Abstract
Estimates of dopamine D2/3 receptor occupancy by endogenous dopamine using positron emission tomography (PET) in animals have varied almost threefold. This variability may have been caused by incomplete depletion of dopamine or by the use of antagonist radioligands, which appear less sensitive than agonist radioligands to changes in endogenous dopamine. PET scans were performed in rats with the agonist PET radioligand [11C]MNPA ([O-methyl-11C]2-methoxy-N-propylnorapomorphine). [11C]MNPA was injected as a bolus plus constant infusion to achieve steady-state concentration in the body and equilibrium receptor binding in the brain. Radioligand binding was compared at baseline and after treatment with reserpine plus α-methyl-para-tyrosine, which cause ~95% depletion of endogenous dopamine. Depletion of dopamine increased radioligand binding in striatum but had little effect in cerebellum. Striatal [11C]MNPA binding potential was 0.93 ± 0.12 at baseline and increased to 1.99 ± 0.25 after dopamine depletion. Occupancy of D2/3 receptors by endogenous dopamine at baseline was calculated to be ~53%. Striatal binding was displaceable with raclopride, but not with BP 897 (a selective D3 compound), thus confirming the D2 receptor specificity of [11C]MNPA binding. Radioactivity extracted from rat brain contained only 8–10% radiometabolites and was insignificantly altered by administration of reserpine plus α-methyl-para-tyrosine. Hence, dopamine depletion did not increase the PET measurements via an effect on radiotracer metabolism. Our in vivo estimate of dopamine’s occupancy of D2/3 receptors at baseline is higher than that previously reported using antagonist radioligands and PET, but is similar to that reported using agonist radioligands and ex vivo measurements.
Keywords: dopamine depletion, reserpine, α-methyl-para-tyrosine, PET, D2/3 receptor, [11C]MNPA
INTRODUCTION
Occupancy of dopamine D2/3 receptors by endogenous dopamine under basal conditions have been estimated with PET and single photon emission computed tomography in human and monkey brain. Dopamine depletion induced by reserpine and/or α-methyl-para-tyrosine decreases competition by endogenous dopamine and increases radioligand binding to the D2/3 receptor (Abi-Dargham et al., 2000; Ginovart et al., 1997; Laruelle et al., 1997; Verhoeff et al., 2002). The increase in binding provides an estimate of the number of D2/3 receptors occupied by endogenous dopamine under basal conditions. Estimates of receptor occupancy by endogenous dopamine in humans and monkey have varied almost threefold between subjects, from 10 to 30% (Abi-Dargham et al., 2000; Ginovart et al., 1997; Laruelle et al., 1997; Verhoeff et al., 2002). The variability in these estimates could have been caused by incomplete depletion of dopamine or by the use of both antagonist and agonist radioligands.
In this study, we used a combination of α-methyl-para-tyrosine and reserpine in rats to achieve uniform and near maximal depletion of endogenous dopamine. The dopamine depleting agents α-methyl-para-tyrosine and reserpine are typically administered at low doses to humans and monkeys because of side effects. For example, α-methyl-para-tyrosine, a reversible inhibitor of tyrosine hydroxylase (Bennett and Sundberg, 1981; Mignot and Laude, 1985), can crystallize in urine and thereby damage the kidney (Engelman et al., 1968). Reserpine irreversibly inhibits vesicular uptake of catecholamines and thereby depletes endogenous dopamine (Guldberg and Broch, 1971; Ponzio et al., 1984). This irreversible inhibitor causes prolonged side effects, such as hypotension and sedation, which restrict the use of high doses in human subjects. Because of these generally unacceptable side effects for primates, we used rodents in the present study to obtain a maximal and fairly uniform depletion of dopamine. Specifically, we used both reserpine (5 mg/kg, i.p.) and α-methyl-para-tyrosine (400 mg/kg, i.p.) together to reduce dopamine in rat striatum by 95% (Guo et al., 2003).
Previous dopamine depletion imaging studies in healthy subjects have been performed using antagonist radioligands such as [123I]IBZM (iodobenzamide), [11C]raclopride, or [11C]NMSP (N-methylspiperone), all of which bind with equal affinity to both the high- and low-affinity state of the D2/3 receptor. In contrast, agonist radioligands bind preferentially to the high-affinity state, which is representative of functional coupling of the receptor to G proteins in membranes (George et al., 1985; Sibley et al., 1982). Recent PET imaging studies have demonstrated that the dopamine agonist radioligands are more sensitive to competition from endogenous dopamine (Ginovart et al., 2006; Narendran et al., 2004; Seneca et al., 2006; Willeit et al., 2008), presumably due to their preferential binding to the high-affinity state. Furthermore, a shift in the proportion of high-affinity state D2/3 receptors has been correlated with the pathophysiology of psychosis-related disorders (Seeman et al., 2006), and rodent models of human neuropsychiatric disorders tend to have an increased density of the D2/3 high-affinity state (Seeman et al., 2005; Sumiyoshi et al., 2005). Thus, agonist radioligands may provide more useful information on the high-affinity state of the D2/3 receptor than antagonist radioligands, which measure all receptors, in both high- and low-affinity states.
The aim of this study was to estimate the occupancy of D2/3 receptors by endogenous dopamine in rat brain in vivo using PET and the agonist radioligand [11C]MNPA. In addition, ex vivo radiometabolite studies were performed to determine if the increase in radioligand binding after dopamine depletion was due to a change in radiometabolites entering the brain.
MATERIALS AND METHODS
Radioligand preparation
[11C]MNPA was prepared by a two step (Gao et al., 1990) labeling method (Finnema et al., 2007), which entails 11C-methylation of the precursor (R)-2-hydroxy-10,11-acetonide-NPA. The specific activity of [11C]MNPA at the time of injection was 116 ± 21 GBq/μmol (n = 16 syntheses). The radiochemical purity was 97%.
