Abstract
Understanding the evolutionary adaptation of the immune system to the developing fetus and placenta represents one of the most fascinating problems in reproductive biology. Recent work has focused on how the behavior of dendritic cells (DCs) is altered at the maternal–fetal interface to suit the unique requirements of pregnancy. This work has provided a significant new perspective into the long-standing immunological paradox of fetomaternal tolerance, and has opened up a new and intriguing area of research into the potential trophic role of uterine DCs in the peri-implantation period. Further research on the biology of uterine DCs promises to give insight into the pathogenesis of many clinically important disorders of pregnancy.
Keywords: angiogenesis, decidua, dendritic cells, fetomaternal tolerance, lymphatic vessel, myometrium, pregnancy
The anatomical organization of the hemochorial placenta promotes persistent and intimate contact between maternal immune cells and placental trophoblasts. In women, maternal blood continuously flows over the syncytiotrophoblast cells of the placental villi, whereas in mice this situation is mirrored in the labyrinth portion of the placenta by the flow of maternal blood over a trilayer of mononuclear trophoblasts and syncytiotrophoblast cells. In addition, extra villous cytotrophoblasts in humans and trophoblast giant cells in mice anchor the placenta to the uterus and are therefore in close association with the maternal leukocytes that populate the decidua (i.e., the specialized endometrial stroma that encases the conceptus; for review, see [1]). These trophoblast subpopulations all express tissue-specific proteins that are expected to be foreign to the maternal immune system, and several subpopulations express appreciable levels of paternally derived MHC molecules [2,3]. Thus, the maternal immune system becomes exposed to fetal/placental alloantigens at two distinct interfaces: in the uterus by the apposition between maternal leukocytes with the invading trophoblast, and in the secondary lymphoid organs (i.e., the spleen and lymph nodes [LNs]) upon the shedding of placental material into maternal blood and its circulation throughout the body. According to the tenets of transplantation immunology, such an arrangement should lead to rejection of the fetal ‘allograft’ by maternal T cells. Why this does not normally occur remains a major immunological paradox with important clinical implications for human reproduction.
Critically, the activation of maternal T cells with fetal/placental specificity would be expected to be mediated by a potent type of antigen-presenting cell (APC) known as a dendritic cell (DC). DCs are generally divided into two major classes: those that reside within the spleen and LNs themselves (which are referred to as lymphoid organ-resident DCs), and those that are stationed within nonlymphoid peripheral tissues (which are sometimes called migratory DCs). Both classes are specialized for antigen uptake, processing and peptide presentation on MHC molecules, and both can sense the presence of pathogens and tissue damage through their expression of pattern-recognition receptors such as the Toll-like receptors. However, recent evidence has suggested that migratory DCs are particularly important for priming T-cell responses to peripheral tissue antigens, a process that commences after they migrate via the lymphatic vessels to the draining LNs [4,5]. In this context, the uterine DC population present at the time of implantation might be expected to pose the greatest immunological threat to fetal survival since these cells are the same ones whose sole purpose, prior to pregnancy, is to protect the uterus from infection.
Thus, the question of why the fetal allograft is not rejected by the maternal immune system boils down, to a large extent, to why uterine DCs fail to initiate immunogenic T-cell responses to placental antigens. In this article, we will first review recent work on this question using mice as a model organism. Interestingly, this work has also opened up new avenues of investigation concerning the potential nonimmunological function of DCs in the uterine response to implantation itself, which we will also review. We will then discuss various ways these new results will probably be extended in the near future and their implications for disorders of human pregnancy.
Mouse & human uterine DC subsets
In the mouse, uterine DCs with LN-homing and T-cell-stimulating capacity have been well characterized and can be generally identified as cells expressing high levels of the CD11c and MHCII surface markers while lacking expression of the macrophage marker F4/80 [6,7]. This latter parameter is critical as uterine macrophages do not possess LN-homing capacity yet can also express relatively high CD11c and MHCII surface levels. The cells further divide into two subsets based on the differential expression of CD11b and CD103, and thus resemble DCs present at other mucosal surfaces [8]. In both the pregnant and nonpregnant uterus, both subsets appear immature, as evidenced by their submaximal expression of MHCII and activation markers such as CD86 and CCR7, yet can be activated in situ by systemic adjuvant injection [6]. Interestingly, immunohistochemical studies have suggested that the two DC subsets localize in different microdomains of the midgestational decidua [9]. While this has been linked to the regional expression of different sets of vascular addressins by maternal vessels [10], its functional significance has remained unclear. Similarly, whether the two DC subsets of the mouse uterus have different functions during pregnancy is unknown.
In contrast to the mouse, the identity and maturation status of DCs in the human pregnant uterus has remained somewhat unclear as a result of the lengthy protocols necessary for their isolation (which tend to induce DC maturation) and the different ways the cells have been identified in the literature (for review see [11]). In one early study, DCs isolated from first trimester decidua were identified by flow cytometry as expressing CD83, a marker of mature DCs [12]. These cells possessed potent antigen-presenting capacity ex vivo. Alternatively, the cells were identified as expressing the MHC molecule HLA-DR while lacking expression of several other lineage markers such as CD3 (T cells), CD19 (B cells), CD14 (macrophages) and CD56/CD16 (natural killer [NK] cells) [13,14]. In more recent work, use of BDCA markers divided decidual DCs primarily into the myeloid DC type 1 (MDC1; BDCA-1+CD19−CD14−) and myeloid DC type 2 (MDC2; BDCA-3+CD14−) subsets [15]. In general, there is agreement that plasmacytoid DCs and Langerhans cells are scarce within the decidua. The MDC2 subset is most abundant and the only one that shows enrichment in the human decidua as compared with peripheral blood [15]. Interestingly, this subset it likely related to the CD103+ DCs of the mouse uterus [16,17]. Like mouse decidual DCs, human decidual DCs appear to have an immature phenotype characterized by low expression of the CD80, CD86, CD40 and CD205 markers.
