Abstract
Bartonella henselae is the major etiological agent of cat scratch disease in humans. Cats act as the natural reservoir of B. henselae and can transmit the infection to humans by a bite or scratch. The prevalence of B. henselae in cat populations was evaluated by serological and bacteriological tests. A total of 769 stray cats from three urban and three rural areas in northern Italy were sampled between January 1999 and December 2000. The positive and the negative predictive values of serological tests with respect to bacteremic status were evaluated. Tests of a total of 140 cats (18%) resulted in detection of bacteremia. A total of 540 cats were tested by serology; 207 (38%) were seropositive. Of the 531 cats tested by both methods, the results for 65 (12.2%) showed both bacteremia detection and seropositivity. The molecular typing of the isolates showed that 20.6% of bacteremic cats were infected with B. henselae type I strain, 61.1% were infected with B. henselae type II, and 18.3% were coinfected with both. A statistically significant difference in antibody and bacteremia prevalences among geographical areas was detected. Statistical analysis showed no association between characteristics such as seroprevalence-bacteremic status, sex, general health status, and the presence of ectoparasites. The negative predictive value of serological test was 84.7%, and the positive predictive value was 31.8%. Receiving operator characteristic analysis of the data showed that serological tests had a low predictive value in relation to the bacteremic status of a cat; in surveys aimed at assessing the real risk of B. henselae infection in a human population, therefore, we suggest the use of blood culture as the reference test. Nevertheless, both blood culture assays and serological tests for Bartonella infection should be performed for a complete evaluation of the health status of cats.
Although cat scratch disease (CSD) was recognized in 1930 and first reported in 1950 by Debrè et al. (8), the etiological agent was identified only in 1992 when Bartonella henselae was definitely associated with CSD in humans (23, 24). Several years later, another Bartonella species, B. clarridgeiae, was identified as a causative agent of CSD in humans (20, 21). Cats act as a natural reservoir of the bacteria and can transmit Bartonella to humans by a scratch or bite. Bartonella infection in cats is usually not characterized by clinical manifestations, but infected cats can remain bacteremic for long periods, thus playing a major role as a reservoir for the bacteria (5, 19). The cat flea, Ctenocephalides felis felis, plays an important role in cat-to-cat transmission of the infection (7, 10, 18). A similar role may also be played by ticks (Ixodes pacificus) (4).
Epidemiological studies worldwide have shown a high level of variability of Bartonella seroprevalence in cats. Seroprevalence levels ranging from 1 to 8.5% in Germany and Switzerland (1, 12, 15) and up to 56% in The Netherlands (2) and 81% in California (6) have been found. In Europe, the highest prevalence of bacteremia was reported from The Netherlands, where 22% of 113 domestic cats were found positive with blood cultures. In France, 16.5% of domestic cats in Paris were found to be bacteremic and 41.1% were seropositive (14).
Relatively little data on Bartonella infection in stray cats are available. Bartonella was isolated in 53% of urban stray cats in France, where 34% of isolates were identified as B. henselae type I, 34% were identified as B. henselae type II, and 30% were identified as B. clarridgeiae (16). Interestingly, a high level of B. clarridgeiae (32%) in domestic cats in France was reported by Gurfield et al. (14). In the same epidemiological study, however, the bacteremic status of cats was significantly associated with the stray origin of adopted cats. Furthermore, Arvand and coworkers reported that 18.7% of stray cats in Berlin, Germany, were infected with B. henselae type II (1). These data underline the role of stray cats (mainly in urbanized areas) as a reservoir of B. henselae. Furthermore, contact with stray cats can be the cause of infection in pet cats.
Here we present the results of an epidemiological survey of Bartonella spp. in stray cats in northern Italy. Since the risk of infection for humans is posed by bacteremic animals, the positive predictive values (PPV) and negative predictive values (NPV) of serology with respect to bacteremic status have also been evaluated.