PET studies
A total of 16 PET scans were performed with [11C]MNPA in male Sprague–Dawley rats (375 ± 94 g, n = 16) (Taconic Farm, Germantown, NY). [11C]MNPA was administered through a catheter in the penile vein (Intramedic PE-10 catheter; Aster Industries, Harmony, PA), as a bolus (24 ± 4 MBq) followed by constant infusion (47 ± 5 MBq/h) using a syringe pump (Harvard PhD 2000, Harvard Apparatus, Holliston, MA). To determine the effect of endogenous dopamine, the binding of [11C]MNPA was compared at baseline (n = 5 rats) and after depletion of dopamine (n = 5 rats). Dopamine was depleted by administration of reserpine and α-methyl-para-tyrosine. Reserpine (5 mg/kg, i.p.) was given 24 h before radioligand injection; α-methyl-para-tyrosine (200 mg/kg, i.p.) was administered twice, at 4 and 1 h before radioligand injection. The specificity of binding was investigated by displacement studies. Raclopride (2 mg/kg, i.v.) was injected during steady-state receptor binding (n = 3 rats). BP897 (0.25 and 0.5 mg/kg, i.v.) was injected during steady-state receptor binding (n = 3 rats). BP 897 has high affinity in vitro at the D3 receptor (Ki = 0.9 nM) and a 70 times lower affinity at the D2 receptor (Ki = 61 nM) (Pilla et al., 1999). In vivo binding experiments in mouse striatum indicate that selective D3 receptor occupancy is at doses below an ED50 of 0.5 mg/kg (Pilla et al., 1999; Sokoloff et al., 2006). Previous PET imaging studies in baboon have used BP 897 at a dose of 0.25 mg/kg (i.v.) to determine the in vivo specificity of D2 vs. D3 receptor binding of [11C]raclopride and [11C]PHNO (Narendran et al., 2006).
For all studies, anesthesia was induced with 5% isoflurane and was maintained with 1–1.5% isoflurane via a noise cone throughout the scan. Body temperature was monitored with a rectal thermometer and maintained at 36.5–37.5°C with a heating lamp and heating pad.
Rats were imaged with the Advanced Technology Laboratory Animal Scanner, which has a reconstructed resolution of 1.6 mm full-width at half maximum (Johnson et al., 2002; Liow et al., 2003). Emission data were collected continuously for 90 min, with scans of increasing duration from 20 s to 20 min. No correction for attenuation or scatter was applied.
Image analysis
The density of available D2/3 receptors was quantified with two reference tissue models, kinetic and equilibrium. In both methods, the outcome measure was binding potential expressed relative to nondisplaceable uptake, BPND, which is the ratio at equilibrium of specific to nondisplaceable uptake (Innis et al., 2007). For [11C]MNPA, BPND was operationally defined as the ratio at equilibrium of (striatum − cerebellum)/cerebellum. For the kinetic method, we used the two-parameter multilinear reference tissue model (Ichise et al., 2003), which fits the entire time–activity curves of striatum and cerebellum from time 0 to the end of the scan (90 min). For the equilibrium method, we calculated the average radioactivity in striatum and cerebellum from 45 to 90 min, when relatively stable concentrations of radioactivity were achieved with the constant infusion. Regional time–activity curves were normalized to injected activity as follows: (% injected activity per hour) per cm3 brain.
For both reference tissue models, the regions of interest (i.e., striatum and cerebellum) were identified from images created by the two-parameter multilinear reference tissue model. This model generates two parametric images from each scanning session: one shows BPND and the other shows blood flow relative to cerebellum. Left and right striatum (total striatum 50 mm3) were identified on the image of BPND, and cerebellum (35 mm3) was identified on the image of relative blood flow.
We assumed that the combination of α-methyl-para-tyrosine plus reserpine caused complete depletion of dopamine—i.e., no endogenous dopamine remained to compete with binding of radioligand. In fact, this dose and regimen of α-methyl-para-tyrosine plus reserpine has been reported to cause ~95% depletion of tissue concentrations of dopamine (Guo et al., 2003). The increase in BPND after dopamine depletion is an indirect measurement of endogenous dopamine occupancy of D2/3 receptors. Similar to other investigators, we estimated the occupancy of endogenous dopamine by the following equation: (depleted BPND − baseline BPND)/depleted BPND (Ginovart et al., 1997; Laruelle et al., 1997). Baseline and dopamine depleted states were compared with a two-tailed t test, with a minimum level of significance designated as P < 0.05.
Group data are expressed as mean ± SD.
Ex vivo experiments
Ex vivo studies were performed in rats during baseline (316 ± 54 g, n = 4) and after dopamine depletion (312 ± 21 g, n = 4), using the same anesthesia conditions as described above. Rats were injected i.v. with [11C]MNPA (41.8 ± 4.1 MBq, injected mass of 1.1 ± 0.3 nmol/kg, n = 8). At 30 min after injection, blood samples (~2 ml) were removed by cardiac puncture, and the rats were euthanized by decapitation. Whole brains were removed, weighed, and placed on ice until high-performance liquid chromatography (HPLC) analysis. Plasma and brain samples were prepared for analysis as previously described (Zoghbi et al., 2006). In brief, plasma samples (450 μl) were isolated from whole blood by centrifugation at 1800g for 1.5 min and added to MeCN (700 μl) containing 5 μg carrier (i.e., nonradioactive) MNPA. The whole brain was placed in MeCN (1–2 ml) with 50 μg carrier MNPA, and total activity was measured in a γ-counter. Brain tissues were homogenized with a Tissue Tearor (model 985–370, BioSpec Products, Bartlesville, OK) and centrifuged at 10,000g for 1 min. The supernatant was used for radio-chromatography, and the precipitate was used to calculate percent recovery. The supernatants were injected on an HLPC C18 Xterra column (Waters Corporation, Milford, MA; 3.9 × 300 mm2) using a mobile phase composed of MeOH/10 mM H3PO4 (50/50, v/v) at a flow rate of 1.0 ml/min. The concentration of radioactivity in brain and plasma was expressed as percent standardized uptake value (%SUV), calculated as: [(activity per gram of tissue)/injected activity] × gram of body weight.
