators. The median year of publication was 2004 (range 1991–2010). The median duration of follow-up was two years (range 0.5–5.3 years). Details of the studies are summarized in Table 1.
Figure 1:
Selection of studies for inclusion in the meta-analysis. RCT = randomized controlled trial.
Table 1:
Description of studies included in the systematic review
| Study, year, location | Population | Sample size in statin/no-statin groups (mean follow-up, yr) | Statin and starting dose, mg/d | Cardiovascular history (%) | 10-yr risk of NFMI or CV death in controls, % | D’Agostino CVD risk,* % | DM HTN, % | Mean TC, LDL-C, HDL-C and TG levels, mmol/L | Mean age, yr (% male) |
|---|---|---|---|---|---|---|---|---|---|
| ASTRONOMER47 2010 Canada |
Men and women 18–82 yr; asymptomatic mild to moderate aortic stenosis (maximum aortic value velocity 2.5–4 m/s); no CAD, CVD, PVD or diabetes | 134/135 (3.5) | Rosuvastatin 40 | NA | 16 | 15 | 0 NR |
5.3 3.2 1.6 1.3 |
58 (62) |
| ESPLANADE49 2010 Italy |
>16 yr; BP >140/90 mm Hg or concomitant antihypertensive therapy; 24-hour proteinuria persistently >1 g (later changed to > 0.5 g) after 2-mo washout from previous treatment with RAS inhibitors or statins; no evidence of urinary tract infections or heart failure | 87/93 (0.5) | Fluvastatin 40–80 | NR | 0 | 18 | 17 NR |
5.6 3.6 1.2 1.4 |
51 (76) |
| LEADe46 2010 10 countries |
Men and women 50–90 yr; diagnosis of probable Alzheimer disease; taking donepezil 10 mg for ≥ 3 mo; LDL-C 2.5–3.5 mmol/L unless DM present (LDL-C < 10%); 2.5–3.5 mmol/L, blood sugar stable and HbA1C no clinically significant or unstable medical condition | 314/325 (1.4) | Atorvastatin 80 | NA | 13 | 28 | NR NR |
5.8 3.7 1.6 1.5 |
74 (48) |
| LORD48 2010 Australia |
18–85 yr with chronic kidney disease (SCr > 120 μmol/L); all levels of proteinuria and serum cholesterol; no previous lipid-lowering therapy | 58/65 (2.5) | Atorvastatin 10 | NR | 12 | 26 | 8 NR |
5.6 3.4 1.2 2.3 |
60 (65) |
| JUPITER4 2008 26 countries |
Men ≥ 50 yr and women ≥ 60 yr; no history of CVD; LDL-C < 3.4 mmol/L, TG < 5.6 mmol/L, high-sensitivity CRP ≥ 2.0 mg/dL; no previous/current use of lipid- lowering therapy | 8901/8901 (median 1.9) | Rosuvastatin 20 | NA | 6 | 22 | 0 NR |
4.8 2.8 1.3 1.3 |
66 (62) |
| Yun et al.50 2008 Korea |
Chest pain at rest; no significant CAD; no acute MI within 6 mo; no previous coronary intervention | 37/37 (0.5) | Rosuvastatin 10 | NR | 0 | 18 | 9 51 |
5.0 3.0 1.2 1.7 |
57 (48) |
| Bone et al.23 2007 USA |
Postmenopausal women 40–75 yr, LDL-C ≥ 3.4 < 4.9 mmol/L; no history of diabetes or CHD | 485/119 (1) | Atorvastatin 10, 20, 40, 80 | NA | 0 | 15 | 0 NR |
6.3 4.0 1.5 1.6 |
59 (0) |
| METEOR34 2007 USA |
Men 40–70 yr and women 55–70 yr; LDL-C < 4.1 mmol/L, HDL-C ≤ 1.6 mmol/L, TG < 5.7 mmol/L; 10-yr risk of CAD events < 10%; no evidence of CAD or other peripheral atherosclerotic disease; no previous revascularization; no diabetes | 702/282 (2) | Rosuvastatin 40 | NA | 0 | 14 | 0 28 |
5.9 4.0 1.3 1.4 |
57 (60) |
| MEGA24 2006 Japan |
Men and postmenopausal women 40–70 yr; TC 5.69– 6.98 mmol/L; no history of CHD or stroke | 3866/3966 (5.3) | Pravastatin 10–20 | NA | 2 | 18 | 21 42 |
6.3 4.1 1.5 1.4 |
58 (31) |
| Dernellis et al.35 2005 Greece |
Adults with CRP 0.8–1.3 mg/dL; at least one episode of PAF on ambulatory monitoring | 40/40 (0.5) | Atorvastatin 20–40 | NR | 0 | 20 | 20 65 |
5.8 4.0 1.2 NR |
52 (65) |
| Di Lullo et al.25 2005 Italy |
Men and women 18–80 yr; mild/moderate CRF for ≥ 5 yr; CRP 3–14 mg/dL, TC 6.5–9.1 mmol/L, HDL-C 1.3–1.8 mmol/L, LDL-C 2.6–4.9 mmol/L, TG 1.8–5.1 mmol/L; no diagnosis of severe heart failure or familial hypercholesterolemia; no cardiac illness | 80/50 (0.7) | Fluvastatin XL 80 | NA | 0 | 32 | 35 65 |
7.6 4.0 1.7 2.6 |
59 (54) |
| Holmberg et al.26 2005 Sweden |
≥ 18 yr; GFR < 30 mL/min per body surface area of 1.73 m2 | 70/73 (1.8) | Atorvastatin 10 | NR | 0 | 40 | 31 NR |
5.8 3.5 1.1 2.4 |
69 (69) |
| HYRIM28 2005 Norway |
Drug-treated hypertensive men 40–75 yr; TC 4.5–8 mmol/L, TG < 4.5 mmol/L; no symptomatic CVD, CHF, type I DM or history of coronary intervention | 283/285 (4) | Fluvastatin† 40 | NA | 8 | 26 | NR 100 |
5.9 3.9 1.3 1.8 |
57 (100) |
| UK-HARP-I27 2005 UK |
Men and women ≥ 18 yr; 1) pre-dialysis with PCr or SCr ≥ 1.7 mg/dL; under dialysis; or functioning renal transplant, and 2) no definite indication or contraindication for cholesterol-lowering therapy or ASA | 224/224 (1) | Simvastatin† 20 | Vascular disease‡ (9) | 9 | 22 | 12 NR |
5.2 3.2 1.0 2.1 |
53 (79) |
| Muldoon et al.29 2004 USA |
Hypercholesterolemic men and women 35–70 yr; LDL-C 4.1–5.7 mmol/L; no CAD, stroke, diabetes or current lipid-lowering medication | 206/102 (0.5) | Simvastatin 10, 40 | NA | 0 | 15 | 0 NR |
6.8 4.7 1.3 1.7 |
54 (48) |