MATERIALS AND METHODS
The survey was carried out as part of a national program for the control of stray animals. A nonprobabilistic sampling procedure of convenience was used to sample cats from January 1999 to December 2000. Cats were captured from courtyards of urban areas (Milan, Brescia, and Pavia) and from neighborhoods of developed rural areas (Lomellina, Oltrepò Pavese, and Garda Lake environs) in the Lombardia region (northern Italy). For each animal, sex, general health status, and (when possible) the presence or absence of ectoparasites were recorded. Age was estimated according to the level of erosion of the permanent teeth. Approximately 4 ml of blood (2 ml in a EDTA-containing sterile tube and 2 ml in a sterile tube without anticoagulant) was collected aseptically from each cat by jugular phlebotomy. The blood samples were kept at 4°C when analyzed within 3 to 4 days; otherwise, they were stored at −20°C until processing.
Blood culture.
Culture assays of refrigerated blood samples were performed with approximately 1.5 to 2 ml of blood previously centrifuged at 1,500 × g for 10 min.
The pelleted erythrocytes were osmotically lysed by adding sterile water. The frozen blood samples were directly plated onto the medium without previous osmotic lysis. Bartonella recovery from erythrocytes lysed by freezing was previously compared to that achieved by adding sterile water; the two methods showed comparable levels of efficiency (unpublished data). The medium used for culture assays was a blood agar base heart infusion (Biolife Italiana S.r.l., Milan, Italy) containing 5% defibrinated fresh rabbit blood. Rabbit blood was collected aseptically by intracardiac puncture. Incubation of the plates was carried out at 37°C for at least 1 month in a moist atmosphere containing 5% CO2. Enumeration of bacterial colonies was performed for each bacteremic cat; results are expressed as CFU per milliliter of blood.
Serological analysis.
The B. henselae indirect immunofluorescence antibody test was performed according to the method described by Chomel and coworkers (6). HEP-2 cells (human larynx carcinoma cells; provided by Maura Ferrari, Laboratorio Substrati cellulari, Istituto Zooprofilattico Sperimentale della Lombardia e dell'Emilia Romagna, Brescia, Italy) were used for the cultivation of B. henselae. Sera were diluted at 1:64 (cutoff value), 1:128, 1:256, 1:512, and 1:1,024 in phosphate-buffered saline. The intensity of specific fluorescence was evaluated subjectively (with scores from 1 to 4 assigned), and the antibody titer was defined by the major dilution with a score of ≥2.
PCR subtyping.
Bartonella strains isolated by blood culture techniques were characterized by PCR for specific Bartonella species by the method of Norman and coworkers (22) on the basis of a restriction fragment length polymorphism of the TaqI enzyme of a 400-bp amplified region of a gltA gene. Binding patterns were compared with those of reference strains B. henselae Huston 1 (ATCC 49882), B. henselae type II (University of California, Davis), and B. clarridgeiae (ATCC 51694) (all kindly supplied by B. B. Chomel, Department of Population Health and Reproduction, School of Veterinary Medicine, University of California, Davis).
B. henselae subtyping was performed according to the method of Bergmans and coworkers (3). Briefly, the assay is based on the variation in the 16S rRNA gene between subtypes I and II of B. henselae and has produced species-specific results. The PCRs were performed directly on a pool of three colonies for each isolate after a 10-min incubation at 94°C.
Statistical analysis.
