Summary
Delta/Serrate/Lag2 (DSL) ligands and their Notch family receptors have profound and pervasive roles in development. They are also expressed in adult tissues, notably in mature neurons and glia in the brain, where their roles are unknown. Here, focusing on the sense of smell in adult Drosophila, we show that Notch is activated in select olfactory receptor neurons (ORNs) in an odorant specific fashion. This response requires olfactory receptor activity and the Notch ligand Delta. We present evidence that Notch activation depends on synaptic transmission by the ORNs in which the receptors are active, and is modulated by the activity of local interneurons in the antennal lobe. It is also subject to regulatory inputs from olfactory receptor activity in other ORNs. These findings identify a new correlate of stimulus-dependent brain activity, and potentially new forms of neural integration and plasticity.
Introduction
Notch family receptors are single-pass transmembrane proteins that mediate intercellular communication in all metazoans. In canonical Notch signaling, Notch is activated by transmembrane ligands of the Delta/Serrate/Lag2 (DSL) family. DSL ligands function by inducing sequential cleavages of the juxtamembrane and transmembrane domains of Notch, allowing the Notch cytosolic domain to gain access to the nucleus where it acts as a transcriptional co-activator. Thus, Notch proteins alter cell behavior in response to membrane tethered DSL signals by acting directly to regulate the transcription of downstream target genes. DSL-Notch signaling constitutes a mechanism for the control of cell fate and pattern throughout animal development (reviewed in (Greenwald, 1998; Kopan and Ilagan, 2009; Lai, 2004; Louvi and Artavanis-Tsakonas, 2006)).
In addition to their many, diverse roles in development, Notch and DSL proteins are also found in the adult brain, in terminally differentiated neurons as well as glia (reviewed in (Louvi and Artavanis-Tsakonas, 2006)). Such observations have long posed the question of whether DSL-Notch signaling plays significant roles in the functioning of the mature brain, and if so, what these roles might be. The general capacity of DSL-Notch signaling to amplify small differentials in other signaling inputs to alter cell behavior is particularly intriguing. Within the context of brain function, such amplification could serve any number of purposes in neural feedbacks circuits that modulate perception or behavior. Indeed, recent findings are consistent with the possibility that Notch activity may influence synaptic structure and memory (Berezovska et al., 1999; Conboy et al., 2007; Costa et al., 2003; Dahlhaus et al., 2008; Ge et al., 2004; Matsuno et al., 2009; Presente et al., 2004; Sestan et al., 1999; Wang et al., 2004a). However, none of these studies have allowed direct visualization of the effects of neural activity on the pattern of DSL-Notch signaling in the brain, nor have they indicated how such signaling might alter brain function.
Here, we have adapted an in vivo assay for transmembrane cleavage of Notch (Lecourtois and Schweisguth, 1998; Struhl and Adachi, 1998) to monitor DSL-Notch signaling in the intact Drosophila brain. This assay entails the expression of chimeric forms of Notch in which the cytosolic domain is replaced by either the Gal4-VP16 or LexA-VP16 transcriptional activator. Cleavage and nuclear import of such heterologous transcription factors is strictly ligand dependent and can be visualized in the intact animal by assaying for the expression of appropriate reporter proteins such as GFP.
Applying this approach, we find that Notch is activated in olfactory, gustatory and auditory peripheral neurons in adult flies as revealed by striking patterns of GFP fluorescence in their axonal projections. Concentrating on the olfactory receptor neurons (ORNs), we find that long-term exposure to different odorants leads to elevated Notch activity in distinct constellations of ORNs, demonstrating that environmental stimuli modulate the pattern of Notch activity in the brain. Activation of Notch depends on odorant receptor activity as well as on signaling by the DSL ligand Delta. It also depends on synaptic transmission by the ORN and is modulated by the activity of local interneurons in the antennal lobe. Finally, Notch activation in any given population of ORNs depends on olfactory receptor activity in other ORNs, indicating that it reflects cross-talk between different ORN populations. Thus, DSL-Notch signaling in ORNs appears to represent a new correlate of stimulus-dependent brain activity, potentially involved in neural processing of sensory input.
Results
Monitoring Notch activation in the adult nervous system of Drosophila
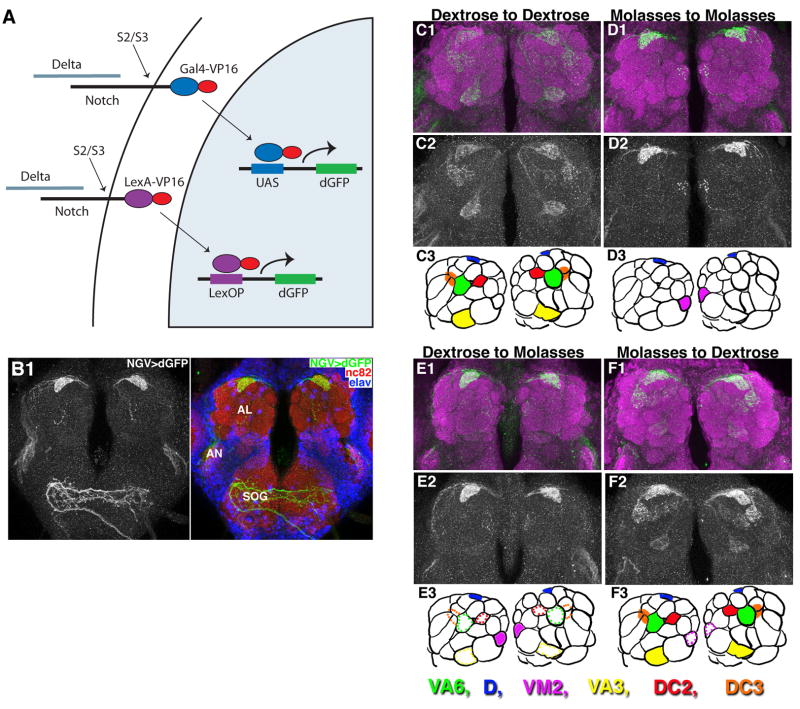
We have adapted an in vivo assay for transmembrane cleavage of Notch (Lecourtois and Schweisguth, 1998; Struhl and Adachi, 1998) to monitor DSL-Notch signaling in the intact Drosophila brain. This assay entails the expression of chimeric forms of Notch in which the cytosolic domain is replaced by either the Gal4-VP16 (N-GV) or LexA-VP16 (N-LV) transcriptional activator under the control of the Tubulinα1 promoter (Basler and Struhl, 1994) which is active in most or all cells (Fig. 1A). Cleavage and nuclear import of such heterologous transcription factors is strictly ligand dependent, and can be visualized in the intact animal by assaying for the expression of appropriate reporter proteins such as GFP. We used UAS and LexOP reporters that express a destabilized form of GFP, dGFP, to increase the likelihood that reporter protein accumulation reflects the recent level of Notch activation. When assayed in the imaginal wing disc, both N-GV and N-LV systems yield patterns of dGFP expression that are similar to those of standard Notch reporters (Suppl. Fig. 1A), and both are Presenilin dependent (Suppl. Fig. 1B), corroborating previous evidence that these assay systems are bona fide indicators of ligand-dependent Notch activity (Lecourtois and Schweisguth, 1998; Struhl and Adachi, 1998). The N-LV assay system appears to recapitulate the expected pattern of Notch activity more extensively than the N-GV system (Suppl. Fig. 1A), suggesting that it is the more sensitive of the two.
Figure 1. The olfactory environment modulates the pattern of Notch activation.
