Abstract
The intrauterine growth restricted (IUGR) fetus develops unique metabolic adaptations in response to exposure to reduced nutrient supply. These adaptations provide survival value for the fetus by enhancing the capacity of the fetus to take up and use nutrients, thereby reducing the need for nutrient supply. Each organ and tissue in the fetus adapts differently, with the brain showing the greatest capacity for maintaining nutrient supply and growth. Such adaptations, if persistent, also have the potential in later life to promote nutrient uptake and storage, which directly lead to complications of obesity, insulin resistance, reduced insulin production, and type 2 diabetes.
Keywords: Fetus, placenta, pregnancy, intrauterine growth restriction (IUGR), nutrition
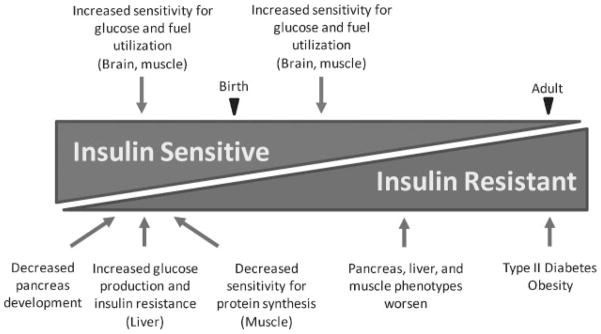
Intrauterine growth restriction (IUGR) is associated with increased risk for development of obesity, insulin resistance, diabetes, and reduced lean body mass in adulthood.1–4 The fetal adaptations to undernutrition that produce these long-term outcomes are not fully understood. Glucose is the major energy substrate for fetal oxidative metabolism.5,6 A variety of mechanisms relating to glucose and fuel metabolism, including increased peripheral insulin sensitivity for glucose utilization, decreased insulin sensitivity for protein synthesis in muscle, decreased pancreatic development, and increased hepatic glucose production, are fetal adaptations to IUGR. Each of these mechanisms has apparent survival value for the IUGR fetus by promoting energy uptake and utilization, reducing the demand for amino acids for growth, reducing anabolic hormone production, and increasing glucose production to maintain glucose supply to vital organs, primarily the brain and heart.7–11 These adaptations also result in asymmetrical growth restriction of the fetus, with greatest restriction of subcutaneous tissue and muscle, less of bone, and least of the brain. Collectively, these adaptations allow IUGR fetal organs and tissues to maintain their energy-dependent basal metabolic functions at the expense of body growth in response to reduced nutrient supply. If these adaptive mechanisms persist, or are more readily inducible later in life, they have the potential to promote energy uptake beyond metabolic capacity when energy supplies increase, producing obesity, insulin resistance, and type 2 diabetes.3 Data from ovine models using nutrient restriction or placental insufficiency2,12–14 and human epidemiological data1,15 indicate a prevailing model whereby IUGR has tissue-specific effects on insulin sensitivity that evolve as offspring mature (Figure 1). This review highlights fetal data from studies in animal models, particularly the sheep, that support this transition from insulin-sensitive to insulin-resistant conditions.
Figure 1.
Transition from insulin sensitive to insulin resistant in intrauterine growth-restricted offspring.
Because short stature and reduced lean mass tend to persist after birth in IUGR offspring,16–18 it appears that even excess protein later in life cannot compensate for the programmed reduction in muscle and bone growth. The same appears to be true for the brain. Even into adolescence, early life nutritional deficiencies are associated with smaller brain size, reduced neuronal development, and impairments in cognition, behavior, and motor capacity.19 These deficiencies occur regardless of energy or protein nutrition in childhood. Encouraging recent studies, however, indicate that providing more nutrition at sensitive periods of early development following fetal nutritional deprivation, or in place of continued poor nutrition in the preterm newborn infant, can improve growth and development, particularly of the central nervous system.20,21 IUGR infants who display catch-up growth by 3 years of age have neurodevelopmental outcomes at 8 years of age equivalent to those without IUGR.21 Additionally, nearly all preterm infants in the neonatal intensive care unit (NICU), whether they are growth restricted in utero or born with growth parameters that are appropriate for gestational age, become relatively growth restricted during their hospitalization. Many of the adverse outcomes are mimicked by postnatal undernutrition in infants born preterm. When nutritional supplies are maximized early in their postnatal NICU course, they have larger brains and improved cognitive performance.22 This is important justification, therefore, for better understanding the changes in fetal development in IUGR infants in response to placental insufficiency and nutritional deprivation. This knowledge will then provide better information about which nutrients might be provided to such infants and when to provide them, to promote their survival and/or prevent the later life complications of undernutrition before the adaptations become irreversible.
Several different models of IUGR in fetal sheep have allowed the comprehensive study of the IUGR fetal phenotype as a result of placental insufficiency, including in vivo metabolic changes. Some of these models include exposure of the pregnant mother to hyperthermic environmental conditions, overfeeding of adolescent ewes still in their growth phase, prepregnancy uterine carun-culectomy, and restriction of the normal increase in uterine blood flow in the third trimester of pregnancy.23–27 These models all produce placental insufficiency using very different mechanisms yet demonstrate common fetal metabolic adaptations to chronic reduced nutrient transport to the fetus. Fetal data from these various models of ovine placental insufficiency are similar to data obtained from IUGR human neonates within the first 48 hours of life. This makes each ovine model useful for studying fetal adaptations to placental insufficiency and nutrient restriction. In this review, we summarize animal model and human data that describe the fetal IUGR phenotype. We specifically focus on fetal glucose and amino acid utilization during IUGR, followed by a review of fetal organ-specific mechanisms that develop in response to chronic nutrient deprivation in utero.
INTRAUTERINE GROWTH RESTRICTION FETAL PHENOTYPE: GLUCOSE SENSITIVITY
Placental insufficiency and IUGR reduces placental glucose transport capacity. This is due to the placenta being simply smaller and having overall fewer glucose transporters, selective transporter insufficiency, or reduced transport surface area relative to the size of the placenta.28,29 Placental insufficiency ultimately results in fetal plasma hypoglycemia, an essential adaptation that promotes glucose transport across the placenta by increasing the transplacental plasma glucose concentration gradient.30,31 This adaptation occurs in response to experimentally reduced glucose supply alone and in IUGR and placental insufficiency.28,32
Expression of Glucose Transporters
IUGR fetal tissues respond in common ways, despite the different mechanisms resulting in placental insufficiency, with adaptations that tend to increase or maintain glucose uptake capacity and rates of glucose utilization. As shown specifically in both IUGR fetal sheep and those made chronically hypoglycemic (e.g., by infusion of insulin into the mother over many days to weeks), concentrations of glucose transporters tend to increase or at least stay at normal levels despite the prevailing low glucose and insulin concentrations.11,33 This adaptation includes both the ubiquitous GLUT1 transporter responsible for glucose uptake in nearly all cells and the insulin-regulated GLUT4 transporter that is increased in expression at cell membrane in response to insulin stimulation. GLUT1 expression is normal in the liver, skeletal muscle, and heart of IUGR fetal sheep, yet increased in the brain.11 GLUT4 concentrations are not different in the skeletal muscle of the IUGR fetus but are significantly increased in the plasma membrane fraction of the IUGR fetal sheep heart.34 Even in the brain, fetal hypoglycemia tends to increase GLUT1 and GLUT3, perhaps in that way helping to maintain rates of cerebral glucose uptake and utilization for energy and growth needs and contributing to the lesser reduction in brain size compared with the fetal body and other organs (liver, muscle, subcutaneous tissue), producing asymmetrical growth restriction.33 These changes in glucose transporters have the effect of maintaining fetal glucose metabolism despite low insulin concentrations.
Upregulation of the Proximal Insulin Signaling Pathway and Glucose Utilization
Completing the adaptation to low glucose and insulin are increases in other regulatory proteins in the glucose metabolic pathways. The expression of the insulin receptor is increased in IUGR skeletal muscle and tends to be increased at both the α- and β-mRNA transcript and alpha and beta chain protein level in the liver.9 Upregulation of the insulin receptor is expected, as it is a natural compensatory response to low levels of insulin in all insulin-responsive cells and organisms, including the IUGR fetus. There also is a decrease in the p85α regulatory subunit of phosphoinositide 3-kinase (PI3K) in IUGR fetal skeletal muscle;9 the p85α regulatory subunit normally is a negative regulator of PI3K and its activation of GLUT 4 translocation. These changes in the insulin receptor and p85α regulatory subunit, therefore, would promote GLUT4 translocation to the cell membrane. These data are consistent with increased peripheral insulin sensitivity to glucose utilization observed in the same IUGR fetal sheep model.11 Chronic glucose deficiency (alone or with IUGR) upregulates the capacity for maintaining glucose utilization rate (GUR) and insulin sensitivity,11,35 specifically GUR expressed per kilogram body weight is normal at less than normal glucose and insulin concentrations (Table 1). This adaptation represents an example of the “thrifty phenotype” that Hales and others ascribed to metabolic adaptation that can aid survival in the presence of nutrient deprivation.1
Table 1.