| PHYLLIS30 2004 Italy |
Men and postmenopausal women 45–70 yr; untreated or uncontrolled HTN, hypercholesterolemia, asymptomatic carotid atherosclerosis; no previous CVA events; LDL-C 4.14–5.17 mmol/L, TG ≤ 3.39 mmol/L | 254/254 (2.6) | Pravastatin† 40 | NA | 5 | 21 | NR 100 |
6.8 4.7 1.4 1.6 |
58 (40) |
| PREVEND-IT31 2004 Netherlands |
28–75 yr; persistent microalbuminuria; BP < 160/100 mm Hg without use of antihypertensive medication; TC < 8 mmol/L (< 5 mmol/L if previous MI); no use of lipid-lowering medication | 433/431 (3.8) | Pravastatin† 40 | MI (1) CVA (1) PVD (1) | 11 | 14 | 3 NR |
5.8 4.1 1.0 1.4 |
51 (65) |
| Rejnmark et al.32 2004 Denmark |
Healthy, postmenopausal women < 76 yr; no previous statin use within 2 yr | 41/41 (1.5) | Simvastatin 40 | NA | 0 | 15 | NR NR |
6.5 4.0 1.9 1.2 |
64 (0) |
| ASCOT–LLA36 2003 Europe |
Men and women 40–79 yr; TC ≤ 6.5 mmol/L; not taking lipid-lowering therapy; ≥ 3 CVD risk factors; no previous MI or heart failure | 5168/5137 (median 3.3) | Atorvastatin 10 | Stroke/TIA (10) PVD (5) Other CVD (4) | 11 | 42 | 25 80 |
5.5 3.4 1.3 1.7 |
63 (81) |
| Bruckert et al.33 2003 France, Italy, Spain, Belgium, Israel |
Men and women 70–85 yr; primary hypercholesterolemia (TC ≥ 6.5 mmol/L, TG ≥ 4.6 mmol/L, LDL-C ≥ 4.1 mmol/L after dietary intevention); no symptomatic CHF, history of MI, angina or stroke | 607/622 (1) | Fluvastatin XL 80 | CHF (0.4) TIA (2) | 2 | 33 | 7 56 |
7.3 5.2 1.4 1.5 |
76 (25) |
| ALLHAT–LLT40 2002 Canada, USA, Puerto Rico, Virgin Islands |
Age ≥ 55 yr; stage I or II HTN; ≥ 1 additional CHD risk factor; fasting LDL-C 3.1–4.9 mmol/L (if no known CHD) or 2.6–3.3 mmol/L (if known CHD); fasting TG < 3.9 mmol/L | 5170/5185 (4.8) | Pravastatin 20–40 | CHD (14) | 18 | 36 | 35 100 |
5.8 3.8 1.2 1.7 |
66 (51) |
| KLIS37 2000 Japan |
Men 45–75 yr; primary hypercholesterolemia (TC ≥ 5.69 mmol/L) in at least two measurements; no history of MI or coronary bypass surgery | 2219/1634 (5) | Pravastatin 10–20 | NA | 3 | 33 | 23 43 |
6.4 4.3 1.3 1.9 |
58 (100) |
| Bak et al.44 1998 Netherlands |
Men 40–70 yr; TC 6.5–8 mmol/L, TG ≤ 4 mmol/L; no CVD; no prior use of lipid-lowering drugs | 106/109 (0.5) | Pravastatin† 20 | NA | 0 | 24 | NR NR |
7.3 5.2 1.1 2.1 |
55 (100) |
| AFCAPS/TexCAPS39 1998 USA |
Men 45–73 yr and postmenopausal women 55–73 yr; no history of MI, angina, claudication, CVA or TIA; TC 4.65–6.82 mmol/L, LDL-C 3.36–4.91 mmol/L, HDL-C ≤ 1.16 mmol/L for men and ≤ 1.22 for women, TG ≤ 4.53 mmol/L | 3304/3301 (5.2) | Lovastatin 20–40 | NA | 7 | 28 | 4 22 |
5.7 3.9 1.0 1.8 |
58 (85) |
| CAIUS42 1996 Italy |
Men and women 45–65 yr; three baseline determinations of LDL-C 3.88–6.47 mmol/L and TG < 2.82 mmol/L; no CAD; at least one carotid artery lesion detected by ultrasound imaging | 151/154 (3) | Pravastatin 40 | NA | 4 | 18 | 0 NR |
6.8 4.7 1.4 1.6 |
55 (53) |
| KAPS41 1995 Finland |
Men 44–65 yr; LDL-C > 4.25 mmol/L, TC < 8.0 mmol/L, BMI < 32 kg/m2, ALT/AST ≤ 1.5-fold the laboratory upper normal limit | 224/223 (3) | Pravastatin 40 | MI (8) | 13 | 29 | 2 33 |
6.7 4.9 1.2 1.7 |
57 (100) |
| WOSCOPS43 1995 Scotland |
Men 45–64 yr; hypercholesterolemia (LDL-C 4.5– 6 mmol/L); no history of MI or other serious illness | 3302/3293 (4.9) | Pravastatin 40 | Vascular disease** (16) | 17 | 31 | 1 16 |
7.0 5.0 1.1 1.8 |
55 (100) |
| ACAPS38 1994 USA |
Men and women 40–79 yr; early carotid atherosclerosis; moderately elevated LDL-C; no history of stroke, TIA, angina or MI | 460/459 (2.8) | Lovastatin† 20–40 | NA | 8 | 21 | 2 29 |
6.1 4.0 1.3 3.6 |
62 (52) |
| EXCEL45 1991 USA |
Men and women (postmenopausal or surgically sterile) 18–70 yr; fasting plasma TC 6.2–7.6 mmol/L, LDL-C ≥ 4.1 mmol/L; no unstable medical conditions, impaired hepatic or renal function | 6582/1663 (0.9) | Lovastatin 20, 40, 80 | CHD (29) | 13 | 21 | NR 40 |
6.7 4.7 1.2 1.8 |
56 (59) |
Note: ALT = alanine aminotransferase, ASA = acetylsalicylic acid, AST = aspartate aminotransferase, BMI = body mass index, BP = blood pressure, CAD = coronary artery disease, CHD = coronary heart disease, CHF = congestive heart failure, CRF = chronic renal failure, CRP = C-reactive protein, CV = cardiovascular, CVA = cerebrovascular accident, CVD = cardiovascular disease, DM = diabetes mellitus, GFR = glomerular filtration rate, HDL-C = high-density lipoprotein cholesterol, HTN = hypertension, LDL-C = low-density lipoprotein cholesterol, MI = myocardial infarction, NA = not applicable, NFMI = nonfatal myocardial infarction, NR = not reported, PAF = paroxysmal atrial fibrillation, PCr = plasma creatinine, PVD = peripheral vascular disease, SCr = serum creatinine, TC = total cholesterol, TG = triglycerides, TIA = transient ischemic attack, UK = United Kingdom, USA = United States of America. For complete study names, see Box 1.