To distinguish between the metabolite stability of [11C]MNPA in whole blood and brain tissue, we measured its stability in perfused brain homogenates. Whole blood sample was drawn by cardiac puncture and the rat (384 g) was then perfused with 0.9% NaCl (30 ml). The blood and excised perfused brain were immediately placed on ice. The perfused brain was homogenized as described earlier. [11C]MNPA (525 μCi) was added to blood and to brain homogenates. Tissue samples were incubated in a water bath at 37°C for 75 min. Samples (50 μl) of blood or brain homogenates were added to CH3CN and analyzed as mentioned earlier.
RESULTS
Baseline [11C]MNPA scans and displacement studies
After bolus plus constant infusion of [11C]MNPA, brain uptake achieved a relatively constant level by 20 min in striatum and cerebellum (Fig. 1). Striatum and cerebellum activities from 45 to 90 min increased by 7% ± 5%/h and 13% ± 12%/h, respectively. Radioactivity concentrated in striatum, with lower levels in cerebellum (Figs. 1 and 2A). Striatal BPND values at baseline were similar when analyzed by kinetic and equilibrium reference tissue models, 0.93 ± 0.12 and 0.83 ± 0.13, respectively.
Fig. 1.
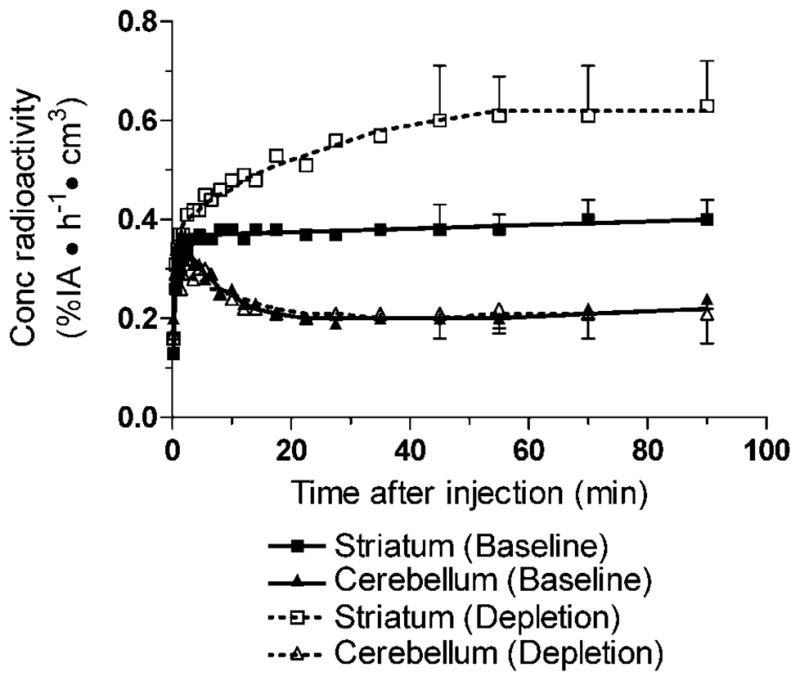
Time–activity curves of [11C]MNPA at baseline (solid lines) and after dopamine depletion (dashed lines). Symbols are the mean of five scans each at baseline and after dopamine depletion. Bars for standard deviation are shown for time frames used for calculating the equilibrium reference tissue model (i.e., 45–90 min). The concentration of radioactivity is expressed as % injected activity (IA) per hour per cm3 brain. Symbols: (■) striatum baseline; (□) striatum depletion; (▲) cerebellum baseline; (△) cerebellum depletion. The triangle symbols (open and filled) indicate cerebellum overlap at several time points.
Fig. 2.
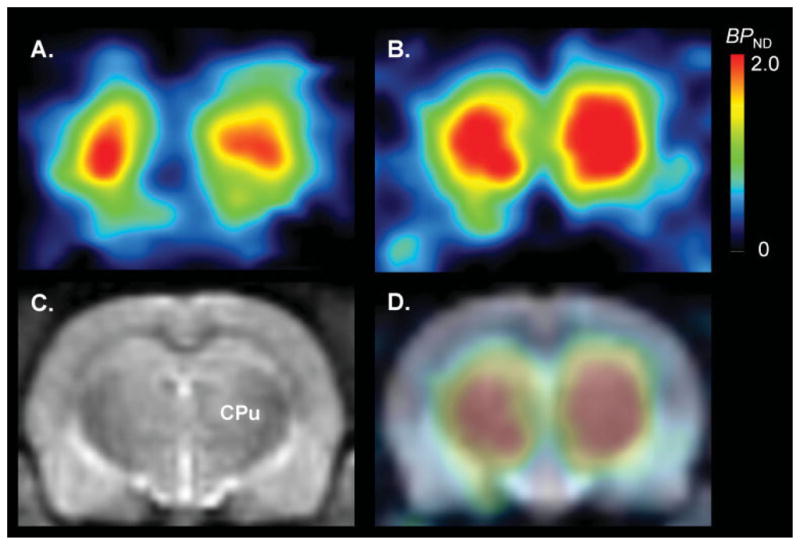
Parametric images of [11C]MNPA BPND calculated with a kinetic reference tissue model at baseline (A) and after dopamine depletion (B). Depletion of endogenous dopamine induced by reserpine plus α-methyl-para-tyrosine increased striatal [11C]MNPA BPND by twofold. An image of a T-2 weighted MRI (C) [CPu: caudate putamen (striatum)] and fused PET-MRI image (D) are shown for clearer orientation of striatum.
Raclopride (2 mg/kg, i.v.), when injected during steady state, displaced striatal activity by ~83% (Fig. 3A). BP 897 (0.25 (data not shown) and 0.5 mg/kg, i.v.), when injected during steady state, displaced striatal activity by <10% (Fig. 3B).
Fig. 3.