The statistical unit of interest was the cat, and all variables (dependent and independent) were measured at this level. Overall crude prevalence values (95% confidence interval [CI] values) for blood culture-positive cats and serologically positive cats were calculated; then, prevalence values for each geographic area and 95% CI values were calculated. The significance of prevalence within the geographic areas was tested using a Pearson χ2 test. Blood culture and serological tests were designated the dependent variables. Both of the dependent variables were treated as binary outcomes and coded 0 (negative or failure) or 1 (positive or success). All of the independent variables (age, sex, presence of ectoparasites, and health status) were treated as categorical and cross-categorized according to the blood culture and serological results. Crude prevalence ratios of association and 95% CI values were calculated for all variables (11). Mantel-Haenszl's method was used to estimate an adjusted prevalence ratio for geographical areas (9). The accuracy of serological tests compared to that of blood culture assays (considered the “gold standard”) was first analyzed using a simple two-by-two table to calculate the specificity, the sensitivity, the PPV, and the NPV. Because the PPV and the NPV depend on the level of the prevalence of disease in the population, different estimations of these parameters were assessed using Bayes's formula (17):
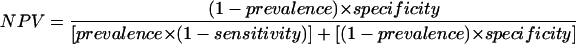 |
(1) |
 |
(2) |
The distribution of the serological titers in bacteremic and nonbacteremic cats was assessed by the receiving operator characteristic (ROC) table method (13). Associations between serological results and bacteremic status were tested with a McNemar test. All statistical analysis were performed using STATA software, version 7.0 (27).
RESULTS
Cat population.
A total of 769 stray cats were sampled. Of these, 213 were from urban areas (114 from Milan, 56 from Brescia, and 43 from Pavia) and 556 were from rural areas (178 from Lomellina, 292 from Oltrepò, and 86 from the Garda Lake environs). The sex was recorded for 595 cats; 76% (451) were female and 24% (144) were male. The age was recorded for 552 cats. The average age was 24 months (the range was from 3 to 120 months). Of 538 cats examined, 93 (17%) were affected by ectoparasites (ticks and fleas). The status of health was recorded for 539 cats; 47 (9%) were found to be affected by minor clinical conditions (mainly otoacariasis, conjunctivitis, and dermatomycoses).
Bacteriological and serological data.
B. henselae was isolated from the blood cultures of 140 cats (18%; 95% CI = 15.5 to 21.0). A total of 131 isolates were typed by PCR and identified as B. henselae. A total of 27 (21%) isolated strains were B. henselae type I, and 80 (61%) were B. henselae type II; 24 bacteremic cats (18%) were found coinfected by both subtypes. In this study no B. clarridgeiae was found. The prevalence of bacteremia was significantly different in cats from different geographical locations [Pearson χ2 df(5) = 82,4761; P < 0.001, where df is degrees of freedom] and ranged from 5.3% in cats from Milan to 48.3% in cats from Garda Lake (Table 1 and Table 2). Bacteremia levels ranged from 1 to 18,000 CFU/ml of whole blood.
TABLE 1.
Distribution of B. henselae isolates from stray cats in northern Italy between bacteremic and seropositive cats
| Location | Results
|
|||||
|---|---|---|---|---|---|---|
| Blood culture
|
Serology
|
|||||
| No. of cats examined | No. (%) of cats testing positive | 95% CI | No. of cats examined | No. (%) of cats testing positive | 95% CI | |
| Milan | 114 | 6 (5.39) | 1.9-11.1 | 87 | 14 (16.1) | 9.1-25.5 |
| Brescia | 56 | 5 (8.9) | 3.0-19.6 | 56 | 6 (10.7) | 4.0-21.8 |
| Pavia | 43 | 16 (37.2) | 23.0-53.3 | 60 | 29 (48.3) | 35.2-61.6 |
| Lomellina | 178 | 25 (14.1) | 9.3-20.1 | 89 | 48 (53.9) | 43.0-64.6 |
| Oltrepò | 292 | 46 (15.7) | 11.8-20.4 | 172 | 83 (48.3) | 40.5-56.0 |
| Garda Lake environs | 86 | 42 (48.3) | 37.4-59.2 | 84 | 27 (31.8) | 22.1-42.7 |
| Total | 769 | 140 | 548 | 207 | ||
TABLE 2.
