Abstract
Background
S-nitrosothiols (SNO) release nitric oxide (NO) through interaction with ascorbic acid (AA). However, little is known about their combined effect in the vasculature. The aim of this study is to investigate the effect of AA on SNO-mediated NO release, proliferation, cell cycle progression, cell death and oxidative stress in vascular cells.
Methods
VSMC and adventitial fibroblasts (AF) harvested from the aortae of Sprague Dawley rats were treated with AA, ± S-nitrosoglutathione (GSNO), or ± diethylenetriamine NONOate (DETA/NO). NO release, proliferation, cell cycle progression, cell death, and oxidative stress were determined by the Greiss reaction, [3H]-thymidine incorporation, flow cytometry, trypan blue exclusion, and DCF staining, respectively.
Results
AA increased NO release from GSNO 3-fold (p<0.001). GSNO and DETA/NO significantly decreased proliferation, but AA abrogated this effect (p<0.05). Mirroring the proliferation data, changes in cell cycle progression induced by GSNO and DETA/NO were reversed by addition of AA. GSNO- and DETA/NO-mediated increases in oxidative stress were significantly decreased by addition of AA (p<0.001).
Conclusion
Despite causing increased NO release from GSNO, AA reduced the antiproliferative and cell cycle effects of GSNO and DETA/NO through modulation of oxidative stress.
Keywords: Atherosclerosis, peripheral arterial disease, vascular smooth muscle cell proliferation, ascorbic acid, reactive oxygen species, nitric oxide
INTRODUCTION
Atherosclerosis is prevalent in all developed nations and is the leading cause of death and disability in the United States.(1) Treatments for severe atherosclerosis include angioplasty, stenting, bypass grafting, and endarterectomy. However, these therapies are subject to high failure rates due to the formation of neointimal hyperplasia. It has been well established that nitric oxide (NO) inhibits the formation of neointimal hyperplasia.(2) Thus, the clinical application of NO to current therapies would greatly improve their durability and reduce the need for repeat interventions.
The two classes of compounds that release NO are diazeniumdiolates and S-nitrosothiols (SNO). Both groups of compounds readily release NO when in contact with physiologic solutions, but only SNO occur endogenously in the form of S-nitrosoalbumin, S-nitrosoglutathione (GSNO) and S-nitrosocysteine.(3) NO release is accelerated from SNO by light, copper (Cu), or ascorbic acid (AA).(3) In addition, after NO release, SNO have the potential to be regenerated endogenously, thereby representing a potentially unlimited source of NO. These advantages of SNO have led some investigators to explore these compounds as potential therapeutic targets.(4) Thus, in anticipation of bioengineering therapeutics that capitalize on this unlimited endogenous pool of NO from SNO, our group evaluated the effect of SNO with and without AA on vascular smooth muscle cells (VSMC) and adventitial fibroblasts (AF). We assessed these groups for changes in NO production, proliferation, cell cycle, cell death, and oxidative stress. Our hypothesis was that AA will increase NO production from GSNO, and that this increase in NO production will lead to heightened antiproliferative effects in VSMC and AF.
METHODS
Cell Culture
VSMC and AF were cultured and maintained as previously described.(5) Cells were plated in 12-well plates (4–5 × 104 cells/well), 48-plates (1.0–1.5 × 105 cells/well), 96 well plates (4–5 × 104 cells/well), or 10 cm dishes (8 × 105 cells/plate) for 24 hours, after which they were growth arrested for 24 hours. Cells were then treated with media containing ± AA (0.5–2.0 mM), ± GSNO (0.5 mM), or ± diethylenetriamine NONOate (DETA/NO) (0.5 mM) simultaneously for 20–24 hours.
Nitric Oxide Release
Media from the 48-well plates were collected to quantify NO release utilizing the Griess reaction as previous described.(5)
Cell Proliferation
Proliferation was assessed in 12-well plates using 3H-thymidine incorporation as previously described.(5)
Cell Cycle Analysis
Flow cytometry was performed on cells collected from 10 cm dishes as previously described.(5)
Cell Death
Cell death was determined using trypan blue exclusion.
Oxidative Stress
24 hours after treatment, 96-well plates were treated with 5-(and-6)chloromethyl-2’,7’dichlorodihydrofluorescein (DCF, 5–10μM) for 5 hours. Fluorescence was quantified with the MP-5 plate reader using excitation and emission wavelengths of 485 nm and 538 nm.
