Abstract
Functional neuroimaging studies have implicated the involvement of the amygdala and ventrolateral prefrontal cortex (vlPFC) in the pathophysiology of bipolar disorder. Hyperactivity in the amygdala and hypoactivity in the vlPFC have been reported in manic bipolar patients scanned during the performance of an affective faces task. Whether this pattern of dysfunction persists during euthymia is unclear. Using functional magnetic resonance imaging (fMRI), 24 euthymic bipolar and 26 demographically matched healthy control subjects were scanned while performing an affective task paradigm involving the matching and labeling of emotional facial expressions. Neuroimaging results showed that, while amygdala activation did not differ significantly between groups, euthymic patients showed a significant decrease in activation of the right vlPFC (BA47) compared to healthy controls during emotion labeling. Additionally, significant decreases in activation of the right insula, putamen, thalamus and lingual gyrus were observed in euthymic bipolar relative to healthy control subjects during the emotion labeling condition. These data, taken in context with prior studies of bipolar mania using the same emotion recognition task, could suggest that amygdala dysfunction may be a state-related abnormality in bipolar disorder, whereas vlPFC dysfunction may represent a trait-related abnormality of the illness. Characterizing these patterns of activation is likely to help in understanding the neural changes related to the different mood states in bipolar disorder, as well as changes that represent more sustained abnormalities. Future studies that assess mood-state related changes in brain activation in longitudinal bipolar samples would be of interest.
Keywords: bipolar disorder, amygdala, prefrontal cortex, fmri, emotion
Introduction
Studies utilizing functional MRI (fMRI) in combination with neuropsychological task paradigms have deepened our understanding of the functional abnormalities that characterize bipolar disorder. FMRI studies of mania, including work from our group, have demonstrated decreased functioning of the ventrolateral prefrontal cortices (vlPFC) (Altshuler et al., 2005a; Altshuler et al., 2005b; Blumberg et al., 2003; Elliott et al., 2004; Foland et al., 2008; Mazzola-Pomietto et al., 2009), as well as increased activation of the amygdala (Altshuler et al., 2005a; Bermpohl et al., 2009; Foland et al., 2008). Whether this fronto-limbic dysfunction persists between episodes (i.e. during euthymia), however, is unknown.
Until recently, it was believed that between episodes of depression and mania, patients with bipolar disorder experience symptom resolution and return to a state of relative “normality” (Malhi et al., 2004). However, three independent studies have observed blunted vlPFC activation in euthymic patients relative to healthy controls during the performance of a cognitive interference (Stroop) task (Blumberg et al., 2003; Kronhaus et al., 2006; Malhi et al., 2005). Recent data from our group has also shown diminished activation of the vlPFC in euthymic bipolar subjects scanned during the performance of a behavioral inhibition (Go/No-Go) task (Townsend et al., 2007). These reductions may relate to residual symptoms of impulsivity (Olley et al., 2005; Swann et al., 2001) and emotional dysregulation (Harmer et al., 2002) that have been reported to persist during euthymia.
In contrast, functional neuroimaging studies using emotion perception or emotion regulation tasks that probe activation level of the amygdala have found no evidence for a persistent functional abnormality of this region during euthymia (Hassel et al., 2008; Malhi et al., 2007a; Malhi et al., 2007b; Robinson et al., 2008; Wessa et al., 2007). It is possible therefore, that, whereas vlPFC hypoactivation might represent a trait-related neural disturbance in bipolar disorder, amygdala hyperactivation could be state-related. In the current study, we aimed to further elucidate patterns of neural function in fronto-limbic networks of euthymic bipolar adults using a task that directly probes functioning in these regions (Hariri et al., 2000). Based on prior evidence suggesting that a normalization of amygdala response occurs during remission (Kaladjian et al., 2009) and data from functional neuroimaging studies showing dysfunction of the vlPFC occurs both in mania (Altshuler et al., 2005; Foland et al., 2008) and euthymia (Blumberg et al., 2003; Kronhaus et al., 2006; Malhi et al., 2005; Townsend et al., 2007), we hypothesized that there would be an abnormality in activation of the vlPFC, but not amygdala, in euthymic bipolar subjects compared to healthy controls during an emotion identification task.
Materials and Methods
Subjects
The Institutional Review Boards at UCLA and the VA Greater Los Angeles Healthcare System approved the study. Each subject provided written informed consent. Subjects with bipolar I disorder were recruited through the outpatient UCLA Mood Disorders Clinic, and the outpatient Bipolar Disorders Clinic of the Veterans Affairs Greater Los Angeles Health Care System. Control subjects were recruited by advertisement in local newspapers and campus flyers. All subjects were evaluated using the Structured Clinical Interview for DSM-IV (SCID) to confirm diagnosis or absence thereof. Illness duration and medication information for patients was obtained by self-report and by reference to medical records when available. Exclusion criteria for all subjects included left-handedness, hypertension, neurological illness, metal implants and a history of skull fracture or head trauma with loss of consciousness >5 minutes. Bipolar subjects with other active Axis I co-morbidities were also excluded. Other exclusionary criteria for healthy controls included current or past psychiatric diagnoses (including history of substance abuse) or current medication use. Euthymia was defined by lack of meeting criteria for a current manic, hypomanic or depressive mood episode according to the SCID, as well as having a Young Mania Rating Scale (YMRS; Young et al., 1978) score of ≤7, and a 21-item Hamilton Depression Rating Scale (HAM-D; Hamilton, 1960) score of ≤7 on the day of scanning.