Hypertension is assumed to be treated, unless inclusion criteria indicated untreated or uncontrolled hypertension, or no use of antihypertensive medications.
Factorial design: statin treatment groups combined, control groups combined.
Includes angina, myocardial infarction, revascularization, stroke and peripheral arterial disease.
Includes angina, intermittent claudication, stroke, transient ischemic attack and abnormality on electrocardiogram.
Risk-of-bias assessment
The 29 trials generally exhibited moderate risk of bias (Table 2). A weighted regression test20 using all-cause mortality results detected no statistical evidence of publication bias (bias = 0.03, p = 0.92).
Table 2:
Risk-of-bias assessment of studies included in the systematic review
| Study | Study design | Statistical analysis | Presentation of results | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Description of participant selection | Concealment of allocation to treatment* | Description of therapeutic regimen | Was trial described as double-blind?* | Was blinding assessment described?/ Was double-blinding method appropriate? | Were withdrawals and dropouts reported?* | Was sample size calculation described? | Was design described as intention-to- treat? | Was this a preliminary analysis/Was trial stopped early? | Were adverse events reported? | Was discussion adequate? | Source of funding* | |
| ASTRONOMER47 | Adequate | Adequate | Adequate | Yes | Yes/Yes | Yes | Yes | Yes | No/NR | Yes | Yes | Mixed |
| ESPLANADE49 | Adequate | Adequate | Adequate | No | NA/NA | Yes | Yes | Yes | No/NR | Yes | Yes | Mixed |
| LEADe46 | Adequate | Adequate | Adequate | Yes | Yes/Yes | Partial | Yes | Yes | No/NR | Yes | Yes | Private |
| LORD48 | Adequate | Adequate | Adequate | Yes | No/NA | Partial | Yes | Yes | No/NR | Yes | Yes | Mixed |
| JUPITER4 | Adequate | Adequate | Adequate | Yes | Yes/Yes | No | Yes | Yes | No/Yes | Yes | Yes | Private |
| Yun et al.50 | Adequate | Unclear | Partial | Yes | No/NA | Partial | No | No | No/NR | Yes | Yes | Foundation |
| Bone et al.23 | Adequate | Unclear | Adequate | Yes | Yes/Yes | Yes | Yes | Yes | No/NR | Yes | Yes | Private |
| METEOR34 | Adequate | Unclear | Adequate | Yes | No/NA | Yes | Yes | Yes | No/NR | Yes | Yes | Private |
| MEGA24 | Adequate | Unclear | Adequate | No | NA/NA | Yes | Yes | Yes | No/NR | Yes | Yes | Mixed |
| Dernellis et al.35 | Adequate | Unclear | Adequate | No | NA/NA | Yes | No | Yes | No/NR | Yes | Yes | NR |
| Di Lullo et al.25 | Adequate | Unclear | Adequate | No | NA/NA | No | No | No | No/NR | Yes | Yes | NR |
| Holmberg et al.26 | Adequate | Unclear | Adequate | No | NA/NA | Yes | No | No | No/NR | Yes | Yes | Public |
| HYRIM28 | Adequate | Unclear | Adequate | Yes | No/NA | No | No | Yes | No/NR | Yes | Yes | Mixed |
| UK-HARP-I27 | Adequate | Adequate | Adequate | Yes | Yes/Yes | Yes | Yes | Yes | No/NR | Yes | Yes | Private |
| Muldoon et al.29 | Adequate | Unclear | Adequate | Yes | Yes/Yes | Yes | Yes | No | No/NR | No | NA | Public |
| PHYLLIS30 | Adequate | Unclear | Adequate | Yes | No/NA | Partial | Yes | Yes | No/NR | Yes | Yes | Private |
| PREVEND-IT31 | Adequate | Unclear | Adequate | Yes | Yes/Yes | Yes | Yes | Yes | No/NR | No | NA | Mixed |
| Rejnmark et al.32 | Adequate | Adequate | Adequate | Yes | Yes/Yes | Yes | Yes | Yes | No/NR | Yes | Yes | Public |
| ASCOT–LLA36 | Adequate | Unclear | Adequate | Yes | Yes/Yes | Yes | Yes | Yes | No/Yes | Yes | No | Private |
| Bruckert et al.33 | Adequate | Unclear | Adequate | Yes | No/NA | Yes | No | Yes | No/NR | Yes | Yes | Private |
| ALLHAT–LLT40 | Adequate | Adequate | Adequate | No | NA/NA | Yes | Yes | Yes | No/NR | Yes | Yes | Mixed |
| KLIS37 | Adequate | Adequate | Adequate | No | NA/NA | No | Yes | No | No/NR | Yes | Yes | Private |
| Bak et al.44 | Adequate | Unclear | Adequate | Yes | Yes/Yes | Partial | No | Yes | No/NR | Yes | Yes | Private |
| AFCAPS/TexCAPS39 | Adequate | Unclear | Adequate | Yes | Yes/Yes | Partial | Yes | Yes | No/NR | Yes | Yes | Private |
| CAIUS42 | Adequate | Adequate | Adequate | Yes | Yes/Yes | Partial | Yes | Yes | No/NR | Yes | Yes | Mixed |
| KAPS41 | Adequate | Unclear | Adequate | Yes | Yes/Yes | Yes | No | Yes | No/NR | Yes | Yes | Mixed |
| WOSCOPS43 | Adequate | Unclear | Adequate | Yes | No/NA | Partial | No | Yes | No/NR | Yes | Yes | Private |
| ACAPS38 | Adequate | Unclear | Adequate | Yes | No/NA | No | Yes | Yes | No/NR | Yes | Yes | Mixed |
| EXCEL45 | Adequate | Unclear | Adequate | Yes | No/NA | Yes | No | Yes | No/NR | Yes | Yes | Private |
Note: NA = not applicable, NR = not reported, mixed = public and private sources of funding.
Items have empirical evidence.
Characteristics of participants
The median age of the participants was 58 years (range 51–76), and the proportion who were male ranged from 0% to 100% (median 62%). The median proportion of participants who had diabetes was 7% (range 0%–35%), and hypertension 47% (range 16%–100%) (Table 1).