Glucose Utilization Rates in Control and Intrauterine Growth-Restricted Fetal Sheep in Relation to Fetal Arterial Plasma Glucose and Insulin Concentrations11
| Control | IUGR | |
|---|---|---|
| Glucose utilization rate (mg/kg/min) | 5 | 5 |
| Glucose (mg/dL) | 20 | 10 |
| Insulin (μU/mL) | 12 | 5 |
Impact on Glycogen Synthesis and Deposition
Another pathway for glucose utilization is storage as glycogen, a process stimulated by insulin. Glycogen synthase kinase, normally a negative regulator of glycogen synthase and thus glycogen synthesis, is reduced in liver and skeletal muscle in response to reduced glucose supply to the fetus.9 This finding, along with the coordinated changes in other insulin-signaling molecules discussed, allows increased insulin action to promote glucose uptake into cells and storage into glycogen. As a result, IUGR fetuses characteristically have increased muscle glycogen stores despite their reduced supply of glucose and low plasma glucose concentrations. Specifically, cardiac glycogen content is increased 70% and skeletal muscle glycogen is increased >100%.11,34 In the late gestation IUGR fetal sheep, however, hepatic glycogen content is maintained despite increased hepatic glucose production.9,11
INTRAUTERINE GROWTH RESTRICTION FETAL PHENOTYPE: AMINO ACID UTILIZATION
Intrauterine Growth Restriction Is Associated with Reduced Transplacental Transport and Fetal Uptake of Amino Acids
Studies in both humans and sheep have consistently found that the placental transport of amino acids is limited during IUGR pregnancies.8,36–39 The severity of IUGR correlates with reduced placental amino acid transport.40,41 Also depending on the degree of severity of IUGR, net fetal (umbilical) uptake rates of the branched chain amino acids and subsequent fetal plasma amino acid concentrations are either unchanged or decreased.8,9,36 Maintenance of normal fetal arterial amino acid concentrations likely represents a balance among increased amino acid release from protein breakdown, increased amino acid oxidation, and reduced amino acid utilization for protein synthesis, again ultimately depending on severity of IUGR, oxygen, and energy availability.
Impact on Amino Acid Metabolism and Protein Balance
Adaptations in protein balance have been found in the fetus that adjust amino acid utilization and metabolism in response to glucose and amino acid (energy) deprivation. Several studies of acutely induced energy deprivation to the fetus (<2 weeks) have shown that fetal protein breakdown increases and amino acid oxidation increases.42 Acute fasting in pregnant sheep that produces maternal and thus fetal hypoglycemia results in a near doubling of leucine oxidation as a fraction of leucine disposal.43 Leucine is an essential amino acid; thus increases in its oxidation prevent its net synthesis into protein and contribution to net protein balance. Two weeks of fetal hypoglycemia induced using a maternal insulin infusion resulted in increased protein breakdown in addition to increased rates of amino acid oxidation. Two weeks of fetal glucose deprivation also increased skeletal muscle expression of the ubiquitin ligases F-box-only protein 32 (FBXO32; also known as muscle atrophy F-box-1 or MAFbx-1) and ring finger protein 28 (RFP28; also known as muscle ring finger protein 1 or MuRF1), which in time return to control values once fetal glucose is replaced (recovery period).42 These data indicate that the increase in gene expression that would promote proteolysis during hypoglycemia might allow for greater amino acid release from fetal tissue for oxidation and energy production. In conditions of acute nutrient deprivation, protein and amino acids function in this way as a body store of energy, helping to maintain normal energy production rates and oxygen consumption.
Such strategies, however, have limited capacity for long-term survival. Instead, a more successful long-term strategy for adaptation to chronic hypoglycemia takes over, involving the normalization of amino acid oxidation rates combined with slowing of the rate of protein accretion (growth).39 This longer term strategy conserves energy that normally might be used to synthesize amino acids into proteins. When even longer periods of reduced glucose and insulin from sustained maternal hypoglycemia (8-week maternal insulin infusion) are induced, leucine oxidation as a fraction of leucine disposal rate returns to normal and the fetus slows its rate of growth by decreasing fetal protein accretion rates and increasing fetal protein breakdown.39 These adaptations allow survival of the fetus at the expense of growth when energy is reduced, producing a viable fetus at term gestation but one that is smaller.
INTRAUTERINE GROWTH RESTRICTION FETAL PHENOTYPE: REDUCED AMINO ACID UPTAKE, SYNTHESIS INTO PROTEIN, AND MUSCLE GROWTH AND DEVELOPMENT
Fetal Nutrient Supply and Lifelong Skeletal Muscle Growth
The human IUGR fetus and neonate are characterized by reduced lean mass.44–46 Small for gestational age infants demonstrate relatively decreased lean mass growth compared with increases in percentage body fat up to 2 years of age.47 Abnormal skeletal muscle development as a result of IUGR is important because muscle fiber number is fixed at birth. This limits postnatal muscle growth to increases in fiber size only, either by protein accretion or satellite cell fusion to existing fibers.48–50 When nutrient supply to the fetus is restricted, both cell division and protein accretion in skeletal muscle are downregulated.51,52 Sheep models of IUGR induced by placental insufficiency, uterine crowding, and maternal nutrient restriction resulted in fewer muscle fibers and fiber-type shifts in the fetus and newborn, implicating defects in primary and secondary myogenesis early in development.53–56 In fact, maternal nutrient restriction during early gestation affects fetal fiber number and type, whereas restriction during late gestation primary affects muscle fiber size.54 Thus timing of the insult during critical periods of fiber formation is important in determining the fetal response. Furthermore, relative decreases in type I slow fibers and increases in type IIb fast glycolytic fibers occurred as a result of maternal nutrient restriction in pregnant sheep, which might contribute to a more insulin-resistant phenotype in IUGR offspring.53,56 In both human and sheep studies, reduced lean mass persists beyond the fetal and neonatal period despite accelerated postnatal growth, indicating persistent impairments in the ability of skeletal muscle to hypertrophy postnatally.11,47,57,58 Adults who were born with low birthweight for gestational age have reduced muscle mass, reduced muscle-to-fat ratios, and reduced muscle strength.17,18,59–61 Given the immediate and persistent deficits in skeletal muscle growth as a result of IUGR, it is important to understand the mechanisms responsible for abnormal muscle growth when nutrient deficiencies produce IUGR.
Hormonal Regulation of Fetal Skeletal Muscle Development
IUGR in humans and sheep is characterized by decreased insulin and insulin-like growth factor (IGF)-1 concentrations,9,62,63 which might contribute to reduced lean mass. Both insulin and IGF-1 promote net protein accretion in the fetus.64–68 In fetal skeletal muscle specifically, IGF-1 is an anabolic growth factor that stimulates myoblast proliferation, differentiation, and myofiber hypertrophy both in vivo and in vitro.54,69Igf1 (−/−) and Igf receptor (−/−) mouse mutants have muscle hypoplasia from decreased myocyte number.70 In subconfluent myoblasts, IGF-1 acts initially to promote proliferation and inhibit differentiation.69,71 In differentiated myotubes that have exited the cell cycle, IGF-1 and insulin function as maintenance factors by promoting protein synthesis.62,72 Other hormones that play a key role in promoting skeletal muscle growth and development such as epidermal and fibroblast growth factors might also be affected by IUGR.73 A limited pool of myoblasts due to low circulating anabolic hormones and mature myofibers that develop resistance to hypertrophic growth could contribute to reduced fetal skeletal muscle mass in IUGR.