A. N-GV and N-LV assays for Notch activation. Binding of Delta to the ectodomain of Notch-GAL4-VP16 (N-GV, upper) and Notch-LexA-VP16 (N-LV, lower) proteins induces juxtamembrane (S2) followed by intramembrane (S3) cleavages, releasing GAL4-VP16 and LexA-VP16 from the membrane, allowing them to activate transcription from UAS.dGFP and LexOP.dGFP reporters in the nucleus. dGFP encodes a destabilized form of GFP. See also Figs. S1A, B.
B. Visualization of Notch activity in the adult brain using the N-GV system. N-GV UAS.dGFP/+ (“NGV>dGFP”) flies were raised on cornmeal food supplemented with molasses as a sugar source. dGFP can be detected in the D glomerulus in the antennal lobe (AL), in the antennal nerve (AN) and in neurons that project to the subesophageal ganglion (SOG). Here, and in all of the remaining figures, glomerular morphology has been visualized using nc82 antisera directed against Bruchpilot, a presynaptic marker (Wagh et al., 2006). Glomeruli were identified using the maps of Stocker et al., 1990 and Couto et al., 2005. Neuronal cell bodies are stained with anti-ELAV. AL neurons mediate olfaction, gustatory neurons project to the SOG (Wang et al., 2004b) and Johnston’s organ neurons project through the AN to the antennomechanosensory center (Sivan-Loukianova and Eberl, 2005). See also Fig. S1C.
C–F. N-GV UAS.dGFP/+ flies raised to eclosion and then aged for four days on cornmeal food supplemented with dextrose or molasses as a sugar source (hence forth “dextrose” or “molasses” food) were transferred for four days to food with the same sugar source (C & D) or to standard food with the other sugar source (E & F). The pattern of dGFP accumulation in response to dextrose food differs from that in response to molasses; changing the food source causes a corresponding change in the dGFP response. Here, and in subsequent figures, the results are also depicted in cartoon form using a standard diagram of left and right antennal lobes, with relevant glomeruli indicated by color (as in the color key): solid coloring indicates a positive dGFP response, and colored dotted outlines indicates a negative response. Some glomeruli (e.g., DC3 in this panel), are located beneath the plane of focus shown in the cartoon, and hence do not correspond with the black outlines depicting the morphology of the glomeruli located at the surface of the antennal lobe.
Using either the N-GV or N-LV system, we detect dGFP accumulation in the axons of three sensory systems that innervate the adult brain: those that mediate smell, hearing and taste (Fig. 1B). As we observed in the wing disc, the level of dGFP appears generally higher for the N-LV response: using the N-LV system, we can detect dGFP in additional brain neurons, as well as in glia, not seen using the N-GV assay (e.g., Fig. 6; see also Suppl. Fig. 2). We focus on Notch activation in the olfactory system because this system is well characterized and easy to manipulate.
Figure 6. Notch activation in odorant receptor neurons depends on synaptic transmission.
A–F. tubP-gal80ts20; N-LV LexOP.dGFP flies carrying an Or82a.Gal4 driver, and either the UAS.IMPTNT-Q4A (IMP; A–C) or UAS.TNT-E (TNT; D–F) transgene, as indicated, were raised at 18°C on molasses food and shifted to dextrose food upon eclosion. The 18°C set (A and D) were aged at 18°C for 11 days and then exposed to a 1:100 dilution of geranyl acetate oil for 4 days at 18°C. The 18°C->30°C set (B and E) were aged at 18°C for 4 days and shifted to 30°C for 4 days prior to being exposed to exposed to geranyl acetate at 30°C for an additional 4 days. The 30°C->18°C set (C and F) were aged at 18°C for 2 days, shifted to 30°C for 4 days, shifted back to 18°C for 4 days and then exposed to geranyl acetate for 4 days at 18°. As depicted in D3, E3 and F3, expression of tetanus toxin (TNT) for eight days at 30°C significantly reduces dGFP accumulation in VA6 in response to geranyl acetate relative to control flies expressing inactive toxin (IMP) under the same conditions. Moreover, the effect is reversible following return to 18° C, which blocks expression of the toxin. (The phenotype we see cannot be due to a direct effect of TNT on Notch, because while Notch is required throughout development, TNT expression has been shown not to affect embryonic development (Sweeney et al., 1995).)
G. The graph depicts box plots of the integrated density of fluorescence in VA6. Only the 30°C set differed significantly. The number of samples analyzed was: 18°C IMP, 22; 18°C TNT, 24; 30°C IMP, 22; 30°C TNT, 24; 30>18°C IMP, 22; 30>18°C TNT, 28.
H. The graph depicts box plots of the integrated density of fluorescence in VA6 normalized to the IMP control in each data set. N-LV IMP (IMP N-LV) and N-LV TNT (TNT N-LV) flies of the same genotype as in A-F and dGFP/tubP-gal80ts20 UAS.IMPTNT-Q4A; 82a GAL4/+ (IMP trafficking) and dGFP/tubP-gal80ts20 UAS.TNT-E; 82a GAL4/+ (TNT trafficking) flies were raised at 18°C on molasses food and shifted to dextrose food upon eclosion. They were aged at 18°C for 4 days and shifted to 30°C for one day prior to being exposed to a 1:100 dilution of geranyl acetate for 4 days. While both the reduction in Notch activity and dGFP trafficking are significant, the reduction in Notch activity is greater (61%) than the reduction in dGFP trafficking (26%).
Distinct patterns of Notch activation in olfactory receptor neurons in response to different food sources
Flies detect odorants via the activation of around 60 distinct sub-populations of olfactory receptor neurons (ORNs) that decorate the antenna and maxillary palps. For the most part, each ORN sub-population is defined by the expression a single olfactory receptor and extends axons to a single, morphologically identifiable glomerulus in the antennal lobe where they synapse with local interneurons (LNs) and second order projection neurons (PNs) (Gao et al., 2000; Hallem and Carlson, 2006; Vosshall et al., 2000).
Both N-GV and N-LV flies maintained on standard cornmeal food, with dextrose as the sugar source, show strong dGFP accumulation in a few glomeruli (principally D, VA3, DC3, DC2 and VA6, Fig. 1C). Comparison of this pattern with that seen by driving dGFP expression with ORN, LN, PN, or glia specific Gal4 drivers confirms that N-GV and N-LV are being activated in ORNs (data not shown). Further confirmation was obtained by directly co-localizing N-GV dependent GFP expression with specific OR reporter transgenes in antennae (Gr21a.dsRed and OR82a.dsRed; Fig. 3A and data not shown) and by killing specific ORNs with diphtheria toxin (data not shown). As can be seen in Suppl. Fig. 1C, endogenous Notch is expressed in ORNs. Given the relative ease with which individual glomeruli can be identified, we use dGFP accumulation in axons innervating glomeruli rather than in cell bodies in antennae in most of the following experiments.
Figure 3. Kinetics of Notch activation in response to increasing or decreasing exposure to CO2.
