Abstract
Aims/hypothesis
Aldosterone concentrations increase in obesity and predict the onset of diabetes. We investigated the effects of aldosterone on glucose homeostasis and insulin secretion in vivo and in vitro.
Methods
We assessed insulin sensitivity and insulin secretion in aldosterone synthase-deficient (As [also known as Cyp11b2]−/−)and wild-type mice using euglycaemic-hyperinsulinaemic and hyperglycaemic clamps, respectively. We also conducted studies during high sodium intake to normalise renin activity and potassium concentration in As−/− mice. We subsequently assessed the effect of aldosterone on insulin secretion in vitro in the presence or absence of mineralocorticoid receptor antagonists in isolated C57BL/6J islets and in the MIN6 beta cell line.
Results
Fasting glucose concentrations were reduced in As−/−mice compared with wild-type. During hyperglycaemic clamps, insulin and C-peptide concentrations increased to a greater extent in As−/− than in wild-type mice. This was not attributable to differences in potassium or angiotensin II, as glucose-stimulated insulin secretion was enhanced in As−/− mice even during high sodium intake. There was no difference in insulin sensitivity between As−/− and wild-type mice in euglycaemic-hyperinsulinaemic clamp studies. In islet and MIN6 beta cell studies, aldosterone inhibited glucose and isobutylmethylxanthine-stimulated insulin secretion, an effect that was not blocked by mineralocorticoid receptor antagonism, but was prevented by the superoxide dismutase mimetic tempol.
Conclusions/interpretation
We demonstrated that aldosterone deficiency or excess modulates insulin secretion in vivo and in vitro via reactive oxygen species and in a manner that is independent of mineralocorticoid receptors. These findings provide insight into the mechanism of glucose intolerance in conditions of relative aldosterone excess.
Keywords: Aldosterone, Aldosterone synthase, Diabetes mellitus, Glucose clamp technique, Insulin, Mineralocorticoid receptor, Pancreatic beta cells, Renin–angiotensin–aldosterone system
Introduction
Aldosterone classically stimulates sodium reabsorption in the distal nephron via the mineralocorticoid receptor (MR), although many extra-renal effects are now appreciated. Aldosterone contributes to myocardial fibrosis, nephrosclerosis and vascular injury even in the absence of severe hypertension [1]. Although the renin-angiotensin-aldosterone system (RAAS) is normally activated during conditions of low sodium or fluid intake, it may be inappropriately elevated in obese individuals. In particular, elevated aldosterone concentrations are associated with obesity and the metabolic syndrome [2–5]. This correlation may be due to the stimulation of aldosterone synthesis by oxidised fatty acids or other circulating aldosterone-stimulatory factors, which act on the adrenal gland independently of angiotensin II [6]. This relationship has led investigators to implicate aldosterone in the development of diabetes, although a causal role has not been firmly established [7].
Aldosterone excess produces insulin resistance in animals and correlates with insulin resistance in humans [8, 9]. Insulin resistance progresses to overt type 2 diabetes when pancreatic beta cells can no longer secrete sufficient insulin to maintain normoglycaemia [10]. The effect of aldosterone on insulin secretion has not been extensively studied; however, an inverse relation between insulin secretion and aldosterone tertiles was observed in a cross-sectional study of hypertensive patients, suggesting an effect of aldosterone on beta cell function [11]. Furthermore, blockade of the RAAS with either ACE inhibitors or angiotensin receptor blockers (ARBs) has beneficial effects on glucose homeostasis, but the mechanism remains uncertain [12, 13]. Because ACE inhibitors or ARBs decrease aldosterone concentrations at least transiently, and the aldosterone and angiotensin pathways are interrelated, the beneficial effects of these drugs may be mediated by alterations in aldosterone signalling.
Aldosterone synthase is the primary enzyme responsible for production of aldosterone by conversion of corticosterone to aldosterone. Aldosterone synthase-deficient mice (As [also known as Cyp11b2]−/−) are incapable of producing aldosterone and provide a tool to investigate the role of endogenous aldosterone in glucose metabolism [14, 15]. Using As−/− mice and glucose clamp techniques, we investigated the role of endogenous aldosterone in the regulation of insulin sensitivity and pancreatic beta cell function in mice. Because potassium and renin activity are increased in As−/− mice during normal sodium intake [14], we also performed studies during high sodium intake, which normalises these measures. We also explored the direct effects of aldosterone and MR antagonism in isolated wild-type murine islets and clonal beta cells.
Methods
Animals
All experiments were approved by the Vanderbilt Institutional Animal Care and Use Committee. As−/− mice were generated on a 129 background [16] and were backcrossed over ≥10 generations on to the C57BL/6J strain obtained from Jackson Laboratory (Bar Harbor, ME, USA). Mice were genotyped as previously described [15]. We studied 12- to 16-week-old male mice. As−/− mice and their wild-type littermates had free access to standard mouse chow (Rodent 5001; Purina Laboratory, Richmond, IN, USA) and water, and were housed in a temperature-controlled facility with a 12 h light/dark cycle. During high-sodium experiments, mice drank 0.9% saline (wt/vol.) for 1 week prior to study.
General clamp procedures
A surgically implanted gastric feeding tube and carotid arterial catheter were implanted for glucose tolerance tests (GTTs). Carotid arterial and jugular venous catheters were implanted for euglycaemic and hyperglycaemic clamps 3–5 days prior to study and clamp protocols were conducted as previously described [17–19]. Basal samples were taken between t= −15 and 0 min in each clamp protocol after a 5 h fast, which started at 08:00 hours. During hyperglycaemic clamps, a variable glucose infusion rate was used to increase and maintain blood glucose at 13.9–16.7 mmol/l during the experimental period. Human insulin was used during hyperinsulinaemic clamps (Humulin R; Eli Lilly, Indianapolis, IN, USA).
Islet isolation, culture and perifusion
Murine islets were isolated from 12- to 18-week-old C57BL/6 J male mice and islet perifusion performed as previously described [19]. Since steroids are present in FBS, which is typically used in islet culture, we performed all cultures and assessments of insulin secretion by isolated islets using steroid-free FBS (Sigma Aldrich, St Louis, MO, USA). Purified islets were cultured for 16–24 h and at 37°C in RPMI-1640 with 5.6 mmol/l glucose + 10% steroid-free FBS, in the presence or absence of aldosterone, spironolactone, eplerenone and tempol (all Sigma-Aldrich), and a combination of the treatments. After overnight culture, insulin secretion was assessed using static incubation or a cell perifusion system. Islets were matched for size and number, and assessed as islet equivalents (IEQ). Static incubation studies were performed for 30 min at 37°C in fresh 2 ml RPMI-1640 containing 16.7 mmol/l glucose with or without treatment. Dynamic islet insulin secretion was evaluated in a cell perifusion apparatus consisting of four parallel columns, with insulin extracted as described previously [20].
Clonal beta cell culture and static insulin secretion studies
MIN6 cells originally obtained from J. Miyazaki [21] were cultured in DMEM containing 25 mmol/l glucose with 10% steroid-free FBS, 100 g/ml streptomycin, 100 units/ml penicillin sulphate (Invitrogen, Carlsbad, CA, USA) and 70 µmol/l beta mercaptoethanol (Sigma). Cells used in these studies were maintained at passage 24–30. Upon reaching 80% confluence, cells were seeded into 24-well plates at 1×106 cells/well and maintained for 3–4 days. Fresh medium containing the indicated concentration of aldosterone, RU-28318 (Tocris Bioscience, Bristol, UK) or DMSO vehicle was added 12–16 h before studies. During static incubation, cells were pre-incubated for 2 h with KRB without glucose, then challenged for 1 h at 37°C with 500 µl KRB containing 0 or 23 mmol/l glucose. Treatment or control drugs were present throughout static incubations. Insulin was measured using an ELISA kit (ALPCO, Salem, NH, USA).
Laboratory assays
In all clamp protocols, arterial whole-blood glucose was measured using the glucose oxidase method (Accu-Chek; Roche Diagnostics, Basel, Switzerland). Plasma collected during the clamp studies or fractions from the perifusion studies were used for insulin and C-peptide assays. Insulin and C-peptide concentrations were determined by RIA (Millipore, St. Charles, MO, USA). In a separate group of animals during normal and high sodium intake (10 wild-type, 12 As−/−), blood was collected via the saphenous vein into heparin-coated tubes and immediately analysed for potassium (iSTAT EC8+ cartridge; Heska, Loveland, TX, USA). Pancreatic insulin content and islet area were determined as previously described [19].