Evidence for Skeletal Muscle Resistance to Stimuli for Growth
Evidence indicates that normal and IUGR fetal skeletal muscle is relatively more resistant to increasing protein accretion in response to additional amino acid supply compared with postnatal muscle. In normally grown fetal sheep a 2-hour mixed amino acid infusion failed to activate signal transduction proteins that upregulate messenger RNA (mRNA) translation in skeletal muscle, independently of physiological increases in insulin.74 Because the fetus receives an uninterrupted supply of amino acids from the placenta resulting in fetal amino acid concentrations higher than those of the mother, additional amino acid supplementation might simply drive oxidative pathways without being used for protein synthesis and accretion.75
mTOR (mammalian target of rapamycin) is a key protein in the insulin signal transduction pathway that responds to upstream nutrient and hormonal signals to coordinate subsequent gene activation, protein synthesis, and cell growth.76,77 mTOR phosphorylates eukaryotic translation initiation factor 4E-binding protein 1 (4EBP1) and ribosomal S6 kinase (S6K), which increase mRNA translation.76,77 Interestingly, mTOR, 5′ adenosine monophosphate-activated protein kinase (AMPK), and AKT activation (AKT or protein kinase beta, PKB, a serine/threonine protein kinase that plays a key role in multiple cellular processes such as glucose metabolism, cell proliferation, apoptosis, transcription, and cell migration) are unchanged in IUGR skeletal muscle, indicating that mechanisms that directly maintain these pathways are intact in IUGR fetal tissues. The initiation phase of mRNA translation is a pivotal site of regulation for global rates of protein synthesis, as well as a site through which the synthesis of specific proteins is controlled. There is no change in elongation initiation factor 4 (eIF4) complex or 4EBP1 expression in IUGR fetal skeletal muscle, despite increased eIF4e and decreased 4EBP1 in IUGR liver, indicating that impaired mRNA translation in IUGR fetal tissues may result from tissue-specific mechanisms or that other sites of restriction of the insulin signal transduction pathway are involved. Skeletal muscle from midgestation nutrient-restricted fetal sheep also had no change in total levels of mTOR or AMPK protein, yet they had reduced phosphorylation of mTOR and the downstream target, ribosomal protein S6.55 Further studies clearly are needed to evaluate the effect of insulin and nutrient stimulation of these pathways.
Impact on Myogenesis
Expression of AKT2 is reduced in the IUGR fetal skeletal muscle, which is consistent with studies showing a distinct role of AKT2 on skeletal muscle differentiation and myogenesis.78–80 During differentiation in vitro and during regeneration in vivo, AKT2 expression and activity are markedly induced, indicating a possible role of AKT2 during muscle proliferation.79,81,82 Indeed in human skeletal muscle, insulin receptor substrate (IRS)-1 and AKT2 are required for myoblast differentiation and glucose metabolism, whereas IRS-2 and AKT1 were used for lipid metabolism.83 Similarly, in AKT2 knockout mice there is mild growth deficiency but also age-dependent loss of adipose tissue and severe diabetes, with neither AKT1 nor AKT3 knockout mice showing similar defects.84 These studies suggest that AKT2 down-regulation in fetal skeletal muscle in the IUGR model could be an important factor limiting skeletal muscle growth in utero.
INTRAUTERINE GROWTH RESTRICTION FETAL PHENOTYPE: REDUCED PANCREATIC B-CELL MASS, PROLIFERATION, AND INSULIN PRODUCTION
Because insulin is a central regulator of fetal growth, the fetal β-cell can be seen as functioning to match fetal nutrient supply with hormonal signals for growth. Decreased insulin secretion may therefore be viewed as an adaptive mechanism to limit fetal growth during placental insufficiency and restriction of nutrient supply to the fetus. Low insulin concentrations are widely reported in human IUGR, as is decreased glucose stimulated insulin secretion.85,86 The adaptations to IUGR that limit insulin secretion are not fully known, but a morphological study demonstrated smaller pancreatic islets and a decrease in β-cell mass following severe growth restriction.87 These findings contrast with a second human morphological study that did not show decreased β-cell mass; however, these subjects were not as severely growth restricted.88 The normal compensation to insulin resistance in adults is an increase in β-cell mass and insulin secretion.89,90 Any restriction of this compensation as an individual ages and develops obesity and insulin resistance will result in hyperglycemia and type 2 diabetes. This makes reduced β-cell mass and insulin secretion one of the most important morbidities of IUGR for long-term health. These basic findings in human IUGR, a progressive decrease in insulin secretion and β-cell mass, have been replicated in many animal models of IUGR. Similar to human IUGR, the more severe the experimental growth restriction, the more severe the β-cell phenotype.91
Severe experimental placental insufficiency–induced IUGR fetal sheep have both decreased baseline and glucose-stimulated fetal insulin arterial plasma concentrations.92 As early as day 90 of gestation (term is 148 days) in such fetuses, umbilical venous plasma insulin concentrations were found to be 43% of control values, although due to large variance the differences were not statistically significant.10 Later in gestation (133 days), baseline arterial plasma concentrations were 32% of control values and glucose-stimulated concentrations were 23% of control values. Pancreatic islets isolated from these fetal sheep were used to determine responsiveness to glucose in vitro. The fraction of insulin present that was released in response to glucose stimulation was actually increased, but the total amount of insulin present within the isolated pancreatic islet was reduced by >80%, whether expressed per islet or per islet DNA mass, indicating that reduced cell number was not the cause of reduced insulin content. Thus when insulin secretion is normalized to islet number, it is significantly decreased in these islets, as expected from the in vivo data.92 Further work has shown a specific reduction in the β-cell population of the pancreas of severely growth-restricted fetal sheep in late gestation,93 similar to human IUGR fetuses. Although total pancreas weight is reduced to the same extent as total fetal weight (60%) in these fetal sheep, the β-cell mass (percentage insulin-positive area of the pancreas determined by immunohistochemistry multiplied by the weight of the pancreas) is reduced further, by 76%, yet β-cell size is not different in the IUGR fetuses, indicating there are fewer β-cells in the IUGR pancreas. β-cell apoptotic rates are not different between placental insufficiency–induced IUGR fetuses and controls, but the IUGR β-cells were found to be progressing through the cell cycle at rates slower than control fetuses, and the proportion of β-cells actually undergoing mitosis was reduced by 72%. Lower rates of β-cell differentiation also might contribute to this reduction because β-cells in the extra-islet compartment, which might represent newly formed β-cells, are decreased in the IUGR pancreas; changes in islet genesis, however, also could also explain the slightly lower rates of differentiation in this population. Together, these results indicate that nutrient deficits caused by placental insufficiency decrease the population of pancreatic β-cells by lengthening cell cycle progression and slowing rates of mitosis;93 mechanisms for these changes in IUGR pancreatic islets and β-cells have not yet been determined. In addition both total pancreatic insulin content and expression are reduced in the placental insufficiency–induced IUGR fetus. Although asymmetrical fetal growth is apparent and demonstrates fetal adaptations to spare nutrients for critical organs, the β-cells appear to be targeted for greater reductions than other pancreatic cells types. This is true in both severe human and sheep IUGR.93 There also is a relative sparing of pancreatic alpha, delta, and pancreatic poly-peptide (PP) producing cells.87,93
Fetal hypoglycemia and hypoxemia are two consistent features of IUGR that likely are important for impaired β-cell function. Both conditions can independently lower insulin release. Chronic hypoglycemia without hypoxemia results in moderate reduction of both in vivo and in vitro nutrient-stimulated insulin secretion.7,94–96 However, when glucose is infused directly into experimentally growth-restricted fetuses and glucose concentrations are normalized for 2 weeks at the end of gestation, this normalization of glucose concentrations is not well tolerated and fails to increase insulin secretion or β-cell mass. Such results support the concept that hypoxemia also is responsible for decreased insulin secretion in IUGR, raising the question of how hypoxemia suppresses in vivo β-cell function. Certainly, in vitro low oxygen conditions inhibit isolated islet insulin release.97 In vivo, however, other mechanisms besides a direct effect of low oxygen concentrations might be important, such as the increased catecholamine response. Acute bouts of hypoxia (from hypoxemia, ischemia, or a combination) increase plasma norepinephrine concentrations in fetal sheep.98,99 In fetal sheep, as in many other species, catecholamines act on the β-cells predominantly via the α2-adrenergic receptors to suppress insulin release.100 This indicates that fetal hypoxemia acts in conjunction with catecholamines to chronically suppress β-cell function. The important role for catecholamines to suppress in vivo insulin secretion in the setting of IUGR has been experimentally confirmed.101
INTRAUTERINE GROWTH RESTRICTION FETAL PHENOTYPE: INCREASED HEPATIC GLUCOSE PRODUCTION
Late gestation IUGR fetal sheep show normal to increased whole body insulin sensitivity to peripheral glucose disposal,11,58 despite lower than normal glucose and insulin concentrations. By contrast, in the liver of IUGR and hypoglycemic fetal sheep, there is increased hepatic phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6PASE) expression and increased hepatic glucose production (HGP) compared with control fetuses.11,35,102–104 This is not enough, however, to meet the increased demand for glucose utilization in the fetus, and the fetus remains hypoglycemic. Upon delivery, postnatal IUGR animals undergo rapid “catch-up” growth and, at least for a short period of early life, continue to demonstrate increased whole-body insulin sensitivity.57,105 The early increases in PEPCK and HGP, however, contrast with increased peripheral insulin sensitivity in fetal and early postnatal life and indicate that chronically increased HGP in IUGR offspring may contribute to the development of total body insulin resistance later in postnatal animals (Figure 1). In support of this possibility, insulin mediated suppression of PEPCK and HGP is blunted in the liver of IUGR rats studied 20 weeks postnatally.106,107 Similarly, postnatal IUGR rodents have increased PEPCK and HGP.108,109 A block in proximal insulin signaling has been suggested as a possible mechanism in the IUGR rodent liver.106,107,110 Future studies addressing hepatic insulin sensitivity in the fetal and postnatal IUGR sheep are needed. Lastly, increasing human epidemiological data indicate the adults who were IUGR are at an increased risk for developing uncontrolled hepatic glucose production and diabetes.1,4 Collectively, these data indicate that the IUGR fetal liver may be developing insulin resistance in terms of glucose production that likely plays an important early role in leading to type 2 diabetes in IUGR offspring.