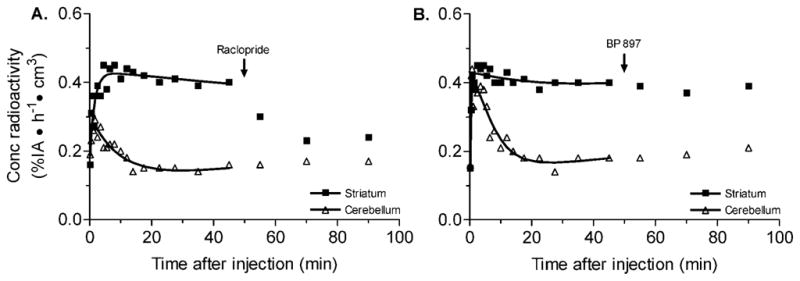
Displacement studies to determine the specificity of [11C]MNPA binding in rat brain. A: The effect of nonradioactive raclopride (2 mg/kg, i.v.) on [11C]MNPA binding in striatum of rat brain. Raclopride was injected 50 min after radioligand injection, resulting in a significant displacement in striatal activity. B: The effect of nonradioactive BP 897 (0.5 mg/kg, i.v.) on [11C]MNPA binding in striatum of rat brain. BP897 was injected 50 min after radio-ligand injection, resulting in little or no changes in striatal activity. The concentration of radioactivity is expressed as % injected activity (IA) per h per cm3 brain. Symbols: (■) striatum; (△) cerebellum.
Effect of dopamine depletion
The depletion of endogenous dopamine induced by reserpine plus α-methyl-para-tyrosine increased activity in striatum but had little effect in cerebellum (Fig. 1). Striatal [11C]MNPA BPND calculated by the kinetic reference tissue model was 0.93 ± 0.12 (n = 5) at baseline and increased to 1.99 ± 0.25 (n = 5; P = 0.0001) after dopamine depletion (Figs. 2 and 4). Similarly, BPND calculated by the equilibrium reference tissue model was 0.83 ± 0.13 (n = 5) at baseline and increased to 1.97 ± 0.09 (n = 5; P = 0.0001) after dopamine depletion. Occupancy of D2/3 receptors by endogenous dopamine was calculated to be 53%, which equals the difference between the binding potential after dopamine depletion compared to that at baseline. This increase in binding potential reflects the amount of endogenous dopamine occupying D2/3 receptors during basal conditions.
Fig. 4.
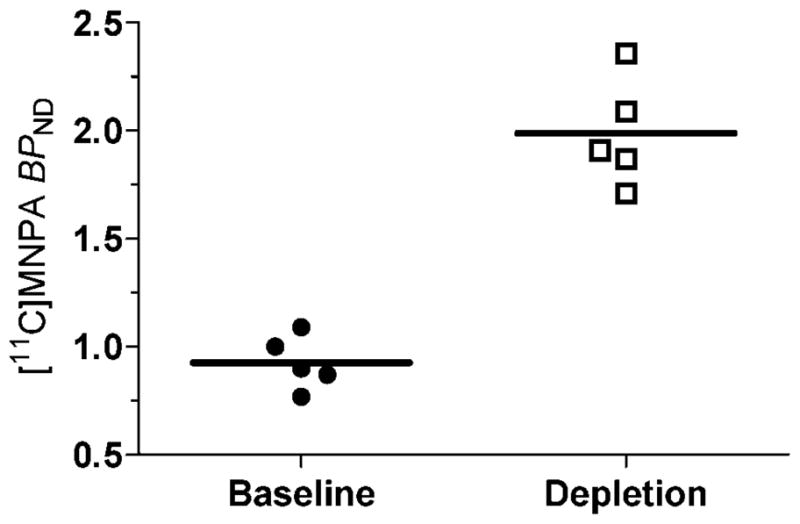
[11C]MNPA BPND calculated with a kinetic reference tissue model at baseline and after dopamine depletion (induced by reserpine and α-methyl-para-tyrosine). Dopamine depletion resulted in a significant increase in striatal [11C]MNPA binding potential (two-tailed P = 0.0001).
Ex vivo experiments
We considered the possibility that administration of reserpine plus α-methyl-para-tyrosine might alter the metabolism of [11C]MNPA, to give radiometabolites that might increase the total radioactivity in brain. Thus, we determined the percentages of radiometabolites and radioligand in brain and whole blood at baseline and after dopamine depletion. Three radio-metabolites in whole brain samples were detected with HPLC analysis (Fig. 5). Two radiometabolites eluted earlier than MNPA and one eluted later, indicating that two of the three radiometabolites are less lipophilic than MNPA. At 30 min after injection of [11C]MNPA, the three radiometabolites in brain were 10.4% ± 1.8% of total radioactivity at baseline and were not significantly different after dopamine depletion (8.0% ± 1.7%; t = 3.2, df = 6, P = 0.57) (Table I). Radiometabolites in plasma constituted 71.4% ± 16.4% of total radioactivity at baseline and were not significantly different after dopamine depletion (61.3% ± 19.3%; t = 0.72, df = 6, P = 0.95) (Table I).
Fig. 5.
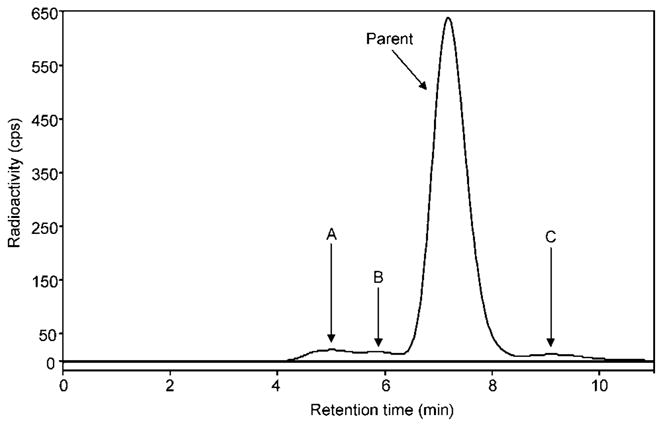
Radiochromatogram of activity extracted from rat brain at 30 min after injection of [11C]MNPA. Parent in rat brain constituted 91% of total radioactivity. Radiometabolites A and B have lower lipophilicity than that of [11C]MNPA. Radiometabolite C eluted later than MNPA, but represented ~3% of the total radioactivity. The radiochromatogram is from the baseline condition as a representative profile seen in both conditions (i.e., baseline and dopamine depletion). Values of the percentage of parent and radio-metabolite in both conditions can be found in Table I.