Statistical analysis and calculations
Data are presented as mean±SEM unless otherwise specified. For comparisons between genotype, Wilcoxon’s signed-rank test and Wilcoxon’s rank-sum test were used for paired and unpaired comparisons respectively. Repeated-measures ANOVA was used with post hoc least significant difference analysis to compare treatment effects between groups for plasma measurements. Islet perifusion and static culture results were analysed using ANOVA with study day as a covariate. All statistical analyses were performed using SPSS for Windows (version 17.0; SPSS, Chicago, IL, USA), with a two-tailed value of p<0.05 considered to be significant.
Additional details
For other details on methods, see electronic supplementary material (ESM), Methods.
Results
Fasting blood glucose is reduced in As−/− mice without alteration of glucose tolerance
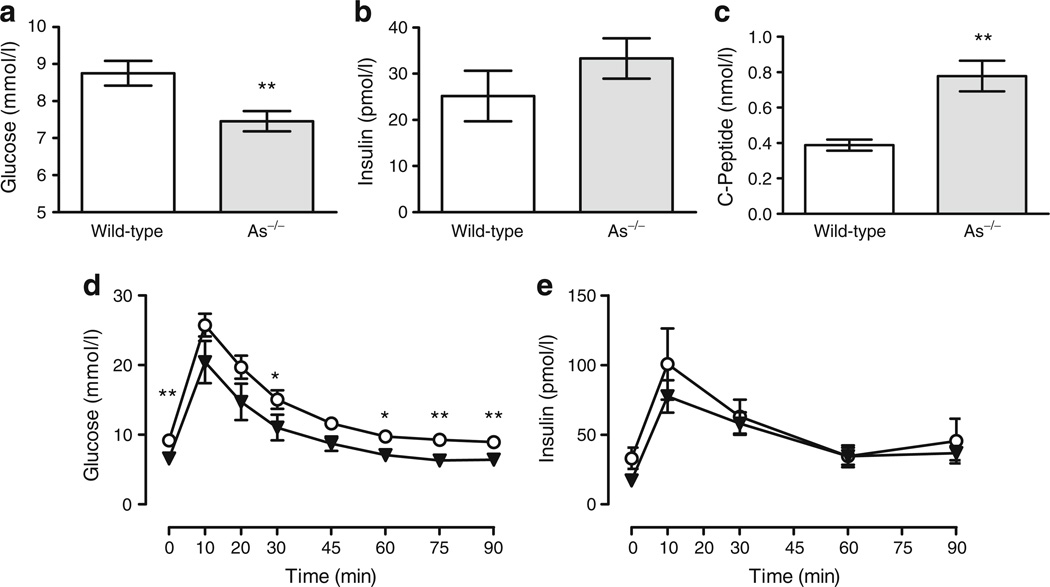
After a 5 h morning fast, arterial glucose was significantly lower in As−/− mice than in wild-type controls (7.46±0.27 vs 8.75±0.33 mmol/l, p= 0.003) (Fig. 1a). Insulin tended to be higher in As−/− mice (Fig. 1b). Because the circulating half-life of C-peptide is longer than that of insulin, we also assessed C-peptide concentrations. C-peptide was significantly increased in As−/− mice (Fig. 1c). Body weight, fat mass and gonadal fat weight were similar in a separate set of age-matched wild-type and As−/− mice (see ESM, Results).
Fig. 1.
Fasting glucose is lower and oral glucose tolerance is normal in As−/− mice. a Data from pooled studies demonstrate that fasting plasma glucose was significantly lower in As−/− than in wild-type (WT) mice (n≥25 per each genotype). Fasting insulin (b) tended to increase (p=0.07, n≥25) and C-peptide (c) was significantly higher in As−/− mice (n≥17). d During the GTTs, glucose was reduced in As−/− (triangles) compared with wild-type (circles) mice, although the changes in glucose and baseline-corrected AUC glucose results were similar. e The baseline-corrected AUC insulin was also similar. *p≤ 0.05, **p≤0.01 between genotypes
To assess whether fasting glucose, gastrointestinal glucose absorption and incretin signalling were similar in As−/− and wild-type mice, we performed GTTs in wild-type (n=7) and As−/− (n=7) mice via a previously implanted percutaneous gastric feeding catheter, with blood sampling via arterial catheters. Whole-blood arterial glucose after a 5 h fast (08:00 to 13:00 hours) was higher in wild-type than in As−/− mice (9.21±0.44 vs 6.56±0.44 mmol/l, respectively, p=0.004) (Fig. 1d), while fasting plasma insulin concentrations were 33.0±7.7 pmol/l in wild-type and 17.1±3.8 pmol/l in As−/−mice (p=0.09) (Fig. 1e). Glucose increased significantly in both groups during the GTT, with peak values occurring at 10 min (Fig. 1d). Although glucose remained higher in wild-type mice, the magnitude of change in glucose was similar in wild-type and As−/− mice (16.6±1.39 vs 13.83±2.78 mmol/l, p=0.75). The area under the glucose–time curve, corrected for baseline glucose, was also similar between the two groups (p=0.32). Likewise, the absolute increase in insulin was similar in each group (wild-type 66.7±26.1 vs 60.9± 14.5 pmol/l for As−/−, p=0.95) (Fig. 1e), despite higher glucose concentrations in wild-type mice.
Glucose-stimulated insulin secretion is increased in As−/−mice independently of potassium concentration and RAAS activation
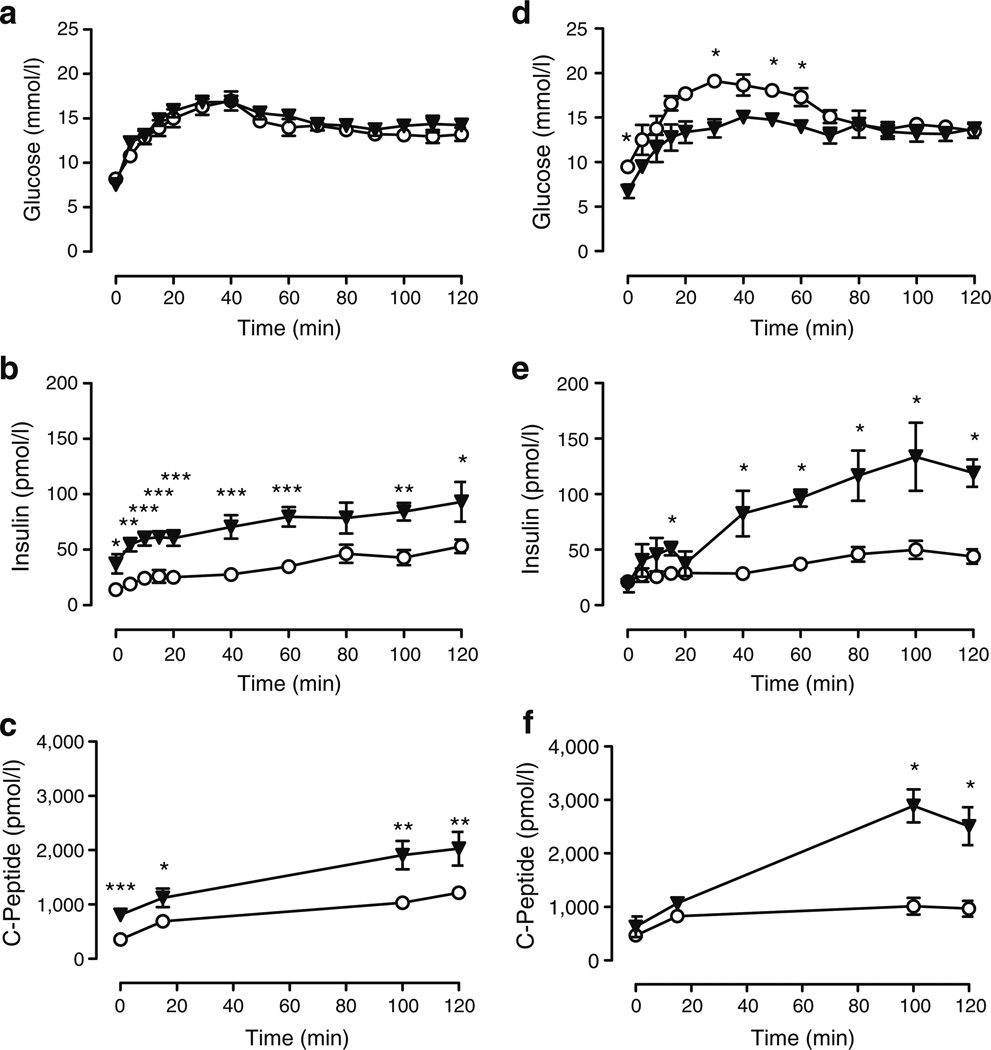
To assess the effect of aldosterone deficiency on insulin secretion and to equalise confounding differences in glucose concentrations between wild-type and As−/− mice, we conducted hyperglycaemic clamps in nine wild-type and nine As−/− mice aged 12 to 18 weeks. Intravenous glucose was infused at variable rates as necessary to maintain plasma glucose at similar steady-state levels (terminal 30 min of the study) in each genotype (13.17±0.56 vs 14.11±0.44 mmol/l in wild-type vs As−/− mice, p=0.22) (Fig. 2a). Insulin concentrations during steady-state glucose were significantly higher in As−/− mice than in wild-type controls (47.9±4.64 vs 89.9±11.3 pmol/l wild-type vs As−/−, p=0.001) (Fig. 2b). C-peptide concentrations were also significantly higher in As−/−mice, demonstrating that increased insulin concentrations in As−/− mice resulted from insulin secretion, rather than altered insulin metabolism (p<0.01)(Fig. 2c). The terminal glucose infusion rate was similar in the two groups (1.45±0.43 vs 1.50±0.37 mmol kg−1 min−1, p=0.67).