In total, 24 subjects with bipolar type I disorder (9f; 38.8±12.8 years), currently euthymic, and 26 demographically comparable healthy control subjects (11f; 37.9±13.3 years) were included in the study. Demographic and clinical characteristics for all subjects are presented in Table 1. No patients were taking lithium medication; 8 (33%) were taking no medications. The remaining 16 bipolar subjects were on a range of medications including anticonvulsants (n=10, 42%; divalproex sodium, lamotrigine, oxcarbazepine), antipsychotics (n=11, 46%; aripiprazole, olanzapine, quetiapine, risperidone) or antidepressants (n=6, 25%; bupropion, SSRIs) to treat their bipolar illness.
Table 1.
Subject demographics
| Demographic variable | Subjects with bipolar disorder (N=24) |
Normal controls (N=26) |
Group difference |
|---|---|---|---|
| Age (Mean ± SD) | 38.8±12.8 | 37.9±13.4 | p=0.774 |
| % Female (n) | 37.5 (9) | 42.3 (11) | p=0.779 |
| Education levela | 3.19±0.63 | 3.04±0.55 | p=0.486 |
| Race | |||
| % Caucasian (n) | 83.3 (20) | 65.4 (17) | p=0.076 |
| % Asian (n) | 16.7 (4) | 11.5 (3) | - |
| % African American (n) | 0 (0) | 19.3 (5) | - |
| % Other (n) | 0 (0) | 3.8 (1) | - |
| % right handed | 100 (24) | 100 (26) | p=1.00 |
| HAMDb | 4.6±2.1 | - | - |
| YMRSc | 1.5±1.9 | - | - |
| Duration of euthymia (months) | 15.0±19.1 | - | - |
| Duration of Illness (years) | 21.2±14.8 | - | - |
| Age at Onset | 16.9±7.4 | - | - |
| Prior Manias | 6.3±8.7 | - | - |
| Prior depressions | 9.6±10.7 | - | - |
| Prior hospitalizations for mania | 1.7±1.8 | - | - |
| Prior hospitalizations for depression |
1.0±1.4 | - | - |
| Duration of illness untreated | 9.4±11.0 | - | - |
| Months Euthymic | 14.9±19.6 | - | - |
Educational level for each subject was rated on a four-point scale (1, grade 8 or less; 2, grade 9–12; 3, 1–4 years of college or university; 4, four or more years of college or university);
Hamilton Depression Rating Scale;
Young Mania Rating Scale. Months euthymic indicated time euthymic prior to scanning. All p values indicate 2-tailed significance levels.
Experimental paradigm
The affective task paradigm used during fMRI scanning has been detailed previously (Hariri et al., 2000; Lieberman et al., 2007) and used in prior studies by our group examining bipolar subjects scanned during the manic mood state (Altshuler et al., 2005a; Altshuler et al., 2008; Foland et al., 2008). Briefly, this task includes several conditions, one of which robustly activates the amygdala (Hariri et al., 2000; Lieberman et al., 2007) and another of which robustly activates the vlPFC (Hariri et al., 2000; Lieberman et al., 2007). The amygdala activating, or “match emotion” condition requires subjects choose one of two emotional faces at the bottom of the screen that best matches the emotional expression of a face at the top of the screen. The vlPFC activating, or “label emotion” condition requires subjects choose one of two words at the bottom of the screen that best describes the expression of the emotional face at the top of the screen. The control (“match forms”) condition requires subjects to match one of two geometric shapes at the bottom of the screen to a target shape at the top of the screen. A fourth “label gender” condition requires subjects choose one of two names on the bottom of the screen that best matches the gender of the emotional face at the top of the screen. The entire behavioral paradigm included 2 “match emotion” blocks, 2 “label emotion” blocks, 2 “label gender” blocks and 6 “control” blocks. Each block lasted 35 seconds and began with a 3 second instruction cue, followed by 8 4-second trials. The order of task presentation was balanced across subjects and subjects responded by pressing one of two buttons with their right hand.
In the current study, the contrast of “match emotion” versus “match forms” was used for the analysis of between-group differences in activation of the amygdala, given that this task robustly activates this structure (Hariri et al., 2000; Lieberman et al., 2007). The “label emotion” versus “match forms” contrast was used for our analysis of between-group differences in activation of the vlPFC, given that several studies (Hariri et al., 2000; Lieberman et al., 2007) have shown that labeling emotional faces, but not matching the emotion or labeling the gender of emotional faces drives activation in this region.
Image acquisition
Functional MRI data were collected using a 3.0 Tesla (T) Siemens Allegra MRI scanner (Milwaukee, WI) using a gradient echo EPI sequence (28 slices; FOV=200mm, slice thickness=3mm, TR/TE=2500/25ms, FA=90°). A structural T2-weighted volume (FOV=200mm, slice thickness=3mm, TR/TE=5000/34ms, FA=90°) was acquired coplanar to functional scans.