A,B. N-GV/Gr21a.dsRed; UAS.GFP/+ flies were raised to eclosion and aged on dextrose food for four to seven days, then shifted to agar and exposed to 5–10% CO2 for the indicated time periods. The nuclear GFP (green) and dsRed (magenta) fluorescence were visualized in whole mount antennae (coincident fluorescence appears white); note that some non-Gr21a expressing ORNs have accumulated GFP, presumably in response to other odorants (A). The depicted 72hr antenna was exposed to 7% CO2. All other images of CO2 exposed antennae are from flies exposed to 5% CO2. The graph (B) depicts box plots of the % Gr21a.dsRed cells that expressed GFP at the indicated times of exposure and % CO2 (in parentheses). Here and in all box plots presented below, the horizontal line in the box is the median of the entire data set; the lines at the top and bottom of the box are the medians of the higher and lower 50% respectively; the whiskers at the top and bottom are the maximum and minimum values excluding the outliers (dots), which are more than 3/2 times the upper quartile or less than 3/2 times the lower quartile. The Mann-Whitney test was applied to data from pairs of time points. ns: not significant; *: P≤ 0.05; **: P≤ 0.01; ***: P≤ 0.001. The number of samples analyzed was: 0hrs, 17; 6hrs (5%), 12; 6hrs (10%), 19; 12hrs (5%), 11; 18hrs (5%), 14; 24hrs (5%), 18; 24hrs (7%), 19; 72hrs (7%), 13.
C. As in A, except that N-GV UAS.dGFP/+ flies were used, and exposed to 5% CO2 on dextrose food for 3 days. Flies were removed from CO2 for the indicated lengths of time, and the Notch response assayed by quantitating dGFP accumulation in the V glomerulus. The graph depicts box plots of the integrated density of fluorescence in V. The number of samples analyzed was: 0 days, 32; 1day, 28; 2 days, 28; 3 days, 23; 4 days, 26; no CO2, 17.
When flies were maintained on an alternative food source with molasses rather than dextrose as the sugar source, dGFP accumulated in a distinct, albeit overlapping, subset of ORNs and associated glomeruli (in D to high levels, and occasionally in other glomeruli such as VM2 as seen in Figure 1D). Given that the food olfactory environment is complex, it is noteworthy that the pattern of Notch activation we detect is sparse. A similarly sparse response to complex (natural) odorant environments has been observed using intrinsic signal imaging in rodents, by ensemble electrophysiology in moths and by calcium imaging in flies (Lin da et al., 2006; Riffell et al., 2009; Semmelhack and Wang, 2009).
The different patterns of Notch activation observed on “dextrose” and “molasses” food are not irreversibly fixed. Instead, transferring the flies from one food to the other results in a corresponding change in the pattern of dGFP accumulation within four days (Figs. 1E, F). Thus, Notch activation in ORNs depends on the olfactory environment and can generate distinct, stereotyped transcriptional responses to given odorant ensembles.
Distinct patterns of Notch activation in response to defined odorants
Several laboratories have identified the ORNs that are activated by transient puffs of specific odors (de Bruyne et al., 2001; Hallem and Carlson, 2006; Hallem et al., 2004; Suh et al., 2004; Wang et al., 2003). Two such odors, geranyl acetate and CO2, evoke dedicated responses: geranyl acetate principally activates Or82a receptor expressing ORNs, which project to VA6 (Couto et al., 2005; Fishilevich and Vosshall, 2005), and CO2 activates Gr21a receptor expressing ORNs that project to V (Couto et al., 2005; Scott et al., 2001; Suh et al., 2004). We asked if each of these odors elicits a Notch response in the appropriate dedicated population of ORNs.
N-GV flies were maintained for at least three days in the presence of geranyl acetate or CO2. As shown in Figure 2, both odorants altered the pattern of dGFP accumulation relative to that of controls, and each induced high levels of dGFP accumulation in the same glomerulus that responds in transient physiological assays: i.e., in VA6 (Figs. 2A–D) and V (Figs. 2E, F), respectively. Further, the response is dose dependent (Figs. 2A–D; data not shown).
Figure 2. Notch is activated in response to defined odors.

N-GV/UAS.dGFP flies raised to eclosion and aged for four days on dextrose food were exposed for four days to different concentrations of geranyl acetate diluted in paraffin oil (A–D), or to atmospheric CO2 (0.03%) (E) or 1% CO2 for three days (F). Exposure to geranyl acetate results in dGFP accumulation predominantly in the VA6 glomerulus and exposure to CO2 results in predominant dGFP accumulation the V glomerulus. All brains in A–D were processed at the same time and imaged at the same microscope settings. To ensure that the geranyl acetate signal was in the linear range, the settings were such that the Notch food response is not visible.
Thus, for both geranyl acetate and CO2, prolonged exposure to odorant activates Notch in the same ORNs that show a physiological response to transient exposure.
Kinetics of Notch activation in response to odorant
The ORN response to puffs of odorants as measured electrophysiologically or by Ca2+ imaging occurs in milliseconds. In contrast, our standard assay involves exposing flies to odorants for at least three days before assaying dGFP accumulation in glomeruli. To assess the minimum time required to elicit a Notch response in ORNs, we asked how quickly Gr21a expressing ORNs respond to CO2 exposure. To reduce any time delay in detecting the GFP signal, we used a UAS.GFP NLS reporter (encoding a stable, nuclear localized form of GFP, rather than dGFP) and assayed for the response in nuclei of Gr21a expressing ORNs (identified by co-expression of a Gr21a.dsRed transgene) to avoid any time lag required for GFP to diffuse down ORN axons.
At normal ambient levels of CO2 (0.03%), the great majority of Gr21a expressing ORNs are devoid of GFP (Fig. 3A, 0hr). Following exposure to 5% CO2, we first detect a significant increase in the percentage GFP positive Gr21a expressing ORNs between 6 and 12 hours, after which the percentage rises rapidly to near peak levels by 24 hours (Figs. 3A, B). By 72hrs, GFP accumulation is apparent in most (>85%) of Gr21a.dsRed expressing ORNs (Fig. 3B).
To assess the rate at which the Notch response decays upon removal of odorant, we exposed N-GV UAS.dGFP flies to 5% CO2 for three days, transferred them to normal ambient CO2 conditions, and monitored how long dGFP persists in the V glomerulus. As shown in Figure 3C, median Notch activity is reduced 41% by one day and 64% by 2 days after odor removal. Thus high levels of Notch activity are tightly coupled to odor exposure.
Notch activation by odorants depends on the DSL ligand Delta
To determine whether odorant evoked Notch activation in ORNs is dependent on DSL signaling, we assayed N-GV and N-LV responses to geranyl acetate and CO2 under conditions in which DSL signaling is limiting. Only two DSL ligands, Delta and Serrate, have been identified in Drosophila (D’Souza et al., 2008; Kopan and Ilagan, 2009), and only Delta appears to be abundantly expressed in the adult head (data not shown). Because Delta is required during development, we reduced Delta activity in adults by using a trans-heteroallelic combination of two temperature sensitive Delta mutations, DlRF (Parks and Muskavitch, 1993; Parody and Muskavitch, 1993; Xu et al., 1992) and a stronger allele, Dl6B (Lehmann, 1983; Parks and Muskavitch, 1993). Flies were raised to adulthood at the permissive temperature, 18°C, and then shifted to the restrictive temperature, 30°C, one day prior to, and for the duration of, exposure to odorant. For CO2, induction of dGFP accumulation using either the N-GV (Figs. 4A-D) or N-LV system (Suppl. Fig. 2) was reduced by heterozygosity for each of the two Dl alleles (more severely for the stronger allele), and abolished for the trans-heterozygous Dl6B/DlRF condition. Similar results were obtained for geranyl acetate, using the N-GV system, except that we could not detect an effect of heterozygosity for the weaker allele, DlRF, and the response was severely reduced, but not abolished, in the transheterozygous condition (Fig. 4I).
Figure 4. Notch activation in response to odor is Delta dependent.