Induction of the Gluconeogenic Pathway in the Intrauterine Growth Restriction Liver
The molecular basis for chronic increased glucose production may be the induction of nuclear factors that increase gluconeogenic gene expression. PGC1α (peroxisome proliferator-activated receptor [PPAR]γ coactivator-1α), a major coactivator that encodes a transcriptional pathway for increased gluconeogenesis, is increased by threefold in the IUGR fetal liver.9 The IUGR liver also has increased expression of CREB (phosphorylated cyclic adenosine monophosphate [cAMP] response element-binding protein),9,11 which binds directly to the PEPCK promoter via the cAMP response element (CRE) binding site and can activate PGC1α expression. The expression of CCAAT/enhancer binding protein α (C/EBPα) and C/EBPβ, which also bind to the CRE, tend to be increased in IUGR fetal liver.9 This indicates that cAMP-dependent regulatory pathways may drive early gluconeogenic gene expression patterns in the PI-IUGR fetal liver. Indeed, the IUGR fetus has been reported to have increased circulating concentrations of glucagon and norepinephrine that activate cAMP-dependent signaling pathways.92 These results are similar to those in another fetal sheep model of short-term (10-day) hypoglycemia, in which the levels of PEPCK, phosphorylated CREB, and glucose production are increased during late gestation.104 These various observations implicate glucose deprivation as an important causative factor for IUGR-induced gluconeogenesis through a mechanism involving cAMP-driven signals, such as PGC1α, CREB, and C/EBP. Whether these are permanently modified as master regulatory factors in the postnatal period is the subject of ongoing investigation.
Role of Epigenetics in the Intrauterine Growth Restriction Liver Phenotype
The mechanisms responsible for chronic tissue-specific changes in metabolism and insulin sensitivity in IUGR may be a consequence of epigenetic changes resulting in altered gene transcription.2,12,111,112 DNA demethylation occurs slowly during replication, due to inhibition of maintenance methylases, specifically DNA methyltransferases (DNMTs), and is associated with actively transcribed genes. Chromatin remodeling occurs with changes in histone modifications.113 The PEPCK gene promoter undergoes changes in chromatin modification and demethylation associated with activation of gene expression during the transition from fetal to postnatal life.114–118 Recently, decreased PEPCK promoter methylation was found in the fetal liver of baboons from a maternal nutrient restriction model.119 Global DNA hypomethylation and increased histone acetylation have been found in postnatal rat IUGR livers.120–123 Future studies are needed to address how these events are modified in utero during IUGR and the role of epigenetic modifications in development of hepatic insulin resistance.
CONCLUSIONS
If fetal adaptations to IUGR (increased peripheral glucose and insulin sensitivity, decreased β-cell mass and insulin secretion, diminished skeletal muscle cell number and capacity for net protein synthesis, and increased glucose production) persist into postnatal life and into late childhood and adulthood, they could underlie the increased risk of IUGR infants for developing obesity and type 2 diabetes as adults. Increased insulin sensitivity might predispose the formerly IUGR infant to have abnormally increased rates of fatty acid deposition, leading to obesity and eventually insulin resistance. If a β-cell defect persists that limits the insulin response to peripheral insulin resistance, then type 2 diabetes would follow. If myocytes are diminished in number, this could reduce whole body insulin action. If increased glucose hepatic production capacity persists, this could augment hyperglycemia caused by the development of peripheral insulin resistance and pancreatic β-cell failure to produce insulin in response to the insulin resistance and relative hyperglycemia.
Such problems lead to two important areas for future research. One area is in the prenatal treatment of IUGR. Previous human studies using nutritional interventions have demonstrated variable results with potential fetal toxicity. However, mechanisms of fetal toxicity are unknown. Animal models will be particularly useful for determining such mechanisms and investigating safe as well as effective interventions for attempting to improve metabolic functions and growth among fetuses with established IUGR. In addition, future research is needed to determine how postnatal feeding practices should be modified in the previously IUGR infant during the period of increased insulin sensitivity with a goal of preventing future insulin resistance and obesity without sacrificing long-term neurodevelopmental outcomes. Improved understanding of the mechanisms that produce the IUGR fetal phenotype as well as those that underlie how such mechanisms and their resulting changes in metabolism are affected by reintroduction of nutrients and anabolic hormones should provide fertile ground for future research into improved outcome of IUGR fetuses.