High-resolution T1-weighted scans were also collected for each subject on a separate 1.5T Siemens Sonata MRI scanner (Milwaukee, WI; 160 slices; FOV=256mm, slice thickness=1mm, TR/TE=1900/4.38ms, FA=15°) for an anatomy-based region of interest (ROI) analysis of the amygdala (see below).
Analysis of demographic variables
Statistical analysis of demographic variables was performed using the STATA statistical software package (www.stata.com). Group differences in categorical and continuous demographic variables were computed using 2-tailed Fisher’s exact and independent t-tests, respectively. The threshold for statistical significance was set to α=0.05.
Behavioral data analyses
Response times and accuracy of performance were recorded for patients and control subjects for the “match emotion”, “label emotion” and “match forms” conditions. Differences between groups were assessed separately for each condition using individual 2-tailed t-tests. The threshold for statistical significance was set to α=0.05.
fMRI data analyses
Analyses of functional neuroimaging data were performed using FEAT (FMRI Expert Analysis Tool) Version 4.1.4, part of FSL (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl). The first two volumes of each subject’s functional scans were discarded to allow for T1 equilibrium effects. The remaining images were realigned to compensate for small head movements (Jenkinson et al., 2002). Data were spatially smoothed using a 5 mm full-width-half maximum Gaussian kernel, temporally high-pass filtered to correct for baseline drifts, and prewhitened (Woolrich et al., 2001). Functional datasets were individually registered to participants’ coplanar anatomical images using a 6-parameter transformation. Functional data were then registered into Montreal Neurological Institute (MNI) space using a 12-parameter transformation (Jenkinson and Smith, 2001). Once in common space, functional imaging data were analyzed statistically using the general linear model (GLM), and a design matrix was generated with a synthetic hemodynamic response function and its first derivative. This provided individual contrast maps for the “match emotion” compared with the “match forms” condition and “label emotion” compared with the “match forms” condition. Higher-level group analyses were performed using FLAME (FMRIB’s Local Analysis of Mixed Effects) (Beckmann et al., 2003; Woolrich et al., 2004). For the voxel-wise analysis of activation within- and between-groups, we report brain regions visible at a height threshold of Z>1.96 and a cluster probability of p<0.05, corrected for whole-brain multiple comparisons using Gaussian random field theory (GRFT). MNI voxel coordinates of peak activations were transformed to Tailarach space using the MNI to Talairach Conversion Applet (www.bioimagesuite.org).
Given our a priori hypotheses regarding the amygdala and vlPFC, ROI-based analyses of these regions were also conducted to obtain more sensitive measures of activation, and to facilitate additional analyses exploring the association between activation in these regions and task type (“match emotion” versus “label emotion”), medication status (on versus off medications and medication type) and other clinical variables (further detail on each of these separate analyses is provided below). Amygdala ROIs were manually delineated on each subject’s high-resolution MPRAGE 1.5T scan by a trained neuroanatomist (C.P.), using a previously validated protocol (Bartzokis et al., 1993). These ROIs were spatially registered to subjects’ fMRI data using a 6-parameter linear transformation to the T2-weighted 3T structural scan, acquired coplanar to functional images (Jenkinson and Smith, 2001). vlPFC ROIs consisted of 8-mm spheres centered on the peak voxel showing a between-group difference in activation of this region in a previous study of bipolar mania using this task (Foland et al., 2008; left −34, 26, −8; right: 36, 30, −10). Percent signal change, visible at a height threshold of Z>1.96 and a corrected cluster probability of p<0.05, was extracted from each region using featquery (part of FSL, www.fmrib.ox.ac.uk/fsl/feat5/featquery.html); percent signal change was extracted from each non-zero voxel within each region, then averaged across voxels to provide a regional activation value for each region of every subject, for each condition. Mean percentage signal change for the “match emotion” and “label emotion” conditions relative to the “match forms” condition was then entered into a repeated measures GLM modeling the effects of both within subjects factors (hemisphere, region, condition) and the between subjects factor (diagnostic group) to assess the main effects of each variable and their interactions.
Examination of medication effects
The impact of current medications on functional activation was explored through direct comparisons between patients who were not treated with medications (n=8) and those who were treated (n=16) at the time of scanning using a voxel-wise analysis in FSL. A height threshold of Z>1.96 and a corrected cluster probability of p<0.05 were used to identify significant group differences. Additional analyses exploring both the main effect of medication (on versus off medication) and medication type (anticonvulsants, antipsychotics, antidepressants) were conducted using 2-tailed t-tests on ROI-based estimates of percent signal change. The threshold for statistical significance was set to α=0.05.
Correlation with prior course of illness
Associations between activation and prior course of illness variables (prior number of depressive episodes, prior number of manic episodes, prior number of hospitalizations for depression, prior number of hospitalizations for mania, duration of time between diagnosis and first medication treatment, age of onset and illness duration) and mood symptoms (HAMD and YMRS score) were explored within patients using correlation analyses with ROI-based estimates of amygdala and vlPFC percent signal change. The threshold for statistical significance was set to α=0.05.
Results
Subject demographics
Bipolar subjects did not differ significantly from healthy controls in age, gender, educational level or race (Table 1).