A–D. N-GV UAS.dGFP/+ flies that are genotypically +/+, DlRF/+, Dl6B/+, or DlRF/Dl6B, were raised at 18°C on molasses food and shifted to dextrose food upon eclosion. Flies were aged at 18°C for 5–6 days and then shifted to 30° C for one day prior to exposure to 5% CO2 on agar at 30° C for three days. The dGFP response is reduced progressively in the weaker DlRF/+ and stronger Dl6B/+ genotypes, and is absent in DlRF/Dl6B transheterozygotes. See also Fig. S2.
E–H. N-GV UAS.dGFP/+ flies that are genotypically +/+ (E,F) or DlRF/Dl6B (G,H) were raised and aged as in A–D. Flies were then shifted to 30°C for 4 days, shifted back to 18°C for one day and exposed to 5% CO2 on agar at 18°C for 3 days. The dGFP response is restored upon return to the permissive temperature.
I. The graph presents an experiment analogous to that shown in A–D, except the flies were exposed to a 1:1000 dilution of geranyl acetate, rather than 5% CO2. The box plots depict dGFP accumulation in the VA6 glomerulus. The results are the same as for CO2, except that there is no significant decline in the geranyl acetate response in the weaker DlRF/+ genotype. The number of samples analyzed was: wt, 20; DlRF/+, 22; Dl6B/+, 14; DlRF/Dl6B, 16.
To confirm that the loss of Notch activation is due to lowered Delta function in adults, and not a consequence of inadequate Delta activity during development, we repeated the CO2 experiment using the N-GV assay for the DlRF/Dl6B genotype, only this time shifting the flies back to 18°C after incubation at 30°C, and assaying dGFP accumulation after an additional four days. Shifting down to the permissive temperature restored dGFP accumulation in the V glomerulus (Figs. 4E-H). We conclude that odorant induced activation of Notch in ORNs requires DSL signal.
Notch activation depends on odorant receptor activity
To determine if odor-induced elevations in Notch activity depend on activity of the olfactory receptors expressed in the responding ORNs, we used an available null mutation in the gene encoding the Or43b receptor (Elmore et al., 2003), which responds to ethyl butyrate in electrophysiological assays and is expressed in ORNs that project to the VM2 glomerulus (Couto et al., 2005; Fishilevich and Vosshall, 2005; Hallem and Carlson, 2006). When we expose wild type flies, or flies that are heterozygous for the Or43b mutation to ethyl butyrate, we detect strong accumulation of dGFP in VM2 (Fig. 5A and data not shown). In contrast, we cannot detect dGFP accumulation in VM2 when we expose homozygous Or43b mutant flies to ethyl butyrate (Fig. 5B). Thus, induction of the Notch response to ethyl butyrate in OR43b expressing ORNs requires activity of the OR43b receptor.
Figure 5. Notch activation in response to odor requires odorant receptor.

A,B. wild type (A) and Or43b− (B) flies, that are also N-GV/UAS.dGFP, were raised and maintained on dextrose food for three to five days and then exposed to a 1:100 dilution of ethyl butyrate for 4 days. Note the absence of the ethyl butyrate response in VM2 in Or43b mutants, as well as the increase in dGFP accumulation in VA6, DM5, DM6 and D compared to the wild type control.
C. Or43b− flies (C) flies, that are also N-GV/UAS.dGFP, were exposed to a 1:100 dilution of geranyl acetate. Predominant dGFP accumulation is in VA6, as in control (wild type) flies (Fig. 2A).
D–F. Or43b− flies carrying the Or43b.Or82a transgene (Or43b−; 43b>Or82a), as well as N-GV and UAS.dGFP, were exposed to paraffin oil (D), or to 1:100 dilutions of ethyl butyrate (E) or geranyl acetate (F). Ectopic expression of the Or82a receptor in Or43b ORNs innervating VM2 is associated with an ectopic dGFP response to geranyl acetate in VM2 (F), but no rescue of dGFP accumulation in VM2 in response to ethyl butyrate (E). Similarly, it is associated with the loss of the prominent dGFP response seen in VA6 and DM6 in Or43b mutant (B), but not wild type flies (A).
The ethyl butyrate response is, however, more complex than the geranyl acetate and CO2 responses, in that it induces electrophysiological responses in several different classes of ORNs (in addition to Or43b expressing ORNs) (Hallem and Carlson, 2006). When we expose wild type flies to ethyl butyrate, we detect accumulation of dGFP in two of these classes of ORNs, those that project to glomeruli DM5 and DM6 (Fig. 5A). We also detect food induced Notch activation, albeit at low levels, in ORNs that project to VA6 and D (Fig. 5A). Strikingly, the failure of Or43b mutant ORNs to activate Notch in response to ethyl butyrate appears to be coupled to an increase in the accumulation of dGFP in DM5, DM6, VA6 and D (Fig. 5B). Hence, it appears that activation of Or43b ORNs by ethyl butyrate normally suppresses Notch activation that would otherwise occur in ORNs that innervate DM5, DM6, VA6, and D.
As a further test of the requirement for odorant receptor activity in evoking the Notch response, we used an Or43b>Or82a transgene to ectopically express the Or82a (geranyl acetate) receptor in place of the missing Or43b (ethyl butyrate) receptor in Or43b mutant ORNs. In wild type flies (Figs. 2A–D), as well as in OR43b mutant flies (Fig. 5C), Or82a expressing ORNs exhibit the primary Notch response to geranyl acetate, as visualized by the strong accumulation of dGFP in VA6 (Fig. 5C); no dGFP accumulation is detected in VM2, the glomerulus normally innervated by Or43b expressing ORNs. In contrast when Or43b mutant flies that carry the Or43b>Or82b transgene are exposed to geranyl acetate, dGFP accumulation can now be detected in VM2 as well as in VA6 (Fig. 5F). As expected, these flies show no Notch activity in VM2 when exposed to ethyl butyrate (Fig. 5E). Thus, odorant induced activity of the ectopic Or82a receptor is sufficient to evoke Notch activation in mutant Or43b ORNs lacking the Or43b receptor.
We note that in contrast to simple OR43b mutant flies (Figure 5B), OR43b mutant flies that ectopically express Or82a in OR43b neurons show no dGFP accumulation in VA6 or DM6 in response to ethyl butyrate (Fig. 5E). Perhaps the presence of the ectopic Or82a receptor allows for spontaneous firing in the OR43b neurons that suppresses Notch activation in the OR82a and Or67a expressing ORNs.
In sum, these experiments establish that activity of the resident olfactory receptor in a given ORN is necessary, and in some cases sufficient, to induce Notch activity in the same ORN. At the same time, they also show that the Notch response to olfactory receptor activity in any given ORN population can be modulated by olfactory receptor activity in other populations.
Notch activation by odorants depends on synaptic transmission by the responding olfactory receptor neurons
In the case of OR43b ORNs, long term exposure to odorant elevates Notch activity by activating the resident odorant receptor, whether endogenous OR43b or ectopically expressed OR82a. Yet, in canonical Notch signaling, Notch is activated in trans by Delta presented by neighboring cells, posing the question of how olfactory receptor activation stimulates Notch activation in the same ORNs. One possibility is that upon activation of their resident olfactory receptor, ORNs communicate with other neurons or glia, via synaptic transmission, to induce them to elevate their production of a retrograde Delta signal. To assess this, we asked whether synaptic transmission from ORNs is required for odorant induced Notch activation, using the expression of tetanus toxin (Sweeney et al., 1995) to block evoked synaptic vesicle release in Or82a (geranyl acetate responsive) or Gr21a (CO2 responsive) ORNs.