The baseline lipid levels in the included trials were: total cholesterol, median 6.0 (range 4.8–7.6) mmol/L; low-density lipoprotein cholesterol, median 4.0 (range 2.8–5.2) mmol/L; high-density lipoprotein cholesterol, median 1.3 (range 1.0–1.9) mmol/L; and triglycerides, median 1.7 (range 1.2–3.6) mmol/L. Seven studies reported baseline C-reactive protein levels: median 0.17 (range 0.15–7.7) mg/dL (16.6 [range 13.8–732.4] nmol/L). The mean 10-year risk of cardiovascular-related death or nonfatal myocardial infarction was 6% (range 0%–18%).
Outcomes
Table 3 presents a summary of the relative risks (overall and stratified by high- and low-potency statins), number needed to treat and absolute risk reduction for all outcomes.
Table 3:
Relative risk of serious outcomes associated with the use of statins in patients at low cardiovascular risk*
| Outcome | Overall RR (95% CI) | RR for high- potency statins (95% CI) | RR for low- potency statins (95% CI) | Number needed to treat (95% CI)† | Absolute risk reduction, % (95% CI)† |
|---|---|---|---|---|---|
| Death from any cause | 0.90 (0.84–0.97) | 0.85 (0.74–0.96) | 0.90 (0.79–1.03) | 239 (149–796) | 0.42 (0.13–0.67) |
| Myocardial infarction | 0.63 (0.50–0.79) | 0.47 (0.31–0.71) | 0.68 (0.53–0.87) | 216 (160–381) | 0.46 (0.26–0.63) |
| Fatal myocardial infarction | 0.96 (0.50–1.85) | 1.54 (0.61–3.89) | 0.59 (0.23–1.51) | – | – |
| Nonfatal myocardial infarction | 0.64 (0.49–0.84) | 0.47 (0.34–0.67) | 0.77 (0.59–1.00) | 153 (108–343) | 0.66 (0.29–0.93) |
| Stroke | 0.83 (0.74–0.93) | 0.70 (0.55–0.87) | 0.89 (0.77–1.03) | 291 (190–707) | 0.34 (0.14–0.53) |
| Fatal stroke | 0.91 (0.65–1.29) | 0.50 (0.13–2.0) | 0.95 (0.67–1.35) | – | – |
| Nonfatal stroke | 0.81 (0.68–0.96) | 0.51 (0.33–0.79) | 0.88 (0.73–1.06) | 335 (199–1592) | 0.30 (0.06–0.50) |
| Unstable angina | 0.71 (0.55–0.92) | 0.73 (0.48–1.11) | 0.70 (0.51–0.97) | 431 (278–1563) | 0.23 (0.06–0.36) |
| Angina, type unspecified | 0.83 (0.57–1.22) | 1.05 (0.12–9.23) | 0.83 (0.56–1.22) | – | – |
| Revascularization | 0.66 (0.57–0.77) | 0.74 (0.39–1.40) | 0.66 (0.55–0.78) | 131 (103–193) | 0.77 (0.52–0.97) |
| Serious adverse event | 1.01 (0.96–1.07) | 0.96 (0.86–1.09) | 1.03 (0.98–1.10) | – | – |
| Rhabdomyolysis | 1.29 (0.25–6.68) | 2.99 (0.31–28.75) | 0.50 (0.05–5.51) | – | – |
| Cancer | 1.00 (0.93–1.08) | 0.95 (0.81–1.11) | 1.02 (0.93–1.11) | – | – |
| New diabetes | 1.05 (0.84–1.32) | 1.17 (1.04–1.32) | 0.76 (0.56–1.02) | – | – |
Note: CI = confidence interval, RR = relative risk.
Low cardiovascular risk was defined as an observed 10-year risk of less than 20% for cardiovascular-related death or nonfatal myocardial infarction.
Number needed to treat and absolute risk reduction for each outcome were calculated on the basis of the pooled risk in the control arm from all included trials. Number needed to treat and absolute risk reduction were calculated only for outcomes with a statistically significant estimate of effect.
All-cause mortality
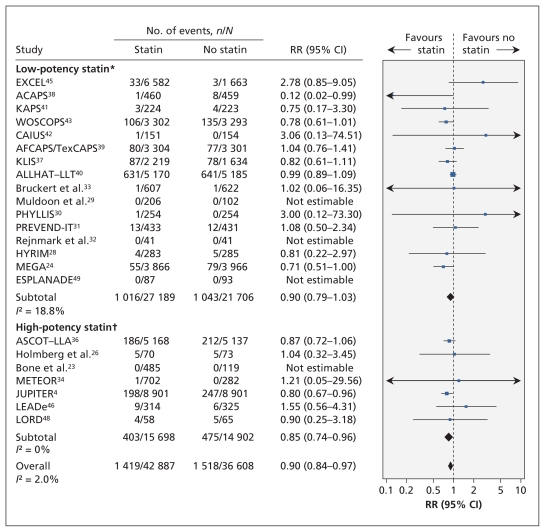
Twenty-three trials (n = 79 495) reported all-cause mortality (Figure 2). The trial-level relative risk could not be estimated for four trials that reported no events in either group.51 From the remaining 19 trials (n = 78 321), the pooled relative risk of death was significantly lower among statin recipients than among controls (RR 0.90, 95% confidence interval [CI] 0.84–0.97; I2 = 2.0%).
Figure 2:
Risk of death from any cause associated with the use of statins (versus no statins) in patients at low cardiovascular risk (observed 10-year risk of cardiovascular-related death or nonfatal myocardial infarction < 20%). A relative risk (RR) of less than 1.0 indicates fewer deaths with the use of statins. CI = confidence interval. *Lovastatin, fluvastatin, pravastatin and simvastatin. †Rosuvastatin and atorvastatin. For complete study names, see Box 1.
In the metaregression analyses, none of the characteristics listed in the methods section (including baseline cholesterol levels or change in lipid levels during the trials) significantly modified the association between statin use and all-cause mortality at the level of p < 0.05 (Appendix 2, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.101280/-/DC1).
Myocardial infarction
Thirteen trials (n = 48 023) reported the number of participants with fatal, nonfatal or unspecified myocardial infarctions. The pooled relative risk was significantly lower among statin recipients than among controls (RR 0.63, 95% CI 0.50–0.79; I2 = 13%].
From eight trials (n = 31 424) that provided data on fatal myocardial infarctions, we found that the pooled relative risk did not differ significantly between treatment groups (RR 0.96, 95% CI 0.5–1.85; I2 = 0%).