Behavioral responses
No significant between-group differences were found for response times or accuracy of responses during performance of the “match emotion,” “label emotion” or “match forms” conditions. Accuracy of responses for the control and euthymic bipolar groups were 93±7% and 92±6% (p=0.77), respectively, for the “match emotion” condition, 98±4% and 98±6% (p=0.98), respectively, for the “label emotion condition, and 92±2% and 92±3% (p=0.93) respectively, for the “match forms” (control) condition. Reaction time of responses for the control and euthymic bipolar groups were 1.88±0.29 and 1.82±0.26 seconds (p=0.50), respectively, for the “match emotion” condition, 1.66±0.30 and 1.77±0.31 seconds (p=0.21), respectively, for the “label emotion” condition, and 1.08±0.18 and 1.11±0.24 seconds (p=0.58), respectively, for the “match forms” (control) condition.
fMRI responses: within groups
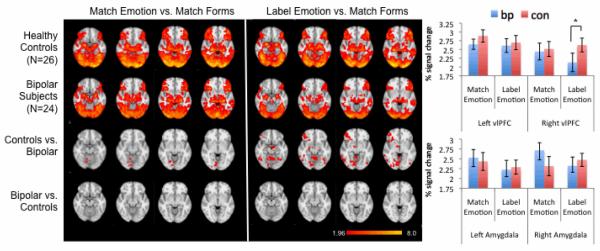
Figure 1 displays the within-group regional patterns of activation for both the “match emotion” versus “match forms” and “label emotion” versus “match forms” contrasts. Consistent with previous studies using this paradigm, healthy controls demonstrated robust activation of the amygdala during the “match emotion” condition and robust activation of the vlPFC during the “label emotion” condition. These patterns of activation were further reflected in ROI-based analyses (Figure 1), although no significant differences in activation were observed within regions, between task types (“match emotion” versus “label emotion”) for either group. Other brain regions activated by both control and bipolar subjects are shown in Figure 1 and included areas typically involved in the higher-order processing of emotional face stimuli such as the anterior cingulate cortex (ACC; BA24/32; x/y/z Talairach coordinates: 8, 20, 41; Z=5.41) and fusiform gyrus (x/y/z Talairach coordinates −38, −51, −11; Z=5.64; 40,−62,−10; Z=6.30).
Figure 1.
Left: within- and between-group activation patterns during the matching and labeling of emotional facial expressions. Images are shown in radiological convention (left is right). Color bar indicates Z score. Right: Percent signal change, extracted from the amygdala and vlPFC during these task conditions are shown in bar graphs. Error bars represent standard error of the mean (s.e.m.).
fMRI responses: between groups
No significant between-group differences were observed in the voxel-wise analysis of brain function during the contrast of “match emotion” versus “match forms” (Figure 1). This pattern was further confirmed in ROI-based analyses of the amygdala and vlPFC. Significant between-group differences in activation, however, were observed in the voxel-wise analysis of “label emotion” versus “match forms.” Specifically, reductions in activation were present in euthymic patients relative to healthy controls in right ventrolateral PFC (BA47), as well as in right insula, putamen, thalamus and lingual gyrus (Figure 1, Table 2). No areas of increased activation were observed in euthymic patients relative to healthy controls for either contrast. These voxel-based findings were confirmed in ROI-based analyses of the amygdala and vlPFC; a significant interaction of diagnosis by hemisphere by region by condition (p=0.0072) was observed that was driven by a significant decrease in percent signal change in the right vlPFC, but not amygdala, of euthymic patients relative to healthy controls for the “label emotion” versus “match forms” contrast (but not the “match emotion” versus “match forms” contrast).
Table 2.
Between-group differences in activation for the “label emotion” versus “match forms” contrast.
| Region | BA | maximally activated voxel coordinates | Z score | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Inferior frontal gyrus | 47 | 42 | 40 | −8 | 4.35 |
| 47 | 34 | 30 | −5 | 3.17 | |
| 47 | 30 | 28 | −4 | 3.15 | |
| Lingual gyrus | 18 | 6 | −75 | 6 | 4.35 |
| Middle frontal gyrus | 8 | 36 | 14 | 44 | 3.51 |
| insula | 32 | 15 | 2 | 3.21 | |
| putamen | 21 | 3 | 5 | 3.12 | |
| thalamus | 9 | −7 | 5 | 3.18 | |
| hypothalamus | 7 | 8 | −3 | 2.96 | |
BA, Brodmann Areas; Z values indicate significance levels; voxel coordinates are in Talairach space.
Examination of medication effects
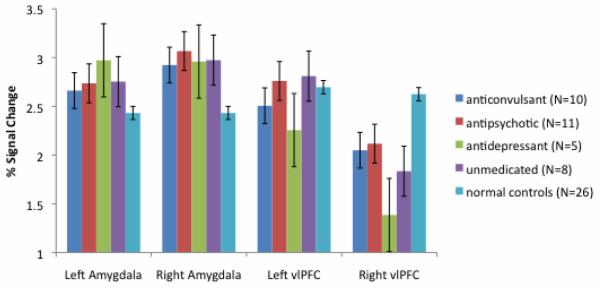
No significant differences in activation were observed between medicated and unmedicated patients in either voxel-based or ROI-based analyses. Additionally, statistical analyses showed medication type (anticonvulsants, antipsychotics or antidepressants) did not significantly impact activation level in the amygdala or vlPFC (all p’s>0.05; Figure 2).