Statistical Analysis
Results are expressed as mean ± SEM. Differences between multiple groups were analyzed using one-way analysis of variance with the Student-Newman-Keuls post hoc test for all pairwise comparisons (SigmaStat Chicago, IL). Statistical significance was assumed when P < 0.05.
RESULTS
Addition of AA increases NO release in a dose-dependent fashion
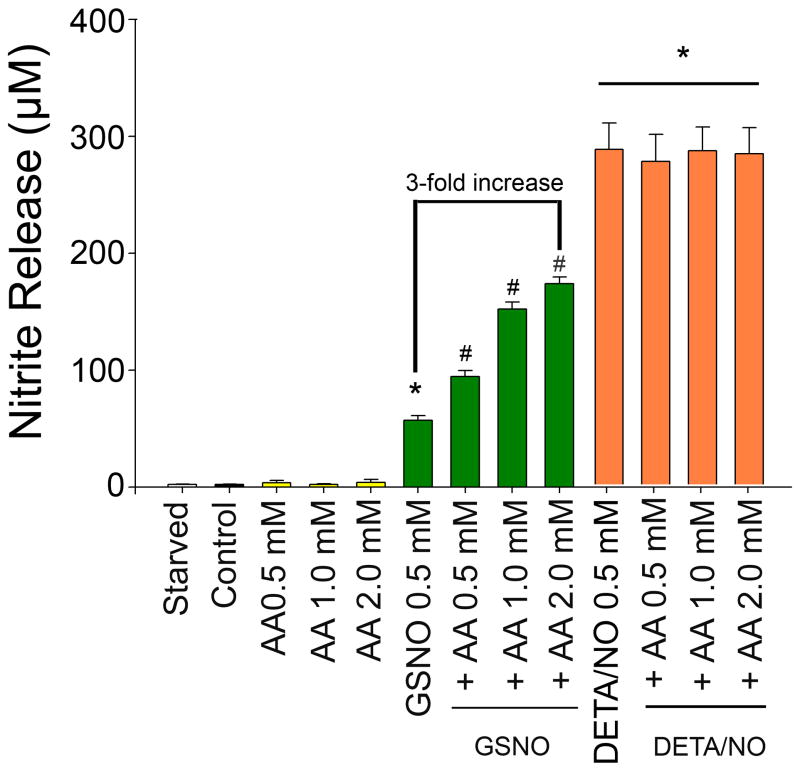
VSMC were exposed to media, AA, GSNO, DETA/NO, GSNO + AA, or DETA/NO + AA and nitrite release was assessed using the Griess reaction. While media and AA resulted in negligible levels of nitrite, GSNO (0.5 MM) and DETA/NO (0.25 mM) resulted in predictable increases in nitrite (Figure 1). The addition of AA to GSNO resulted in a further dose-dependent increase in nitrite, with 2.0 mM of AA increasing nitrite 3-fold over GSNO alone (57 μM to 173 μM, p<0.001). Conversely, the addition of AA to DETA/NO did not result in further increase of nitrite levels.
Figure 1.
Nitrite release as measured using the Griess reaction was assessed in vascular smooth muscle cells (VSMC) following exposure S-nitrosoglutathione (GSNO 0.5 mM), or ± DETA/NO (0.25 mM) ± ascorbic acid (AA) (*p< 0.001 versus control, **p< 0.001 versus Control and GSNO alone. Data are representative of 10 separate experiments. n=3/treatment group.