References
- 1.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Simmons RA. Developmental origins of beta-cell failure in type 2 diabetes: the role of epigenetic mechanisms. Pediatr Res. 2007;61(5 Pt 2):64R–67R. doi: 10.1203/pdr.0b013e3180457623. [DOI] [PubMed] [Google Scholar]
- 3.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85(2):571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 4.Gluckman PD, Hanson MA, Buklijas T, Low FM, Beedle AS. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nat Rev Endocrinol. 2009;5(7):401–408. doi: 10.1038/nrendo.2009.102. [DOI] [PubMed] [Google Scholar]
- 5.Hay WW., Jr Recent observations on the regulation of fetal metabolism by glucose. J Physiol. 2006;572(Pt 1):17–24. doi: 10.1113/jphysiol.2006.105072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Battaglia FC, Meschia G. Principal substrates of fetal metabolism. Physiol Rev. 1978;58(2):499–527. doi: 10.1152/physrev.1978.58.2.499. [DOI] [PubMed] [Google Scholar]
- 7.Rozance PJ, Limesand SW, Hay WW., Jr Decreased nutrient-stimulated insulin secretion in chronically hypoglycemic late-gestation fetal sheep is due to an intrinsic islet defect. Am J Physiol Endocrinol Metab. 2006;291(2):E404–E411. doi: 10.1152/ajpendo.00643.2005. [DOI] [PubMed] [Google Scholar]
- 8.Ross JC, Fennessey PV, Wilkening RB, Battaglia FC, Meschia G. Placental transport and fetal utilization of leucine in a model of fetal growth retardation. Am J Physiol. 1996;270(3 Pt 1):E491–E503. doi: 10.1152/ajpendo.1996.270.3.E491. [DOI] [PubMed] [Google Scholar]
- 9.Thorn SR, Regnault TR, Brown LD, et al. Intrauterine growth restriction increases fetal hepatic gluconeogenic capacity and reduces messenger ribonucleic acid translation initiation and nutrient sensing in fetal liver and skeletal muscle. Endocrinology. 2009;150(7):3021–3030. doi: 10.1210/en.2008-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Vrijer B, Davidsen ML, Wilkening RB, Anthony RV, Regnault TR. Altered placental and fetal expression of IGFs and IGF-binding proteins associated with intrauterine growth restriction in fetal sheep during early and mid-pregnancy. Pediatr Res. 2006;60(5):507–512. doi: 10.1203/01.PDR.0000242364.78002.71. [DOI] [PubMed] [Google Scholar]
- 11.Limesand SW, Rozance PJ, Smith D, Hay WW., Jr Increased insulin sensitivity and maintenance of glucose utilization rates in fetal sheep with placental insufficiency and intrauterine growth restriction. Am J Physiol Endocrinol Metab. 2007;293(6):E1716–E1725. doi: 10.1152/ajpendo.00459.2007. [DOI] [PubMed] [Google Scholar]
- 12.Ozanne SE, Constância M. Mechanisms of disease: the developmental origins of disease and the role of the epigenotype. Nat Clin Pract Endocrinol Metab. 2007;3(7):539–546. doi: 10.1038/ncpendmet0531. [DOI] [PubMed] [Google Scholar]
- 13.Martin-Gronert MS, Ozanne SE. Experimental IUGR and later diabetes. J Intern Med. 2007;261(5):437–452. doi: 10.1111/j.1365-2796.2007.01800.x. [DOI] [PubMed] [Google Scholar]
- 14.Morrison JL, Duffield JA, Muhlhausler BS, Gentili S, McMillen IC. Fetal growth restriction, catch-up growth and the early origins of insulin resistance and visceral obesity. Pediatr Nephrol. 2010;25(4):669–677. doi: 10.1007/s00467-009-1407-3. [DOI] [PubMed] [Google Scholar]
- 15.Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301(6761):1111. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paz I, Seidman DS, Danon YL, Laor A, Stevenson DK, Gale R. Are children born small for gestational age at increased risk of short stature? Am J Dis Child. 1993;147(3):337–339. doi: 10.1001/archpedi.1993.02160270099030. [DOI] [PubMed] [Google Scholar]
- 17.Kensara OA, Wootton SA, Phillips DI, Patel M, Jackson AA, Elia M Hertfordshire Study Group. Fetal programming of body composition: relation between birth weight and body composition measured with dual-energy X-ray absorptiometry and anthropometric methods in older Englishmen. Am J Clin Nutr. 2005;82(5):980–987. doi: 10.1093/ajcn/82.5.980. [DOI] [PubMed] [Google Scholar]
- 18.Sayer AA, Syddall HE, Dennison EM, et al. Birth weight, weight at 1 y of age, and body composition in older men: findings from the Hertfordshire Cohort Study. Am J Clin Nutr. 2004;80(1):199–203. doi: 10.1093/ajcn/80.1.199. [DOI] [PubMed] [Google Scholar]
- 19.Smart JL. Critical periods in brain development. Ciba Found Symp. 1991;156:109–124. doi: 10.1002/9780470514047.ch8. discussion 124–108. [DOI] [PubMed] [Google Scholar]
- 20.Lucas A, Morley R, Cole TJ. Randomised trial of early diet in preterm babies and later intelligence quotient. BMJ. 1998;317(7171):1481–1487. doi: 10.1136/bmj.317.7171.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casey PH, Whiteside-Mansell L, Barrett K, Bradley RH, Gargus R. Impact of prenatal and/or postnatal growth problems in low birth weight preterm infants on school-age outcomes: an 8-year longitudinal evaluation. Pediatrics. 2006;118(3):1078–1086. doi: 10.1542/peds.2006-0361. [DOI] [PubMed] [Google Scholar]
- 22.Isaacs EB, Gadian DG, Sabatini S, et al. The effect of early human diet on caudate volumes and IQ. Pediatr Res. 2008;63(3):308–314. doi: 10.1203/PDR.0b013e318163a271. [DOI] [PubMed] [Google Scholar]
- 23.De Blasio MJ, Gatford KL, McMillen IC, Robinson JS, Owens JA. Placental restriction of fetal growth increases insulin action, growth, and adiposity in the young lamb. Endocrinology. 2007;148(3):1350–1358. doi: 10.1210/en.2006-0653. [DOI] [PubMed] [Google Scholar]
- 24.Wallace JM, Regnault TR, Limesand SW, Hay WW, Jr, Anthony RV. Investigating the causes of low birth weight in contrasting ovine paradigms. J Physiol. 2005;565(Pt 1):19–26. doi: 10.1113/jphysiol.2004.082032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallace JM, Bourke DA, Aitken RP, Milne JS, Hay WW., Jr Placental glucose transport in growth-restricted pregnancies induced by overnourishing adolescent sheep. J Physiol. 2003;547(Pt 1):85–94. doi: 10.1113/jphysiol.2002.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barry JS, Rozance PJ, Anthony RV. An animal model of placental insufficiency-induced intrauterine growth restriction. Semin Perinatol. 2008;32(3):225–230. doi: 10.1053/j.semperi.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Eremia SC, de Boo HA, Bloomfield FH, Oliver MH, Harding JE. Fetal and amniotic insulin-like growth factor-I supplements improve growth rate in intrauterine growth restriction fetal sheep. Endocrinology. 2007;148(6):2963–2972. doi: 10.1210/en.2006-1701. [DOI] [PubMed] [Google Scholar]
- 28.Thureen PJ, Trembler KA, Meschia G, Makowski EL, Wilkening RB. Placental glucose transport in heat-induced fetal growth retardation. Am J Physiol. 1992;263(3 Pt 2):R578–R585. doi: 10.1152/ajpregu.1992.263.3.R578. [DOI] [PubMed] [Google Scholar]
- 29.Bell AW, Wilkening RB, Meschia G. Some aspects of placental function in chronically heat-stressed ewes. J Dev Physiol. 1987;9(1):17–29. [PubMed] [Google Scholar]
- 30.Hay WW, Jr, Sparks JW, Wilkening RB, Battaglia FC, Meschia G. Fetal glucose uptake and utilization as functions of maternal glucose concentration. Am J Physiol. 1984;246(3 Pt 1):E237–E242. doi: 10.1152/ajpendo.1984.246.3.E237. [DOI] [PubMed] [Google Scholar]
- 31.Hay WW, Jr, Molina RA, DiGiacomo JE, Meschia G. Model of placental glucose consumption and glucose transfer. Am J Physiol. 1990;258(3 Pt 2):R569–R577. doi: 10.1152/ajpregu.1990.258.3.R569. [DOI] [PubMed] [Google Scholar]
- 32.Aldoretta PW, Carver TD, Hay WW., Jr Ovine uteroplacental glucose and oxygen metabolism in relation to chronic changes in maternal and fetal glucose concentrations. Placenta. 1994;15(7):753–764. doi: 10.1016/0143-4004(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 33.Das UG, Schroeder RE, Hay WW, Jr, Devaskar SU. Time-dependent and tissue-specific effects of circulating glucose on fetal ovine glucose transporters. Am J Physiol. 1999;276 (3 Pt 2):R809–R817. doi: 10.1152/ajpregu.1999.276.3.R809. [DOI] [PubMed] [Google Scholar]