Role of uterine DCs in T-cell recognition of the fetal allograft
Although attempts to document the presentation of fetal/placental antigens during gestation and the induction of alloantigen-specific T cell responses date back to the mid-1990s (for reviews, see [18] and [19]), the origin of the relevant APC, the timing and location of antigen exposure, and the extent of T-cell activation have only recently been clarified. This became possible with the development of a model system based on the use of the Act-mOVA-transgenic mice, which express a transmembrane form of ovalbumin (OVA) under control of the β-actin promoter [20]. The mating of Act-mOVA males to nontransgenic females creates a situation where OVA is expressed exclusively by cells of the conceptus, and thus serves as a surrogate fetal/placental alloantigen. Indeed, invasive and endovascular trophoblasts express particularly high levels of OVA, while expression by labyrinthine trophoblasts is distinctly lower [21]. By using this mating scheme in conjunction with the adoptive transfer of OVA-specific CD4+ (OT-II) and CD8+ (OT-I) T-cell-receptor-transgenic T cells, which allows OVA presentation to be monitored through the induced T-cell-proliferative response, our group and others have directly demonstrated the presentation of fetal/placental antigens to maternal T cells during murine pregnancy [21–23]. Strikingly, OVA-induced T-cell proliferation commenced at midgestation in a relatively synchronous fashion throughout all secondary lymphoid organs, and required that the appropriate MHC restriction elements be present on maternal APCs. Consistent with these results, we additionally showed that maternal T cells are ignorant of intact paternal MHC I molecules over the entire course of gestation, and that OVA-specific T-cell proliferation does not occur within OVA+ implantation sites at a time when presentation has already commenced in the draining LN [21]. Together, these results indicated that the secondary lymphoid organs are the primary sites of fetal/placental alloantigen presentation, and that this presentation is mediated exclusively by APCs of maternal origin.
Provocatively, this initial work also revealed that OVA presentation commenced in the uterine draining LN always approximately 2 days earlier than in the nondraining LN (e.g., the subcutaneous LN) [21]. This observation indicated OVA transport via the regional lymphatics, but left open the question of whether the transport was cell mediated. More recent work from our laboratory indicated that the transport and presentation of fetal/placental antigens is in fact not mediated by uterine DCs [6]. This became apparent through the use of mutant mice lacking the CCR7 chemokine receptor required for DC migration to lymphatic vessels. Thus, when Ccr7-/- females were mated to Act-mOVA males, we observed the same spatiotemporal pattern of OVA presentation to maternal T cells in the draining LN relative to the nondraining LN. Furthermore, we could ascribe this noninvolvement of uterine DCs in T-cell recognition of the fetal allograft to two critical aspects of the pregnant uterus. First, we found that the tissue density of DCs in the decidua progressively declines over the first half of postimplantation development, which leaves very few DCs near the developing placenta and their absence from the placenta itself, at least up to E14.5. Second, we found that those few DCs that were present in the decidua were unable to migrate to the regional LNs, even after we induced their maturation in situ with the strong adjuvant lipopolysaccharide. We furthermore linked the decidual entrapment of uterine DCs to the confinement of uterine lymphatic vessels to the myometrium, the smooth muscle cell layer overlying the decidua. Indeed, myo metrial lymphatic vessels were the only structures expressing CCL21, the CCR7 chemokine ligand critical for directing DC emigration to the draining LNs. This distribution meant that decidual DCs would have to migrate over 1 mm from the periphery of the placenta to reach the nearest lymphatic vessel that would give them access to the draining LN.
Together, these results suggest that the unique immunological status of the fetus and placenta, in comparison to surgical organ transplants, is due in large part to the presence of a specialized stromal tissue (i.e., the decidua) that encases the conceptus and minimizes its immune surveillance by migratory DCs. In the mouse, the DCs in question are exclusively of maternal origin, consistent with the mouse conceptus developing DCs only very late in gestation [24]. However, the migration of human fetal DCs, which may arise as early as week 9 of gestation [25], would also be expected to be limited by the same anatomical and possibly physiochemical barriers that limit the migration of maternal uterine DCs. In the mouse, at least, maternal T cells thus become aware of the conceptus exclusively by spleen- and LN-resident DCs that acquire antigen transported in cell-free form via the blood and regional lymphatic vessels. Presumably, the earlier onset of antigen presentation in the draining versus nondraining LN is due to blood filtration by the spleen, which probably delays access of the shed material to the nondraining LN. The significance of this unusual mode of antigen dissemination will be discussed later.
Lymphatic vasculature of the uterus
If decidual entrapment also represents the normal physiological state of DCs at the human maternal–fetal interface, then pathological alterations that promote their migration to the uterine LN may result in the induction of immunogenic responses towards the developing fetus and thus an adverse pregnancy outcome. Assessing this possibility, however, requires greater insight into the developmental differences between the human and murine decidua. At present, the extent to which the human decidua even contains lymphatic vessels is unclear. Using an in vivo model based on the implantation of chorionic villi under the kidney capsules of severe combined immunodeficiency (SCID) mice, Red-Horse et al. showed that human trophoblast invasion induced the robust formation of vessels expressing the lymphatic marker LYVE-1, suggesting that human placental cells can induce lymphangiogenesis during pregnancy [26]. Consistent with this data, LYVE-1 immunostaining of human uterine sections failed to detect lymphatic vessels in the nonpregnant endometrium but revealed numerous LYVE-1+ vessels in the decidua capsularis and in the regions of the uterus invaded by fetal cytotrophoblasts such as the decidua basalis.
In a more recent study, Volchek et al. provided a detailed histological description of the distribution of the lymphatic vasculature in the human pregnant uterus at different stages of gestation using the lymphatic marker podoplanin [27]. The authors reported abundant lymphatic vessels in areas of nondecidualized endometrium, particularly those surrounding spiral arteries and endometrial glands, and a moderate number of vessels in the myometrium. In contrast to Red-Horse et al., however, this group found the decidua to be largely devoid of lymphatics. Indeed, lymphatic vessels were always absent in areas of decidua surrounding spiral arteries and regressing lymphatic structures were found at the border between nondecidualized endometrium and decidual tissue, suggesting that decidualization may induce the collapse and active destruction of the lymphatic vasculature. Although these discrepancies may in part be explained by the different markers used to define lymphatic endothelial cells, the findings by Volchek et al. suggest a striking parallel in the distribution of the lymphatics between the human and murine uterus [27]. The absence of lymphatic vessels from the decidua may thus represent a universally conserved mechanism to minimize immune surveillance at the maternal–fetal interface.