Figure 2.
Percent signal change values extracted from the amygdala and vlPFC during the matching and labeling of emotional facial expressions, respectively, in euthymic bipolar subjects treated with anticonvulsants (N=10), antipsychotics (N=11), antidepressants (N=5) or no medications (N=8) at the time of scanning. Error bars represent standard error of the mean (s.e.m.). Signal change in unmedicated euthymic subjects did not differ from that of subjects treated with any one medication type (all p’s >0.05).
Correlation with prior course of illness and mood symptoms
No significant correlations were observed between prior course of illness and activation of the amygdala or vlPFC in either voxel-based or ROI-based analyses of these regions. Exploratory ROI –based analyses examining associations between activation and current mood ratings, however, revealed significant positive correlations between HAMD scores and bilateral amygdala activation during emotion matching (left: r=0.81, p<0.001; right: r=0.55, p=0.006), but not emotion labeling (p’s>0.2), and significant negative correlations between YMRS scores and bilateral vlPFC activation during emotion labeling (left: r=−0.51, p=0.012; right: r=−0.42, p=0.046), but not emotion matching (p’s>0.1).
Discussion
To our knowledge, this is one of the first studies to report activation on the amygdala and prefrontal brain regions during both emotion perception and emotion labeling tasks in bipolar euthymic adults. Our findings in healthy controls support previous reports (Foland et al., 2008; Hariri et al., 2000; Lieberman et al., 2007) showing robust bilateral amygdala and vlPFC activation during the matching and labeling of emotional faces, respectively. Our findings in patients are also consistent with previous reports of bipolar disorder showing no evidence for an abnormality in amygdala function during euthymia (Hassel et al., 2008; Malhi et al., 2007a; Malhi et al., 2007b; Robinson et al., 2008; Wessa et al., 2007). And these data extend prior reports by demonstrating, for the first time to our knowledge, decreased activation of the vlPFC in euthymia during an emotion labeling task, a higher-order cognitive task that has been directly linked to the implicit regulation of emotion (Hariri et al., 2000; Lieberman et al., 2007).
Using the same “match emotion” task, our group has previously reported heightened activation of the amygdala in bipolar individuals scanned during mania (Altshuler et al., 2005a; Foland et al., 2008). These findings, taken both in context with the normal amygdala reactivity observed here, and in previous studies (Hassel et al., 2008; Malhi et al., 2007a; Malhi et al., 2007b; Robinson et al., 2008; Wessa et al., 2007) could suggest that there is a state-dependent hyperactivation of this region. Such an interpretation is speculative given the cross-sectional nature of our findings. Nevertheless, such a view is consistent with a recent study by Kaladjian et al. (2009), who tracked patients longitudinally and found significant reductions in amygdala activation when patients transitioned from mania to euthymia. Additional longitudinal studies, therefore, would be helpful in further delineating state- versus trait-related abnormalities in amygdala function in bipolar disorder.
We also found that vlPFC activation was significantly reduced in euthymic bipolar subjects compared to healthy controls. These findings are in line with a previous study by our group that used the same “label emotion” task to probe functioning level of this brain region in bipolar mania (Foland et al., 2008), and thus add to the existing literature suggesting a continued deficit in prefrontal function in bipolar subjects (Altshuler et al., in preparation; Blumberg et al., 2003; Kronhaus et al., 2006; Malhi et al., 2005). As the vlPFC is a brain area that is critical in the integration of emotional information and the regulation of the intensity of emotional responses (Cabeza and Nyberg, 2000; Fuster, 1989), dysfunction in this region could provide a mechanism for the failure of manic and euthymic patients to regulate mood appropriately (Harmer et al., 2002). Deficits in activation of this brain region could also relate to residual symptoms of impulsivity (Olley et al., 2005; Swann et al., 2001) that are reported to persist across mood states. Future neuroimaging studies of bipolar euthymia that involve tasks of motor control, response inhibition, control over risky behavior, the ability to delay gratification, or the effortful regulation of emotion or pain could address whether similar deficits are present in the vlPFC. Indeed, recent unpublished observations from our group show euthymic bipolar individuals have blunted activation in the vlPFC, bilaterally, compared to healthy controls, during the performance of a Go/No-Go task (Townsend et al., submitted). Although activation deficits in this unpublished study and activation deficits in the current study do not show clear associations with task performance, future investigations that assess the relation between vlPFC activation and the self-control behaviors subserved by this brain region, perhaps as measured using experience sampling methods, would be of interest.
Reasons for the persistent reduction in neural activation in the vlPFC during euthymia are not clear. It is possible that structural deficits in vlPFC gray matter may contribute to reduced activation. Indeed, a reduction in gray matter thickness in BA47 has been reported in at least three prior structural neuroimaging studies of bipolar disorder (Lyoo et al., 2004; Lyoo et al., 2006; Stanfield et al., 2009). Whether there is a direct and overlapping relationship between brain structure and function in this region within the same sample of bipolar subjects however is uncertain, and is the current focus of ongoing investigations by our group.