The antiproliferative effect of NO on VSMC and AF is reversed with the addition of AA
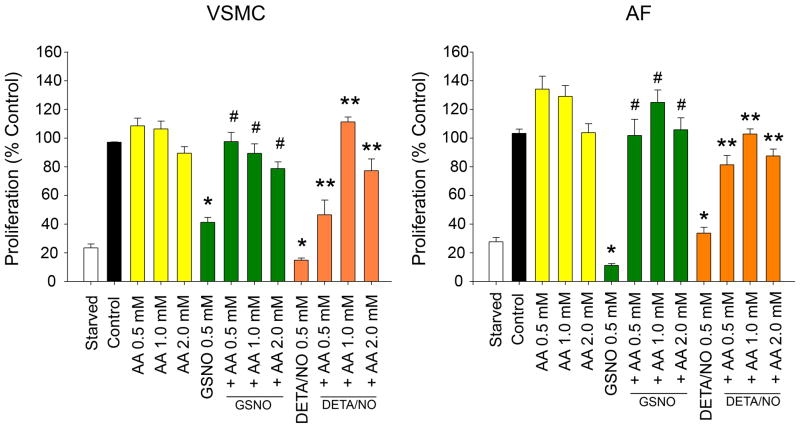
Exposure of VSMC and AF to AA alone resulted in a trend toward an increase in proliferation, however this did not reach statistical significance (Figure 2). Treatment of VSMC and AF with GSNO (0.5 mM) resulted in significant inhibition of both VSMC and AF proliferation (59% inhibition for VSMC, 89% inhibition of AF, p<0.001; Figure 2). Proliferation of both VSMC and AF reverted to control levels with the addition of all concentrations of AA to GSNO (p<0.001), despite the increase in NO release. Treatment of VSMC and AF with DETA/NO (0.5 mM) resulted in significant inhibition of both VSMC and AF proliferation (60% inhibition for VSMC, 65% inhibition of AF, p<0.001; Figure 2). Interestingly, proliferation of both VSMC and AF reverted to control levels with the addition of AA to DETA/NO (VSMC 1.0 mM AA, AF 2.0 mM AA p<0.001). There was no significant difference with respect to cell death (not shown).
Figure 2.
Proliferation of vascular smooth muscle cells (VSMC) and adventitial fibroblasts (AF) were assessed following exposure to ascorbic acid (AA, 0.5 – 2.0 mM), ± S-nitrosoglutathione (GSNO, 0.5 mM), or ± DETA/NO (0.5 mM). In both cell types, addition of GSNO or DETA/NO resulted in a decrease in proliferation (*p<0.01 versus control). In both cell types, addition of AA to GSNO or DETA/NO reversed this decrease in proliferation (##p<0.001 versus GSNO, **p<0.05 versus DETA/NO). Data are representative of 4 separate experiments. n=3/treatment group
AA reverses the effect of GSNO on cell cycle progression in VSMC
Treatment of VSMC with AA resulted in no significant change in the percentage of cells G0/G1, S, or G2/M from control (p=NS). Treatment of VSMC with GSNO (0.5 mM) resulted in an increase of cells in G0/G1 (64% to 74%, p<0.001), a decrease in G2M (11% to 6%, p=0.002), and no significant change in S (21% to 24%, p=NS) vs. control. Treatment of VSMC with both AA and GSNO (0.5 mM) resulted in a dose-dependent reversion of the cell cycle pattern seen with GSNO alone. The percentage of cells in G0/G1 decreased below control levels (65%, 60%, and 58% with AA 0.5, 1.0, and 2.0 mM, resp., p=0.013 vs. control). The percentage of cells in S increased above control levels (22%, 26%, and 33% with AA 0.5, 1.0, and 2.0 mM, resp., p=0.003 vs. control). The percentage of cells in G2M increased to control levels (8%, 10%, and 12%, p=NS vs. control). Of note, these data correlate with the proliferation data.
Addition of GSNO resulted in an increase in oxidative stress which was reversed with the addition of AA
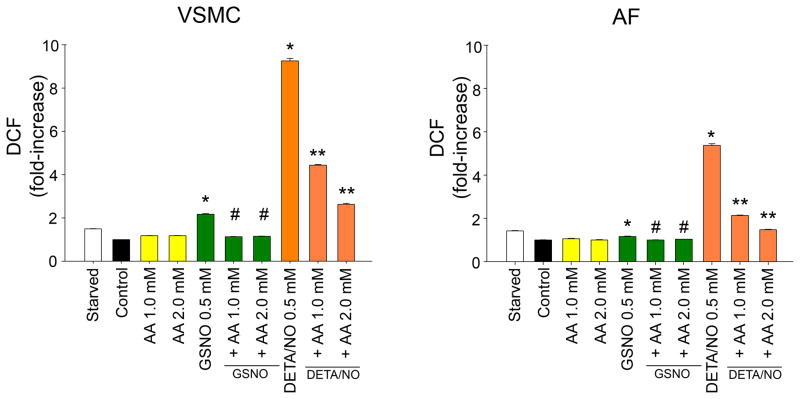
To determine how AA may reverse the antiproliferative effects of GSNO despite increased NO production, we evaluated the effect of AA and GSNO on reactive oxygen species production in VSMC and AF using DCF. Treatment VSMC and AF with AA alone resulted in no significant change in oxidative stress. Treatment of VSMC and AF with GSNO and DETA/NO significantly increased oxidative stress (2.2-fold for GSNO and 9.4-fold for DETA/NO; Figure 3, p<0.05) while the addition of AA to GSNO and DETA/NO in both cell types significantly reduced oxidative stress levels (1.1-fold for GSNO+AA [2.0 mM, p=0.001] and 2.6-fold for DETA/NO+AA [2.0 mM, p=0.001]). The addition of N-acetyl-L-cysteine (NAC) to GSNO also reduced oxidative stress (not shown). These data suggest that one mechanism that AA may be inhibiting the antiproliferative effects of NO on VSMC and AF despite increased NO release is by reducing oxidative stress.