Antigen-specific functions of uterine DCs in the pre- & peri-implantation periods
Although the aforementioned work has focused on the involvement of DCs in T-cell recognition of the fetal allograft in the postimplantation period, DCs have also emerged as playing a role in the immune response to semen. This was demonstrated by the work of Robertson et al., who used the aforementioned Act-mOVA system to show that exposure of maternal APCs to OVA in the seminal fluid leads to the activation and localized expansion of antigen-specific T cells in the uterine draining LNs [22]. Although activated anti-OVA OT-I T cells isolated from the uterine draining LNs expressed detectable levels of IFN-γ and IL-2 upon in vitro restimulation, these levels were quite low and did not significantly affect pregnancy progression, or embryo viability. More recently, these researchers have suggested that the tolerogenic effect of semen is a property of the seminal plasma and is mediated through the expansion of Treg cells. Thus, the authors observed an expansion of FOXP3+ Tregs in the uterine draining LNs that was accompanied by a comparable increase of these cells in the pre-implantation uterus [28]. The authors speculated that the expansion of Tregs in the pre-implantation period was linked to the presentation of paternal alloantigen in the presence of immune-deviating factors, such as TGF-β, in the seminal plasma.
However, is the accumulation of Tregs in the implantation period critical for the maintenance of early pregnancy? Building on a prior finding that reconstitution of T-cell-deficient mice with Treg-depleted T cells leads to early pregnancy failure [29], Shima et al. recently reported that Treg depletion with anti-CD25 monoclonal antibodies not only induced rapid embryo resorption when the antibodies were given on E4.5 and E7.5, but also prevented implantation when the antibodies were given on E2.5 [30]. Importantly, pregnancy failure occurred with allogeneic but not syngeneic mating combination of inbred mice, suggesting that implantation and early embryonic survival requires the semen-induced generation of alloantigen-specific Tregs. Whether this response requires antigen presentation by uterine DCs that have ingested seminal antigens and then migrated to the LN unknown. Furthermore, it is unclear why Treg depletion has such a strong, and allospecific effect when induced early in gestation, when maternal T-cells appear ignorant of the conceptus itself, but no effect on embryo viability when induced at mid-gestation, when fetal/placental antigens are being presented in the spleen and LN [21,30].
Trophic functions of uterine DCs
Aside their immunological functions, uterine DCs have been recently implicated as playing a key developmental role in mediating embryo–uterus interactions in the peri-implantation period [31,32]. These studies relied upon use of CD11c-DTR transgenic mice, in which the CD11c promoter drives expression of the diphtheria toxin receptor (DTR), and thus renders CD11c-expressing cells susceptible to acute ablation following diphtheria toxin (DT) administration [33]. In Plaks et al., the conditional ablation of uterine DCs just before implantation on E3.5 induced pregnancy failure through effects on both embryo implantation and decidualization [31]. This latter effect was associated with the impaired proliferation and differentiation of endometrial stromal cells, as well as defective angiogenesis characterized by increased vascular permeability and attenuated vessel maturation. These observations were in turn linked to the reduced uterine expression of TGF-β1 and soluble Flt1 (sFlt1), a decoy receptor that antagonizes the activity of the proangiogenic factor VEGF-A. Interestingly, sorted uterine DCs expressed detectable levels of sFlt-1, thus suggesting a possible role for these cells in the modulation of local VEGF-A concentrations. In addition, the authors found that DC depletion after implantation on E5.5 had no effect on decidualization or embryo viability, which indicated that uterine DCs were specifically required during the window of embryo implantation.
Using the same mouse model, Krey et al. similarly reported reduced decidual cell proliferation and altered vascular development upon CD11c+ cell ablation [32]. In addition, the authors found that DT treatment affected the accumulation of mature NK cells in the E7.5 pregnant uterus, suggesting that uterine NK differentiation might be directly influenced by DC-derived signals. This is a significant finding given the well-known importance of uterine NK cell-derived IFN-γ in decidual spiral artery remodeling (for review, see [34]). It is important to note, however, that the higher doses of DT used by Krey et al. could have directly impaired NK cell viability since uterine NK cells express appreciable levels of the CD11c-DTR transgene [31]. On the other hand, Karsten et al. showed that various genetic and pharmacological manipulations that impair the trafficking of CD11c+CD11b+ cells to the pregnant uterus were also associated with lower numbers of uterine NK cells and a reduction in their cell size [35], again supporting the idea that DCs might regulate NK cell maturation. Reductions in the number of decidual CD11c+CD11b+ cells also led to decidual hypocellularity, defects in maternal spiral artery remodeling and lower uterine levels of IFN-γ, all consistent with a loss of uterine NK cell function [34]. These effects were in turn linked to reduced uterine levels of IL-15, a key molecule for uterine NK cell differentiation and maturation (for review, see [34]). In pregnant women, IL-15 has been show to be mainly produced by uterine stromal cells and by the endothelial cells lining the spiral arteries. However, the work of Karsten et al. on mice also revealed IL-15 in protein extracts of isolated uterine CD11c+ cells, and localization studies by the same group showed a physical interaction between decidual NK cells and cells with an apparent phenotype of CD11b+ DCs [9]. Together, these findings highlight the potential importance of DC–NK cross-talk in regulating the vascular remodeling that occurs in the postimplantation period.