Using the Gal4/UAS method, we expressed either active (TNT) or inactive (IMP) forms of the tetanus toxin light chain in Or82a or Gr21a expressing ORNs and monitored Notch activity using the N-LV/LexA.dGFP assay. To restrict expression of tetanus toxin to ORNs in the adult, we used a temperature sensitive form of GAL80 to block transcriptional activation by Gal4 at 18°C, but permit it at 30°C (McGuire et al., 2003). Flies were raised to adulthood at 18°C, and then either maintained at 18°C or switched to 30°C for four days prior to, and for the duration of, exposure to odor. At 18°C, neither IMP nor TNT is expressed, and exposure to geranyl acetate leads to dGFP accumulation in VA6, as in wild type flies (Figs. 6A, D; With the N-LV assay we often detect Notch activation in VA1 and DC2 in response to food odors and at 18°C we also detect Notch activation in VM2.). However, flies that express TNT at 30°C show significantly decreased dGFP accumulation in VA6 (Fig. 6E), whereas those expressing IMP do not (Fig. 6B). Similar results were obtained when TNT and IMP were expressed in Gr21a-expressing ORNs: TNT, but not IMP, expression blocked dGFP accumulation in V in response to CO2 (data not shown).
To test whether expression of TNT blocks dGFP accumulation because it irreversibly damages or kills the Or82a-expressing ORNs, we shifted flies expressing TNT at 30°C back to 18°C for 4 days to allow GAL80ts to repress GAL4 driven production of TNT. dGFP accumulation can then again be detected in VA6 (Figs. 6C, F). Quantification of these results is shown in Figure 6G, confirming that there is a highly significant decrease (P<0.0001, Mann Whitney) in Notch activation in VA6 when tetanus toxin is expressed in Or82a-expressing ORNs.
Chiang et al. have reported that long-term expression of tetanus toxin in single ORNs innervating a given glomerulus is associated with axonal degeneration (Chiang et al., 2009). To ensure that the reduction in Notch induced dGFP expression we observed in ORNs expressing TNT was not merely due to degeneration of ORN axons, we assessed the ability of our reporter to traffic down axons by co-expressing dGFP and either TNT or IMP in OR82a- expressing ORNs, using the same temperature shift regime employed for assaying Notch activation in response to geranyl acetate. While we did see a reduction in dGFP accumulation in TNT expressing ORNs (20%), this reduction was not as severe as the reduction in Notch activation (57%). In addition, we shortened the length of time flies were shifted to 30°C prior to being exposed to geranyl acetate to one day. Again, while we could detect a reduction of 26% in dGFP trafficking when tetanus toxin is expressed, this was not as severe as the 61% reduction in Notch activity (Fig. 6H). This reduction in Notch activity is actually an underestimate, as most of the residual Notch activity is in fact not in the ORNs but in other axons that innervate that antennal lobe (data not shown). Taken together with our finding that the block in Notch activation is reversible, this result suggests that the effect of TNT cannot be attributed simply to neural degeneration, downstream of the block in synaptic transmission.
Thus, the elevation of Notch activity induced in ORNs by activation of their resident odorant receptors depends on synaptic transmission by the responding ORNs.
Notch activation in ORNs is modulated by the activity of local interneurons in the antennal lobe
ORNs project to single glomeruli in the antennal lobe, where they synapse on local interneurons (LNs) and projection neurons (PNs) (Gao et al., 2000; Vosshall et al., 2000). LNs are diverse in their connectivity (Chou et al., 2010), synapse on many glomeruli and modulate incoming neural input from many ORNs. This modulation can be either excitatory or inhibitory (reviewed in (Wilson, 2008)). To determine whether the level of Notch activation induced in ORNs by odorants is modulated by LN function in the antennal lobe, we examined the consequences of blocking synaptic transmission in specific subsets of LNs using the same experimental protocol described above for blocking synaptic transmission in chosen ORNs. We employed two drivers, LN1.Gal4 and LN2.Gal4, that are expressed in distinct subsets of primarily GABAergic LNs (Das et al., 2008; Okada et al., 2009; Sachse et al., 2007) and one driver, Kra.GAL4, that is expressed in both GABAergic and cholinergic LNs (Chou et al., 2010; Shang et al., 2007). As summarized below, we could detect changes in the level of dGFP accumulation in VA6 and V induced, respectively, by geranyl acetate and CO2 when TNT was expressed using one or more of these drivers.
Expression of TNT in Kra.Gal4 expressing LNs enhanced the geranyl acetate and CO2 responses in VA6 and V relative to the IMP control (Figs. 7A–F). TNT expression in LN1.Gal4 expressing LNs did not appear to alter the geranyl acetate response (Fig. 7G–I), but repressed the CO2 response (Figs. 7J–L). Finally, we found that TNT expression in LN2.Gal4 expressing LNs caused an increase in the geranyl acetate response (Fig. 7M–O), but had no affect on the CO2 response (Fig. 7P-R). Of the three classes of LNs we examined, only those expressing LN2.GAL4 have been hypothesized to have extensive contacts with ORN terminals (Okada et al., 2009), and our finding that TNT expression in LN2.Gal4 expressing LNs has no effect on the CO2 response is in accord with the observation that Gr21a expressing ORNs do not express GABAB receptors and are not subject to significant presynaptic inhibition (Root et al., 2008). The effect of blocking synaptic transmission by LNs expressing Kra.Gal4 and LN1.Gal4 on Notch activation in ORNs is not necessarily direct. Nevertheless, the level of Notch activation induced in ORNs by odorant appears to depend on the function of LNs in the antennal lobe. This is further evidence that the level of the Notch response reflects cross talk between glomeruli.
Figure 7. Notch activation in olfactory receptor neurons is modulated by synaptic activity of local interneurons in the antennal lobe.
N-LV LexOP.dGFP/tubP-gal80ts20 flies carrying Kra.Gal4, LN1.Gal4, or LN2.Gal4 drivers and either the UAS.IMPTNT-Q4-A (IMP) or UAS.TNT-E (TNT) transgene, as indicated, were raised to eclosion on molasses food and aged for two to four days on dextrose food at 18° C. They were then transferred to 30°C for four days and exposed at 30°C to a 1:100 dilution of geranyl acetate for four more days or to 5% CO2 (Kra.GAL4 and LN2.GAL4) or 10% CO2 (LN1.GAL4) for three more days, as indicated. TNT expression under the control of each of the three LN drivers significantly altered the level of dGFP accumulation in response to either, or both, geranyl acetate and CO2, in the appropriate target glomerulus (VA6 for geranyl acetate and V for CO2). In the cartoons, darker shades of green indicate increased Notch activity. For each set of experiments, the graphs depict box plots of fluorescence in VA6 (geranyl acetate exposed flies) or V (CO2 exposed flies). The number of samples analyzed for each genotype and odor was: Kra>IMP geranyl acetate, 14; Kra>TNT geranyl acetate, 26; Kra>IMP CO2, 33; Kra>TNT CO2, 27; LN1>IMP geranyl acetate, 25; LN1>TNT geranyl acetate, 28; LN1>IMP CO2, 41; LN1>TNT CO2, 33; LN2>IMP geranyl acetate, 30; LN2>TNT geranyl acetate, 26; LN2>IMP CO2, 32; LN2>TNT CO2, 24.
Notch activation by odorants or odorant mixtures that activate more than one olfactory receptor
For CO2 and geranyl acetate, both of which strongly activate single olfactory receptors in dedicated ORN populations, the Notch response to prolonged exposure shows a simple one-to-one correspondence with the electrophysiological response to transient exposure. Our results with ethyl butyrate however, suggest that odorants that activate multiple odorant receptors may induce a more complex Notch response. Ethyl butyrate induces electrophysiological responses in ORNs that project to DM6, DM5, DM2, DM3, VC4, VM5, VM2, VM3, DC1 and VC3 (Hallem and Carlson, 2006). Of these, we only detect Notch activity in DM5, DM6, and VM2 (Fig. 5A).