Although all subjects in the current patient sample were euthymic at the time of scanning, significant correlations were observed between HAMD scores and bilateral amygdala activation during emotion matching (left: r=0.81, p<0.001; right: r=0.55, p=0.006), and between YMRS scores and bilateral vlPFC activation during emotion labeling (left: r=−0.51, p=0.012; right: r=−0.42, p=0.046). These findings could suggest that mood symptoms, including those that are not severe enough to meet diagnostic criteria for a mood episode, could influence fronto-limbic function. These findings could also have important prognostic implications for patients; subthreshold mood symptoms, especially depressive symptoms, are common in bipolar disorder, and can be predictive of relapse into a future mood episode (Marangell, 2004). Future prospective studies, therefore, that examine whether neural activations during remission are associated not only with mood symptoms but with the onset of future mood episodes would be of interest. This is the first report, to our knowledge, to find correlations between mood symptoms and activation in a euthymic bipolar sample; future studies that replicate these findings would be helpful in ensuring their validity.
Only one study to our knowledge has used the emotion identification task in the study of bipolar euthymia. In this study, no differences were observed between groups in activation of the amygdala. Significant increases in activation of the vlPFC, however, were found in euthymic patients relative to controls (Robinson et al., 2008). This result contrasts with our own. Differences in study findings may be due to differences in statistical analyses; the authors of this prior report examined vlPFC activation occurring across the “match emotion” and “label emotion” task conditions, combined against activation occurring during the control condition. These differences may also be due to variations in the clinical profiles of the euthymic subjects; 60% of patients in the prior report were experiencing comorbid anxiety at the time of scanning, whereas only 4% of patients in the current study reported anxiety. Future investigations that directly examine the effects of comorbid anxiety on brain function in a sample of euthymic individuals could more accurately address this latter possibility.
Group differences in functioning of the vlPFC and amygdala were the primary focus of this study. However several other regions were found to be differentially activated between groups during the contrast of “label emotion” versus “match forms.” Decreased right putamen activation, seen here in euthymic bipolar subjects, has been demonstrated in a previous study using the emotional Stroop task (Malhi et al., 2005). Reduced activation in the right thalamus was also previously found in a sample of bipolar manic subjects (Strakowski et al., 2008) suggesting dysfunction of these subcortical structures may persist across different mood states. Our finding of reduced activation in right dorsal frontal cortex (BA8) has previously been reported in euthymic patients during tasks of working memory (Townsend et al., in press) and reduced activation in the right lingual gyrus, a region that is known to be involved in face recognition and processing of visual emotional stimuli (Mobbs et al., 2004), has been previously found in euthymic bipolar subjects as well (Malhi et al., 2005). The meaning of these findings and the relevance of the lateralization to the clinical presentation of bipolar disorder remain to be understood.
Our data should be interpreted in light of several limitations. First, only a small number of patients in the current study were unmedicated at the time of scanning (N=8, 33%), and the impact of medication treatment on brain function is largely unexplored. It is possible that amygdala response, observed here and in our previous studies of mania that have used this task (Altshuler et al., 2005a; Foland et al., 2008), may have been affected by medications. However, given that pharmacologic treatment did not reduce activation globally in our analysis of medicated versus unmedicated patients, and given that medication type did not appear to significantly impact percent signal change in this area (or in the vlPFC), we feel it unlikely that this factor had a significant confounding effect. Nevertheless, because the number of unmedicated subjects in the current study was small, we had limited power however to examine this issue. Second, reduced activation of the vlPFC of euthymic bipolar subjects may be the result of their attending less to the task; however, accuracy and response times suggest that all subjects were attending appropriately. Moreover, activity in the fusiform gyrus, a region that responds to the viewing of faces, and that can be directly modulated by attention (Pessoa et al., 2002), was not significantly different between groups. Third, given prior evidence for functional abnormalities in the amygdala and vlPFC in manic subjects scanned using this task, we have interpreted our findings, in part, to mean that there may be a state-related abnormality in amygdala function, whereas functional abnormalities in the vlPFC may be trait-related. However, only future studies, which measure brain activation within subjects in different mood states would be able to tease apart state- versus trait-effects on brain function in bipolar disorder.
In summary, we found no significant differences in amygdala activation between groups using an emotion perception task, however significant reductions in activation were found in the right vlPFC of euthymic bipolar subjects compared to healthy controls using an emotion labeling task. Taken in context with prior studies of bipolar mania and euthymia, our findings support the possibility that amygdala dysfunction may represent a state-marker of bipolar illness, whereas vlPFC dysfunction may be independent of mood state and represent a trait-marker of the illness. Understanding these state- versus trait-related differences is of profound significance, both in terms of understanding the neuropathophysiology of the changing mood-states in bipolar illness, as well as understanding the stable, enduring (mood independent) abnormalities. Future studies, therefore, that assess the impact of mood state on activation in longitudinal samples are needed.
Highlights.
We compare activation between euthymic bipolar and control subjects using fMRI.
Amygdala reactivity did not differ between euthymic bipolar and control subjects.
PFC activation was lower in euthymic bipolar compared to control subjects.
Dysfunction of the PFC may represent a trait marker of bipolar illness.
Dysfunction of the amygdala may represent a mood-state marker of bipolar illness.