Discussion
The aforementioned data offer evidence that the specialized anatomy of the uterus and the stromal response to embryo implantation decrease local DC densities around the conceptus and inhibit DC emigration from the uterus. Together, these phenomena minimize immunogenic T-cell exposure to fetal/placental antigens and thus provide a major mechanism for preventing the immune rejection of the fetus. Furthermore, the discussed data highlight a previously unappreciated nonimmunological trophic function for uterine DCs in embryo implantation and the ensuing decidual reaction. In light of these data, the entrapment of DCs in the decidua acquires a dual significance: on the one hand it prevents DC surveillance of the maternal–fetal interface and on the other it allows for uterine DCs to perform their trophic function. From this perspective, however, the loss of DC tissue densities within the decidua becomes puzzling. If decidual DCs are important for decidualization, then their loss in density would seem likely to increase the risk of early pregnancy failure. Furthermore, low decidual DC densities and decidual DC entrapment would seem to work at cross-purposes with the sine qua non of DCs, that is, to control infection. Thus, the control of DC behavior within the pregnant uterus is likely the result of a series of interlinked evolutionary compromises that balance the competing needs for preventing immunogenic immune response to the fetal allograft, maintaining some level of immune surveillance for pathogens, and the requirement for uterine DCs to promote decidualization itself. Based upon this viewpoint (Figure 1), we will discuss what we believe to be the critical areas in uterine biology relevant to DC behavior that are likely to see substantial progress in the next several years.
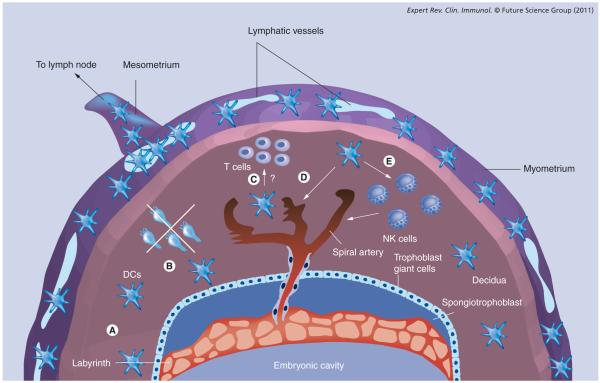
Figure 1. Dendritic cell function at the maternal–fetal interface.
A schematized representation of the pregnant mouse uterus, illustrating the various dimensions of decidual DC function discussed in the text (A–E). Two complementary mechanisms minimize the exposure of fetal/placental alloantigens to T cells: (A) the progressive decline of decidual DC tissue densities over the first half of the postimplantation period; and (B) the inability of the few remaining DCs to migrate to the regional lymph nodes. Decidual entrapment is in turn linked to the lack of lymphatic vessels in the mouse decidua, a feature apparently shared with the human uterus. In mice, uterine lymphatic vessels are confined to the myometrium, at a distance of up to 1 mm away from the surface of the placenta. (C) Interactions between decidual DCs and T cells. In humans, these interactions have been suggested by the clustering of T cells around decidual DCs, but their functional significance is unknown. (D & E) Trophic functions of uterine DCs in decidual vascularization. These functions might include direct effects on decidual vessel maturation (D), or indirect effects, via uterine NK cells, on vascular remodeling. In addition, uterine DCs are thought to be important for initializing the decidual reaction induced by embryo implantation (not depicted).
DC: Dendritic cell; NK: Natural killer.
Uterine lymphangiogenesis & DC trafficking at the maternal–fetal interface
As discussed previously, the absence of lymphatic vessels from both the mouse and human decidua suggests an evolutionarily conserved mechanism for preventing the emigration of decidual DCs to the draining LN, and thus minimizing immunogenic exposure of maternal T cells to fetal/placental antigens. Thus, some of the key immediate goals of reproductive immunology might actually fall into the realm of developmental biology: that is, identifying the pathways that restrict endometrial/decidual lymphangiogenesis and the conditions where dysregulation of these pathways lead to pathology. Interestingly, recent data have suggested that the mouse endometrium lacks lymphatic vessels not merely because it fails to express sufficient levels of the key lymphangiogenesis factors VEGF-C and VEGF-D, rather, when VEGF-D was ectopically expressed by tumor cells introduced into the uterus lumen, myometrial lymphatic vessels hypertrophied but failed to grow into the endometrium [36]. This result suggests that the endometrium either produces a lymphatic vessel chemorepellant, or lacks the appropriate ‘soil’ to sustain lymphatic vessel growth or survival. Distinguishing between these possibilities, as well as identifying underlying molecular players, will have major implications for disorders of human pregnancy, particularly if such disorders are found to be associated with derangements in decidual lymphatics.
Clearly, an overabundance (or the mere presence) of lymphatic vessels in the decidua might allow migratory DCs to initiate immunogenic T-cell responses to fetal/placental antigens. However, as with all organ systems, lymphatic vessels are also likely to have a critical nonimmunological role in removing excess extracellular fluid from the uterus. By extension, it has been speculated that excess amniotic fluid might in part be removed by lymphatic drainage [26]. If this is the case, inadequate numbers of lymphatic vessels in the areas of uterus that neighbor the decidua might be associated with polyhydramnios. This disorder, while frequently associated with congenital abnormalities, in most cases has no known etiology. Taken together, these considerations suggest that the regulation of uterine lymphangiogenesis might be an important new avenue for investigation. Indeed, insight into the molecular basis of the antilymphangiogenic nature of the endometrium/decidua might have broad implications outside the context of pregnancy, as dysregulation of such factors could be easily seen as contributing to tumor metastasis and other pathologies.
Interestingly, subluminal DCs are able to emigrate from the nonpregnant mouse uterus in a CCR7-dependent fashion, even though the endometrium, similar to the decidua, lacks lymphatic vessels [6]. This suggests that CCL21 chemokine gradients can be maintained in the uterus over the approximately 200–400-μm distance that separates the luminal epithelium from the myometrium, or alternatively, that there is a secondary CCR7-independent pathway to first induce the migration of endometrial DCs to the myometrium. Either possibility suggests additional mechanisms that might impair DC emigration from the decidua, aside from its lack of lymphatic vessels. Obviously, if endometrial to myometrial DC migration requires a secondary set of chemo-attractants, the lost production of these factors upon decidualization would limit the set of DCs that are able to exit the decidua to only those submyometrial ones that are still able to sense a CCL21 gradient. Second, decidualization could modify the maintenance of stable CCL21 gradients at the levels of chemokine sequestration by the extracellular matrix, chemokine degradation or chemokine uptake by decoy receptors. Third, even if the necessary chemokine gradients persist in the decidua and can extend all the way to the invasive front of the trophoblast, it is possible that changes in extracellular matrix composition upon decidualization prevent interstitial DC migration. Insight into many of these questions could be gained by the use of live imaging techniques, an experimental modality that has yet to be extensively applied to the study of cell movements within the pregnant uterus.