- 34.Barry JS, Davidsen ML, Limesand SW, et al. Developmental changes in ovine myocardial glucose transporters and insulin signaling following hyperthermia-induced intra-uterine fetal growth restriction. Exp Biol Med (Maywood) 2006;231(5):566–575. doi: 10.1177/153537020623100511. [DOI] [PubMed] [Google Scholar]
- 35.DiGiacomo JE, Hay WW., Jr Fetal glucose metabolism and oxygen consumption during sustained hypoglycemia. Metabolism. 1990;39(2):193–202. doi: 10.1016/0026-0495(90)90075-n. [DOI] [PubMed] [Google Scholar]
- 36.Pardi G, Cetin I, Marconi AM, et al. Diagnostic value of blood sampling in fetuses with growth retardation. N Engl J Med. 1993;328(10):692–696. doi: 10.1056/NEJM199303113281004. [DOI] [PubMed] [Google Scholar]
- 37.Marconi AM, Paolini CL, Stramare L, et al. Steady state maternal-fetal leucine enrichments in normal and intrauterine growth-restricted pregnancies. Pediatr Res. 1999;46(1):114–119. doi: 10.1203/00006450-199907000-00019. [DOI] [PubMed] [Google Scholar]
- 38.Paolini CL, Marconi AM, Ronzoni S, et al. Placental transport of leucine, phenylalanine, glycine, and proline in intrauterine growth-restricted pregnancies. J Clin Endocrinol Metab. 2001;86(11):5427–5432. doi: 10.1210/jcem.86.11.8036. [DOI] [PubMed] [Google Scholar]
- 39.Carver TD, Quick AA, Teng CC, Pike AW, Fennessey PV, Hay WW., Jr Leucine metabolism in chronically hypoglycemic hypoinsulinemic growth-restricted fetal sheep. Am J Physiol. 1997;272(1 Pt 1):E107–E117. doi: 10.1152/ajpendo.1997.272.1.E107. [DOI] [PubMed] [Google Scholar]
- 40.Glazier JD, Cetin I, Perugino G, et al. Association between the activity of the system A amino acid transporter in the microvillous plasma membrane of the human placenta and severity of fetal compromise in intrauterine growth restriction. Pediatr Res. 1997;42(4):514–519. doi: 10.1203/00006450-199710000-00016. [DOI] [PubMed] [Google Scholar]
- 41.de Vrijer B, Regnault TR, Wilkening RB, Meschia G, Battaglia FC. Placental uptake and transport of ACP, a neutral nonmetabolizable amino acid, in an ovine model of fetal growth restriction. Am J Physiol Endocrinol Metab. 2004;287(6):E1114–E1124. doi: 10.1152/ajpendo.00259.2004. [DOI] [PubMed] [Google Scholar]
- 42.Limesand SW, Rozance PJ, Brown LD, Hay WW., Jr Effects of chronic hypoglycemia and euglycemic correction on lysine metabolism in fetal sheep. Am J Physiol Endocrinol Metab. 2009;296(4):E879–E887. doi: 10.1152/ajpendo.90832.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Veen LC, Teng C, Hay WW, Jr, Meschia G, Battaglia FC. Leucine disposal and oxidation rates in the fetal lamb. Metabolism. 1987;36(1):48–53. doi: 10.1016/0026-0495(87)90062-x. [DOI] [PubMed] [Google Scholar]
- 44.Padoan A, Rigano S, Ferrazzi E, Beaty BL, Battaglia FC, Galan HL. Differences in fat and lean mass proportions in normal and growth-restricted fetuses. Am J Obstet Gynecol. 2004;191(4):1459–1464. doi: 10.1016/j.ajog.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 45.Larciprete G, Valensise H, Di Pierro G, et al. Intrauterine growth restriction and fetal body composition. Ultrasound Obstet Gynecol. 2005;26(3):258–262. doi: 10.1002/uog.1980. [DOI] [PubMed] [Google Scholar]
- 46.Lapillonne A, Braillon P, Claris O, Chatelain PG, Delmas PD, Salle BL. Body composition in appropriate and in small for gestational age infants. Acta Paediatr. 1997;86(2):196–200. doi: 10.1111/j.1651-2227.1997.tb08868.x. [DOI] [PubMed] [Google Scholar]
- 47.Hediger ML, Overpeck MD, Kuczmarski RJ, McGlynn A, Maurer KR, Davis WW. Muscularity and fatness of infants and young children born small- or large-for-gestational-age. Pediatrics. 1998;102(5):E60. doi: 10.1542/peds.102.5.e60. [DOI] [PubMed] [Google Scholar]
- 48.Davis TA, Fiorotto ML. Regulation of muscle growth in neonates. Curr Opin Clin Nutr Metab Care. 2009;12(1):78–85. doi: 10.1097/MCO.0b013e32831cef9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ten Broek RW, Grefte S, Von den Hoff JW. Regulatory factors and cell populations involved in skeletal muscle regeneration. J Cell Physiol. 2010;224(1):7–16. doi: 10.1002/jcp.22127. [DOI] [PubMed] [Google Scholar]
- 50.Widdowson EM, Crabb DE, Milner RD. Cellular development of some human organs before birth. Arch Dis Child. 1972;47(254):652–655. doi: 10.1136/adc.47.254.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greenwood PL, Slepetis RM, Hermanson JW, Bell AW. Intrauterine growth retardation is associated with reduced cell cycle activity, but not myofibre number, in ovine fetal muscle. Reprod Fertil Dev. 1999;11(4–5):281–291. doi: 10.1071/rd99054. [DOI] [PubMed] [Google Scholar]
- 52.Greenwood PL, Hunt AS, Hermanson JW, Bell AW. Effects of birth weight and postnatal nutrition on neonatal sheep: II. Skeletal muscle growth and development. J Anim Sci. 2000;78(1):50–61. doi: 10.2527/2000.78150x. [DOI] [PubMed] [Google Scholar]
- 53.Costello PM, Rowlerson A, Astaman NA, et al. Peri-implantation and late gestation maternal undernutrition differentially affect fetal sheep skeletal muscle development. J Physiol. 2008;586(9):2371–2379. doi: 10.1113/jphysiol.2008.150987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fahey AJ, Brameld JM, Parr T, Buttery PJ. The effect of maternal undernutrition before muscle differentiation on the muscle fiber development of the newborn lamb. J Anim Sci. 2005;83(11):2564–2571. doi: 10.2527/2005.83112564x. [DOI] [PubMed] [Google Scholar]
- 55.Zhu MJ, Ford SP, Nathanielsz PW, Du M. Effect of maternal nutrient restriction in sheep on the development of fetal skeletal muscle. Biol Reprod. 2004;71(6):1968–1973. doi: 10.1095/biolreprod.104.034561. [DOI] [PubMed] [Google Scholar]
- 56.Zhu MJ, Ford SP, Means WJ, Hess BW, Nathanielsz PW, Du M. Maternal nutrient restriction affects properties of skeletal muscle in offspring. J Physiol. 2006;575(Pt 1):241–250. doi: 10.1113/jphysiol.2006.112110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Blasio MJ, Gatford KL, Robinson JS, Owens JA. Placental restriction of fetal growth reduces size at birth and alters postnatal growth, feeding activity, and adiposity in the young lamb. Am J Physiol Regul Integr Comp Physiol. 2007;292(2):R875–R886. doi: 10.1152/ajpregu.00430.2006. [DOI] [PubMed] [Google Scholar]
- 58.Wallace JM, Milne JS, Aitken RP, Hay WW., Jr Sensitivity to metabolic signals in late-gestation growth-restricted fetuses from rapidly growing adolescent sheep. Am J Physiol Endocrinol Metab. 2007;293(5):E1233–E1241. doi: 10.1152/ajpendo.00294.2007. [DOI] [PubMed] [Google Scholar]
- 59.Gale CR, Martyn CN, Kellingray S, Eastell R, Cooper C. Intrauterine programming of adult body composition. J Clin Endocrinol Metab. 2001;86(1):267–272. doi: 10.1210/jcem.86.1.7155. [DOI] [PubMed] [Google Scholar]
- 60.Inskip HM, Godfrey KM, Martin HJ, Simmonds SJ, Cooper C, Sayer AA Southampton Women’s Survey Study Group. Size at birth and its relation to muscle strength in young adult women. J Intern Med. 2007;262(3):368–374. doi: 10.1111/j.1365-2796.2007.01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ylihärsilä H, Kajantie E, Osmond C, Forsén T, Barker DJ, Eriksson JG. Birth size, adult body composition and muscle strength in later life. Int J Obes (Lond) 2007;31(9):1392–1399. doi: 10.1038/sj.ijo.0803612. [DOI] [PubMed] [Google Scholar]
- 62.Harper JM, Soar JB, Buttery PJ. Changes in protein metabolism of ovine primary muscle cultures on treatment with growth hormone, insulin, insulin-like growth factor I or epidermal growth factor. J Endocrinol. 1987;112(1):87–96. doi: 10.1677/joe.0.1120087. [DOI] [PubMed] [Google Scholar]