Acknowledgements
This work was supported by grants from the National Institute of Mental Health (MH078556 to LCFR, and MH075944 and MH01848 to LLA). Additional support for algorithm development was provided by the National Institute on Aging (NIA), the National Institute of Biomedical Imaging and Bioengineering (NIBIB), and the National Center for Research Resources (NCRR; AG016570, EB01651, RR019771 to PT). For their generous support, the authors also thank the National Association for Research on Schizophrenia and Affective Disorders (NARSAD), Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, The Ahmanson Foundation, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, Tamkin Foundation, Jennifer Jones-Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family and Northstar Fund. The project was also supported by Grant Numbers RR12169, RR13642 and RR00865 from the NCRR. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no competing financial interests.
References
- Altshuler L, Bookheimer S, Proenza M, Townsend J, Sabb F, Firestine A, et al. Increased amygdala activation during mania: a functional magnetic resonance imaging study. Am J Psychiatry. 2005a;162:1211–1213. doi: 10.1176/appi.ajp.162.6.1211. [DOI] [PubMed] [Google Scholar]
- Altshuler L, Bookheimer S, Townsend J, Proenza M, Eisenberger N, Sabb F, et al. Blunted activation in orbitofrontal cortex during mania: a functional magnetic resonance imaging study. Biol Psychiatry. 2005b;58:763–769. doi: 10.1016/j.biopsych.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Altshuler L, Bookheimer S, Townsend J, Proenza M, Sabb F, Mintz J, et al. Regional brain changes in bipolar I depression: a functional magnetic resonance imaging study. Bipolar Disorders. 2008;10:708–717. doi: 10.1111/j.1399-5618.2008.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler L, Townsend J, Foland-Ross L, Eisenberger N, Fischer J, Cohen M, et al. Deficits in orbitofrontal cortex activation in euthymic bipolar subjects: an fMRI study. (in preparation)
- Bartzokis G, Mintz J, Marx P, Osborn D, Gutkind D, Chiang F, et al. Reliability of in vivo volume measures of hippocampus and other brain structures using MRI. Magn Reson Imaging. 1993;11:993–1006. doi: 10.1016/0730-725x(93)90218-3. [DOI] [PubMed] [Google Scholar]
- Beckmann C, Jenkinson M, Smith S. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Bermpohl F, Dalanay U, Kahnt T, Sajonz B, Heimann H, Ricken R, et al. A preliminary study of increased amygdala activation to positive affective stimuli in mania. Bipolar Disorders. 2009;11:70–75. doi: 10.1111/j.1399-5618.2008.00648.x. [DOI] [PubMed] [Google Scholar]
- Blumberg H, Leung H, Skudlarski P, Lacadie C, Fredericks C, Harris B, et al. A functional magnetic resonance imaging study of bipolar disorder: state- and trait-related dysfunction in ventral prefrontal cortices. Arch Gen Psychiatry. 2003;60:601–609. doi: 10.1001/archpsyc.60.6.601. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Elliott R, Ogilvie A, Rubinsztein J, Calderon G, Dolan R, Sahakian B. Abnormal ventral frontal response during performance of an affective go/no go task in patients with mania. Biol Psychiatry. 2004;55:1163–1170. doi: 10.1016/j.biopsych.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Foland L, Altshuler L, Bookheimer S, Eisenberger N, Townsend J, Thompson P. Evidence for deficient modulation of amygdala response by prefrontal cortex in bipolar mania. Psychiatry Res. 2008;162:27–37. doi: 10.1016/j.pscychresns.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster J. The Prefrontal Cortex. Raven Press; New York: 1989. [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;12:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri A, Bookheimer S, Mazziotta J. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11:43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Harmer C, Grayson L, Goodwin G. Enhanced recognition of disgust in bipolar illness. Biol Psychiatry. 2002;51:1091–1099. doi: 10.1016/s0006-3223(01)01249-5. [DOI] [PubMed] [Google Scholar]
- Hassel S, Almeida J, Kerr N, Nau S, Ladouceur C, Fissell K, et al. Elevated striatal and decreased dorsolateral prefrontal cortical activity in response to emotional stimuli in euthymic bipolar disorder: no associations with psychotropic medication load. Bipolar Disorders. 2008;10:916–927. doi: 10.1111/j.1399-5618.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kaladjian A, Jeanningros R, Azorin J, Nazarian B, Roth M, Anton J, et al. Remission from mania is associated with a decrease in amygdala activation during motor response inhibition. Bipolar Disorders. 2009;11:530–538. doi: 10.1111/j.1399-5618.2009.00722.x. [DOI] [PubMed] [Google Scholar]
- Kronhaus D, Lawrence N, Williams A, Frangou S, Brammer M, Williams S, et al. Stroop performance in bipolar disorder: further evidence for abnormalities in the ventral prefrontal cortex. Bipolar Disorders. 2006;8:28–39. doi: 10.1111/j.1399-5618.2006.00282.x. [DOI] [PubMed] [Google Scholar]