Control of DC tissue densities at the maternal–fetal interface
As mentioned previously, our recent work has revealed that DCs are present in the mouse decidua at much lower densities than in the undecidualized endometrium, and we speculated that this is a complementary mechanism to minimize immunogenic exposure of T cells to fetal/placental antigens in addition to their decidual entrapment [6]. Thus, understanding how the fetus and placenta avoid rejection would benefit from insight into the regulatory pathways that control decidual DC tissue densities. While currently unknown, these pathways would also be expected to have major effects on the ability of decidual DCs, as a population, to fulfill their trophic functions or contribute towards placental bed immunopathologies, as discussed later. Provocatively, immunohistochemical analyses of first trimester human uterine tissues have suggested that CD83+ DC densities are lower in the areas of fully decidualized stroma as compared with the areas near endometrial glands [12]. Clearly, further work is needed to determine whether this reflects a decidualization-induced decrease in absolute DC density in comparison to the nonpregnant endometrium, which would validate using the mouse as a model organism to study its functional significance. However, understanding how DC tissue densities are established in the decidua will probably have broad implications, even if the mouse and human differ in this one regard.
At steady state, DC tissue densities would be expected to be a function of the key parameters of DC population dynamics – that is, rates of DC emigration, recruitment, in situ proliferation and survival. While DC emigration is well-established to be induced by the chemokine CCL21 expressed by lymphatic vessels, the factors that control the other three parameters have remained obscure. Furthermore, which parameter is rate-limiting for establishing DC tissue densities is unknown. For example, DC lifespan within uninflamed peripheral tissues is thought to be quite long and tissue-resident DCs are not thought to divide rapidly in situ [8]. If they are also entrapped with the human decidua as they are within the mouse, then the recruitment rate of bloodborne DC precursors such as pre-DCs would emerge as the key parameter determining decidual DC tissue densities. Unfortunately, the molecular factors that induce DC precursor extravasation into peripheral tissues are currently unknown. Once these factors are identified, however, their level of expression in the decidua during complicated and uncomplicated pregnancies will be of great potential interest.
Trophic functions of uterine DCs
A third major area ripe for exploration is the potential role of DCs in implantation and decidualization. As discussed previously, the experiments raising this possibility involved acute DC ablation in the peri-implantation period and suggested that uterine DCs regulate decidual angiogenesis. Although there are hints at underlying mechanisms, additional work needs to be performed to delineate exact cellular and molecular pathways and thus substantiate this novel DC function. For example, the extent to which DCs regulate uterine vascular cells directly versus indirectly is currently unclear. Indeed, the idea that DC–NK cell interactions are critical for decidual angiogenesis in the peri-implantation period [32] is inconsistent with the observation that alymphoid Rag2-/- Il2rγ-/- mice show normal implantation rates and progress to approximately E7.5 without any apparent defect in decidualization [37]. Conversely, the idea that uterine DC-derived sFlt1 directly stabilizes the decidual vasculature [31] seems to be inconsistent with the thought that DCs typically regulate angiogenesis through their production of VEGF-A itself [38]. Indeed, the absolute amount of sFlt1 produced by uterine DCs as compared with other cell types at the maternal–fetal interface has also not yet been established. Intriguingly, it has been shown that semen induces uterine Treg expansion [28] and that Treg ablation causes implantation defects and early embryo resorption [30]. While Tregs are not commonly thought to be pro-angiogenic, their increased presence in the peri-implantation uterus might dampen inflammatory processes that would otherwise be detrimental to decidualization. Thus, it will be of interest to determine whether impaired DC–Treg interactions in the peri-implantation period might explain any of the observed defects induced upon DC ablation.
Future work on the role of DCs in implantation and decidualization also needs to address the phenotype of mice lacking Flt3L, the primary DC mitogen and differentiation factor that drives the development of all DC subsets in vivo [39]. These mice show severe reductions in the number of uterine DCs, as expected, but no overt reproductive defects [40]. One possibility to explain this apparent discrepancy is that the uteri of Flt3L-/- mice bear a compensatory DC-like population that can act in place of true uterine DCs. Alternatively, it is possible that the DT-induced sudden death of uterine DCs in the peri-implantation period generates enough local cellular debris or inflammation to nonspecifically disrupt the pathways that regulate decidualization, which is an exquisitely sensitive and highly dynamic developmental process. Even more importantly, it is possible that an ‘off-target’ effect of DT administration to CD11c-DTR mice is the cause of implantation/decidualization failure. This concern arises not only because several non-DC hematopoietic lineages can express CD11c, but also because DT injection can kill CD11c-DTR mice through effects on nonhematopoietic cell types [41].
If uterine DCs are confirmed to function in a trophic fashion during implantation and decidualization, future work also needs to address how this specialized function is itself induced in the peri-implantation period. Importantly, acute DC ablation not only inhibits formation of true, embryo-induced deciduae, but also the formation of artificial deciduomas, which are decidua-like structures that can be induced by the intraluminal infusion of materials such as sesame seed oil [31]. The necessary uterine receptivity for such artificial reactions only requires prior mating to vasectomized males. Thus, it is possible that seminal plasma contains the key factor(s) that instructs uterine DCs to express a trophic response when so triggered by oil or the implanting blastocyst. Indeed, examining the oil-induced behavior of uterine DCs when females are either left unmated or mated to vasectomized males might give insight into the importance of induced uterine receptivity per se in instructing the appropriate DC response upon implantation.
Role of DCs in placental bed immunopathologies
Since migratory DCs play a central role in mucosal host defense, it might be expected that their entrapment within the decidua would severely impair the ability of this tissue layer to initiate immune responses to pathogens. However, the maternal–fetal interface in humans, rather than being a primary site of infection (except in iatrogenic cases), is typically colonized through an ascending route (i.e., via the vagina and cervix) or via the blood. In both cases, nonuterine DCs would likely be able to provide all the key antigen-presenting function for T-cell priming so that any lack of contribution from uterine DCs would not significantly limit the generation of effector T cells.