B. henselae types in stray cats from different locations in northern Italy
| Location | No. of isolates | No. (%) of B. henselae type I | No. (%) of B. henselae type II | No. (%) of B. henselae type I and type II |
|---|---|---|---|---|
| Milan | 6 | 2 (33.3) | 3 (50) | 1 (16.6) |
| Brescia | 5 | 0 | 5 (100) | 0 |
| Pavia | 16 | 6 (37.5) | 7 (43.7) | 3 (18.7) |
| Lomellina | 25 | 5 (20) | 10 (40) | 10 (40) |
| Oltrepò Pavese | 38 | 7 (18.4) | 25 (65.8) | 6 (15.8) |
| Garda Lake environs | 41 | 7 (17.1) | 30 (73.2) | 4 (9.7) |
| Total | 131 | 27 (21) | 80 (61) | 24 (18) |
Overall seroprevalence was 38% (CI = 33.6 to 41.9) (207 testing positive out of 548 cats examined). Seroprevalence ranged from 10.7% in Brescia to 48.3% in Pavia and Oltrepò Pavese (Pearson χ2 test) [df(5) = 56.96; P < 0.001] (Table 2).
The independent variables (age, sex, presence of ectoparasites, and health status) were not significantly associated with the crude prevalence (95% CI) and adjusted prevalence (95% CI) of bacteremia and seroprevalence. However, cats older than 12 months showed a significantly higher Bartonella seroprevalence than younger cats.
Comparison between blood culture and serology tests.
A total of 531 cats were analyzed for both bacteremia and seroprevalence (Table 3). Of 115 bacteremic cats, 50 were found to be seronegative (43.5%); 139 out of 416 nonbacteremic cats were seropositive (33%), and tests of 65 out of 531 cats gave both bacteremic and seropositive results (Table 3). Most of the bacteremic cats had antibody titers ranging from 1:128 to 1:256. No bacteremic cat had the highest antibody titer (1:1,024). The association between serological results and bacteremia in cats was highly significant (χ2McNemar = 41.9; P < 0.0001).
TABLE 3.
Comparison between bacteremia and seropositivity results in 531 cats in northern Italy
| Status of cat | Results
|
||
|---|---|---|---|
| Bacteremic | Not bacteremic | Total | |
| Seropositive | 65 | 139 | 204 |
| Seronegative | 50 | 277 | 327 |
| Total | 115 | 416 | 531 |
With the blood culture assay considered the gold standard, the diagnostic sensitivity of the serological test was 56.5% and the specificity was 67%. However, seropositive cats had a 31.8% (65 of 204) probability of also being bacteremic (PPV) and seronegative cats had an 84.7% (277 of 327) probability of being nonbacteremic (NPV). The comparison between antibody titers and blood culture results is shown in Table 4. The NPVs and PPVs of the serological test (using Bayes's formula to estimate the different prevalences of bacteremia found in cats from the different locations) are shown in Table 5. The NPV decreased when prevalence increased.
TABLE 4.
Comparison between antibody titers and bacteremia in cats
| Antibody titer | No. (%) of cats with indicated test result
|
||
|---|---|---|---|
| Bacteremic | Not bacteremic | Total | |
| <64 | 50 (15) | 277 | 327 |
| 64 | 38 (35) | 71 | 109 |
| 128 | 20 (54) | 37 | 57 |
| 256 | 4 (21) | 19 | 23 |
| 512 | 3 (27) | 8 | 11 |
| 1,024 | 0 | 4 | 4 |
| Total | 115 | 416 | 531 |
TABLE 5.