To examine this further, we sampled the Notch response in the glomeruli of N-GV flies maintained for at least three days on dextrose food in the presence of each of thirteen defined odorants (including geranyl acetate, CO2 and ethyl butyrate; Fig. 8A and legend). Approximately two thirds of the odorants tested (ethyl butyrate, pentyl acetate, octanol, geranyl acetate, 1 octen-3-oI, 3-octanol, 2-phenylethanol, ethyl benzoate and CO2) altered the pattern of dGFP accumulation in antennal lobe glomeruli relative to the control dextrose food response. With the exception of CO2, all of these odorants are known to activate two or more receptors (Hallem and Carlson, 2006; Hallem et al., 2004; Wang et al., 2003) and with the exception of geranyl acetate, all induced dGFP accumulation in at least a few, and sometimes several, glomeruli (Fig. 8A; Geranyl acetate strongly activates Or82a and weakly activates Or98a (Hallem and Carlson, 2006). We only detect Notch activation in Or82a expressing ORNs). However, as in the case of ethyl butyrate (Fig. 5A), the correspondence between the Notch response and the previously described electrophysiological response was incomplete, or in the case of 2-phenylethanol (Fig. 8A), non-existent. This lack of correspondence, as well as the failure of some defined odorants to induce a Notch response, could be due to our assaying the consequences of odorant exposure over days, rather than seconds (e.g., some odorants might degrade, or break down into other molecules with different odorant activities). Alternatively, as suggested by our experiments with ethyl butyrate (Fig. 5), Notch activation induced by olfactory receptor activity in any given ORN population may be sensitive to olfactory receptor activation in other ORNs populations.
Figure 8. Notch activation induced by odorant mixtures depends on inhibitory cross talk between responding olfactory receptor neurons.
A. 4 day old N-GV UAS.dGFP/+ flies which had been raised on dextrose food were exposed to 1:100 dilutions of odors (for 4 days) or to 5% CO2 (for 3 days). Seven to twelve flies were assayed for each odor. The fraction of flies with Notch activity in the indicated glomeruli is presented in blue. The activity of the ORNs in response to puffs of the corresponding odor is shown in red. With the exception of 3-octanol this is electrophysiological data (de Bruyne et al., 2001; Hallem and Carlson, 2006) and the height of the red bars reflects spike number. Glomeruli activated by 3-octanol were determined by calcium imaging (Wang et al., 2003). No obvious changes in Notch activation were observed for the following odors: phenethyl acetate, methyl salicylate, cis-vaccenyl acetate, octyl aldehyde.
B–H. N-GV UAS.dGFP/+ flies raised to eclosion and aged for four days on dextrose food were exposed for four days to different concentrations of geranyl acetate and pentyl acetate as indicated, or to paraffin oil alone. Surface plots of Notch activation in an antennal lobe are depicted in B–G, with the height and color of the peaks being proportional to pixel intensity. Orientation of the antennal lobe is depicted in B: M, medial; D, dorsal; L, lateral; V, ventral. Addition of pentyl acetate to geranyl acetate at concentrations of 1:50 and 1:5 caused stepwise reductions in dGFP accumulation in VA6 (asterisk). Conversely, there is an increase in dGFP accumulation in VA3 (arrowhead). Box plots of the fluorescence in VA6 are presented in H, illustrating the reduction in Notch activation in VA6 induced by pentyl acetate (GA, geranyl acetate; PA, pentyl acetate). The number of samples analyzed was: oil, 24; PA 1:50, 24; GA 1:1000, 32; GA 1:1000 PA 1:5, 32; GA 1:1000 PA 1:50, 30.
To test this possibility directly, we assayed the Notch response to a mixture of two odorants, geranyl acetate and pentyl acetate. Pentyl acetate activates distinct olfactory receptors in several different ORN populations, and induces Notch activity in multiple glomeruli in a dose-dependent fashion. Specifically, dGFP accumulation is detected in approximately five glomeruli (VM2, VA4, DM3, VM5 and DC2) in response to low levels of pentyl acetate (a 1:50 dilution, Fig. 8C); exposure to a ten fold higher concentration increases the level of dGFP accumulation in these glomeruli and also induces dGFP accumulation in at least seven additional glomeruli (Fig. 8D; DA4, DC1, DL3, DL4, DL5, DM6 and VM3). This dose-dependent increase in the number of responding glomeruli is in accord with similar results obtained in flies and mice, using various electrophysiological and imaging techniques to assay neural activity (Hallem and Carlson, 2006; Hallem et al., 2004; Lin da et al., 2006; Wang et al., 2003).
When a 1:50 dilution of pentyl acetate is added to a 1:1000 dilution of geranyl acetate the prominent geranyl acetate response induced in VA6 is reduced (compare Figs. 8E and F), whereas conversely, the pentyl acetate response in VA3 and/or VA4 is increased. When the concentration of pentyl acetate is further increased, to a 1:5 dilution, the geranyl acetate VA6 signal is almost completely abolished (compare Figs. 8E and G). The data is presented graphically in Fig. 8H. A 1:50 dilution of pentyl acetate also suppresses the activation of Notch induced in VA6 by exposure to dextrose food alone (compare Figs. 8B and C, graph in H). Equivalent examples of suppression have been reported in both insects and vertebrates when odors are presented as mixtures (Deisig et al., 2006; Lei and Vickers, 2008; Olsen et al., 2010; Silbering and Galizia, 2007; Tabor et al., 2004). Thus, the patterns of Notch activity induced by odorant ensembles that activate multiple receptors appears to reflect some form of neural processing of the input stimuli.
Discussion
Sensory perception depends on experience, as encoded in changes in synapse, neuron and circuit behavior caused by prior sensory input. Using an in vivo assay that translates Notch activity into the expression of GFP, we find that prolonged exposure of adult Drosophila to defined odorants activates Notch in particular ORNs. Here, we summarize the attributes of this response, and speculate about how it occurs and what roles it may play.
Activation of Notch by geranyl acetate and CO2: a simple neural correlate of olfactory experience
Geranyl acetate and CO2 are each known to strongly activate only a single olfactory receptor (de Bruyne et al., 2001; Hallem and Carlson, 2006; Suh et al., 2004). These receptors are expressed in unique populations of ORNs, each of which projects to a single glomerulus (Couto et al., 2005; Fishilevich and Vosshall, 2005; Scott et al., 2001; Suh et al., 2004) and synapses with a unique population of PNs (Marin et al., 2005; Sachse et al., 2007; Schlief and Wilson, 2007). Hence, these odorants appear to be detected by dedicated sensory circuits.
For both circuits, we observe a simple one-to-one correspondence between odorant and the induction of Notch activity: prolonged exposure to geranyl acetate activates Notch in ORNs that express the geranyl acetate receptor (Or82a), and similarly, CO2 activates Notch in ORNs that express the CO2 receptor (Gr21a). Given that there are ~60 different ORN populations, each defined by expression of a unique olfactory receptor, the one-to-one correspondence we observe for the Notch response is highly significant. We infer that the activation of olfactory receptor by each odor is likely specific and responsible for inducing the activation of Notch in the ORNs that express that receptor. This inference is confirmed by experiments in which we ectopically express the Or82a receptor in Or43b expressing ORNs, which are normally responsive to ethyl butyrate: exposure to geranyl acetate now induces Notch activation in the VM2 glomerulus to which these OR82a expressing Or43b ORNs project.