Figure 3.
Quantification of oxidative stress with 5-(and-6)chloromethyl-2’,7’dichlorodihydrofluorescein (DCF) in vascular smooth muscle cells (VSMC) and adventitial fibroblasts (AF) following exposure to ascorbic acid (AA, 0.5 – 2.0 mM), ± S-nitrosoglutathione (GSNO, 0.5 mM), or ± DETA/NO (0.5 mM). In both cell types, addition of GSNO and DETA/NO increased oxidative stress (*p<0.05 versus controls). In both cell types, addition of AA to GSNO or DETA/NO decreased oxidative stress (#p<0.05 versus GSNO, **p<0.05 versus DETA/NO). Data are representative of 3 separate experiments. n=4/treatment group.
DISCUSSION
We found that the addition of AA increases NO release from GSNO but has a paradoxical effect on the antiproliferative and cell cycle effects of GSNO via modulation of oxidative stress. We also observed that VSMC exhibited a larger increase in oxidative stress as compared to AF with either NO donor suggesting cell specific differences. While we used AA, Bundy et al confirmed an increased release of NO from SNO using other antioxidants such as glutathione and (NAC).(6) Similarly, they also showed a paradoxical increase in proliferation with the addition of the antioxidants to GSNO despite confirmed increases in the levels of NO. This challenges the traditional view that proliferation in neointimal hyperplasia is driven in part by increases in ROS.
Several studies have confirmed that neointimal hyperplasia is accompanied with an increase in ROS due to increased NADPH oxidase activity.(7;8) Decreasing the levels of ROS using a NADPH oxidase inhibitor or various isoflavones has inhibited the formation of neointimal hyperplasia.(7;8) The paradox with our results can be partially explained by examining the determinants of the oxidative environment. The oxidative environment is a result of the balance between production of ROS and reactive nitrogen species (RNS) and their metabolism by both enzymatic and non-enzymatic pathways. Antioxidants such as AA and glutathione represent non-enzymatic paths for metabolism of these species. However, AA can also act as a pro-oxidant in the presence of metals such as copper. NO can increase oxidative stress by reacting with superoxide to form peroxynitrite or by inhibiting complex I and IV of the mitochondrial electron transport chain.(9) Conversely, NO can decrease superoxide production by inhibiting NADPH oxidase or lipid peroxidation.(10)(11) Despite mechanisms to both increase and decrease ROS, our data indicate that the net effect of NO is a significant increase in oxidative stress and that AA reduces this NO-mediated increase in oxidative stress possibly through its non-enzymatic pathways. However, these conclusions do not fully explain the paradox we, and others, observed in cellular proliferation.
Another explanation for the alteration of cellular proliferation is that it is not just the presence or absence of ROS but rather the amount. Low levels of ROS may stimulate while high levels inhibit proliferation. Another factor to consider is the type of ROS being produced. While NO increases oxidative stress, it does so though an increase in ROS and RNS. Excess RNS can alter protein functions through S-nitrosylation and/or nitration of regulatory proteins.(12) For example, with respect to the G0/G1 cell cycle arrest observed with the NO donor, AA could act to reverse the S-nitrosylation caused by NO on the cyclin dependent kinase enzymes, thereby releasing the inhibitory effects of NO and permitting cell cycle progression. A limitation of our study is that DCF is a nonspecific probe and does not allow identification of the reactive species involved. In the vasculature, NO may cause a relative increase of RNS compared to ROS, while AA may restore this ratio. This could explain the paradoxical effect of AA on the antiproliferative effects of NO.