- Lieberman M, Eisenberger N, Crockett M, Tom S, Pfeifer J, Way B. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychological Science. 2007;18:421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Lyoo I, Kim M, Stoll A, Demopulos C, Parow A, Dager S, et al. Frontal lobe gray matter density decreases in bipolar I disorder. Biol Psychiatry. 2004;55:648–651. doi: 10.1016/j.biopsych.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Lyoo I, Sung Y, Dager S, Friedman S, Lee J, Kim S, et al. Regional cerebral cortical thinning in bipolar disorder. Bipolar Disorders. 2006;8:65–74. doi: 10.1111/j.1399-5618.2006.00284.x. [DOI] [PubMed] [Google Scholar]
- Malhi G, Ivanovski B, Szekeres V, Olley A. Bipolar disorder: It’s all in your mind? The neuropsychological profile of a biological disorder. Canadian Journal of Psychiatry. 2004:813–819. doi: 10.1177/070674370404901204. [DOI] [PubMed] [Google Scholar]
- Malhi G, Lagopoulos J, Owen A, Ivanovski B, Shnier R, Sachdev P. Reduced activation to implicit affect induction in euthymic bipolar patients: an fMRI study. J Affect Disord. 2007a;97:109–122. doi: 10.1016/j.jad.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Malhi G, Lagopoulos J, Sachdev P, Ivanovski B, Shnier R. An emotional Stroop functional MRI study of euthymic bipolar disorder. Bipolar Disorders. 2005;7:58–69. doi: 10.1111/j.1399-5618.2005.00255.x. [DOI] [PubMed] [Google Scholar]
- Malhi G, Lagopoulos J, Sachdev P, Ivanovski B, Shnier R, Ketter T. Is a lack of disgust something to fear? A functional magnetic resonance imaging facial emotion recognition study in euthymic bipolar disorder patients. Bipolar Disorders. 2007b;9:345–357. doi: 10.1111/j.1399-5618.2007.00485.x. [DOI] [PubMed] [Google Scholar]
- Marangell L. The importance of subsyndromal symptoms in bipolar disorder. J Clin Psychiatry. 2004;65:24–27. [PubMed] [Google Scholar]
- Mazzola-Pomietto P, Kaladjian A, Azorin J, Anton J, Jeanningros R. Bilateral decrease in ventrolateral prefrontal cortex activation during motor response inhibition in mania. Psychiatry Res. 2009;43:432–441. doi: 10.1016/j.jpsychires.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Garrett A, Menon V, Rose F, Bellugi U, AL R. Anomalous brain activation during face and gaze processing in Williams syndrome. Neurology. 2004;62:2070–2076. doi: 10.1212/01.wnl.0000129536.95274.dc. [DOI] [PubMed] [Google Scholar]
- Olley A, Malhi G, Mitchell P, Batchelor J, Lagopoulos J, MP A. When euthymia is just not good enough: the neuropsychology of bipolar disorder. J Nerv Ment Dis. 2005;193:323–330. doi: 10.1097/01.nmd.0000161684.35904.f4. [DOI] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider L. Neural processing of emotional faces requires attention. Proc Natl Acad Sci U S A. 2002;99:11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J, Monkul E, Tordesillas-Gutiérrez D, Franklin C, Bearden C, Fox P, et al. Fronto-limbic circuitry in euthymic bipolar disorder: evidence for prefrontal hyperactivation. Psychiatry Res. 2008;164:106–113. doi: 10.1016/j.pscychresns.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Stanfield A, Moorhead TWJ, Dominic E, McKirdy J, Sussmann J, Hall J, et al. Structural abnormalities of ventrolateral and orbitofrontal cortex in patients with familial bipolar disorder. Bipolar Disorders. 2009;11:135–144. doi: 10.1111/j.1399-5618.2009.00666.x. [DOI] [PubMed] [Google Scholar]
- Strakowski S, Adler C, Cerullo M, Eliassen J, Lamy M, Fleck D, et al. MRI brain activation in first-episode bipolar mania during a response inhibition task. Early Intervention in Psychiatry. 2008;2:225–233. doi: 10.1111/j.1751-7893.2008.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann A, Anderson J, Dougherty D, Moeller F. Measurement of inter-episode impulsivity in bipolar disorder. Psychiatry Res. 2001;101:195–197. doi: 10.1016/s0165-1781(00)00249-3. [DOI] [PubMed] [Google Scholar]
- Townsend J, Altshuler L, Cohen M, Eisenberger N, Foland L, Bookheimer S. Society for Neuroscience. San Diego, CA: 2007. Persistent deficits in orbitofrontal cortex function in euthymic bipolar subjects. [Google Scholar]
- Townsend J, Bookheimer S, Foland-Ross L, Sugar C, Altshuler L. fMRI abnormalities in dorsolateral prefrontal cortex during a working memory task in manic, euthymic and depressed bipolar subjects. Psychiatry Research: Neuroimaging. doi: 10.1016/j.pscychresns.2009.11.010. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessa M, Houenou J, Paillère-Martinot M, Berthoz S, Artiges E, Leboyer M, et al. Fronto-striatal overactivation in euthymic bipolar patients during an emotional go/nogo task. Am J Psychiatry. 2007;164:638–646. doi: 10.1176/ajp.2007.164.4.638. [DOI] [PubMed] [Google Scholar]
- Woolrich M, Behrens T, Smith S. Constrained linear basis sets for HRF modelling using Variational Bayes. Neuroimage. 2004;21:1748–1761. doi: 10.1016/j.neuroimage.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Woolrich M, Ripley B, Brady M, Smith S. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Young R, Biggs J, Ziegler V, Meyer D. A rating scale for mania: Reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]