In addition to their classical role in T-cell priming within lymphoid organs, however, peripheral tissue DCs have also recently been shown to play a local role in perpetuating effector T-cell activity once T cells have reached sites of inflammation [42]. Furthermore, it has been suggested that DC–T-cell interactions within the tissue stroma, if inadequately regulated, might contribute to immunopathology [43]. An evaluation of DC behavior at the maternal–fetal interface from this perspective has not yet been performed, yet might give significant insight into a variety of obstetrical complications. For example, it is possible that the establishment and maintenance of low DC tissue densities in the decidua might be critical not only for minimizing T-cell priming to fetal/placental antigens, as discussed previously, but also for minimizing the effector activity of the sporadic infiltrating T cell. The downside to this arrangement, of course, is that low DC densities might promote the decidua as a sanctuary for pathogens or increase the risk that a pathogen could breach the maternal–fetal interface and infect the fetus and placenta. Indeed, the set-point for decidual DC densities could be viewed as yet another evolutionary compromise between the requirements for reproduction and the requirements for host defense.
By taking this line of reasoning in the opposite direction, it is possible that the overactivity of decidual DCs in either mice or humans might lead to local levels of inflammation that compromise placental development or function. In such cases, DCs could promote immunopathology if they produced more inflammatory mediators on a per cell basis, or simply if their tissue densities became elevated. For example, one recent paper has shown an association between preeclampsia in humans and an increased density of immature DCs in the decidua basalis [44]. It will be worthwhile extending this kind of ana lysis to placental pathologies that involve T-cell accumulation. For example, villitis of unknown etiology is a common pathological finding (5–10% of all human pregnancies) where increased numbers of maternal T cells extend from the basal plate to into the placental villi [45]. This lesion is associated with an increased risk of adverse pregnancy outcome and has been suggested to be a sign of chronic rejection of the fetus and placenta. Thus, it is tempting to speculate that maternal DCs contribute to villitis of unknown etiology through their local activity within the decidua and infiltrated villi. Indeed, decidual T cells have been observed in clusters around decidual DCs in the absence of any placental pathology [12]; however, there is currently little appreciation of the functional consequences of these interactions, and it is unknown whether they involve the presentation of fetal/placental antigens. There is also no direct evidence at present that uterine DCs locally induce antigen-specific tolerogenic T-cell responses in vivo.
Expert commentary & five-year view
Lastly, it is worthwhile considering the implications of the observation that T-cell recognition of placental antigens in the mouse is mediated exclusively by spleen- and LN-resident DCs that have locally acquired these antigens in cell-free form. While this ‘hidden’ maternal–fetal interface (for review, see [46]) should thus be considered as the front line of the adaptive immune response to the fetal allograft, very little is currently known about the properties of the shed placental antigen, or the nature and response of the ingesting DCs. Previously, we showed that the presentation of fetal/placental OVA in the secondary lymphoid organs induced CD8+ T cells to undergo several rounds of division, but not to accumulate in number, produce the cytokines IFN-γ and IL-2, or manifest in vivo cytotoxicity [21]. Instead, the cells underwent clonal deletion. Strikingly, this response was only partially reversed when pregnant mice were treated with strong adjuvants, suggesting that the shed material could induce T-cell tolerance in a dominant fashion. More recently, we linked this property to the ability of the shed placental material to fix complement and hence accumulate on follicular dendritic cells within the B-cell follicles of the spleen and LN, although the ultimate reason for tolerance induction remained obscure [47]. Furthermore, the physical nature of the material remains unclear, although it is tempting to speculate that it shares some commonality with the various species of microvesicle released from human placenta into the maternal blood [48]. Further exploration of this specialized antigen acquisition and presentation pathway, which appears to mediate all T-cell recognition of the conceptus, together with elucidation of the mechanisms that regulate uterine lymphangiogenesis and the roles of DCs in tissue remodeling and placental bed immunopathologies, will have major implications for understanding the overall phenomenon of fetomaternal tolerance and the etiology of compromised reproductive health.
Key issues.
Despite the ample exposure of the maternal immune system to fetal/placental alloantigens during pregnancy, the fetus is not subject to cell-mediated immune rejection as are solid organ transplants. This paradox of fetomaternal immune tolerance remains an outstanding question in reproductive biology and transplantation immunology.
Uterine dendritic cells (DCs) might be expected to pose the greatest immunological threat to fetal survival as the main function of these cells in nonpregnant animals is to protect the uterus from infections.
Recent data from the mouse has suggested that uterine DCs do not initiate immunogenic T-cell responses to fetal/placental antigens, in part because their tissue density progressively declines in the decidua over the course of gestation and, more importantly, because the few remaining DCs at the maternal–fetal interface become entrapped within the tissue. Thus, these cells cannot migrate to the regional lymph node to prime T-cell responses to fetal/placental antigens.
The entrapment of uterine DCs during pregnancy in mice is likely due to the striking lack of lymphatic vessels in the decidua. Decidual lymphatic vessels also appear to be absent from the human decidua, thus suggesting an evolutionarily conserved developmental mechanism for preventing the initiation of immunogenic T-cell responses to fetal and placental antigens.
Aside from their intrinsic immunological function, uterine DCs appear to have a trophic role in early pregnancy through their ability to influence embryo implantation, decidualization and decidual angiogenesis.
Inadequate or inappropriate regulation of decidual DC function or population dynamics might play a role in immunopathologies of the placental bed.
In the absence of DC migration from the maternal–fetal interface, T-cell recognition of the fetus and placenta in the secondary lymphoid organs of the mother becomes mediated exclusively by lymphoid tissue-resident DCs that have ingested placental antigens that are shed into maternal circulation. This pathway is highly tolerogenic for poorly understood reasons.
Acknowledgments
Work in the Erlebacher laboratory was supported by grants from the NIH (RO1AI062980), the American Cancer Society (RSG-10-158-01-LIB), and the Leona M and Harry B Helmsley Charitable Trust.