In addition to the requirements for odor and olfactory receptor, we can define four other properties of the Notch response to geranyl acetate and CO2. First, Notch activation depends on the DSL ligand Delta. Second, it depends on synaptic transmission by the ORN in which the olfactory receptor is active. Third, Notch activity is modulated by synaptic transmission by LNs in the antennal lobe. Fourth, Notch activity in any given ORN population can be modulated by olfactory receptor activity in other populations. Taken together, these findings argue for distinct pre- and post-synaptic components of the Notch response in ORNs.
Activation of Notch by complex odorants: evidence for neural processing
Flies maintained on different food sources show distinct, albeit relatively sparse, patterns of Notch activity in ORNs, and the same was true when flies were exposed to defined odorants or odorant mixtures that activate more than one receptor. Under these conditions, we typically detect Notch activation in the ORNs that project to only a handful of glomeruli in each case. However, in contrast to the Notch response to geranyl acetate and CO2, there is only a partial correlation between the ORNs that show the Notch response to these broader spectrum odorants, and those that respond electrophysiologically to the same odorant.
Given our evidence that both geranyl acetate and CO2 responses depend on synaptic transmission by ORNs as well as LNs in the antennal lobe, we posit that at least some of these more complex Notch responses reflect the consequences of neural integration. In support, we show that olfactory receptor activity in one ORN population can block odorant-induced activation of Notch in another ORN population, as revealed by mutations that abolish receptor activity, or by coincident exposure to a second odorant. Inhibitory interactions uncovered by receptor mutation cannot be easily explained by decay or change in odorant properties under the conditions of long-term odorant exposure. They fit nicely, however, with recent electrophysiological findings that demonstrate cross-regulatory inputs mediated by LNs (reviewed in (Wilson, 2008)), and are in accord with our observation that blocking synaptic transmission in GABAergic LN2 interneurons, that have been hypothesized to have extensive contacts with ORN terminals (Okada et al., 2009), results in elevated odorant-dependent Notch activity in Or82a expressing ORNs. Moreover, they are consistent with our general observation that only a few ORN populations typically show a robust Notch response to any given odorant ensemble.
Activation of Notch by exposure to odorant
A key question posed by our results is how persistent olfactory receptor activity in an ORN alters Notch activity in the same cell. We consider this problem from two perspectives: (i) what is the source of the Delta signal?, and (ii) how does olfactory receptor activity in ORNs lead to the enhanced activation of Notch by this signal?
Notch is activated by its ligands in trans, i.e. by ligands on the surface of neighboring cells. In the antenna, the ORN cell body is surrounded by glia, and its dendrites are enveloped by the thecogen cell (Shanbhag et al., 2000). Glia also envelope the axon as it projects to the antennal lobe via the antennal nerve (Sen et al., 2005). Finally, ORNs synapse with LNs and PNs in the antennal lobe. Delta presented by any of these abutting cells could be responsible for activating Notch in the ORN. Delta is ubiquitously expressed in neurons in the adult fly brain (Suppl. Fig. 1C). In preliminary experiments, we have found that over-expressing Delta along with neuralized (an E3 ubiquitin ligase required for Delta function) in PNs results in increased odor dependent Notch activation in ORNs. Conversely, we have found that expressing a Delta RNAi in these adult PNs decreased odor dependent Notch activation in ORNs. These data implicate PNs as one source of Delta.
We envision two classes of mechanisms by which odorant receptor activity results in enhanced Notch signaling. In the first, olfactory receptor activity acts cell autonomously to sensitize Notch to a tonic Delta signal from other cells. In the second, olfactory receptor activity dictates transmission of a signal to an abutting cell that induces production of a retrograde Delta signal. Given our evidence that the Notch response reflects neural cross talk at the glomerular level, we favor the second class of mechanisms, and specifically, the possibility that synaptic transmission from the ORNs induces responding LNs or PNs to send the Delta signal. Accordingly, we view Notch activation in the ORNs as a reflection of neural integration at the glomerular level.
Possible roles of DSL-Notch signaling in response to olfactory stimulation
The Notch response we have identified in the adult olfactory system appears to initiate and decay over relatively long periods. In light of these kinetics, we can envisage at least two roles for stimulus-dependent DSL-Notch signaling. The first is to mediate adaptive responses to prominent and relatively stable environmental inputs. In nature, Drosophila may spend a significant fraction of time in relatively constant olfactory environments, e.g., in the vicinity of an abundant food source. Under these conditions, it might be useful to adapt the olfactory system so that it remains responsive to novel odorants or to changes in concentrations of one or a few odorants (Dalton, 2000; Devaud, 2003). Most of the olfactory adaptations that have been identified in Drosophila occur and decay within minutes (Stortkuhl et al., 1999), or within hours (Cho et al., 2004). However, at least one kind appears to be generated over days by persistent exposure to odorant (Devaud et al., 2001; Devaud et al., 2003; Sachse et al., 2007). Specifically, flies exposed to chronically elevated levels of CO2 adapt behaviorally and exhibit an increase in the volume of the V glomerulus (Sachse et al., 2007). The relationship of the Notch response to that identified by Sachse et al. is intriguing. Indeed, we have preliminary data that reducing and increasing the level of Notch activity in Gr21a expressing ORNs decreases and enhances respectively the level of long-term behavioral adaptation to CO2.
A second, more speculative, possibility is that Notch activity serves as a memory trace to allow pairings between inputs that have informative value within a given time frame, but are not coincident. Although some learned behaviors require precise pairing of conditioned and unconditioned inputs (Tully and Quinn, 1985), others, such as taste aversion in mammals, involve pairings that occur over time intervals of hours (Bermudez-Rattoni, 2004). The time scale of the Notch response might permit pairing of precepts of a given environmental condition with positive or negative outcomes over such longer periods.
Aside from the olfactory system, we have detected Notch activity in sensory neurons that mediate hearing and taste. Moreover, using the more sensitive N-LV reporter system, we have also detected Notch activation in non-sensory neurons in the brain. Further improvements in detecting Notch activation in vivo may provide new correlates of neural activity at higher levels of brain function.
Experimental Procedures
Transgenes
N-GV has been described by (Struhl and Adachi, 2000). N-LV is a derivative of N-GV in which the GAL4 DNA binding domain of N-GV is replaced with the LexA repressor (Gyuris et al., 1993). Both transgenes are driven by Tubulinα1 promoters (Basler and Struhl, 1994).
UAS.dGFP was generated by inserting the coding sequence for a destabilized form of GFP (Clontech) that contains a single SV40 NLS into the Pelican vector (Barolo et al., 2000). LexOP.dGFP is a derivative of UAS.dGFP in which the Gal4 binding sites have been replaced by eight LexA binding sites (Ebina et al., 1983).
Gr21a, Or82a and Or43b promoters were amplified from genomic DNA, using primers specified by Couto et al. (Couto et al., 2005). The Gr21a and Or82a promoters were sub-cloned into the pRed H-Stinger vector (Barolo et al., 2004) to generate Gr21a.dsRed and Or82a.dsRed. The Or43b>Or82a transgene was generated similarly by replacing the DsRed coding sequence in pRed H-Stinger with that encoding Or82a.