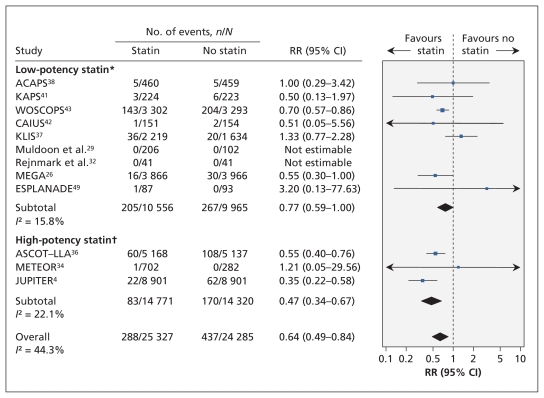
Ten trials (n = 49 222) reported nonfatal myocardial infarctions. The pooled relative risk was significantly lower among statin recipients than among controls (RR 0.64, 95% CI 0.49–0.84; I2 = 44%) (Figure 3). After removing studies that enrolled people with prior myocardial infarction, we found that the point estimate was similar to the estimate from the primary analysis (eight trials, n = 48 595; RR 0.64, 95% CI 0.48–0.86; moderate heterogeneity [I2 = 54%]).
Figure 3:
Risk of nonfatal myocardial infarction associated with the use of statins (versus no statins) in patients at low cardiovascular risk (observed 10-year risk of cardiovascular-related death or nonfatal myocardial infarction < 20%). A relative risk (RR) of less than 1.0 indicates fewer events with the use of statins. CI = confidence interval. *Lovastatin, fluvastatin, pravastatin and simvastatin. †Rosuvastatin and atorvastatin. For complete study names, see Box 1.
Stroke
Fourteen trials (n = 60 841) reported the number of participants with fatal, nonfatal or undefined strokes. The pooled relative risk of stroke was significantly lower among statin recipients than among controls (RR 0.83, 95% CI 0.74–0.93; I2 = 0%).
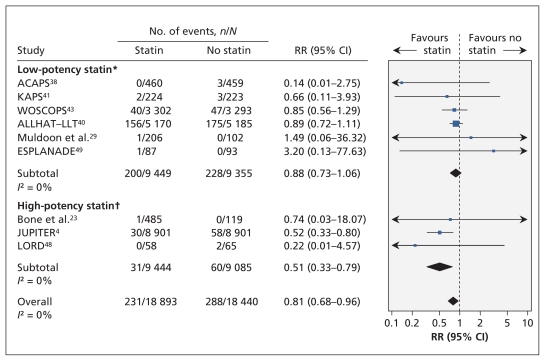
Five trials (n = 36 118) reported on the risk of fatal stroke; the pooled relative risk did not differ significantly between treatment groups (RR 0.91, 95% CI 0.65–1.29; I2 = 0%). Nine trials (n = 37 333) reported the number of participants with nonfatal stroke; the pooled relative risk was significantly lower among statin recipients (relative risk 0.81 [0.68–0.96]; I2 = 0%) (Figure 4).
Figure 4:
Risk of nonfatal stroke associated with the use of statins (versus no statins) in patients at low cardiovascular risk (observed 10-year risk of cardiovascular-related death or nonfatal myocardial infarction < 20%). A relative risk (RR) of less than 1.0 indicates fewer events with the use of statins. CI = confidence interval. *Lovastatin, fluvastatin, pravastatin and simvastatin. †Rosuvastatin and atorvastatin. For complete study names, see Box 1.
Coronary revascularization
Eight trials (n = 43 708) reported the number of participants who underwent percutaneous or surgical coronary revascularization. The incidence of revascularization was significantly lower among statin recipients than among controls (RR 0.66, 95% CI 0.57–0.77; I2 = 7%).
Other outcomes
Four trials (n = 35 017) reported on the risk of unstable angina: the pooled relative risk was significantly lower among statin recipients than among controls (RR 0.71, 95% CI 0.55–0.92; I2 = 0%). Three trials (n = 9082) reported on the risk of unspecified angina: the pooled relative risk did not differ significantly between treatment groups (RR 0.83, 95% CI 0.57–1.22; I2 = 0%). Thirteen trials reported on the proportion of participants who adhered to statin therapy (range 63%–98%). Length of hospital stay, persistence on statin therapy and quality-of-life measures were not reported in any of the studies.
Adverse events
Twenty-one trials (n = 47 589) reported the number of participants who had serious adverse events. The trial-level relative risk could not be estimated for four trials that reported no events in either group. From the remaining 17 trials (n = 47 021), the pooled risk of serious adverse events did not differ significantly between treatment groups (RR 1.01, 95% CI 0.96–1.07; I2 = 8%).
Ten trials (n = 45 557) reported the number of participants who experienced rhabdomyolysis. From the three trials in which this outcome occurred (n = 34 712), the pooled risk of rhabdomyolysis did not differ significantly between groups (RR 1.29, 95% CI 0.25–6.68; I2 = 0%).
Ten trials (n = 62 547) reported the number of participants in whom cancer was diagnosed, and four trials (n = 31 818) reported the proportion with new diabetes. The pooled risk of cancer did not differ significantly between groups (RR 1.00, 95% CI 0.93–1.08; I2 = 0%), nor did the pooled risk of diabetes (RR 1.05, 95% CI 0.84–1.32), although heterogeneity between studies in the latter analysis was large (I2 = 64%).
Subgroup and sensitivity analyses
Indirect comparisons between high- and low-potency statins did not show a significant difference in the risk of all-cause mortality (RR for high- v. low-potency statins: 0.94, 95% CI 0.79–1.13), fatal or nonfatal myocardial infarction (RR 0.69, 95% CI 0.43–1.12) or stroke (RR 0.79, 95% CI 0.61–1.02).
The sensitivity analyses, many of which used alternative definitions of low cardiovascular risk, showed findings that were consistent with the results of the primary analysis across a wide variety of assumptions and conditions (Appendices 3 and 4, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.101280/-/DC1). In particular, use of estimates rather than observed data to classify trials with respect to 10-year cardiovascular risk led to similar conclusions regarding the efficacy of statins in reducing all-cause mortality. In addition, when low cardiovascular risk was defined as a 10-year risk of cardiovascular-related death or nonfatal myocardial infarction of less than 10%, the pooled relative risk was similar to that of the primary analysis (RR 0.83, 95% CI 0.73–0.94). The pooled relative risk of all-cause mortality among statin recipients was statistically significant in virtually all of the sensitivity analyses (28 of 33). In addition, the point estimate for the pooled relative risk was relatively stable in these analyses (range 0.78–0.93, as compared with the point estimate of 0.90 in the primary analysis), except for one analysis that included trials with a follow-up period shorter than the median of two years (pooled RR 0.99).