Footnotes
Financial & competing interests disclosure The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat. Rev. Immunol. 2006;6(8):584–594. doi: 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- 2.Hiby SE, Apps R, Sharkey AM, et al. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J. Clin. Invest. 2010;120(11):4102–4110. doi: 10.1172/JCI43998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madeja Z, Yadi H, Apps R, et al. Paternal MHC expression on mouse trophoblast affects uterine vascularization and fetal growth. Proc. Natl Acad. Sci. USA. 2011 doi: 10.1073/pnas.1005342108. DOI: 10.1073/pnas.1005342108. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Itano AA, McSorley SJ, Reinhardt RL, et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19(1):47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 5.Allenspach EJ, Lemos MP, Porrett PM, Turka LA, Laufer TM. Migratory and lymphoid-resident dendritic cells cooperate to efficiently prime naive CD4 T cells. Immunity. 2008;29(5):795–806. doi: 10.1016/j.immuni.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins MK, Tay CS, Erlebacher A. Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice. J. Clin. Invest. 2009;119(7):2062–2073. doi: 10.1172/JCI38714. •• The authors describe two novel mechanisms for preventing immune surveillance of the maternal–fetal interface: the loss of dendritic cell (DC) tissue density that occurs during decidualization, and the entrapment of decidual DCs.
- 7.Keenihan SN, Robertson SA. Diversity in phenotype and steroid hormone dependence in dendritic cells and macrophages in the mouse uterus. Biol. Reprod. 2004;70(6):1562–1572. doi: 10.1095/biolreprod.103.024794. [DOI] [PubMed] [Google Scholar]
- 8.Helft J, Ginhoux F, Bogunovic M, Merad M. Origin and functional heterogeneity of non-lymphoid tissue dendritic cells in mice. Immunol. Rev. 2010;234(1):55–75. doi: 10.1111/j.0105-2896.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- 9.Behrends J, Karsten CM, Wilke S, Robke A, Kruse A. Identification of ITGA4/ ITGB7 and ITGAE/ITGB7 expressing subsets of decidual dendritic-like cells within distinct microdomains of the pregnant mouse uterus. Biol. Reprod. 2008;79(4):624–632. doi: 10.1095/biolreprod.107.067041. [DOI] [PubMed] [Google Scholar]
- 10.Kruse A, Martens N, Fernekorn U, Hallmann R, Butcher EC. Alterations in the expression of homing-associated molecules at the maternal/fetal interface during the course of pregnancy. Biol. Reprod. 2002;66(2):333–345. doi: 10.1095/biolreprod66.2.333. [DOI] [PubMed] [Google Scholar]
- 11.Kammerer U, Kruse A, Barrientos G, Arck PC, Blois SM. Role of dendritic cells in the regulation of maternal immune responses to the fetus during mammalian gestation. Immunol. Invest. 2008;37(5):499–533. doi: 10.1080/08820130802191334. [DOI] [PubMed] [Google Scholar]
- 12.Kammerer U, Schoppet M, McLellan AD, et al. Human decidua contains potent immunostimulatory CD83(+) dendritic cells. Am. J. Pathol. 2000;157(1):159–169. doi: 10.1016/S0002-9440(10)64527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardner L, Moffett A. Dendritic cells in the human decidua. Biol. Reprod. 2003;69(4):1438–1446. doi: 10.1095/biolreprod.103.017574. [DOI] [PubMed] [Google Scholar]
- 14.Miyazaki S, Tsuda H, Sakai M, et al. Predominance of Th2-promoting dendritic cells in early human pregnancy decidua. J. Leukoc. Biol. 2003;74(4):514–522. doi: 10.1189/jlb.1102566. [DOI] [PubMed] [Google Scholar]
- 15.Ban YL, Kong BH, Qu X, Yang QF, Ma YY. BDCA-1+, BDCA-2+ and BDCA-3+ dendritic cells in early human pregnancy decidua. Clin. Exp. Immunol. 2008;151(3):399–406. doi: 10.1111/j.1365-2249.2007.03576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jongbloed SL, Kassianos AJ, McDonald KJ, et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J. Exp. Med. 2010;207(6):1247–1260. doi: 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edelson BT, Kc W, Juang R, et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8α+ conventional dendritic cells. J. Exp. Med. 2010;207(4):823–836. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moldenhauer LM, Hayball JD, Robertson SA. Utilising T cell receptor transgenic mice to define mechanisms of maternal T cell tolerance in pregnancy. J. Reprod. Immunol. 2010;87(1–2):1–13. doi: 10.1016/j.jri.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Erlebacher A. Immune surveillance of the maternal/fetal interface: controversies and implications. Trends Endocrinol. Metab. 2010;21(7):428–434. doi: 10.1016/j.tem.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ehst BD, Ingulli E, Jenkins MK. Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. Am. J. Transplant. 2003;3(11):1355–1362. doi: 10.1046/j.1600-6135.2003.00246.x. [DOI] [PubMed] [Google Scholar]
- 21.Erlebacher A, Vencato D, Price KA, Zhang D, Glimcher LH. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J. Clin. Invest. 2007;117(5):1399–1411. doi: 10.1172/JCI28214. •• This is the first study that clearly defines the antigen-presentation pathways that mediate T-cell recognition of the fetus and placenta.
- 22.Moldenhauer LM, Diener KR, Thring DM, Brown MP, Hayball JD, Robertson SA. Cross-presentation of male seminal fluid antigens elicits T cell activation to initiate the female immune response to pregnancy. J. Immunol. 2009;182(12):8080–8093. doi: 10.4049/jimmunol.0804018. • First study that shows that paternal antigens present in the seminal fluid can be detected by maternal T cells in an antigen-specific fashion.