PPVs and NPVs of serology estimated with Bayes's formula for different prevalences of B. henselae bacteremia in cats from six locations in northern Italy
| Location | Results
|
||||||
|---|---|---|---|---|---|---|---|
| Blood culture
|
Predictive value for serological result
|
||||||
| No. of cats examined | Prevalence (P) (probability pretest) (%) | PPV (%) | Change in probability (PPV-P) (%) | Prevalence (1 − P) of Bartonella-free cats (pretest probability) (%) | NPV (%) | Change in probability NPV − (1 − P) (%) | |
| Milan | 114 | 5.3 | 8.7 | 3.4 | 94.7 | 96.5 | 1.8 |
| Brescia | 56 | 8.9 | 14.2 | 5.3 | 91.1 | 94.0 | 2.9 |
| Pavia | 43 | 37.2 | 50.1 | 12.9 | 62.8 | 72.1 | 9.3 |
| Lomellina | 178 | 14.1 | 21.8 | 7.7 | 85.9 | 90.3 | 4.4 |
| Oltrepò Pavese | 292 | 15.7 | 24.0 | 8.3 | 84.3 | 89.2 | 4.9 |
| Garda Lake environs | 86 | 48.3 | 61.3 | 13 | 51.7 | 62.1 | 10.4 |
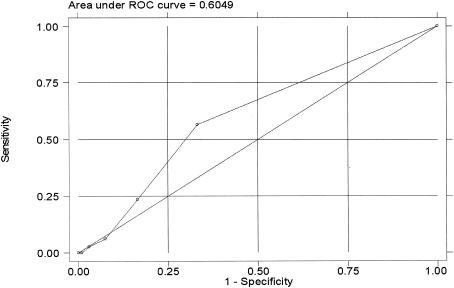
The ROC analysis showed that the area under the curve was 60.5% (95% CI, 55.3 to 66.7%) and was not significantly higher than 50% (the value indicating no diagnostic utility) (Fig. 1).
FIG.1.
Accuracy (shown using an ROC curve) of a serological test for Bartonella diagnosis.
DISCUSSION
In this study, the prevalence of anti-B. henselae antibodies and Bartonella bacteremia in stray cats from various geographic areas in northern Italy has been assessed. Cats were captured from January 1999 to December 2000. A large series of 769 animals was included in the study; 213 cats were from urban areas and 556 were from rural areas. B. henselae bacteremia was detected in 140 (18%) of 769 cats, whereas anti-B. henselae antibodies were detected in 207 (38%) of 548 sera examined. Seroprevalence was higher in cats older than 12 months. No associations between seropositivity, bacteremic status, sex, and general health were found, confirming previous observations (15).
Bacteremic animals were infected with B. henselae type I in 20.6% of cases, with B. henselae type II in 60% of cases, and with both types in 18.3% of cases. Our results confirm the high level of prevalence of B. henselae in stray cats and the spread of type II in western Europe (1, 2, 16, 26). Further, association between B. henselae types I and II appears to be not infrequent in Europe, as recently observed by Gurfield et al. (14) with respect to domestic cats from France. Coinfection was also observed in experimental studies, suggesting the lack of cross-protection between the two B. henselae types (28). B. clarridgeiae was not found in any bacteremic cat. This could be due to the low level of frequency and high level of variability of this species in the cat population (5, 26). Though the number of cats in this study was quite large, cats were sampled from a very large geographical area and the isolation of species with low to very low frequency levels is strongly related to the sample size of a single cat colony. Nevertheless, our results suggest that B. clarridgeiae is likely present at very low frequencies in cat populations in northern Italy.
None of the bacteremic cats had a serum antibody titer of 1:1,024. Most bacteremic cats were either seronegative (<64) or showed lower anti-Bartonella antibody levels (1:128). Furthermore, a decrease in the number of bacteremic cats with higher antibody titers (>256) was observed. Though no statistical association was found for either antibody titers or CFU counts, results suggest that the antibody response to B. henselae might develop slowly and, at least in the first phase of the infection, might not be efficient in controlling bacteremia. Protection may become more efficient when the antibody levels are higher.
Both seroprevalence and bacteremia differed significantly among geographical locations, probably as a consequence of the environmental conditions prevalent where cats were sampled. Bacteremia and seroprevalence levels in flea-infested cats were 1.24 and 1.12 times higher, respectively, than those seen with noninfested cats.