- 63.Economides DL, Nicolaides KH, Campbell S. Metabolic and endocrine findings in appropriate and small for gestational age fetuses. J Perinat Med. 1991;19(1–2):97–105. doi: 10.1515/jpme.1991.19.1-2.97. [DOI] [PubMed] [Google Scholar]
- 64.Boyle DW, Denne SC, Moorehead H, Lee WH, Bowsher RR, Liechty EA. Effect of rhIGF-I infusion on whole fetal and fetal skeletal muscle protein metabolism in sheep. Am J Physiol. 1998;275(6 Pt 1):E1082–E1091. doi: 10.1152/ajpendo.1998.275.6.E1082. [DOI] [PubMed] [Google Scholar]
- 65.Liechty EA, Boyle DW, Moorehead H, Lee WH, Yang XL, Denne SC. Glucose and amino acid kinetic response to graded infusion of rhIGF-I in the late gestation ovine fetus. Am J Physiol. 1999;277(3 Pt 1):E537–E543. doi: 10.1152/ajpendo.1999.277.3.E537. [DOI] [PubMed] [Google Scholar]
- 66.Milley JR. Effects of insulin on ovine fetal leucine kinetics and protein metabolism. J Clin Invest. 1994;93(4):1616–1624. doi: 10.1172/JCI117142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shen W, Wisniowski P, Ahmed L, Boyle DW, Denne SC, Liechty EA. Protein anabolic effects of insulin and IGF-I in the ovine fetus. Am J Physiol Endocrinol Metab. 2003;284(4):E748–E756. doi: 10.1152/ajpendo.00399.2002. [DOI] [PubMed] [Google Scholar]
- 68.Brown LD, Hay WW., Jr Effect of hyperinsulinemia on amino acid utilization and oxidation independent of glucose metabolism in the ovine fetus. Am J Physiol Endocrinol Metab. 2006;291(6):E1333–E1340. doi: 10.1152/ajpendo.00028.2006. [DOI] [PubMed] [Google Scholar]
- 69.Rosenthal SM, Cheng ZQ. Opposing early and late effects of insulin-like growth factor I on differentiation and the cell cycle regulatory retinoblastoma protein in skeletal myoblasts. Proc Natl Acad Sci U S A. 1995;92(22):10307–10311. doi: 10.1073/pnas.92.22.10307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75(1):59–72. [PubMed] [Google Scholar]
- 71.Molkentin JD, Olson EN. Defining the regulatory networks for muscle development. Curr Opin Genet Dev. 1996;6(4):445–453. doi: 10.1016/s0959-437x(96)80066-9. [DOI] [PubMed] [Google Scholar]
- 72.Barton ER. The ABCs of IGF-I isoforms: impact on muscle hypertrophy and implications for repair. Appl Physiol Nutr Metab. 2006;31(6):791–797. doi: 10.1139/h06-054. [DOI] [PubMed] [Google Scholar]
- 73.Karalaki M, Fili S, Philippou A, Koutsilieris M. Muscle regeneration: cellular and molecular events. In Vivo. 2009;23(5):779–796. [PubMed] [Google Scholar]
- 74.Brown LD, Rozance PJ, Barry JS, Friedman JE, Hay WW., Jr Insulin is required for amino acid stimulation of dual pathways for translational control in skeletal muscle in the late-gestation ovine fetus. Am J Physiol Endocrinol Metab. 2009;296(1):E56–E63. doi: 10.1152/ajpendo.90310.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lemons JA, Adcock EW, III, Jones MD, Jr, Naughton MA, Meschia G, Battaglia FC. Umbilical uptake of amino acids in the unstressed fetal lamb. J Clin Invest. 1976;58(6):1428–1434. doi: 10.1172/JCI108598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441(7092):424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 77.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124(3):471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 78.Calera MR, Pilch PF. Induction of Akt-2 correlates with differentiation in Sol8 muscle cells. Biochem Biophys Res Commun. 1998;251(3):835–841. doi: 10.1006/bbrc.1998.9566. [DOI] [PubMed] [Google Scholar]
- 79.Vandromme M, Rochat A, Meier R, et al. Protein kinase B beta/Akt2 plays a specific role in muscle differentiation. J Biol Chem. 2001;276(11):8173–8179. doi: 10.1074/jbc.M005587200. [DOI] [PubMed] [Google Scholar]
- 80.Sumitani S, Goya K, Testa JR, Kouhara H, Kasayama S. Akt1 and Akt2 differently regulate muscle creatine kinase and myogenin gene transcription in insulin-induced differentiation of C2C12 myoblasts. Endocrinology. 2002;143(3):820–828. doi: 10.1210/endo.143.3.8687. [DOI] [PubMed] [Google Scholar]
- 81.Tureckova J, Wilson EM, Cappalonga JL, Rotwein P. Insulin-like growth factor-mediated muscle differentiation: collaboration between phosphatidylinositol 3-kinase-Akt-signaling pathways and myogenin. J Biol Chem. 2001;276(42):39264–39270. doi: 10.1074/jbc.M104991200. [DOI] [PubMed] [Google Scholar]
- 82.Barton ER, Morris L, Musaro A, Rosenthal N, Sweeney HL. Muscle-specific expression of insulin-like growth factor I counters muscle decline in mdx mice. J Cell Biol. 2002;157(1):137–148. doi: 10.1083/jcb.200108071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bouzakri K, Karlsson HK, Vestergaard H, Madsbad S, Christiansen E, Zierath JR. IRS-1 serine phosphorylation and insulin resistance in skeletal muscle from pancreas transplant recipients. Diabetes. 2006;55(3):785–791. doi: 10.2337/diabetes.55.03.06.db05-0796. [DOI] [PubMed] [Google Scholar]
- 84.Yang ZZ, Tschopp O, Baudry A, Dümmler B, Hynx D, Hemmings BA. Physiological functions of protein kinase B/Akt. Biochem Soc Trans. 2004;32(Pt 2):350–354. doi: 10.1042/bst0320350. [DOI] [PubMed] [Google Scholar]
- 85.Nieto-Díaz A, Villar J, Matorras-Weinig R, Valenzuela-Ruìz P. Intrauterine growth retardation at term: association between anthropometric and endocrine parameters. Acta Obstet Gynecol Scand. 1996;75(2):127–131. doi: 10.3109/00016349609033303. [DOI] [PubMed] [Google Scholar]
- 86.Nicolini U, Hubinont C, Santolaya J, Fisk NM, Rodeck CH. Effects of fetal intravenous glucose challenge in normal and growth retarded fetuses. Horm Metab Res. 1990;22(8):426–430. doi: 10.1055/s-2007-1004939. [DOI] [PubMed] [Google Scholar]
- 87.Van Assche FA, De Prins F, Aerts L, Verjans M. The endocrine pancreas in small-for-dates infants. Br J Obstet Gynaecol. 1977;84(10):751–753. doi: 10.1111/j.1471-0528.1977.tb12486.x. [DOI] [PubMed] [Google Scholar]
- 88.Béringue F, Blondeau B, Castellotti MC, Bréant B, Czernichow P, Polak M. Endocrine pancreas development in growth-retarded human fetuses. Diabetes. 2002;51(2):385–391. doi: 10.2337/diabetes.51.2.385. [DOI] [PubMed] [Google Scholar]
- 89.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 90.Polonsky KS, Sturis J, Bell GI. Seminars in Medicine of the Beth Israel Hospital, Boston. Non-insulin-dependent diabetes mellitus—a genetically programmed failure of the beta cell to compensate for insulin resistance. N Engl J Med. 1996;334(12):777–783. doi: 10.1056/NEJM199603213341207. [DOI] [PubMed] [Google Scholar]
- 91.Green AS, Rozance PJ, Limesand SW. Consequences of a compromised intrauterine environment on islet function. J Endocrinol. 2010;205(3):211–224. doi: 10.1677/JOE-09-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Limesand SW, Rozance PJ, Zerbe GO, Hutton JC, Hay WW., Jr Attenuated insulin release and storage in fetal sheep pancreatic islets with intrauterine growth restriction. Endocrinology. 2006;147(3):1488–1497. doi: 10.1210/en.2005-0900. [DOI] [PubMed] [Google Scholar]
- 93.Limesand SW, Jensen J, Hutton JC, Hay WW., Jr Diminished beta-cell replication contributes to reduced beta-cell mass in fetal sheep with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol. 2005;288(5):R1297–R1305. doi: 10.1152/ajpregu.00494.2004. [DOI] [PubMed] [Google Scholar]
- 94.Carver TD, Anderson SM, Aldoretta PW, Hay WW., Jr Effect of low-level basal plus marked “pulsatile” hyperglycemia on insulin secretion in fetal sheep. Am J Physiol. 1996;271(5 Pt 1):E865–E871. doi: 10.1152/ajpendo.1996.271.5.E865. [DOI] [PubMed] [Google Scholar]
- 95.Rozance PJ, Limesand SW, Zerbe GO, Hay WW., Jr Chronic fetal hypoglycemia inhibits the later steps of stimulus-secretion coupling in pancreatic beta-cells. Am J Physiol Endocrinol Metab. 2007;292(5):E1256–E1264. doi: 10.1152/ajpendo.00265.2006. [DOI] [PubMed] [Google Scholar]