- 23.Taglauer ES, Yankee TM, Petroff MG. Maternal PD-1 regulates accumulation of fetal antigen-specific CD8+ T cells in pregnancy. J. Reprod. Immunol. 2009;80(1–2):12–21. doi: 10.1016/j.jri.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dakic A, Shao QX, D’Amico A, et al. Development of the dendritic cell system during mouse ontogeny. J. Immunol. 2004;172(2):1018–1027. doi: 10.4049/jimmunol.172.2.1018. [DOI] [PubMed] [Google Scholar]
- 25.Schuster C, Vaculik C, Fiala C, et al. HLA-DR+ leukocytes acquire CD1 antigens in embryonic and fetal human skin and contain functional antigen-presenting cells. J. Exp. Med. 2009;206(1):169–181. doi: 10.1084/jem.20081747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Red-Horse K, Rivera J, Schanz A, et al. Cytotrophoblast induction of arterial apoptosis and lymphangiogenesis in an in vivo model of human placentation. J. Clin. Invest. 2006;116(10):2643–2652. doi: 10.1172/JCI27306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volchek M, Girling JE, Lash GE, et al. Lymphatics in the human endometrium disappear during decidualization. Hum. Reprod. 2010;25(10):2455–2464. doi: 10.1093/humrep/deq224. • The most comprehensive description of the distribution of lymphatic vessels in the human placenta and decidua.
- 28.Guerin LR, Moldenhauer LM, Prins JR, Bromfield JJ, Hayball JD, Robertson SA. Seminal fluid regulates accumulation of FOXP3+ regulatory T cells in the preimplantation mouse uterus through expanding the FOXP3+ cell pool and CCL19-mediated recruitment. Biol. Reprod. 2011;85(2):397–408. doi: 10.1095/biolreprod.110.088591. [DOI] [PubMed] [Google Scholar]
- 29.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 2004;5(3):266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 30.Shima T, Sasaki Y, Itoh M, et al. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J. Reprod. Immunol. 2010;85(2):121–129. doi: 10.1016/j.jri.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Plaks V, Birnberg T, Berkutzki T, et al. Uterine DCs are crucial for decidua formation during embryo implantation in mice. J. Clin. Invest. 2008;118(12):3954–3965. doi: 10.1172/JCI36682. •• The authors describe a novel role for uterine DCs in implantation, decidualization and decidual angiogenesis in the peri-implantation period.
- 32.Krey G, Frank P, Shaikly V, et al. In vivo dendritic cell depletion reduces breeding efficiency, affecting implantation and early placental development in mice. J. Mol. Med. 2008;86(9):999–1011. doi: 10.1007/s00109-008-0379-2. •• The authors describe a novel role for uterine DCs in implantation, decidualization and decidual angiogenesis in the peri-implantation period.
- 33.Jung S, Unutmaz D, Wong P, et al. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17(2):211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Croy BA, Esadeg S, Chantakru S, et al. Update on pathways regulating the activation of uterine natural killer cells, their interactions with decidual spiral arteries and homing of their precursors to the uterus. J. Reprod. Immunol. 2003;59(2):175–191. doi: 10.1016/s0165-0378(03)00046-9. [DOI] [PubMed] [Google Scholar]
- 35.Karsten CM, Behrends J, Wagner AK, et al. DC within the pregnant mouse uterus influence growth and functional properties of uterine NK cells. Eur. J. Immunol. 2009;39(8):2203–2214. doi: 10.1002/eji.200838844. • The authors identify possible molecular mechanisms by which uterine DCs influence the function of uterine NK cells.
- 36.Girling JE, Donoghue JF, Lederman FL, et al. Vascular endothelial growth factor-D over-expressing tumor cells induce differential effects on uterine vasculature in a mouse model of endometrial cancer. Reprod. Biol. Endocrinol. 2010;8:84. doi: 10.1186/1477-7827-8-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenwood JD, Minhas K, di Santo JP, Makita M, Kiso Y, Croy BA. Ultrastructural studies of implantation sites from mice deficient in uterine natural killer cells. Placenta. 2000;21(7):693–702. doi: 10.1053/plac.2000.0556. [DOI] [PubMed] [Google Scholar]
- 38.Sozzani S, Rusnati M, Riboldi E, Mitola S, Presta M. Dendritic cell–endothelial cell cross-talk in angiogenesis. Trends Immunol. 2007;28(9):385–392. doi: 10.1016/j.it.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 39.McKenna HJ, Stocking KL, Miller RE, et al. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000;95(11):3489–3497. [PubMed] [Google Scholar]
- 40.Tagliani E, Shi C, Nancy P, Tay CS, Pamer EG, Erlebacher A. Coordinate regulation of tissue macrophage and dendritic cell population dynamics by CSF-1. J. Exp. Med. 2011 doi: 10.1084/jem.20110866. DOI: 10.1084/jem.20110866. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zammit DJ, Cauley LS, Pham QM, Lefrancois L. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity. 2005;22(5):561–570. doi: 10.1016/j.immuni.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McLachlan JB, Catron DM, Moon JJ, Jenkins MK. Dendritic cell antigen presentation drives simultaneous cytokine production by effector and regulatory T cells in inflamed skin. Immunity. 2009;30(2):277–288. doi: 10.1016/j.immuni.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hufford MM, Kim TS, Sun J, Braciale TJ. Antiviral CD8+ T cell effector activities in situ are regulated by target cell type. J. Exp. Med. 2011;208(1):167–180. doi: 10.1084/jem.20101850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang SJ, Chen CP, Schatz F, Rahman M, Abrahams VM, Lockwood CJ. Pre-eclampsia is associated with dendritic cell recruitment into the uterine decidua. J. Pathol. 2008;214(3):328–336. doi: 10.1002/path.2257. • The data presented in this paper link preeclampsia to an aberrant accumulation of decidual DCs.
- 45.Benirschke K, Kaufmann P, Baergen RN. Pathology of the Human Placenta. Springer, NY, USA: 2006. [Google Scholar]
- 46.Taglauer ES, Waldorf KM Adams, Petroff MG. The hidden maternal–fetal interface: events involving the lymphoid organs in maternal–fetal tolerance. Int. J. Dev. Biol. 2009;54(2–3):421–430. doi: 10.1387/ijdb.082800et. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCloskey ML, de Lafaille MA Curotto, Carroll MC, Erlebacher A. Acquisition and presentation of follicular dendritic cell-bound antigen by lymph node-resident dendritic cells. J. Exp. Med. 2011;208(1):135–148. doi: 10.1084/jem.20100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Redman CW, Sargent IL. Circulating microparticles in normal pregnancy and pre-eclampsia. Placenta. 2008;(Suppl. 29A):S73–S77. doi: 10.1016/j.placenta.2007.11.016. [DOI] [PubMed] [Google Scholar]