Fig. 2.
Glucose-stimulated insulin secretion is increased in As−/− mice. During hyperglycaemic clamp studies under normal sodium (a–c) and high sodium (d–f) conditions, glucose was infused to achieve equivalent glucose values at the terminal phase of the study. Terminal glucose (a, d) was similar in As−/− (triangles) compared with wild-type (circles) mice. Insulin (b, e) and C-peptide (c, f) concentrations were significantly greater in As−/− than in wild-type mice. *p≤0.05, **p≤0.01 and ***p≤0.001 vs wild-type
In mice ingesting a normal sodium diet, serum potassium was significantly higher in As−/− mice (5.0±0.2 vs 5.8± 0.2 mmol/l, wild-type vs As−/−, p=0.004).Because potassium stimulates insulin secretion, and high sodium intake normalises serum potassium and renin activity in As−/−mice [16], we repeated hyperglycaemic clamps in mice during high sodium intake (five wild-type, four As−/−). During high sodium intake (0.9% saline drinking for 5–7 days), potassium in As−/− mice was similar to that of wild-type, (5.2±0.2 vs 5.3±0.2 mmol/l, wild-type vs As−/−, p= 0.88). Glucose concentration was similar at steady state (13.83±0.56 vs 13.33±0.56 mmol/l, wild-type vs As−/−, p=0.73) (Fig. 2d), while insulin (47.0±6.4 vs 127.6±9.3 pmol/l, wild-type vs As−/−, p=0.016) (Fig. 2e) and C-peptide (514±50 vs 966±121 pmol/l, wild-type vs As−/−, p=0.001) (Fig. 2f) were again significantly higher in As−/−mice. The terminal glucose infusion rate required to maintain equivalent serum glucose concentrations was significantly higher in the As−/− than in the wild-type group (3.26±0.076 vs 1.17±0.073 mmol kg−1 min−1,p=0.016), reflecting the increased insulin concentration.
Pancreatic islet density and insulin content are unaltered in As−/− mice
To determine whether increased glucose-mediated insulin secretion resulted from increased beta cell mass, we assessed pancreatic insulin content and conducted quantitative morphometric analysis of pancreatic tissue from As−/− and wild-type mice. Pancreatic insulin content was similar in both groups (132.7±11.7 and 149.8± 13.9 nmol/g tissue, wild-type [n=5] and As−/− [n=4], p= 0.56). Likewise, pancreatic islet area was similar between the two groups (0.46±0.08 vs 0.50±0.10% of total pancreas area, n=5 each group, p=0.92).
Aldosterone impairs insulin secretion in isolated murine islets and MIN6 beta cells
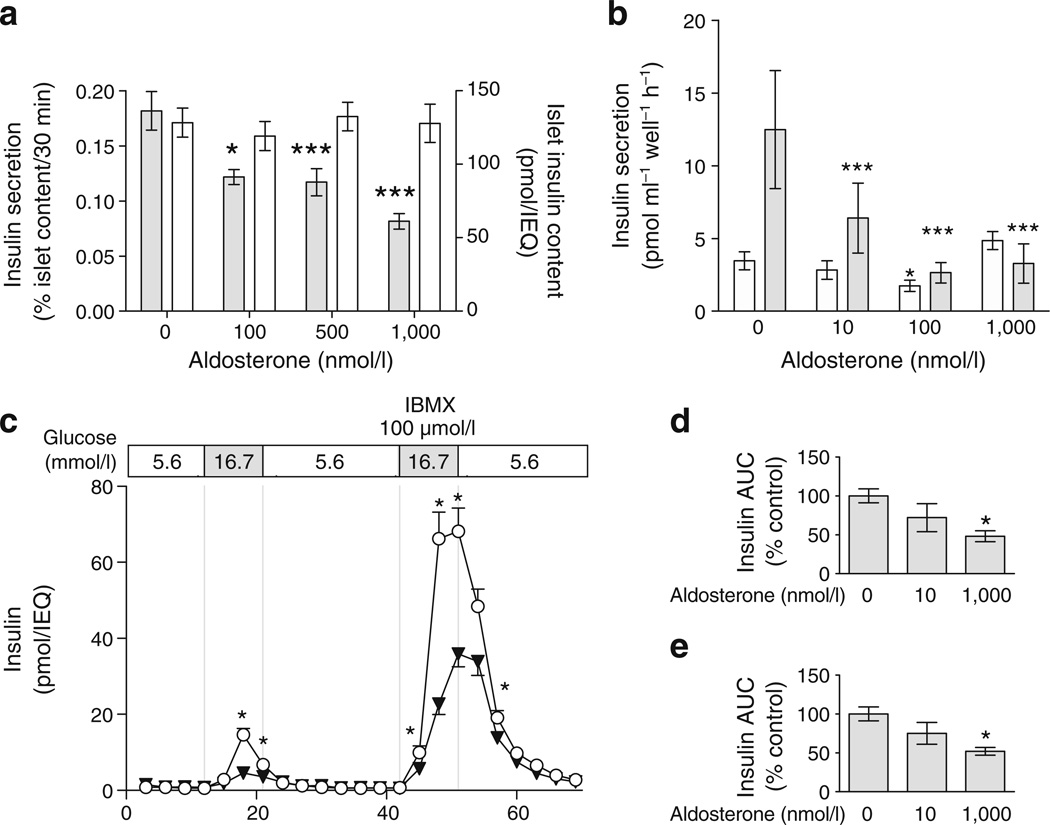
To further exclude confounding by systemic effects of aldosterone and to better assess the direct effect of aldosterone on pancreatic islet cell function, we conducted studies in isolated wild-type murine islets. In a dose-ranging study, islets were cultured for 24 h in the presence of aldosterone and then stimulated with 16.7 mmol/l glucose in static culture for 30 min on the following day (n≥5 replicates each group). Aldosterone impaired glucose-stimulated insulin response in a dose-dependent manner (Fig. 3a). Islet insulin content, assessed after study completion, did not differ between treatment groups (p=0.37) (Fig. 3a). Because the results were not affected by normalisation for islet insulin content, these studies suggest that aldosterone affects glucose sensing or stimulus–response coupling, rather than insulin production. Similar results were obtained in the MIN6 beta cell line during static culture, with maximal inhibition achieved between 10 and 100 nmol/l concentration (Fig. 3b). Under unstimulated conditions (0 mmol/l glucose in MIN6), 100 nmol/l aldoste-rone slightly decreased insulin secretion (p=0.03).
Fig. 3.