TABLE I.
Composition of radioactivity in rat brain and plasma after injection of [11C]MNPA
| Samples | Radiochemical species | Condition
|
|
|---|---|---|---|
| Baseline (%) | Depletion (%) | ||
| Brain | [11C]MNPA | 89.6 ± 0.9 | 92.0 ± 1.2 |
| Radiometabolites | 10.4 ± 1.7 | 8.0 ± 1.8 | |
| Plasma | [11C]MNPA | 28.6 ± 21.6 | 38.7 ± 18.2 |
| Radiometabolites | 71.4 ± 16.4 | 61.3 ± 19.3 | |
Rats were euthanized 30 min after injection of [11C]MNPA, and radioactivity was extracted from brain and plasma. Values are average ± SD of radioHPLC analyses (n = 4 in each condition).
[11C]MNPA was stable in vitro in homogenates of perfused rat brain, but was less stable in whole blood. [11C]MNPA had a radiochemical purity of 97% parent before incubation with tissue. After incubation for 75 min at 37°C, parent radioligand was unchanged in brain homogenates, but decreased to 87% of radioactivity in whole blood.
DISCUSSION
Our PET imaging in rats confirmed prior studies in monkeys that the agonist radioligand [11C]MNPA is more sensitive to changes in endogenous dopamine than antagonist radioligands (Seneca et al., 2006). The binding potential in rat striatum at baseline estimated by both kinetic and equilibrium reference tissue models was similar to that reported for monkey (Seneca et al., 2006). The specificity of binding to dopamine D2 and D3 receptors was confirmed by displacement studies with nonradioactive raclopride, which reduced striatal activity by ~83%. To determine the specificity of radioligand binding to D2 vs. D3 receptors, we performed displacement studies with a selective D3 compound, BP 897. Displacement studies with nonradioactive BP 897 had little or no effect of [11C]MNPA binding in rat striatum. Striatal activity was decreased by <10%. Thus, confirming the D2 receptor specificity of [11C]MNPA binding.
Occupancy of D2/3 receptors in rat brain by endogenous dopamine was calculated to be 53%. Like other investigators (Laruelle, 2000), we made two assumptions to estimate the percentage of D2/3 receptors in the high-affinity state. First, [11C]MNPA at tracer doses bound only to the high-affinity state. Second, dopamine depletion converted all receptors in low affinity to the high-affinity state. We found that striatal binding of [11C]MNPA doubled after dopamine depletion. Based on this finding and the two assumptions, we estimated that at baseline half of all D2/3 receptors are in the high-affinity state and half are in the low-affinity state.
The 53% occupancy of D2/3 receptors by endogenous dopamine is higher than that previously reported using antagonist radioligands in human and monkey, with estimates ranging from ~10 to ~30% (Abi-Dargham et al., 2000; Ginovart et al., 1997; Laruelle et al., 1997; Verhoeff et al., 2002). However, our calculated receptor occupancy by endogenous dopamine was similar to ex vivo data in rodents using the agonist radioligand [3H]NPA, with estimates ranging from ~40 to ~55% (Ross and Jackson, 1989; van der Werf et al., 1983). The greater increase in BPND observed in the present study may be attributed, at least partly, to the use of an agonist radioligand, which is more sensitive than antagonist radioligands to changes in endogenous dopamine (Ginovart et al., 2006; Narendran et al., 2004; Seneca et al., 2006). Another contributing factor may be the combined use of two dopamine depleting agents—namely, reserpine plus α-methyl-para-tyrosine. This dual treatment regimen reduces dopamine concentrations in rat striatum by 95% (Guo et al., 2003). Previous in vivo PET studies using antagonist radioligands in human and monkey used only one depleting agent at much lower doses, presumably depleting dopamine to a lesser degree.
The increase in striatal binding might have been caused by any combination of at least five factors: (1) increase of radiolabeled metabolites in brain, (2) upregulation of the number of D2/3 receptors, (3) trafficking of receptors to the surface membrane, assuming that internalized receptors do not bind MNPA, (4) increase in the percentage of D2/3 receptors in the high-affinity state, and (5) removal of competition by endogenous dopamine. With regard to the first factor, the increase in striatal binding was not due to an increase in brain radiometabolites. Ex vivo measurements of rat brain at 30 min after radioligand injection demonstrated that ~90% of the total brain activity was unchanged radioligand. The percentage of parent (~90%) and radiometabolite (~10%) in rat brain was insignificantly affected by dopamine depletion. Thus, we can assume that 90% of the total radioactivity measured in vivo by PET was parent compound and that administration of dopamine depleting drugs (i.e., reserpine and α-methyl-para-tyrosine) had minimal impact on the metabolism of [11C]MNPA. With regard to the second possibility of receptor upregulation, several homogenate binding studies have found no increase in the density of D2/3 receptors after acute dopamine depletion (Laruelle et al., 1997; Ross and Jackson, 1989). In agreement with the homogenate binding studies, in vivo Scatchard analysis using [11C]raclopride showed an increase in receptor affinity but no change in D2/3 receptor density in monkey brain (Ginovart et al., 1997). Thus, the first (i.e., an increase in radiometabolites in brain) and second possibility (i.e., an upregulation in the number of D2/3 receptors) seem unlikely. Nevertheless, the remaining three mechanisms are plausible: receptor trafficking from intracellular sites, increased percentage of receptors in the high-affinity state, and removal of competition by endogenous dopamine. None of these remaining possibilities can be excluded by our results.