The results of this study show poor performance of a serological test in evaluating the actual prevalence of B. henselae among cats (sensitivity, 56.7%; specificity, 66.7%; area under the curve [ROC analysis], 60.6%). According to several researchers, however, a high NPV (>89%) could make serology a useful screening tool for excluding bacteremia (6). Here, we also observed high NPV values (84.7%). However, predictive values (which are strictly dependent on actual prevalence) may not be reliable indicators for the utility of a diagnostic test. According to Sackett and coworkers (25), the utility of diagnostic tests depends on the values obtained from the difference between pretest (prevalence) and posttest (predictive values) probabilities. In this survey, for example, 5.3% of cats in the Milan area were bacteremic for B. henselae, a result which we consider the actual prevalence or pretest probability. Following serological screening, cats tested either positive or negative. In the case of a positive result, we can see from Table 5 that (using Bayes's formula) the posttest probability that a serologically positive cat was bacteremic is 8.7% (PPV). Therefore, serology increased the predictive value by 3.4% (a change from 8.7 to 5.3%; i.e., a difference of 3.4). In the case of a negative serological result, remembering that the pretest probability that a cat was nonbacteremic is 94.7%, the posttest probability increases by only 1.8% to 96.5% (NPV).
Clearly, serology for use in investigations of cats from the Milan area does not appear to offer increased predictive values with respect to ascertaining the prevalence of nonbacteremic cats. When we compare the pre- and posttest probabilities for positive and negative serological test results, we observe greater utility in the case of positive results. Indeed, the increase in pretest probabilities (prevalence) is larger in the case of positive serology than that seen with negative serology. When we consider the values reported in Table 5, we can see that the highest values for the differences between pre- and posttest probabilities for both positive and negative serological tests are observed in those areas where prevalence is approximately 50%. In fact, according to Sackett and coworkers (25), the utility of a diagnostic test is greatest when pretest probability values range from 40 to 60% and is at its highest when prevalence is 50%. The results of ROC analysis confirm the lack of diagnostic utility of serological testing.
In conclusion, we feel that serological testing for B. henselae in cats to assess the risk of transmission to humans should be used only when the actual prevalence of bacteremic cats in a given area is known and is shown to be approximately 40 to 60%. In all cases in which transmission to humans (in particular, to immune-depressed individuals) is a risk, we recommend both blood culture and serology testing.
Transmission from cats to humans usually occurs via a scratch or bite and is due to the presence of the bacterium on claws and/or in the oral cavity. In our opinion, Bartonella can reach the claws through contact with infected flea feces present on the skin. Moreover, Bartonella can reach the oral cavity directly from bleeding gums or indirectly by the licking of contaminated skin and claws. Flea-infested cats tend to have higher grooming activity than noninfested cats. These circumstances would make a bacteremic cat a major risk for humans. Determination of the presence or absence of bacteremia is crucial in assessing the actual risk of transmission for the cat; however, a nonbacteremic cat with positive serology results should be reevaluated for possible recurrent bacteremia.
Acknowledgments
We are grateful to Bruno Chomel (Department of Population Health and Reproduction, School of Veterinary Medicine, University of California, Davis) for kindly supplying B. henselae type I and type II and B. clarridgeiae and for collaboration in identifying some strains of B. henselae.
We also thank Federico Martinello, Stefano Mersi, Paolo De Masi (ASL Pavia, Public Health Veterinary Service), and Roberta Belli-Blanes (ASL Brescia, Public Health Veterinary Service) for cat recruitment and blood sampling.