Interpretation
We found that the use of statins by people at low cardiovascular risk reduced the relative risk of death from any cause by 10%. Treatment with statins also reduced the risk of stroke, myocardial infarction, unstable angina and coronary revascularization to an extent similar to that seen in trials involving patients with coronary artery disease.52
In a meta-analysis of the efficacy and safety of statins among patients with coronary artery disease, the number needed to treat was 86 to prevent a single death from any cause and 62 to prevent a single nonfatal myocardial infarction.52 The corresponding numbers needed to treat among people at low cardiovascular risk by our primary definition were 239 and 153, which reflect the generally low rates of vascular events in this population. Because statins might be used indefinitely to reduce cardiovascular risk, the absolute benefit may increase (accompanied by corresponding decreases in the number needed to treat) with longer durations of treatment, although this remains speculative.
Although we sought information from all trials on outcomes associated with serious harm from statins, information on serious adverse events was available for only 59% (47 589/ 80 711) of the trial participants. This low proportion is consistent with previously documented deficiencies in reporting harm among participants in randomized controlled trials.53,54 Our experience with soliciting this information from investigators revealed a lack of clarity among trial authors regarding how serious adverse events were reported in published reports. Because the risk of serious morbidity would be expected to decline among statin users, our finding of a lack of significant difference in the rate of serious adverse events between treatment groups and our nonsignificant estimate of elevated risk among statin users should be interpreted with caution. A complete analysis of published and unpublished data from individual patients on the effect of statins among low-risk patients would be a useful addition to the literature, but it would require cooperation among the various stakeholders.
Our metaregression and indirect comparisons did not show statistically significant differences in the efficacy of high- and low-potency statins. However, metaregression has relatively low statistical power. The as-yet unproven potential for high-potency statins to prevent cardiovascular outcomes more effectively must be balanced against their higher costs compared with low-potency statins. The recent availability of generic (lower cost) atorvastatin makes this issue of particular importance for decision-makers and third-party payers.
Unlike a recent systematic review that included statin trials involving both low- and high-risk people,55 we did not find a significantly increased risk of new diabetes among low-risk statin users, perhaps because of differences in study populations or statistical power. If such an effect exists, this would be expected to further reduce the absolute benefit of statin treatment in low-risk populations over the long term.
Our findings also differ from those of another recently published systematic review of 11 trials involving 65 229 people without cardiovascular disease that found no evidence of a benefit of statin use on all-cause mortality.56 The authors reported a relative risk reduction for all-cause mortality that was similar to ours (9% v. 10%), but their estimate was less precise, leading to a 95% CI that crossed unity (0.83–1.01). Eight trials in that review were included in our study.4,24,28,31,36,39,40,43 However, we included an additional 11 trials that reported on all-cause mortality, and we excluded 2 trials57,58 that exclusively enrolled participants with a diagnosis of diabetes at baseline. A third study included in the prior reviews was excluded from our study because the 10-year risk for cardiovascular-related death or nonfatal myocardial infarction was greater than 20%.59
Strengths and limitations
This is an up-to-date, comprehensive systematic review of the clinical implications of statin use among people at low cardiovascular risk. We included studies in which participants were considered to be at low risk according to an accepted definition — a 10-year incidence of less than 20% for cardiovascular-related death or nonfatal myocardial infarction.10 We excluded trials that predominantly enrolled people at higher cardiovascular risk, such as those with diabetes or prior cardiovascular events. Therefore, our relative risk estimates were unlikely to be influenced by higher-risk subgroups within the trial populations.
Our analysis has limitations. First, as with all meta-analyses, our conclusions are limited by the availability of individual trials. We cannot exclude the possibility of publication bias, although it seems unlikely that there are unidentified trials large enough to influence the direction or significance of our findings.
Second, although we used accepted techniques for metaregression in an attempt to identify factors associated with greater or lesser benefit from statin use among low-risk participants, statistical power for these analyses was relatively low given the number of available trials.
Third, the duration of all trials was relatively short given that people at low cardiovascular risk might require treatment with statins for decades.
Fourth, we used the number needed to treat to summarize the benefits of statins across different analyses. Whether the number needed to treat is appropriate for use in meta-analyses is controversial; however, we believe that the simplicity and transparency of this method outweigh its potential disadvantages.21,60
Fifth, although we reported a wide range of clinically relevant outcomes as specified in our protocol, the definition for some (e.g., unstable angina) will have varied across trials.
Sixth, in an attempt at unbiased identification of people at low cardiovascular risk, we based our inclusion criterion on the observed incidence of events among placebo recipients, anticipating that clinicians could apply our findings in practice by using their risk prediction instrument of choice to identify patients with an estimated 10-year risk of less than 20% for cardiovascular-related death or nonfatal myocardial infarction. Because risk prediction tools may overestimate true rates of events in contemporary practice, we repeated analyses after including only trials with an estimated 10-year risk of less than 20% based on mean participant characteristics and two widely used risk equations;11,12 we reached similar conclusions.
Finally, given that most of the trials included in our review were at moderate risk of bias, we cannot exclude the possibility that other factors besides statin use may have influenced the observed differences in health outcomes between treatment groups.
Conclusion
Both low- and high-potency statins were efficacious in preventing death and cardiovascular-related morbidity in people at low risk of cardiovascular events (whose 10-year risk of cardiovascular-related death or nonfatal myocardial infarction is less than 20%), most of whom did not have a history of coronary artery disease or diabetes. However, the number needed to treat to prevent one adverse outcome was relatively high for any statin. Whether high-potency statins improve outcomes to a greater extent than low-potency statins is uncertain based on current data.
Box 1: Full names of trials included in the meta-analysis.
ACAPS: Asymptomatic Carotid Artery Progression Study
AFCAPS/TexCAPS: Air Force/Texas Coronary Atherosclerosis Prevention Study
ALLHAT–LLT: Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial
ASCOT–LLA: Anglo-Scandinavian Cardiac Outcomes Trial – Lipid Lowering Arm
ASTRONOMER: Aortic Stenosis Progression Observation: Measuring the Effects of Rosuvastatin
CAIUS: Carotid Atherosclerosis Italian Ultrasound Study
ESPLANADE: European Study for Preventing by Lipid-lowering Agents aNd ACE-inhibition Dialysis Endpoints
EXCEL: Expanded Clinical Evaluation of Lovastatin Study
HYRIM: Hypertension High-Risk Management Trial
JUPITER: Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin
KAPS: Kuopio Atherosclerosis Prevention Study
KLIS: Kyushu Lipid Intervention Study
LEADe: Lipitor’s Effect in Alzheimer’s Dementia
LORD: Lipid Lowering and Onset of Renal Disease
MEGA: Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese
METEOR: Measuring Effects on Intima-Media Thickness: an Evaluation of Rosuvastatin
PHYLLIS: Plaque Hypertension Lipid-Lowering Italian Study
PREVEND-IT: Prevention of Renal and Vascular Endstage Disease Intervention Trial
UK-HARP-I: First United Kingdom Heart and Renal Protection
WOSCOPS: West of Scotland Coronary Prevention Study
Supplementary Material
Acknowledgements
The authors thank Dr. Steven Grover for his helpful comments on an earlier draft of this manuscript.