Occupancy of D2/3 receptors by radioligand
The mass dose of MNPA coadministered with [11C]MNPA in this study may have been large enough to occupy a significant percentage of D2/3 receptors, and such occupancy would have caused us to underestimate binding potential. We analyzed the results using the typical assumptions of radiotracer imaging, in which the radioligand occupies an insignificant percentage (often defined as <5–10%) of the target receptor. Although such conditions commonly apply in animals with relatively large brains (e.g., human and monkey), they may not apply in animals with small brains (e.g., rat and mouse). That is, the mass of carrier injected in a monkey will have a higher concentration in rat and may occupy >10% of its receptors. Thus, rodent imaging would typically require radioligand to have higher specific activity and lower mass dose than for imaging in larger animals or humans. We estimated [11C]MNPA’s occupancy of D2/3 receptors in rat brain based on the concentration of specifically bound radioligand in brain at 45–90 min and the published value of D2/3 receptor density (Bmax) in rats. Specific binding, defined as striatum minus cerebellum, was 0.78 nM. Assuming the density of D2/3 receptors in rat striatum is 22.7 nM (Seeman et al., 2002), [11C]MNPA occupied about 3.4% (0.78/22.7) of all D2/3 receptors. Finally, if we assume that about 60% of all D2/3 receptors in vivo are in the high-affinity state (Narendran et al., 2004; Seneca et al., 2006), then [11C]MNPA occupied ~6% ( = 3.4%/0.6) of high-affinity D2/3 receptors. Furthermore, this minor underestimation of binding potential would be almost equal under baseline and dopamine depleted conditions. That is, the injected mass dose of MNPA was similar for baseline (0.46 ± 0.22 μg) and dopamine depleted conditions (0.44 ± 0.01 μg). Thus, the underestimation of true binding potential would have had insignificant effect on our estimate of occupancy of D2/3 receptors by dopamine.
Furthermore, specific and displaceable binding in the reference region may cause an error in the calculation of binding potential. Binding potential calculated using reference tissue models compare the concentration of radioligand in a receptor-rich to receptor-free region. For dopamine D2/3 receptor radioligands, the receptor-rich region is striatum and receptor-free region is cerebellum. Autoradiographic studies in animal brain have shown the highest density of dopamine D2/3 receptors in striatum, with low to negligible density in cerebellum (Hall et al., 1996; Hall et al., 1988). Radioligands with high affinity to D2/3 receptors such as [11C]FLB-457 and [123I]epidepride, may have specific and displaceable binding to low density of receptors in cerebellum (Asselin et al., 2007; Pinborg et al., 2007). However, [11C]MNPA has a 7-fold lower affinity (Kihigh = 0.14 nM) than [11C]FLB-457 (Ki = 0.02 nM) to D2 receptors and may not bind specifically to the low density of receptors in cerebellum.
Anesthesia effects on radioligand binding
The present study used isoflurane anesthesia, which may have affected radioligand uptake at baseline and after dopamine depletion. For example, isoflurane anesthesia decreases brain uptake of [18F]FDG (Toyama et al., 2004), increases brain uptake of [11C]PHNO and [11C]β-CFT (McCormick et al., 2006; Tsukada et al., 1999), but has no effect on brain uptake of [3H/11C]raclopride (McCormick et al., 2006; Tsukada et al., 1999). We have not directly assessed the effect of isoflurane anesthesia on brain uptake of [11C]MNPA. Nevertheless, our values of binding potential at baseline (and under isoflurane anesthesia) are only slightly higher (~10%) than those of (Tokunaga et al., 2007) in awake rats. In addition, we used the same anesthesia at baseline and after dopamine depletion.
In summary, we confirm the utility of [11C]MNPA as a radioligand for PET imaging of the dopamine free state of D2/3 receptors. The specificity of [11C]MNPA binding to D2 receptors was confirmed by displacement studies using raclopride and BP 897. We estimate that dopamine occupies ~53% of D2/3 receptors in brains of rats under gaseous anesthesia. Chronic depletion of dopamine (as in Parkinson disease) or elevated subcortical dopamine transmission (as hypothesized in schizophrenia) may alter the percentage of D2/3 receptors in the high-affinity state. Thus, the agonist radioligand [11C]MNPA may be useful to examine the effects on D2/3 receptors of altered dopamine neurotransmission in neurodegenerative and neuropsychiatric disorders.
Acknowledgments
This research was supported in part by the Intramural Program (project #Z01-MH-002795-06) of the National Institute of Mental Health. We thank Edward Tuan and Pavitra Kannan for assistance with the ex vivo radiometabolite experiments; PMOD Technologies (Adliswil, Switzerland) for providing its image analysis and modeling software; and John Neumeyer (Mclean Hospital/Harvard Medical School) for providing an authentic sample of MNPA.
Contract grant sponsor: Intramural Program of the National Institute of Mental Health; Contract grant number: Z01-MH-002795-06.