REFERENCES
- 1.Arvand, M., A. J. Klose, D. Schwartz-Porsche, H. Hahn, and C. Wendt. 2001. Genetic variability and prevalence of Bartonella henselae in cats in Berlin, Germany, and analysis of its genetic relatedness to a strain from Berlin that is pathogenic for humans. J. Clin. Microbiol. 39:743-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergmans, A. M. C., C. M. A. De Jong, G. Van Amerongen, C. S. Schot, and L. M. Schouls. 1997. Prevalence of Bartonella species in domestic cats in The Netherlands. J. Clin. Microbiol. 35:2256-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergmans, A. M. C., J. F. P. Schellekens, J. D. A. Van Embden, and L. M. Schouls. 1996. Predominance of two Bartonella henselae variants among cat-scratch disease patients in The Netherlands. J. Clin. Microbiol. 34:254-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang, C. C., B. B. Chomel, R. W. Kasten, V. Romano, and N. Tietze. 2001. Molecular evidence of Bartonella spp. in questing adult Ixodes pacificus ticks in California. J. Clin. Microbiol. 39:1221-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chomel, B. B. 2000. Cat-scratch disease. Rev. Sci. Tech. Off. Int. Epizoot. 19:136-150. [DOI] [PubMed] [Google Scholar]
- 6.Chomel, B. B., R. C. Abbott, R. W. Kasten, K. A. Floyd-Hawkins, P. H. Kass, C. A. Glaser, N. C. Pedersen, and J. E. Koehler. 1995. Bartonella henselae prevalence in domestic cats in California: risk factors and association between bacteremia and antibody titers. J. Clin. Microbiol. 33:2445-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chomel, B. B., R. W. Kasten, K. Floyd-Hawkins, B. Chi, K. Yamamoto, J. Roberts-Wilson, A. N. Gurfield, R. C. Abbott, N. C. Pedersen, and J. E. Koehler. 1996. Experimental transmission of Bartonella henselae by cat fleas. J. Clin. Microbiol. 34:1952-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Debrè, R., M. Lamy, M.-L. Jammet, L. Costil, and P. Mozziconacci. 1950. La maladie des griffes de chat. Bull. Mem. Soc. Med. Hop. Paris 66:76-79. [PubMed] [Google Scholar]
- 9.Fleiss, J. L. 1981. Statistical methods for rates and proportions. Wiley Series in probability and mathematical statistics, 2nd ed. John Wiley & Sons, Inc., New York, N.Y.
- 10.Foil, L., E. Andress, R. L. Freeland, A. F. Roy, R. Rutledge, P. C. Triche, and K. L. O'Reilly. 1998. Experimental infection of domestic cats with Bartonella henselae by inoculation of Ctenocephalides felis (Siphonaptera: Pulicidae) feces. J. Med. Entomol. 35:625-628. [DOI] [PubMed] [Google Scholar]
- 11.Frankena, K., and M. V. Thrusfield. 1997. Basic of observational studies, p. 101-134. In J. P. T. M. Noordhuizen, K. Frankena, C. M. van der Hoofd, and E. A. M. Graat (ed.), Application of quantitative methods in veterinary epidemiology. Wageningen, Wageningen Pers, The Netherlands.
- 12.Glaus, T., R. Hofmann-Lebmann, C. Greene, B. Glaus, C. Wolfensberger, and H. Luitz. 1997. Seroprevalence of Bartonella henselae infection and correlation with disease status in cats in Switzerland. J. Clin. Microbiol. 35:2883-2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greiner, M., D. Pfeiffer, and R. D. Smith. 2000. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev. Vet. Med. 45:23-41. [DOI] [PubMed] [Google Scholar]
- 14.Gurfield, A. N., H. J. Boulouis, B. B. Chomel, R. W. Kasten, R. Heller, C. Bouillin, C. Gandoin, D. Thibault, C. C. Chang, F. Barrat, and Y. Piedmont. 2001. Epidemiology of Bartonella infection in domestic cats in France. Vet. Microbiol. 80:185-198. [DOI] [PubMed] [Google Scholar]
- 15.Haimerl, M., A. M. Tenter, K. Simon, M. Rommel, J. Hinger, and I. B. Autenrieth. 1999. Seroprevalence of Bartonella henselae in cats in Germany. J. Med. Microbiol. 48:849-856. [DOI] [PubMed] [Google Scholar]
- 16.Heller, R., M. Artois, V. Xemar, D. De Briel, H. Gehin, B. Jaulhac, H. Monteil, and Y. Piedmont. 1997. Prevalence of Bartonella henselae and Bartonella clarridgeiae in stray cats. J. Clin. Microbiol. 35:1327-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henken, A. M., E. A. M. Graat, and J. Casal. 1997. Measurement of disease frequency, p. 65-97. In J. P. T. M. Noordhuizen, K. Frankena, C. M. van der Hoofd, and E. A. M. Graat (ed.), Application of quantitative methods in veterinary epidemiology. Wageningen, Wageningen Pers, The Netherlands.