Aldosterone decreases insulin secretion in a dose-dependent manner in isolated islets and the MIN6 beta cell line. a Aldosterone reduced glucose-stimulated insulin concentrations, assessed as insulin secreted after 16.7 mmol/l glucose stimulus for 30 min in static culture (grey bars). Total islet insulin content was unaltered by aldosterone (white bars), suggesting an effect on insulin secretion rather than insulin content. b In MIN6 cells, aldosterone decreased insulin secretion in response to 23 mmol/l glucose (grey bars), reaching maximal inhibition at 100 nmol/l. c In isolated islet perifusion studies, insulin secretion in response to glucose (16.7 mmol/l) and isobutyl-methylxanthine (IBMX; 100 µmol/l) was diminished in the presence of aldosterone 1000 nmol/l (triangles) compared with control (circles). d Insulin secretion quantified as insulin AUC was significantly lower in islets treated with aldosterone (1000 nmol/l) in response to glucose (16.7 mmol/l) and (e) to glucose (16.7 mmol/l)+IBMX (100 µmol/l). *p≤0.05 and ***p≤0.001 vs 0 nmol/l aldosterone
We performed perifusion studies of isolated islets using aldosterone at concentrations of 10 and 1,000 nmol/l after overnight culture (Fig. 3c, d). Aldosterone decreased glucose- and isobutylmethylxanthine (IBMX)-stimulated insulin secretion in a dose-dependent manner (Fig. 3d, e), with a significant effect of 10 nmol/l aldosterone evident during peak IBMX stimulation (ESM Fig. 1) and a trend towards significance during peak glucose stimulation (p= 0.06). Aldosterone also impaired tolbutamide-induced insulin secretion (10.5±0.9 vs 15.8±1.7 pmol min−1 IEQ−1 in the presence and absence of aldosterone, respectively, p<0.01). Aldosterone did not affect insulin secretion during the baseline period (5.5 mmol/l glucose).
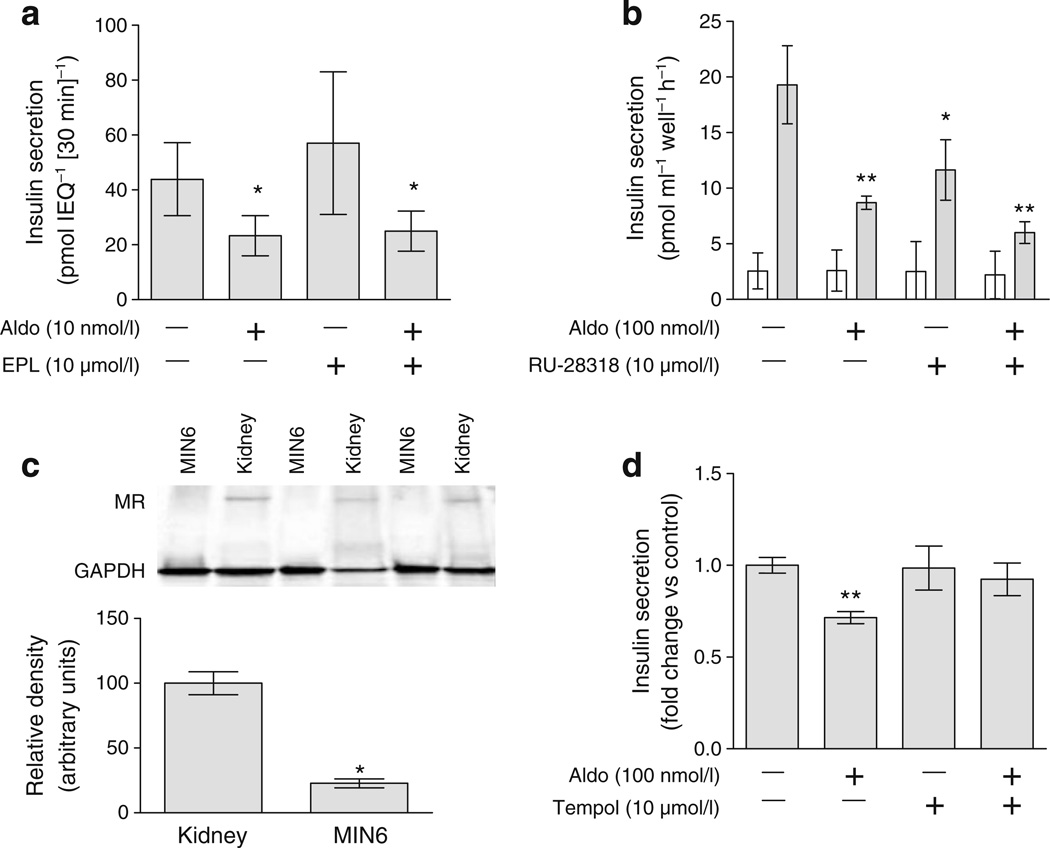
Aldosterone impairs insulin secretion in an MR-independent manner
We tested the effect of the specific MR antagonist eplerenone at 10 µmol/l, a concentration greater than the established IC50 of 360 nmol/l [22, 23], on insulin secretion in perifused islets. Aldosterone significantly decreased insulin secretion in response to glucose and IBMX. Eplerenone alone did not affect insulin secretion or insulin content, or prevent the effect of aldosterone in perifused islets. Results were similar in response to glucose and IBMX (ESM Fig. 2a, b). We also tested a higher molar ratio of eplerenone to aldosterone by lowering the aldosterone concentration in isolated islet static culture: results were similar, suggesting that inadequate competition alone could not explain these results (Fig. 4a). Results were similar during spironolactone treatment (ESM Fig. 2c, d). In MIN6 cells, the water-soluble MR antagonist RU-28318 (10 µmol/l) alone reduced insulin secretion and did not prevent the effect of aldosterone (Fig. 4b). Furthermore, MR protein abundance was markedly lower in MIN6 cells than in renal cortex (Fig. 4c). Glucocorticoid receptor antagonism with mifepristone (10 µmol/l) did not prevent the effect of aldosterone within isolated islets. However, pretreatment with the superoxide dismutase mimetic, tempol, prevented the effect of aldosterone on insulin secretion (Fig. 4d). These studies suggest that aldosterone alters insulin secretion in pancreatic islets and clonal beta cells via a mechanism that is independent of MR and glucocorticoid receptor, and involves generation of reactive oxygen species.
Fig. 4.
Aldosterone impairs insulin secretion via an MR-independent mechanism and oxidative stress. a Eplerenone (EPL) did not prevent the effect of aldosterone (Aldo) on insulin secretion in isolated islet static culture. b The water-soluble MR antagonist RU-28318 did not prevent the effect of aldosterone in the clonal MIN6 cell line (0 mmol/l glucose, white bars; 23 mmol/l glucose, grey bars). c MR protein abundance was markedly reduced in MIN6 cells compared with renal cortex. d The antioxidant tempol prevented the effect of aldosterone in isolated islet studies. *p<0.05 and **p≤0.01 vs control
Mr mRNA is expressed in murine pancreatic islets
To determine whether the MR is expressed in pancreatic islets, we assessed relative mRNA concentrations in pancreatic islets (n=4) and MIN6 cells (n=3), compared with renal cortex (n=4) from wild-type mice (Table 1). Mr (also known as Nr3c2) gene expression in isolated murine islets was similar to that in the renal cortex, with a trend towards being reduced in MIN6 cells. When compared within pancreatic islets, Mr mRNA was expressed at a much lower level than the beta cell-specific transcription factor Mafa (Table 2). Because 11beta-hydroxysteroid dehydrogenase types I and II affect the ability of glucocorticoids to activate the MR, we also assessed 11 βHsd1 and 11βHsd2 (also known as Hsd11b1 and Hsd11b2, respectively) mRNA concentration. mRNA of 11βHsd1 and 11βHsd2 was detectable at much lower levels in the pancreatic islets and MIN6 cell line than with the respective concentrations in kidney (fold change p≤0.02 for each) (Table 1).
Table 1.
Relative mRNA expression
| Tissue | Mr | 11βHsd1 | 11βHsd2 |
|---|---|---|---|
| Renal cortex | 1.01±0.09 | 1.00±0.06 | 1.03±0.10 |
| Pancreatic islets | 1.12±0.19 | 0.02±0.007*** | 0.002±0.0006*** |
| MIN6 cell line | 0.68±0.08† | 0.0033±0.001** | 0.0067±0.001*** |
Data are expressed as mean±SEM fold-changes vs renal cortex in each gene of interest; 18S was used as endogenous control
p<0.01
p<0.001 vs renal cortex
p=0.07 vs pancreatic islets
Table 2.