References
- Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, Weiss R, Cooper TB, Mann JJ, van Heertum RL, Gorman JM, Laruelle M. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci USA. 2000;97:8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselin MC, Montgomery AJ, Grasby PM, Hume SP. Quantification of PET studies with the very high-affinity dopamine D2/D3 receptor ligand [11C]FLB 457: Re-evaluation of the validity of using a cerebellar reference region. J Cereb Blood Flow Metab. 2007;27:378–392. doi: 10.1038/sj.jcbfm.9600340. [DOI] [PubMed] [Google Scholar]
- Bennett BA, Sundberg DK. Catecholamine biosynthesis in specific brain areas of the rat as determined by liquid chromatography and amperometric detection. Life Sci. 1981;28:2811–2817. doi: 10.1016/0024-3205(81)90096-5. [DOI] [PubMed] [Google Scholar]
- Engelman K, Horwitz D, Jequier E, Sjoerdsma A. Biochemical and pharmacologic effects of alpha-methyltyrosine in man. J Clin Invest. 1968;47:577–594. doi: 10.1172/JCI105754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnema SJ, Jia ZS, Steiger C, Brinkman IH, Bang-Andersen BB, Wikström HV, Halldin C. Radiosynthesis of [11C]MNPA and [3H]MNPA from the precursor (R)-2-hydroxy-10,11-acetonide-NPA. J Label Compd Radiopharm V. 2007;50:S201. [Google Scholar]
- Gao YG, Baldessarini RJ, Kula NS, Neumeyer JL. Synthesis and dopamine receptor affinities of enantiomers of 2-substituted apomorphines and their N-n-propyl analogues. J Med Chem. 1990;33:1800–1805. doi: 10.1021/jm00168a040. [DOI] [PubMed] [Google Scholar]
- George SR, Watanabe M, di Paolo T, Falardeau P, Labrie F, Seeman P. The functional state of the dopamine receptor in the anterior pituitary is in the high affinity form. Endocrinology. 1985;117:690–697. doi: 10.1210/endo-117-2-690. [DOI] [PubMed] [Google Scholar]
- Ginovart N, Farde L, Halldin C, Swahn CG. Effect of reserpine-induced depletion of synaptic dopamine on [11C]raclopride binding to D2-dopamine receptors in the monkey brain. Synapse. 1997;25:321–325. doi: 10.1002/(SICI)1098-2396(199704)25:4<321::AID-SYN2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Ginovart N, Galineau L, Willeit M, Mizrahi R, Bloomfield PM, Seeman P, Houle S, Kapur S, Wilson AA. Binding characteristics and sensitivity to endogenous dopamine of [11C]-(+)-PHNO, a new agonist radiotracer for imaging the high-affinity state of D2 receptors in vivo using positron emission tomography. J Neurochem. 2006;97:1089–1103. doi: 10.1111/j.1471-4159.2006.03840.x. [DOI] [PubMed] [Google Scholar]
- Guldberg HC, Broch OJ., Jr On the mode of action of reserpine on dopamine metabolism in the rat striatum. Eur J Pharmacol. 1971;13:155–167. doi: 10.1016/0014-2999(71)90146-4. [DOI] [PubMed] [Google Scholar]
- Guo N, Hwang DR, Lo ES, Huang YY, Laruelle M, Abi-Dargham A. Dopamine depletion and in vivo binding of PET D1 receptor radioligands: Implications for imaging studies in schizophrenia. Neuropsychopharmacology. 2003;28:1703–1711. doi: 10.1038/sj.npp.1300224. [DOI] [PubMed] [Google Scholar]
- Hall H, Kohler C, Gawell L, Farde L, Sedvall G. Raclopride, a new selective ligand for the dopamine-D2 receptors. Prog Neurpsychopharmacol Biol Psychiatry. 1988;12:559–568. doi: 10.1016/0278-5846(88)90001-2. [DOI] [PubMed] [Google Scholar]
- Hall H, Farde L, Halldin C, Hurd YL, Pauli S, Sedvall G. Autoradiographic localization of extrastriatal D2-dopamine receptors in the human brain using [125I]epidepride. Synapse. 1996;23:115–123. doi: 10.1002/(SICI)1098-2396(199606)23:2<115::AID-SYN7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Ichise M, Liow JS, Lu JQ, Takano A, Model K, Toyama H, Suhara T, Suzuki K, Innis RB, Carson RE. Linearized reference tissue parametric imaging methods: Application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab. 2003;23:1096–1112. doi: 10.1097/01.WCB.0000085441.37552.CA. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Johnson CA, Seidel J, Vaquero JJ, Pascau M, Desco M, Green MV. Exact positioning for OSEM reconstructions on the ATLAS depth-of-interaction small animal scanner. Mol Imaging Biol. 2002;4:S85. [Google Scholar]
- Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: A critical review. J Cereb Blood Flow Metab. 2000;20:423–451. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- Laruelle M, D’souza CD, Baldwin RM, Abi-Dargham A, Kanes SJ, Fingado CL, Seibyl JP, Zoghbi SS, Bowers MB, Jatlow P, Charney DS, Innis RB. Imaging D2 receptor occupancy by endogenous dopamine in humans. Neuropsychopharmacology. 1997;17:162–174. doi: 10.1016/S0893-133X(97)00043-2. [DOI] [PubMed] [Google Scholar]
- Liow JS, Johnson CA, Toyama H, Green MV, Innis RB. A single slice rebinning/2D exact positioning OSEM reconstruction for the NIH ATLAS small animal PET scanner. J Nucl Med. 2003;44:163P. [Google Scholar]
- McCormick PN, Ginovart N, Vasdev N, Seeman P, Kapur S, Wilson AA. Isoflurane increases both the specific binding ratio and sensitivity to amphetamine challenge of [11C]-(+)PHNO. Neuro-Image. 2006;31:T20. [Google Scholar]
- Mignot E, Laude D. Study of dopamine turnover by monitoring the decline of dopamine metabolites in rat CSF after alpha-methyl-p-tyrosine. J Neurochem. 1985;45:1527–1533. doi: 10.1111/j.1471-4159.1985.tb07223.x. [DOI] [PubMed] [Google Scholar]
- Narendran R, Hwang DR, Slifstein M, Talbot PS, Erritzoe D, Huang Y, Cooper TB, Martinez D, Kegeles LS, Abi-Dargham A, Laruelle M. In vivo vulnerability to competition by endogenous dopamine: Comparison of the D2 receptor agonist radiotracer (−)-N-[11C]propyl-norapomorphine ([11C]NPA) with the D2 receptor antagonist radiotracer [11C]-raclopride. Synapse. 2004;52:188–208. doi: 10.1002/syn.20013. [DOI] [PubMed] [Google Scholar]