- 18.Higgins, J. A., S. Radulovic, D. C. Jaworski, and A. F. Azod. 1996. Acquisition of the cat scratch disease agent Bartonella henselae by cat fleas (Siphonaptera: Pulicidae). J. Med. Entomol. 33:490-495. [DOI] [PubMed] [Google Scholar]
- 19.Kordick, D. L., K. M. Wilson, D. J. Sexton, T. L. Hadfield, H. A. Berkhoff, and E. B. Breitschwerdt. 1995. Prolonged Bartonella bacteremia in cats associated with cat-scratch disease patients. J. Clin. Microbiol. 33:3245-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kordick, D. L., E. J. Hilyard, K. H. Hadfield, T. L. Wilson, A. G. Steigerwalt, D. J. Brenner, and E. B. Breitschwerdt. 1997. Bartonella clarridgeiae, a newly recognized zoonotic pathogen causing inoculation papules, fever, and lymphadenopathy (cat scratch disease). J. Clin. Microbiol. 35:1813-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawson, P. A., and M. D. Collins. 1996. Description of Bartonella clarridgeiae sp. nov. isolated from the cat of a patient with Bartonella henselae septicemia. Med. Microbiol. Lett. 5:64-73. [Google Scholar]
- 22.Norman, A. F., R. Regnery, P. Jameson, C. Greene, and D. C. Krause. 1995. Differentiation of Bartonella-like isolates at the species level by PCR restriction fragment length polymorphism in the citrate synthase gene. J. Clin. Microbiol. 33:1797-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Regnery, R. L., B. E. Anderson, J. E. Clarridge III, M. C. Rodriguez-Barradas, D. C. Jones, and J. H. Carr. 1992. Characterization of a novel Rochalimaea species, R. henselae sp. nov., isolated from blood of a febrile, human immunodeficiency virus-positive patient. J. Clin. Microbiol. 30:265-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Regnery, R. L., M. Martin, and J. G. Olson. 1992. Naturally occurring Rochalimaea henselae infection in domestic cats. Lancet 340:557-558. [DOI] [PubMed] [Google Scholar]
- 25.Sackett, D. L., B. R. Haynes, G. H. Guyatt, and P. Tugwell. 1991. Clinical epidemiology: a basic science for clinical medicine, 2nd ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 26.Sander, A., C. Buhler, K. Pelz, E. von Cramm, and W. Bredt. 1997. Detection and identification of two Bartonella henselae variants in domestic cats in Germany. J. Clin. Microbiol. 35:584-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Statacorp. 2001. Stata statistical software: release 7.0. Stata Corporation, College Station, Tex.
- 28.Yamamoto, K., B. B. Chomel, R. W. Kasten, C. C. Chang, T. Tseggai, P. R. Decker, M. Mackowiak, K. A. Floyd-Hawkins, and N. C. Pedersen. 1998. Homologous protection but lack of heterologous-protection by various species and types of Bartonella in specific pathogen-free cats. Vet. Immunol. Immunopathol. 65:671-673. [DOI] [PubMed] [Google Scholar]