Marcello Tonelli, Finlay McAlister and Braden Manns are supported by Alberta Innovates — Health Solutions (formerly the Alberta Heritage Foundation for Medical Research (AHFMR) Health Scholar Awards. Brenda Hemmelgarn and Scott Klarenbach were supported by Population Health Investigator Awards from Alberta Innovates — Health Solutions. Marcello Tonelli was supported by a Government of Canada Research Chair in the optimal care of people with chronic kidney disease. Fiona Clement was supported by a postdoctoral fellowship award from the Canadian Health Services Research Foundation and AHFMR. Marcello Tonelli, Brenda Hemmelgarn, Scott Klarenbach, Finlay McAlister and Braden Manns were supported by an alternative funding plan from the Government of Alberta and the Universities of Alberta and Calgary.
See related research article by Conly and colleagues at www.cmaj.ca/lookup/doi/10.1503/cmaj.101281 and related commentary by Gupta at www.cmaj.ca/lookup/doi/10.1503/cmaj.111674
Footnotes
Competing interests: Marcello Tonelli has received research support from Pfizer; in 2011 he served on an advisory board for Merck, providing expert opinion on global chronic kidney disease and cardiovascular disease. Brenda Hemmelgarn has received research support from Merck and Amgen. Braden Manns has received research support from Merck Canada. No competing interests declared by the other authors.
This article has been peer reviewed.
Contributors: All the authors contributed to the conception and design. Marcello Tonelli, Fiona Clement, Braden Manns, Anita Lloyd and Jonathan Conly contributed to the acquisition of data and drafted the report. All of the authors contributed to the analysis and interpretation of data, critically revised the report for important intellectual content and approved the final version submitted for publication.
Funding: This study was jointly funded by the Canadian Agency for Drugs and Technology in Health and the Alberta Heritage Foundation for Medical Research Interdisciplinary Team Grants Program (which supports the Interdisciplinary Chronic Disease Collaboration). The study sponsors had no role in the design of the study, the collection, analysis or interpretation of data, the writing of the report or the decision to submit the article for publication.
References
- 1.Cholesterol Treatment Trialists’ (CTT) Collaborators, Kearney PM, Blackwell L, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008;371:117–25 [DOI] [PubMed] [Google Scholar]
- 2.National Cholesterol Education Program (NCEP) Expert Panel Detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). Bethesda (MD): National Institutes of Health; 2002 [PubMed] [Google Scholar]
- 3.Genest J, McPherson R, Frohlich J, et al. 2009 Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult — 2009 recommendations. Can J Cardiol 2009;25: 567–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ridker PM, Danielson E, Fonseca FA, et al. ; JUPITER Study Group Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195–207 [DOI] [PubMed] [Google Scholar]
- 5.Canadian Agency for Drugs and Technologies in Health Requirements for health technology assessment template for reports. Ottawa (ON): The Agency; 2008 [Google Scholar]
- 6.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward S, Lloyd Jones M, Pandor A, et al. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technol Assess 2007;11:1–160 [DOI] [PubMed] [Google Scholar]
- 8.Pletcher MJ, Lazar L, Bibbins-Domingo K, et al. Comparing impact and cost-effectiveness of primary prevention strategies for lipid-lowering. Ann Intern Med 2009;150:243–54 [DOI] [PubMed] [Google Scholar]
- 9.Pearson ES. The application of statistical methods to industrial standardization and quality control. London (UK): British Standards Institution; 1960 [Google Scholar]
- 10.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol 2004; 44:720–32 [DOI] [PubMed] [Google Scholar]
- 11.D’Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117:743–53 [DOI] [PubMed] [Google Scholar]
- 12.Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection. Evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001;285:2486–97 [DOI] [PubMed] [Google Scholar]
- 13.Juni P, Altman DG, Egger M. Assessing the quality of randomised controlled trials. In: Egger M, Davey Smith G, Altman DG, editors. Systematic reviews in health care. Vol 2 London (UK): BMJ Books; 2001. p. 87–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulz KF, Chalmers I, Hayes RJ, et al. Empirical evidence of bias. JAMA 1995;273:408–12 [DOI] [PubMed] [Google Scholar]
- 15.Jadad AR, Moore RA, Carrol D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials 1996;17:1–12 [DOI] [PubMed] [Google Scholar]
- 16.Cho MK, Bero LA. The quality of drug studies published in symposium proceedings. Ann Intern Med 1996;124:485–9 [DOI] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88 [DOI] [PubMed] [Google Scholar]
- 18.Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med 2002;21:1559–73 [DOI] [PubMed] [Google Scholar]
- 19.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schunemann HJ, Oxman AD, Vist GE, et al. Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Oxford (UK): The Cochrane Collaboration; 2011 [Google Scholar]
- 22.Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997;50:683–91 [DOI] [PubMed] [Google Scholar]
- 23.Bone HG, Kiel DP, Lindsay RS, et al. Effects of atorvastatin on bone in postmenopausal women with dyslipidemia: a double-blind, placebo-controlled, dose-ranging trial. J Clin Endocrinol Metab 2007;92:4671–7 [DOI] [PubMed] [Google Scholar]
- 24.Nakamura H, Arakawa K, Itakura H, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet 2006;368: 1155–63 [DOI] [PubMed] [Google Scholar]
- 25.Di Lullo L, Addesse R, Comegna C, et al. Effects of fluvastatin treatment on lipid profile, C-reactive protein trend, and renal function in dyslipidemic patients with chronic renal failure. Adv Ther 2005;22:601–12 [DOI] [PubMed] [Google Scholar]
- 26.Holmberg B, Brannstrom M, Bucht B, et al. Safety and efficacy of atorvastatin in patients with severe renal dysfunction. Scand J Urol Nephrol 2005;39:503–10 [DOI] [PubMed] [Google Scholar]
- 27.Baigent C, Landray M, Leaper C, et al. First United Kingdom Heart and Renal Protection (UK-HARP-I) study: biochemical efficacy and safety of simvastatin and safety of low-dose aspirin in chronic kidney disease. Am J Kidney Dis 2005;45:473–84 [DOI] [PubMed] [Google Scholar]