- Narendran R, Slifstein M, Guillin O, Hwang Y, Hwang DR, Scher E, Reeder S, Rabiner E, Laruelle M. Dopamine (D2/3) receptor agonist positron emission tomography radiotracer [11C]-(+)-PHNO is a D3 receptor preferring agonist in vivo. Synapse. 2006;60:485–495. doi: 10.1002/syn.20325. [DOI] [PubMed] [Google Scholar]
- Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, Schwartz JC, Everitt BJ, Sokoloff P. Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature. 1999;400:371–375. doi: 10.1038/22560. [DOI] [PubMed] [Google Scholar]
- Pinborg LH, Videbaek C, Ziebell M, Mackeprang T, Friberg L, Rasmussen H, Knudsen GM, Glenthoj BY. [123I]epidepride binding to cerebellar dopamine D2/D3 receptors is displaceable: Implications for the use of cerebellum as a reference region. Neuro-Image. 2007;34:1450–1453. doi: 10.1016/j.neuroimage.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Ponzio F, Achilli G, Calderini G, Ferretti P, Perego C, Toffano G, Algeri S. Depletion and recovery of neuronal monoamine storage in rats of different ages treated with reserpine. Neurobiol Aging. 1984;5:101–104. doi: 10.1016/0197-4580(84)90038-1. [DOI] [PubMed] [Google Scholar]
- Ross SB, Jackson DM. Kinetic properties of the in vivo accumulation of 3H-(−)-N-n-propylnorapomorphine in mouse brain. Naunyn Schmiedebergs. Arch Pharmacol. 1989;340:13–20. doi: 10.1007/BF00169200. [DOI] [PubMed] [Google Scholar]
- Seeman P, Tallerico T, Ko F, Tenn C, Kapur S. Amphetamine-sensitized animals show a marked increase in dopamine D2 high receptors occupied by endogenous dopamine, even in the absence of acute challenges. Synapse. 2002;46:235–239. doi: 10.1002/syn.10139. [DOI] [PubMed] [Google Scholar]
- Seeman P, Weinshenker D, Quirion R, Srivastava LK, Bhardwaj SK, Grandy DK, Premont RT, Sotnikova TD, Boksa P, El-Ghundi M, O’Dowd BF, George SR, Perreault ML, Mannisto PT, Robinson S, Palmiter RD, Tallerico T. Dopamine supersensitivity correlates with D2High states, implying many paths to psychosis. Proc Natl Acad Sci USA. 2005;102:3513–3518. doi: 10.1073/pnas.0409766102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P, Schwarz J, Chen JF, Szechtman H, Perreault M, McKnight GS, Roder JC, Quirion R, Boksa P, Srivastava LK, Yanai K, Weinshenker D, Sumiyoshi T. Psychosis pathways converge via D2High dopamine receptors. Synapse. 2006;60:319–346. doi: 10.1002/syn.20303. [DOI] [PubMed] [Google Scholar]
- Seneca N, Finnema SJ, Farde L, Gulyas B, Wikstrom HV, Halldin C, Innis RB. Effect of amphetamine on dopamine D2 receptor binding in nonhuman primate brain: A comparison of the agonist radioligand [11C]MNPA and antagonist [11C]raclopride. Synapse. 2006;59:260–269. doi: 10.1002/syn.20238. [DOI] [PubMed] [Google Scholar]
- Sibley DR, De Lean A, Creese I. Anterior pituitary dopamine receptors. Demonstration of interconvertible high and low affinity states of the D-2 dopamine receptor. J Biol Chem. 1982;257:6351–6361. [PubMed] [Google Scholar]
- Sokoloff P, Diaz J, Le Foll B, Guillin O, Leriche L, Bezard E, Gross C. The dopamine D3 receptor: A therapeutic target for the treatment of neuropsychiatric disorders. CNS Neurol Disord Drug Targets. 2006;5:25–43. doi: 10.2174/187152706784111551. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, Seeman P, Uehara T, Itoh H, Tsunoda M, Kurachi M. Increased proportion of high-affinity dopamine D2 receptors in rats with excitotoxic damage of the entorhinal cortex, an animal model of schizophrenia. Brain Res Mol Brain Res. 2005;140:116–119. doi: 10.1016/j.molbrainres.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Tokunaga M, Seneca N, Maeda J, Shin RM, Okauchi T, Maruyama M, Innis RB, Halldin C, Suzuki K, Higuchi M, Suhara T. Glutamate-dopamine interaction in awake rats and monkeys assessed by PET and dopamine D2/3 receptor agonist radiotracer [11C]MNPA. Soc Neurosci. 2007;471.26/M13 [Google Scholar]
- Toyama H, Ichise M, Liow JS, Vines DC, Seneca NM, Modell KJ, Seidel J, Green MV, Innis RB. Evaluation of anesthesia effects on [18F]FDG uptake in mouse brain and heart using small animal PET. Nucl Med Biol. 2004;31:251–256. doi: 10.1016/S0969-8051(03)00124-0. [DOI] [PubMed] [Google Scholar]
- Tsukada H, Nishiyama S, Kakiuchi T, Ohba H, Sato K, Harada N, Nakanishi S. Isoflurane anesthesia enhances the inhibitory effects of cocaine and GBR12909 on dopamine transporter: PET studies in combination with microdialysis in the monkey brain. Brain Res. 1999;849:85–96. doi: 10.1016/s0006-8993(99)02018-1. [DOI] [PubMed] [Google Scholar]
- van der Werf JF, Sebens JB, Vaalburg W, Korf J. In vivo binding of N-n-propylnorapomorphine in the rat brain: Regional localization, quantification in striatum and lack of correlation with dopamine metabolism. Eur J Pharmacol. 1983;87:259–270. doi: 10.1016/0014-2999(83)90336-9. [DOI] [PubMed] [Google Scholar]
- Verhoeff NP, Hussey D, Lee M, Tauscher J, Papatheodorou G, Wilson AA, Houle S, Kapur S. Dopamine depletion results in increased neostriatal D2, but not D1, receptor binding in humans. Mol Psychiatry. 2002;7:322–238. doi: 10.1038/sj.mp.4001062. [DOI] [PubMed] [Google Scholar]
- Willeit M, Ginovart N, Graff A, Rusjan P, Vitcu I, Houle S, Seeman P, Wilson AA, Kapur S. First human evidence of d-amphetamine induced displacement of a D2/3 agonist radioligand: A [11C]-(+)-PHNO positron emission tomography study. Neuropsychopharmacology. 2008;33:279–289. doi: 10.1038/sj.npp.1301400. [DOI] [PubMed] [Google Scholar]
- Zoghbi SS, Shetty HU, Ichise M, Fujita M, Imaizumi M, Liow JS, Shah J, Musachio JL, Pike VW, Innis RB. PET imaging of the dopamine transporter with 18F-FECNT: A polar radiometabolite confounds brain radioligand measurements. J Nucl Med. 2006;47:520–527. [PubMed] [Google Scholar]