In conclusion we have shown that AA increases NO release from GSNO. GSNO and DETA/NO decrease proliferation with a concurrent increase in oxidative stress. Addition of AA decreases oxidative stress with a paradoxical increase of proliferation, despite increased levels of NO. Further studies are needed to elucidate the mechanism by which AA effects NO in the vasculature so that AA-based vascular therapies can be developed that capitalize on the limitless endogenous supply of NO.
Acknowledgments
Funding Sources: This work was supported by funding from the National Institutes of Health (K08HL084203, MRK), (T32 HL094293-01, EKG), the Society for Vascular Surgery Foundation (MRK), and Northwestern Memorial Foundation Collaborative Development Initiative, Center for Limb Preservation, Bluhm Cardiovascular Institute
Footnotes
This work was presented at the Association of VA Surgeons 35th Annual Meeting in Irvine, California.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De SG, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010 Feb 23;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Ahanchi SS, Tsihlis ND, Kibbe MR. The role of nitric oxide in the pathophysiology of intimal hyperplasia. J Vasc Surg. 2007 Jun;45( Suppl A):A64–A73. doi: 10.1016/j.jvs.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 3.Hogg N. The biochemistry and physiology of S-nitrosothiols. Annu Rev Pharmacol Toxicol. 2002;42:585–600. doi: 10.1146/annurev.pharmtox.42.092501.104328. [DOI] [PubMed] [Google Scholar]
- 4.Hogg N. Biological chemistry and clinical potential of S-nitrosothiols. Free Radic Biol Med. 2000 May 15;28(10):1478–86. doi: 10.1016/s0891-5849(00)00248-3. [DOI] [PubMed] [Google Scholar]
- 5.Tsihlis ND, Murar J, Kapadia MR, Ahanchi SS, Oustwani CS, Saavedra JE, et al. Isopropylamine NONOate (IPA/NO) moderates neointimal hyperplasia following vascular injury. J Vasc Surg. 2010 May;51(5):1248–59. doi: 10.1016/j.jvs.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bundy RE, Marczin N, Chester AH, Yacoub M. A redox-based mechanism for nitric oxide-induced inhibition of DNA synthesis in human vascular smooth muscle cells. Br J Pharmacol. 2000 Apr;129(7):1513–21. doi: 10.1038/sj.bjp.0703240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dourron HM, Jacobson GM, Park JL, Liu J, Reddy DJ, Scheel ML, et al. Perivascular gene transfer of NADPH oxidase inhibitor suppresses angioplasty-induced neointimal proliferation of rat carotid artery. Am J Physiol Heart Circ Physiol. 2005 Feb;288(2):H946–H953. doi: 10.1152/ajpheart.00413.2004. [DOI] [PubMed] [Google Scholar]
- 8.Kanellakis P, Nestel P, Bobik A. Angioplasty-induced superoxide anions and neointimal hyperplasia in the rabbit carotid artery: suppression by the isoflavone trans-tetrahydrodaidzein. Atherosclerosis. 2004 Sep;176(1):63–72. doi: 10.1016/j.atherosclerosis.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Brune B, von Knethen A, Sandau KB. Nitric oxide and its role in apoptosis. Eur J Pharmacol. 1998 Jun 26;351(3):261–72. doi: 10.1016/s0014-2999(98)00274-x. [DOI] [PubMed] [Google Scholar]
- 10.Harrison CB, Drummond GR, Sobey CG, Selemidis S. Evidence that nitric oxide inhibits vascular inflammation and superoxide production via a p47phox-dependent mechanism in mice. Clin Exp Pharmacol Physiol. 2010 Apr;37(4):429–34. doi: 10.1111/j.1440-1681.2009.05317.x. [DOI] [PubMed] [Google Scholar]
- 11.Rubbo H, Radi R, Trujillo M, Telleri R, Kalyanaraman B, Barnes S, et al. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J Biol Chem. 1994 Oct 21;269(42):26066–75. [PubMed] [Google Scholar]
- 12.Stamler JS. Redox Signaling - Nitrosylation and Related Target Interactions of Nitric-Oxide. Cell. 1994 Sep 23;78(6):931–6. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]