- 28.Anderssen SA, Hjelstuen AK, Hjermann I, et al. Fluvastatin and lifestyle modification for reduction of carotid intima-media thickness and left ventricular mass progression in drug-treated hypertensives [Hypertension High Risk Management trial (HYRIM)]. Atherosclerosis 2005;178:387–97 [DOI] [PubMed] [Google Scholar]
- 29.Muldoon MF, Ryan CM, Sereika SM, et al. Randomized trial of the effects of simvastatin on cognitive functioning in hypercholesterolemic adults. Am J Med 2004;117:823–9 [DOI] [PubMed] [Google Scholar]
- 30.Zanchetti A, Crepaldi G, Bond MG, et al. Different effects of antihypertensive regimens based on fosinopril or hydrochlorothiazide with or without lipid lowering by pravastatin on progression of asymptomatic carotid atherosclerosis: principal results of PHYLLIS — a randomized double-blind trial. Stroke 2004;35:2807–12 [DOI] [PubMed] [Google Scholar]
- 31.Asselbergs FW, Diercks GFH, Hillege HL, et al. ; Prevention of Renal and Vascular Endstage Disease Intervention Trial (PREVEND IT) Investigators Effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria. Circulation 2004;110:2809–16 [DOI] [PubMed] [Google Scholar]
- 32.Rejnmark L, Buus NH, Vestergaard P, et al. Effects of simvastatin on bone turnover and BMD: a 1-year randomized controlled trial in postmenopausal osteopenic women. J Bone Miner Res 2004;19:737–44 [DOI] [PubMed] [Google Scholar]
- 33.Bruckert E, Lievre M, Giral P, et al. Short-term efficacy and safety of extended-release fluvastatin in a large cohort of elderly patients. Am J Geriatr Cardiol 2003;12:225–31 [DOI] [PubMed] [Google Scholar]
- 34.Crouse JR, III, Raichlen JS, Riley WA, et al. Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR trial. JAMA 2007;297:1344–53 [DOI] [PubMed] [Google Scholar]
- 35.Dernellis J, Panaretou M. Effect of C-reactive protein reduction on paroxysmal atrial fibrillation. Am Heart J 2005;150:1064. [DOI] [PubMed] [Google Scholar]
- 36.Sever PS, Dahlof B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial – Lipid Lowering Arm (ASCOT–LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–58 [DOI] [PubMed] [Google Scholar]
- 37.Pravastatin use and risk of coronary events and cerebral infarction in japanese men with moderate hypercholesterolemia: the Kyushu Lipid Intervention Study [KLIS]. J Atheroscler Thromb 2000;7:110–21 [DOI] [PubMed] [Google Scholar]
- 38.Furberg CD, Adams HP, Jr, Applegate WB, et al. Effect of lovastatin on early carotid atherosclerosis and cardiovascular events. Asymptomatic Carotid Artery Progression Study (ACAPS) Research Group. Circulation 1994;90:1679–87 [DOI] [PubMed] [Google Scholar]
- 39.Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998;279:1615–22 [DOI] [PubMed] [Google Scholar]
- 40.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT–LLT). JAMA 2002;288:2998–3007 [DOI] [PubMed] [Google Scholar]
- 41.Salonen R, Nyyssonen K, Porkkala E, et al. Kuopio Atherosclerosis Prevention Study (KAPS) A population-based primary preventive trial of the effect of LDL lowering on atherosclerotic progression in carotid and femoral arteries. Circulation 1995;92:1758–64 [DOI] [PubMed] [Google Scholar]
- 42.Mercuri M, Bond MG, Sirtori CR, et al. Pravastatin reduces carotid intima-media thickness progression in an asymptomatic hypercholesterolemic mediterranean population: the Carotid Atherosclerosis Italian Ultrasound Study [CAIUS]. Am J Med 1996;101:627–34 [DOI] [PubMed] [Google Scholar]
- 43.Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med 1995; 333:1301–7 [DOI] [PubMed] [Google Scholar]
- 44.Bak AA, Huizer J, Leijten PA, et al. Diet and pravastatin in moderate hypercholesterolaemia: a randomized trial in 215 middle-aged men free from cardiovascular disease. J Intern Med 1998; 244:371–8 [DOI] [PubMed] [Google Scholar]
- 45.Bradford RH, Shear CL, Chremos AN, et al. Expanded Clinical Evaluation of Lovastatin (EXCEL) study results. I. Efficacy in modifying plasma lipoproteins and adverse event profile in 8245 patients with moderate hypercholesterolemia. Arch Intern Med 1991;151:43–9 [DOI] [PubMed] [Google Scholar]
- 46.Feldman HH, Doody RS, Kivipelto M, et al. Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology 2010;74:956–64 [DOI] [PubMed] [Google Scholar]
- 47.Chan KL, Teo K, Dumesnil JG, et al. Effect of lipid lowering with rosuvastatin on progression of aortic stenosis: results of the Aortic Stenosis Progression Observation: Measuring Effects of Rosuvastatin (ASTRONOMER) Trial. Circulation 2010;121:306–14 [DOI] [PubMed] [Google Scholar]
- 48.Fassett RG, Robertson IK, Ball MJ, et al. Effect of atorvastatin on kidney function in chronic kidney disease: a randomised double-blind placebo-controlled trial [LORD study]. Atherosclerosis 2010;213:218–24 [DOI] [PubMed] [Google Scholar]
- 49.Ruggenenti P, Perna A, Tonelli M, et al. Effects of add-on fluvastatin therapy in patients with chronic proteinuric nephropathy on dual renin–angiotensin system blockade: the ESPLANADE trial. Clin J Am Soc Nephrol 2010;5:1928–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yun KH, Shin IS, Park EM, et al. Effect of additional statin therapy on endothelial function and prognosis in patients with vasospastic angina. Korean Circ J 2008;38:638–43 [Google Scholar]
- 51.Higgins JPT, Deeks JJ, Altman DG. Special topics in statistics. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. 5.1.0 ed Oxford (UK): The Cochrane Collaboration; 2011 [Google Scholar]
- 52.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet 2005;366:1267–78 [DOI] [PubMed] [Google Scholar]
- 53.Chan AW, Hrobjartsson A, Haahr MT, et al. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA 2004;291:2457–65 [DOI] [PubMed] [Google Scholar]
- 54.Ioannidis JP, Lau J. Completeness of safety reporting in randomized trials: an evaluation of 7 medical areas. JAMA 2001;285:437–43 [DOI] [PubMed] [Google Scholar]
- 55.Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 2010;375:735–42 [DOI] [PubMed] [Google Scholar]
- 56.Ray KK, Seshasai SR, Erqou S, et al. Statins and all-cause mortality in high-risk primary prevention: a meta-analysis of 11 randomized controlled trials involving 65,229 participants. Arch Intern Med 2010;170:1024–31 [DOI] [PubMed] [Google Scholar]
- 57.Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004; 364:685–96 [DOI] [PubMed] [Google Scholar]
- 58.Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN). Diabetes Care 2006;29:1478–85 [DOI] [PubMed] [Google Scholar]
- 59.Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002;360:1623–30 [DOI] [PubMed] [Google Scholar]
- 60.McAlister FA. The “number needed to treat” turns 20 — and continues to be used and misused. CMAJ 2008;179:549–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.