Relative mRNA expression in pancreatic islets
| Variable | Mafa | Mr | 11βHsd1 | 11βHsd2 |
|---|---|---|---|---|
| Relative expression | 1.03±0.15 | 0.15±0.02*** | 0.06±0.02*** | 0.01±0.002*** |
Data are expressed as mean±SEM fold-changes vs Mafa for each gene of interest; 18S was used as endogenous control
p<0.001 vs Mafa
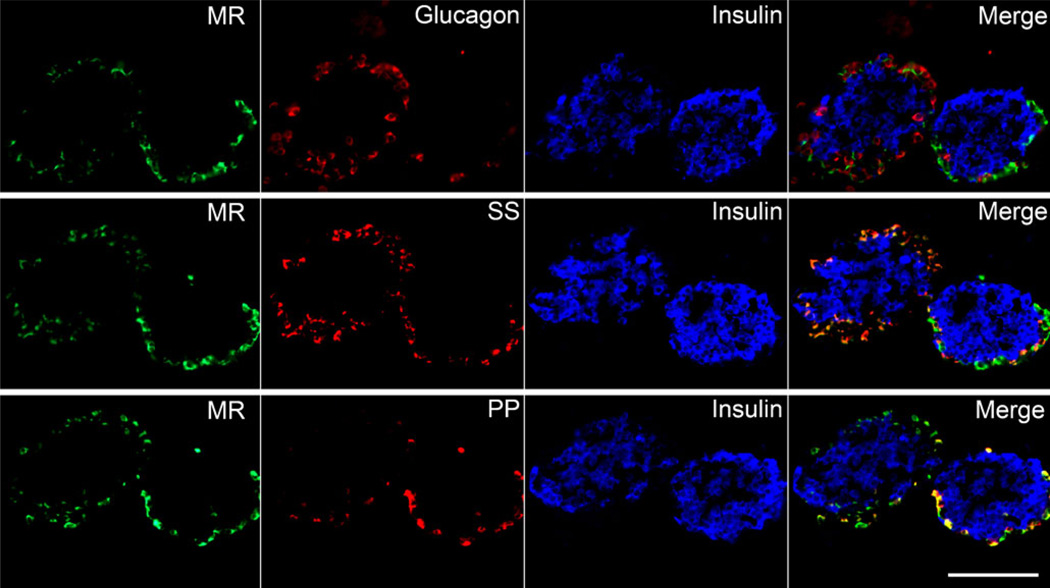
We performed immunohistochemistry on pancreatic islet sections to localise the MR, using a monoclonal antibody targeting the MR N-terminal amino acids 1–18 [24]. The MR co-localised with somatostatin- and pancreatic polypeptide-containing cells at the periphery of the islets (Fig. 5). However, the MR did not co-localise with insulin or glucagon. Because the pancreatic islet is a highly vascularised structure, we also stained for the blood vessel endothelial marker CD31, but found no co-localisation (ESM Fig. 3).
Fig. 5.
The MR is produced in pancreatic delta and pancreatic polypeptide-positive cells in isolated murine islets. Immunofluorescence staining was performed on three consecutive sections of isolated murine islets embedded in collagen (40× objective). The MR (green) localised to the islet periphery and did not co-localise with insulin (blue pseudocolour) or glucagon (red, top row). The MR also co-localised with somatostatin- (SS, red, middle row) and pancreatic polypeptide-positive (PP) cells (red, bottom row). Scale bar 100 µm
Insulin sensitivity is unchanged in As−/− mice
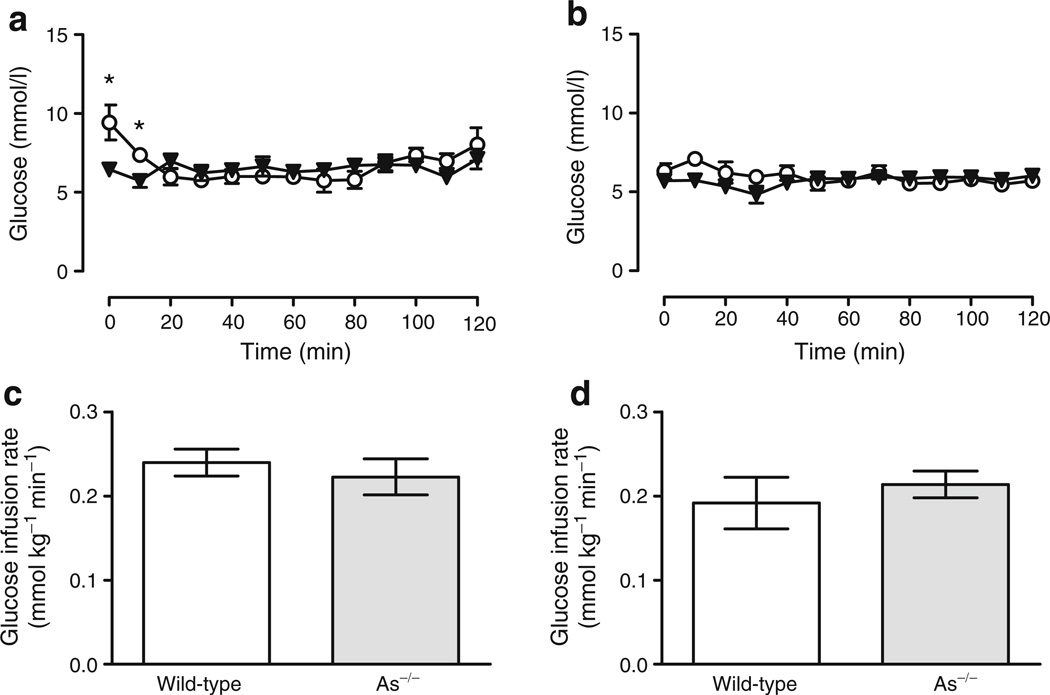
To determine whether endogenous aldosterone affects insulin sensitivity, we performed euglycaemic–hyperinsulinaemic clamps in three wild-type and six As−/− mice. Insulin (4 mU kg−1 min−1) and glucose (variable rate) were infused intravenously to normalise glucose concentrations and the glucose infusion rate at the terminal portion of the study was used as an estimate of insulin sensitivity. Glucose concentrations during steady state (i.e. the terminal 30 min of the study) were maintained at 7.33± 0.58 mmol/l in wild-type and 6.64±0.35 mmol/l in As−/− mice (p=0.26) (Fig. 6a). The average terminal glucose infusion rate was similar in both groups (0.240±0.016 vs 0.222± 0.021 mmol kg−1 min−1, wild-type vs As−/−, p=0.71) (Fig. 6c). Insulin concentrations were also statistically similar at steady state (67.47±8.4 vs 80.9±12.3 pmol/l, wild-type vs As−/−, respectively, p=0.79). Tissue-specific glucose uptake, determined from the accumulation of 2-deoxy-[14C]glucose phosphate in tissue (brain, adipose and muscle) from 2-deoxy-[14C]glucose injected intravenously during the clamp, was similar in the two genotype groups, with the exception of adipose tissue (Table 3), indicating that overall insulin action was similar in both mouse groups. Euglycaemic–hyperinsulinaemic clamps were also performed during high sodium intake in order to suppress endogenous aldosterone in wild-type mice and to control for alterations of potassium and angiotensin in As−/− mice. Insulin sensitivity was unchanged by sodium intake and was similar among groups. No differences in tissue-specific glucose uptake or endogenous glucose production were observed (Fig. 6b, d, Table 3).
Fig. 6.
Insulin sensitivity is similar in wild-type and As−/−mice. Blood glucose (a, b) and glucose infusion rate (c, d) during euglycaemic-hyperinsulinaemic clamp studies in wild-type (circles) and As−/− (triangles) mice while under normal (a, c) and high sodium intake (b, d). *p≤0.05 vs wild-type
Table 3.