- 96.Limesand SW, Hay WW., Jr Adaptation of ovine fetal pancreatic insulin secretion to chronic hypoglycaemia and euglycaemic correction. J Physiol. 2003;547(Pt 1):95–105. doi: 10.1113/jphysiol.2002.026831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dionne KE, Colton CK, Yarmush ML. Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes. 1993;42(1):12–21. doi: 10.2337/diab.42.1.12. [DOI] [PubMed] [Google Scholar]
- 98.Milley JR. Ovine fetal metabolism during norepinephrine infusion. Am J Physiol. 1997;273(2 Pt 1):E336–E347. doi: 10.1152/ajpendo.1997.273.2.E336. [DOI] [PubMed] [Google Scholar]
- 99.Cheung CY. Fetal adrenal medulla catecholamine response to hypoxia—direct and neural components. Am J Physiol. 1990;258(6 Pt 2):R1340–R1346. doi: 10.1152/ajpregu.1990.258.6.R1340. [DOI] [PubMed] [Google Scholar]
- 100.Jackson BT, Piasecki GJ, Cohn HE, Cohen WR. Control of fetal insulin secretion. Am J Physiol Regul Integr Comp Physiol. 2000;279(6):R2179–R2188. doi: 10.1152/ajpregu.2000.279.6.R2179. [DOI] [PubMed] [Google Scholar]
- 101.Leos RA, Anderson MJ, Chen X, Pugmire J, Anderson KA, Limesand SW. Chronic exposure to elevated norepinephrine suppresses insulin secretion in fetal sheep with placental insufficiency and intrauterine growth restriction. Am J Physiol Endocrinol Metab. 2010;298(4):E770–E778. doi: 10.1152/ajpendo.00494.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gentili S, Morrison JL, McMillen IC. Intrauterine growth restriction and differential patterns of hepatic growth and expression of IGF1, PCK2, and HSDL1 mRNA in the sheep fetus in late gestation. Biol Reprod. 2009;80(6):1121–1127. doi: 10.1095/biolreprod.108.073569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Narkewicz MR, Carver TD, Hay WW., Jr Induction of cytosolic phosphoenolpyruvate carboxykinase in the ovine fetal liver by chronic fetal hypoglycemia and hypoinsulinemia. Pediatr Res. 1993;33(5):493–496. doi: 10.1203/00006450-199305000-00014. [DOI] [PubMed] [Google Scholar]
- 104.Rozance PJ, Limesand SW, Barry JS, et al. Chronic late-gestation hypoglycemia upregulates hepatic PEPCK associated with increased PGC1alpha mRNA and phosphorylated CREB in fetal sheep. Am J Physiol Endocrinol Metab. 2008;294(2):E365–E370. doi: 10.1152/ajpendo.00639.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ford SP, Hess BW, Schwope MM, et al. Maternal undernutrition during early to mid-gestation in the ewe results in altered growth, adiposity, and glucose tolerance in male offspring. J Anim Sci. 2007;85(5):1285–1294. doi: 10.2527/jas.2005-624. [DOI] [PubMed] [Google Scholar]
- 106.Vuguin P, Raab E, Liu B, Barzilai N, Simmons R. Hepatic insulin resistance precedes the development of diabetes in a model of intrauterine growth retardation. Diabetes. 2004;53(10):2617–2622. doi: 10.2337/diabetes.53.10.2617. [DOI] [PubMed] [Google Scholar]
- 107.Ozanne SE, Smith GD, Tikerpae J, Hales CN. Altered regulation of hepatic glucose output in the male offspring of protein-malnourished rat dams. Am J Physiol. 1996;270(4 Pt 1):E559–E564. doi: 10.1152/ajpendo.1996.270.4.E559. [DOI] [PubMed] [Google Scholar]
- 108.Lane RH, Flozak AS, Ogata ES, Bell GI, Simmons RA. Altered hepatic gene expression of enzymes involved in energy metabolism in the growth-retarded fetal rat. Pediatr Res. 1996;39(3):390–394. doi: 10.1203/00006450-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 109.Desai M, Byrne CD, Zhang J, Petry CJ, Lucas A, Hales CN. Programming of hepatic insulin-sensitive enzymes in offspring of rat dams fed a protein-restricted diet. Am J Physiol. 1997;272(5 Pt 1):G1083–G1090. doi: 10.1152/ajpgi.1997.272.5.G1083. [DOI] [PubMed] [Google Scholar]
- 110.Ozanne SE, Wang CL, Coleman N, Smith GD. Altered muscle insulin sensitivity in the male offspring of protein-malnourished rats. Am J Physiol. 1996;271(6 Pt 1):E1128–E1134. doi: 10.1152/ajpendo.1996.271.6.E1128. [DOI] [PubMed] [Google Scholar]
- 111.Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature. 2007;447(7143):433–440. doi: 10.1038/nature05919. [DOI] [PubMed] [Google Scholar]
- 112.Burdge GC, Hanson MA, Slater-Jefferies JL, Lillycrop KA. Epigenetic regulation of transcription: a mechanism for inducing variations in phenotype (fetal programming) by differences in nutrition during early life? Br J Nutr. 2007;97(6):1036–1046. doi: 10.1017/S0007114507682920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang T, Fu M, Pestell R, Sauve AA. SIRT1 and endocrine signaling. Trends Endocrinol Metab. 2006;17(5):186–191. doi: 10.1016/j.tem.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 114.Yang J, Reshef L, Cassuto H, Aleman G, Hanson RW. Aspects of the control of phosphoenolpyruvate carboxykinase gene transcription. J Biol Chem. 2009;284(40):27031–27035. doi: 10.1074/jbc.R109.040535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Benvenisty N, Mencher D, Meyuhas O, Razin A, Reshef L. Sequential changes in DNA methylation patterns of the rat phosphoenolpyruvate carboxykinase gene during development. Proc Natl Acad Sci U S A. 1985;82(2):267–271. doi: 10.1073/pnas.82.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Benvenisty N, Reshef L. Developmental acquisition of DNase I sensitivity of the phosphoenolpyruvate carboxykinase (GTP) gene in rat liver. Proc Natl Acad Sci U S A. 1987;84(5):1132–1136. doi: 10.1073/pnas.84.5.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Benvenisty N, Szyf M, Mencher D, Razin A, Reshef L. Tissue-specific hypomethylation and expression of rat phosphoenolpyruvate carboxykinase gene induced by in vivo treatment of fetuses and neonates with 5-azacytidine. Biochemistry. 1985;24(19):5015–5019. doi: 10.1021/bi00340a009. [DOI] [PubMed] [Google Scholar]
- 118.Trus M, Benvenisty N, Cohen H, Reshef L. Developmentally regulated interactions of liver nuclear factors with the rat phosphoenolpyruvate carboxykinase promoter. Mol Cell Biol. 1990;10(5):2418–2422. doi: 10.1128/mcb.10.5.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nijland MJ, Mitsuya K, Li C, et al. Epigenetic modification of fetal baboon hepatic phosphoenolpyruvate carboxykinase following exposure to moderately reduced nutrient availability. J Physiol. 2010;588(Pt 8):1349–1359. doi: 10.1113/jphysiol.2009.184168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.MacLennan NK, James SJ, Melnyk S, et al. Uteroplacental insufficiency alters DNA methylation, one-carbon metabolism, and histone acetylation in IUGR rats. Physiol Genomics. 2004;18(1):43–50. doi: 10.1152/physiolgenomics.00042.2004. [DOI] [PubMed] [Google Scholar]
- 121.Fu Q, McKnight RA, Yu X, Wang L, Callaway CW, Lane RH. Uteroplacental insufficiency induces site-specific changes in histone H3 covalent modifications and affects DNA-histone H3 positioning in day 0 IUGR rat liver. Physiol Genomics. 2004;20(1):108–116. doi: 10.1152/physiolgenomics.00175.2004. [DOI] [PubMed] [Google Scholar]
- 122.Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr. 2005;135(6):1382–1386. doi: 10.1093/jn/135.6.1382. [DOI] [PubMed] [Google Scholar]
- 123.Lillycrop KA, Slater-Jefferies JL, Hanson MA, Godfrey KM, Jackson AA, Burdge GC. Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications. Br J Nutr. 2007;97(6):1064–1073. doi: 10.1017/S000711450769196X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Owens JA, Gatford KL, De Blasio MJ, Edwards LJ, McMillen IC, Fowden AL. Restriction of placental growth in sheep impairs insulin secretion but not sensitivity before birth. J Physiol. 2007;584(Pt 3):935–949. doi: 10.1113/jphysiol.2007.142141. [DOI] [PMC free article] [PubMed] [Google Scholar]