Endogenous glucose production and tissue-specific glucose uptake during eugly-caemic–hyperinsulinaemic clamp during normal and high sodium intake
| Variable | Normal sodium |
High sodium |
||
|---|---|---|---|---|
| Wild-type n=3 |
As−/− n=6 |
Wild-type n=4 |
As−/− n=10 |
|
| EndoRa (mg kg−1 min−1) | NA | NA | 15.4±2.0 | 15.1 ±0.9 |
| Tissue Rg (µg min−1[mg tissue]−1) | ||||
| Gastrocnemius | 14.6±2.1 | 9.4±2.2 | 16.5±4.8 | 12.8±2.2 |
| Vastus lateralis | 14.0±2.3 | 8.7±2.1 | 14.3±2.2 | 10.9±1.9 |
| Soleus | 79.8±19.7 | 54.4±15.2 | 85.3±24.2 | 46.0±8.4 |
| Diaphragm | 133.7±43.8 | 152.9±15.0 | 107.3±20.0 | 80.9±15.3 |
| Heart | 394.1±45.3 | 349.5±40.2 | 239.3±68.7 | 206.2±29.9 |
| Adipose | 3.35±1.0 | 9.06±1.9* | 6.3±1.8 | 5.9±1.0 |
| Brain | 60.6±8.7 | 49.1±3.0 | 53.7±11.9 | 38.6±4.8 |
Data are expressed as mean± SEM
EndoR was not assessed during normal sodium
EndoRa, endogenous glucose production;Rg, glucose uptake
p<0.05 vs wild-type
Discussion
The present study demonstrates that aldosterone deficiency enhances glucose-stimulated insulin secretion in vivo and that this effect is independent of electrolyte disturbance, insulin sensitivity or renin activity in As−/−mice. Excess aldosterone, at a concentration consistent with that seen in primary aldosteronism and the metabolic syndrome, reduced insulin secretion in isolated perifused murine islets and in clonal beta cells through a mechanism that is independent of MR. To our knowledge, this is the first study to demonstrate that endogenous aldosterone regulates insulin secretion and that perturbation of aldosterone directly alters insulin secretion within beta cells.
Mouse and rat models harbouring renin transgenes exhibit altered glucose homeostasis [25, 26]. Supra-physiological concentrations of angiotensin II and aldo-sterone impair insulin sensitivity in skeletal muscle of rodents [27, 28]. Recent studies demonstrating the presence of RAAS components in pancreatic islets suggest that renin or angiotensin II also play a physiological role in insulin secretion [29, 30]. Clinical studies in humans suggest that interruption of the RAAS may reduce diabetes risk [31]. The ARB valsartan prevented progression to diabetes in participants with impaired glucose tolerance [13]. In the Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication (DREAM) trial, the ACE inhibitor ramipril improved the incidence of normoglycaemia, but not the development of diabetes [12]. Because previous observational studies of the effect of RAAS blockade on diabetes risk have been performed in patients with congestive heart failure and during diuretic use, lack of diuretic-induced RAAS activation in the DREAM trial may have diminished the effect of ramipril [31].
Most human studies on the effect of hyperaldosteronism have focused on insulin sensitivity, while none has assessed insulin secretion using hyperglycaemic clamps. Sindelka et al. demonstrated that surgical removal, but not spironolactone treatment, improved insulin sensitivity in patients with aldosterone-producing adenomas [32]. Other studies have yielded mixed findings in patients with primary aldosteronism [32, 33]. Furthermore, studies that have estimated insulin sensitivity by HOMA may be confounded by differences in insulin secretion [34].
To date, few studies have addressed the effects of aldosterone on insulin secretion in pancreatic islets. Pierluissi et al. mentioned that aldosterone (8 nmol/l) reduced insulin secretion by 18% in isolated rat islets compared with controls, but did not assess the effect of aldosterone on the beta cell, the dose–response or the effect of MR antagonism [35]. Our studies expand substantially on these preliminary findings, providing direct evidence that aldosterone affects insulin secretion in vivo and in vitro, and demonstrating that this effect is independent of MR and the glucocorticoid receptor, and is mediated via production of reactive oxygen species. Moreover, studies in the MIN6 cell line suggest that aldosterone exerts this effect directly within beta cells.
In vivo, aldosterone synthase deficiency could affect insulin secretion due to indirect effects of increased angiotensin II or potassium. During normal sodium intake, relative volume depletion and increased angiotensin II in As−/− mice would be expected to decrease islet blood flow and insulin secretion. However, potassium is also elevated under these conditions in As−/− mice and directly stimulates insulin secretion in isolated islets. We controlled for these possible confounders in two ways. First, we studied As−/− mice treated with a high sodium diet, which reduces plasma renin activity, angiotensin II and plasma potassium [14]. In this setting, we found that glucose-stimulated insulin secretion was enhanced further in As−/− mice, even though potassium and plasma renin activity were comparable in wild-type and As−/−mice under these conditions. Second, aldosterone reduced insulin secretion in isolated perifused islets and MIN6 cells, during which potassium concentration and perfusion pressure were held constant. Although circulating glucocorticoids could affect insulin secretion in As−/− mice, we have previously determined that corticosterone is unchanged compared with wild-type littermates [15]. In addition, we used steroid-free culture media during the in vitro studies specifically to avoid the confounding effect of glucocorticoids.
To assess the contribution of the MR to effects of aldosterone on insulin secretion, we examined the abundance and localisation of the MR within pancreatic islets. Chuang et al. have characterised the nuclear hormone receptor mRNA profile in islets and demonstrated expression of MR mRNA within isolated murine and human islets [36]. We also found that Mr mRNA is expressed in pancreatic islets, and localised to a subset of cells in the islet periphery (delta and pancreatic polypeptide cells), rather than to beta cells. The effect of aldosterone on insulin secretion in cultured MIN6 cells demonstrates that aldosterone exerts a direct effect on beta cells, while the low abundance of MR protein in beta cells also suggests an MR-independent mechanism. Our pharmacological studies also confirm that aldosterone regulates insulin secretion via an MR-independent mechanism.
Specifically, the MR antagonists spironolactone, eplerenone and RU-28318 did not prevent inhibition of insulin secretion by aldosterone, suggesting that aldosterone affects insulin secretion via an MR-independent mechanism. The classic aldosterone signalling pathway within the principal cells of the kidney involves MR activation, homo-dimerisation, translocation to the nucleus and increased transcription of target genes. However, aldosterone may also exert non-genomic and MR-independent effects in non-epithelial tissues such as the vasculature, cardiomyocytes and other tissues [37]. The non-genomic effects of aldosterone do not involve DNA transcription, and in some cases are not blocked by MR antagonists [37–41]. The finding that aldosterone attenuated insulin secretion in response to glucose, IBMX and the sulfonylurea tolbutamide suggests that aldosterone exerted an effect downstream from KATP. Moreover, our data suggest that aldosterone impairs insulin secretion by increasing the production of reactive oxygen species. Aldosterone is known to induce oxidative stress in other settings [42], and reactive oxygen species impair insulin secretion [43].
The finding that aldosterone impairs insulin secretion in vitro and in vivo may have broad implications for patients with insulin resistance and impaired glucose tolerance, because impaired beta cell function is a necessary step in progression to type 2 diabetes [10]. Since plasma aldosterone concentrations are inappropriately elevated in obesity [2], aldosterone may be a critical link between obesity and the insulin resistance and impaired insulin secretion that produce overt diabetes. Although previous studies have demonstrated that aldosterone and the MR promote adipocyte differentiation in vitro [44], As−/− mice exhibited no difference in adiposity measures, suggesting that aldosterone deficiency alone may not reduce adipose mass. Rather, it appears that fatderived factors promote aldosterone secretion in the setting of obesity and the metabolic syndrome [6]. We therefore hypothesise that aldosterone could further contribute to diabetes progression by impairing the insulin secretory response. Interestingly, insulin secretion was impaired in a cross-sectional study of patients with aldosterone-producing adenomas, although this correlated with altered serum potassium [45]. More recently, Mosso et al. applied HOMA-2 to a group of patients with primary aldosteronism and found evidence of impaired beta cell function compared with patients with essential hypertension that was independent of serum potassium [11].
Our data may have particular relevance to the use of MR antagonists in diabetic participants and to the ongoing development of aldosterone synthase inhibitors. Treatment with either spironolactone or eplerenone increases plasma aldosterone concentrations, regardless of whether an ACE inhibitor or ARB is co-administered [46]. Glycaemic control worsened during spironolactone treatment in a randomised, placebo-controlled study in diabetic participants [47]. An additional observational study detected an increased incidence of diabetes in spironolactone-treated patients compared with losartan-treated patients [48]. The observation that aldosterone decreases insulin secretion through an MR-independent mechanism may explain these findings [47, 49], and further studies investigating this effect are warranted. During long-term ACE inhibitor or ARB administration, aldosterone increases back to baseline values, despite an initial suppression. Pharmacological agents that specifically target aldosterone synthase reduce plasma aldosterone in humans and could provide a therapeutic alternative in the future [50].
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the excellent technical assistance of T. Ansari and C. Malabanan. We thank A. Golovin at the Vanderbilt University Islet Procurement and Analysis Core for performing islet isolation and assessing islet insulin secretion. We thank the Vanderbilt University Hormone Assay Core for performing insulin assays. We also thank C. Gomez-Sanchez for generously providing anti-MR antibodies. This work was supported by the Juvenile Diabetes Research Foundation International, the VA Research Service, the NIH (DK081662, DK66636, DK69603, DK68854, HL067308, HL060906, HL07738, DK072473, DK089572),the Vanderbilt Mouse Metabolic Phenotyping Center (DK59637) and the Vanderbilt Diabetes Research and Training Center (DK20593).
Abbreviations
- ARB
Angiotensin receptor blocker
- DREAM
Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication
- GTT
Glucose tolerance test
- IBMX
Isobutylmethylxanthine
- IEQ
Islet equivalent
- MR
Mineralocorticoid receptor
- RAAS
Renin–angiotensin–aldosterone system
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00125-011-2158-9) contains supplementary material, which is available to authorised users.
Duality of interest The authors declare that there is no duality of interest associated with this manuscript.
Contributor Information
J. M. Luther, Email: James.Luther@Vanderbilt.edu, Department of Medicine, Division of Clinical Pharmacology, Vanderbilt University Medical Center, 2200 Pierce Ave, 560 RRB, Nashville, TN 37232-6602, USA; Department of Medicine, Division of Nephrology and Hypertension, Vanderbilt University Medical Center, Nashville, TN, USA.
P. Luo, Department of Medicine, Division of Clinical Pharmacology, Vanderbilt University Medical Center, 2200 Pierce Ave, 560 RRB, Nashville, TN 37232-6602, USA
M. T. Kreger, Department of Medicine, Division of Clinical Pharmacology, Vanderbilt University Medical Center, 2200 Pierce Ave, 560 RRB, Nashville, TN 37232-6602, USA
M. Brissova, Department of Medicine Division of Diabetes Endocrinology and Metabolism, Vanderbilt University Medical Center, Nashville, TN, USA
C. Dai, Department of Medicine Division of Diabetes Endocrinology and Metabolism, Vanderbilt University Medical Center, Nashville, TN, USA
T. T. Whitfield, University of Tennessee School of Medicine, Memphis, TN, USA
H. S. Kim, Department of Pathology and Laboratory Medicine, University of North Carolina, Chapel Hill, NC, USA
D. H. Wasserman, Department of Molecular Physiology and Biophysics, Vanderbilt University Medical Center, Nashville, TN, USA NIH – Mouse Metabolic Phenotyping Center, Vanderbilt University Medical Center, Nashville, TN, USA; Diabetes Research Training Center, Vanderbilt University Medical Center, Nashville, TN, USA.
A. C. Powers, Department of Medicine, Division of Diabetes, Endocrinology and Metabolism, Vanderbilt University Medical Center, Nashville, TN, USA Department of Molecular Physiology and Biophysics, Vanderbilt University Medical Center, Nashville, TN, USA; Diabetes Research Training Center, Vanderbilt University Medical Center, Nashville, TN, USA; VA Tennessee Valley Healthcare System, Nashville, TN, USA.
N. J. Brown, Department of Medicine, Division of Clinical Pharmacology, Vanderbilt University Medical Center, 2200 Pierce Ave, 560 RRB, Nashville, TN 37232-6602, USA
References
- 1.Rocha R, Stier CT, Jr, Kifor I, et al. Aldosterone: a mediator of myocardial necrosis and renal arteriopathy. Endocrinology. 2000;141:3871–3878. doi: 10.1210/endo.141.10.7711. [DOI] [PubMed] [Google Scholar]
- 2.Goodfriend TL, Egan BM, Kelley DE. Plasma aldosterone, plasma lipoproteins, obesity and insulin resistance in humans. Prostaglandins Leukot Essent Fatty Acids. 1999;60:401–405. doi: 10.1016/s0952-3278(99)80020-9. [DOI] [PubMed] [Google Scholar]
- 3.Bochud M, Nussberger J, Bovet P, et al. Plasma aldosterone is independently associated with the metabolic syndrome. Hypertension. 2006;48:239–245. doi: 10.1161/01.HYP.0000231338.41548.fc. [DOI] [PubMed] [Google Scholar]
- 4.Ingelsson E, Pencina MJ, Tofler GH, et al. Multimarker approach to evaluate the incidence of the metabolic syndrome and longitudinal changes in metabolic risk factors: the Framingham Offspring Study. Circulation. 2007;116:984–992. doi: 10.1161/CIRCULATIONAHA.107.708537. [DOI] [PubMed] [Google Scholar]
- 5.Tuck ML, Sowers J, Dornfeld L, Kledzik G, Maxwell M. The effect of weight reduction on blood pressure, plasma renin activity, and plasma aldosterone levels in obese patients. N Engl J Med. 1981;304:930–933. doi: 10.1056/NEJM198104163041602. [DOI] [PubMed] [Google Scholar]
- 6.Goodfriend TL, Ball DL, Egan BM, Campbell WB, Nithipatikom K. Epoxy-keto derivative of linoleic acid stimulates aldosterone secretion. Hypertension. 2004;43:358–363. doi: 10.1161/01.HYP.0000113294.06704.64. [DOI] [PubMed] [Google Scholar]
- 7.Conn JW. Hypertension, the potassium ion and impaired carbohydrate tolerance. N Engl J Med. 1965;273:1135–1143. doi: 10.1056/NEJM196511182732106. [DOI] [PubMed] [Google Scholar]
- 8.Colussi G, Catena C, Lapenna R, Nadalini E, Chiuch A, Sechi LA. Insulin resistance and hyperinsulinemia are related to plasma aldosterone levels in hypertensive patients. Diabetes Care. 2007;30:2349–2354. doi: 10.2337/dc07-0525. [DOI] [PubMed] [Google Scholar]
- 9.Catena C, Lapenna R, Baroselli S, et al. Insulin sensitivity in patients with primary aldosteronism: a follow-up study. J Clin Endocrinol Metab. 2006;91:3457–3463. doi: 10.1210/jc.2006-0736. [DOI] [PubMed] [Google Scholar]
- 10.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 11.Mosso LM, Carvajal CA, Maiz A, et al. A possible association between primary aldosteronism and a lower β-cell function. J Hypertens. 2007;25:2125–2130. doi: 10.1097/HJH.0b013e3282861fa4. [DOI] [PubMed] [Google Scholar]
- 12.Bosch J, Yusuf S, Gerstein HC, et al. Effect of ramipril on the incidence of diabetes. N Engl J Med. 2006;355:1551–1562. doi: 10.1056/NEJMoa065061. [DOI] [PubMed] [Google Scholar]
- 13.McMurray JJ, Holman RR, Haffner SM, et al. Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010;362:1477–1490. doi: 10.1056/NEJMoa1001121. [DOI] [PubMed] [Google Scholar]
- 14.Makhanova N, Lee G, Takahashi N, et al. Kidney function in mice lacking aldosterone. Am J Physiol Renal Physiol. 2006;290:F61–F69. doi: 10.1152/ajprenal.00257.2005. [DOI] [PubMed] [Google Scholar]
- 15.Luther JM, Wang Z, Ma J, Makhanova N, Kim HS, Brown NJ. Endogenous aldosterone contributes to acute angiotensin II-stimulated plasminogen activator inhibitor-1 and preproendothelin-1 expression in heart but not aorta. Endocrinology. 2009;150:2229–2236. doi: 10.1210/en.2008-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee G, Makhanova N, Caron K, et al. Homeostatic responses in the adrenal cortex to the absence of aldosterone in mice. Endocrinology. 2005;146:2650–2656. doi: 10.1210/en.2004-1102. [DOI] [PubMed] [Google Scholar]
- 17.Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes. 2006;55:390–397. doi: 10.2337/diabetes.55.02.06.db05-0686. [DOI] [PubMed] [Google Scholar]
- 18.Fueger PT, Hess HS, Bracy DP, et al. Regulation of insulin-stimulated muscle glucose uptake in the conscious mouse: role of glucose transport is dependent on glucose phosphorylation capacity. Endocrinology. 2004;145:4912–4916. doi: 10.1210/en.2004-0465. [DOI] [PubMed] [Google Scholar]
- 19.Berglund ED, Li CY, Poffenberger G, et al. Glucose metabolism in vivo in four commonly used inbred mouse strains. Diabetes. 2008;57:1790–1799. doi: 10.2337/db07-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brissova M, Shiota M, Nicholson WE, et al. Reduction in pancreatic transcription factor PDX-1 impairs glucose-stimulated insulin secretion. J Biol Chem. 2002;277:11225–11232. doi: 10.1074/jbc.M111272200. [DOI] [PubMed] [Google Scholar]
- 21.Miyazaki J, Araki K, Yamato E, et al. Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology. 1990;127:126–132. doi: 10.1210/endo-127-1-126. [DOI] [PubMed] [Google Scholar]
- 22.Brown NJ. Eplerenone: cardiovascular protection. Circulation. 2003;107:2512–2518. doi: 10.1161/01.CIR.0000071081.35693.9A. [DOI] [PubMed] [Google Scholar]
- 23.Garthwaite SM, McMahon EG. The evolution of aldosterone antagonists. Mol Cell Endocrinol. 2004;217:27–31. doi: 10.1016/j.mce.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Gomez-Sanchez CE, de Rodriguez AF, Romero DG, et al. Development of a panel of monoclonal antibodies against the mineralocorticoid receptor. Endocrinology. 2006;147:1343–1348. doi: 10.1210/en.2005-0860. [DOI] [PubMed] [Google Scholar]
- 25.Uehara S, Tsuchida M, Kanno T, Sasaki M, Nishikibe M, Fukamizu A. Late-onset obesity in mice transgenic for the human renin gene. Int J Mol Med. 2003;11:723–727. [PubMed] [Google Scholar]
- 26.Gratze P, Boschmann M, Dechend R, et al. Energy metabolism in human renin-gene transgenic rats: does renin contribute to obesity? Hypertension. 2009;53:516–523. doi: 10.1161/HYPERTENSIONAHA.108.124966. [DOI] [PubMed] [Google Scholar]
- 27.Hitomi H, Kiyomoto H, Nishiyama A, et al. Aldosterone suppresses insulin signaling via the downregulation of insulin receptor substrate-1 in vascular smooth muscle cells. Hypertension. 2007;50:750–755. doi: 10.1161/HYPERTENSIONAHA.107.093955. [DOI] [PubMed] [Google Scholar]
- 28.Lastra G, Whaley-Connell A, Manrique C, et al. Low-dose spironolactone reduces reactive oxygen species generation and improves insulin-stimulated glucose transport in skeletal muscle in the TG(mRen2)27 rat. Am J Physiol Endocrinol Metab. 2008;295:E110–E116. doi: 10.1152/ajpendo.00258.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carlsson PO, Berne C, Jansson L. Angiotensin II and the endocrine pancreas: effects on islet blood flow and insulin secretion in rats. Diabetologia. 1998;41:127–133. doi: 10.1007/s001250050880. [DOI] [PubMed] [Google Scholar]
- 30.Leung PS, Chan WP, Wong TP, Sernia C. Expression and localization of the renin-angiotensin system in the rat pancreas. J Endocrinol. 1999;160:13–19. doi: 10.1677/joe.0.1600013. [DOI] [PubMed] [Google Scholar]
- 31.Scheen AJ. Prevention of type 2 diabetes mellitus through inhibition of the renin-angiotensin system. Drugs. 2004;64:2537–2565. doi: 10.2165/00003495-200464220-00004. [DOI] [PubMed] [Google Scholar]
- 32.Sindelka G, Widimsky J, Haas T, Prazny M, Hilgertova J, Skrha J. Insulin action in primary hyperaldosteronism before and after surgical or pharmacological treatment. Exp Clin Endocrinol Diabetes. 2000;108:21–25. doi: 10.1055/s-0032-1329211. [DOI] [PubMed] [Google Scholar]
- 33.Voiculescu A, Hollenbeck M, Kutkuhn B, Grabensee B, Plum J. Successful treatment of renovascular hypertension has no effect on insulin sensitivity. Eur J Clin Investig. 2003;33:848–854. doi: 10.1046/j.1365-2362.2003.01236.x. [DOI] [PubMed] [Google Scholar]
- 34.Festa A, Williams K, Hanley AJ, Haffner SM. Beta-cell dysfunction in subjects with impaired glucose tolerance and early type 2 diabetes: comparison of surrogate markers with first-phase insulin secretion from an intravenous glucose tolerance test. Diabetes. 2008;57:1638–1644. doi: 10.2337/db07-0954. [DOI] [PubMed] [Google Scholar]
- 35.Pierluissi J, Navas FO, Ashcroft SJ. Effect of adrenal steroids on insulin release from cultured rat islets of Langerhans. Diabetologia. 1986;29:119–121. doi: 10.1007/BF00456122. [DOI] [PubMed] [Google Scholar]
- 36.Chuang JC, Cha JY, Garmey JC, Mirmira RG, Repa JJ. Research resource: nuclear hormone receptor expression in the endocrine pancreas. Mol Endocrinol. 2008;22:2353–2363. doi: 10.1210/me.2007-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lemarie CA, Simeone SM, Nikonova A, et al. Aldosterone-induced activation of signaling pathways requires activity of angiotensin type 1a receptors. Circ Res. 2009;105:852–859. doi: 10.1161/CIRCRESAHA.109.196576. [DOI] [PubMed] [Google Scholar]
- 38.Boldyreff B, Wehling M. Non-genomic actions of aldosterone: mechanisms and consequences in kidney cells. Nephrol Dial Transplant. 2003;18:1693–1695. doi: 10.1093/ndt/gfg265. [DOI] [PubMed] [Google Scholar]
- 39.Hirasawa G, Sasano H, Ki T, et al. Colocalization of 11β-hydroxysteroid dehydrogenase type II and mineralocorticoid receptor in human epithelia. J Clin Endocrinol Metab. 1997;82:3859–3863. doi: 10.1210/jcem.82.11.4337. [DOI] [PubMed] [Google Scholar]
- 40.Losel R, Schultz A, Boldyreff B, Wehling M. Rapid effects of aldosterone on vascular cells: clinical implications. Steroids. 2004;69:575–578. doi: 10.1016/j.steroids.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Gekle M, Grossmann C. Actions of aldosterone in the cardiovascular system: the good, the bad, and the ugly? Pflugers Arch. 2009;458:231–246. doi: 10.1007/s00424-008-0616-0. [DOI] [PubMed] [Google Scholar]
- 42.Briet M, Schiffrin EL. Aldosterone: effects on the kidney and cardiovascular system. Nat Rev Nephrol. 2010;6:261–273. doi: 10.1038/nrneph.2010.30. [DOI] [PubMed] [Google Scholar]
- 43.Drews G, Krippeit-Drews P, Dufer M. Oxidative stress and beta-cell dysfunction. Pflugers Arch. 2010;460:703–718. doi: 10.1007/s00424-010-0862-9. [DOI] [PubMed] [Google Scholar]
- 44.Caprio M, Feve B, Claes A, Viengchareun S, Lombes M, Zennaro MC. Pivotal role of the mineralocorticoid receptor in corticosteroid-induced adipogenesis. FASEB J. 2007;21:2185–2194. doi: 10.1096/fj.06-7970com. [DOI] [PubMed] [Google Scholar]
- 45.Shimamoto K, Shiiki M, Ise T, et al. Does insulin resistance participate in an impaired glucose tolerance in primary aldosteronism? J Hum Hypertens. 1994;8:755–759. [PubMed] [Google Scholar]
- 46.Weinberger MH, White WB, Ruilope LM, et al. Effects of eplerenone vs losartan in patients with low-renin hypertension. Am Heart J. 2005;150:426–433. doi: 10.1016/j.ahj.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 47.Swaminathan K, Davies J, George J, Rajendra NS, Morris AD, Struthers AD. Spironolactone for poorly controlled hypertension in type 2 diabetes: conflicting effects on blood pressure, endothelial function, glycaemic control and hormonal profiles. Diabetologia. 2008;51:762–768. doi: 10.1007/s00125-008-0972-5. [DOI] [PubMed] [Google Scholar]
- 48.Arase Y, Suzuki F, Suzuki Y, et al. Losartan reduces the onset of type 2 diabetes in hypertensive Japanese patients with chronic hepatitis C. J Med Virol. 2009;81:1584–1590. doi: 10.1002/jmv.21572. [DOI] [PubMed] [Google Scholar]
- 49.Strauch B, Widimsky J, Sindelka G, Skrha J. Does the treatment of primary hyperaldosteronism influence glucose tolerance? Physiol Res. 2003;52:503–506. [PubMed] [Google Scholar]
- 50.Amar L, Azizi M, Menard J, Peyrard S, Watson C, Plouin PF. Aldosterone synthase inhibition with LCI699: a proof-of-concept study in patients with primary aldosteronism. Hypertension. 2010;56:831–838. doi: 10.1161/HYPERTENSIONAHA.110.157271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.