Other transgenes employed are: UAS.IMPTNT and UAS.TNT (Sweeney et al., 1995); Or82a.GAL4 (Fishilevich and Vosshall, 2005); Kra.GAL4 (Dubnau et al., 2003); LN1.GAL4 and LN2.GAL4 (Sachse et al., 2007); Gr21a.GAL4 (Suh et al., 2004); tubP-gal80ts (McGuire et al., 2003). Mutant alleles are: Or43b null allele (Elmore et al., 2003); DlRF (Xu et al., 1990); Dl6B (Lehmann, 1983).
Odorant exposure
Unless otherwise specified, flies were exposed to odors at room temperature. 0.5ml of odorant in paraffin oil was placed in a 2.0ml microcentrifuge tube covered with Nitex. The microcentrifuge tube, along with approximately 20–30 flies, was placed into a 25 X 95mm polystyrene Drosophila vial containing approximately 10ml of dextrose food. Flies were exposed to CO2 in a CO2 incubator by placing them in a vial containing agar and sucrose that was covered with Nitex.
Histology and immuno-fluorescence
Drosophila adults were dipped in ethanol, their brains dissected in PBS and fixed in 4% paraformaldehyde in PBS containing 0.001% Triton X-100 for 15 minutes. Subsequent steps were as described (Lieber et al., 1993). The brains were mounted in 90% glycerol, 0.1M TrisHCl (pH 8.1), 2.5% DABCO. Primary antisera used are: rabbit polyclonal anti-GFP (Invitrogen); mouse monoclonal anti-Bruchpilot, nc82 (Wagh et al., 2006); rat monoclonal anti-ELAV, 7E8A10 (O’Neill et al., 1994); rabbit polyclonal anti-Notch (Lieber et al., 1993); mouse monoclonal anti-Delta, C594.9B (Qi et al., 1999); mouse monoclonal anti-Repo, 8D12 (Alfonso and Jones, 2002). All monoclonal antisera were obtained from the Developmental Studies Hybridoma Bank. Secondary antibodies were Alexa Fluor 488 donkey anti-rabbit, Alexa Fluor 647 goat anti-rat (Invitrogen), Cy3 donkey anti-mouse, Rhodamine Red X donkey anti-rabbit, Rhodamine Red X donkey anti-mouse and Cy5 anti-mouse (Jackson Laboratories).
Image analysis
Brains were imaged in the linear range, using a Leica SP5 confocal microscope. All brains within one experiment were imaged using the same green channel settings. Z-stacks were collected at approximately 1uM spacing. Maximum projections of the stacks were analyzed using ImageJ (Rasband, 1997–2008). Two tailed Mann-Whitney tests were performed using GraphPad Prism version 5.0b for Mac OS X, GraphPad Software, San Diego California USA, www.graphpad.com.
Supplementary Material
Acknowledgments
We thank A. Adachi for obtaining germ-line transformants, D. Smith, C. O’Kane, L. Vosshall, J. Dubnau, L. Vasconcelos and the Bloomington stock center for Drosophila stocks, and R. Axel for much helpful advice and discussion. S.K and T.L thank M. Young for his support in their initial foray into the role of Notch in the fly brain. S.K. and T.L. are Research Scientists, and G.S. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alfonso TB, Jones BW. gcm2 promotes glial cell differentiation and is required with glial cells missing for macrophage development in Drosophila. Dev Biol. 2002;248:369–383. doi: 10.1006/dbio.2002.0740. [DOI] [PubMed] [Google Scholar]
- Barolo S, Carver LA, Posakony JW. GFP and beta-galactosidase transformation vectors for promoter/enhancer analysis in Drosophila. Biotechniques. 2000;29:726, 728, 730, 732. doi: 10.2144/00294bm10. [DOI] [PubMed] [Google Scholar]
- Barolo S, Castro B, Posakony JW. New Drosophila transgenic reporters: insulated P-element vectors expressing fast-maturing RFP. Biotechniques. 2004;36:436–440. 442. doi: 10.2144/04363ST03. [DOI] [PubMed] [Google Scholar]
- Basler K, Struhl G. Compartment boundaries and the control of Drosophila limb pattern by hedgehog protein. Nature. 1994;368:208–214. doi: 10.1038/368208a0. [DOI] [PubMed] [Google Scholar]
- Berezovska O, McLean P, Knowles R, Frosh M, Lu FM, Lux SE, Hyman BT. Notch1 inhibits neurite outgrowth in postmitotic primary neurons. Neuroscience. 1999;93:433–439. doi: 10.1016/s0306-4522(99)00157-8. [DOI] [PubMed] [Google Scholar]
- Bermudez-Rattoni F. Molecular mechanisms of taste-recognition memory. Nat Rev Neurosci. 2004;5:209–217. doi: 10.1038/nrn1344. [DOI] [PubMed] [Google Scholar]
- Chiang A, Priya R, Ramaswami M, Vijayraghavan K, Rodrigues V. Neuronal activity and Wnt signaling act through Gsk3-{beta} to regulate axonal integrity in mature Drosophila olfactory sensory neurons. Development. 2009;136:1273–1282. doi: 10.1242/dev.031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho W, Heberlein U, Wolf FW. Habituation of an odorant-induced startle response in Drosophila. Genes Brain Behav. 2004;3:127–137. doi: 10.1111/j.1601-183x.2004.00061.x. [DOI] [PubMed] [Google Scholar]
- Chou YH, Spletter ML, Yaksi E, Leong JC, Wilson RI, Luo L. Diversity and wiring variability of olfactory local interneurons in the Drosophila antennal lobe. Nat Neurosci. 2010;13:439–449. doi: 10.1038/nn.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy L, Seymour CM, Monopoli MP, O’Sullivan NC, Murphy KJ, Regan CM. Notch signalling becomes transiently attenuated during long-term memory consolidation in adult Wistar rats. Neurobiol Learn Mem. 2007;88:342–351. doi: 10.1016/j.nlm.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Costa RM, Honjo T, Silva AJ. Learning and memory deficits in Notch mutant mice. Curr Biol. 2003;13:1348–1354. doi: 10.1016/s0960-9822(03)00492-5. [DOI] [PubMed] [Google Scholar]
- Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol. 2005;15:1535–1547. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- D’Souza B, Miyamoto A, Weinmaster G. The many facets of Notch ligands. Oncogene. 2008;27:5148–5167. doi: 10.1038/onc.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlhaus M, Hermans JM, Van Woerden LH, Saiepour MH, Nakazawa K, Mansvelder HD, Heimel JA, Levelt CN. Notch1 signaling in pyramidal neurons regulates synaptic connectivity and experience-dependent modifications of acuity in the visual cortex. J Neurosci. 2008;28:10794–10802. doi: 10.1523/JNEUROSCI.1348-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton P. Psychophysical and behavioral characteristics of olfactory adaptation. Chem Senses. 2000;25:487–492. doi: 10.1093/chemse/25.4.487. [DOI] [PubMed] [Google Scholar]
- Das A, Sen S, Lichtneckert R, Okada R, Ito K, Rodrigues V, Reichert H. Drosophila olfactory local interneurons and projection neurons derive from a common neuroblast lineage specified by the empty spiracles gene. Neural Dev. 2008;3:33. doi: 10.1186/1749-8104-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruyne M, Foster K, Carlson JR. Odor coding in the Drosophila antenna. Neuron. 2001;30:537–552. doi: 10.1016/s0896-6273(01)00289-6. [DOI] [PubMed] [Google Scholar]
- Deisig N, Giurfa M, Lachnit H, Sandoz JC. Neural representation of olfactory mixtures in the honeybee antennal lobe. Eur J Neurosci. 2006;24:1161–1174. doi: 10.1111/j.1460-9568.2006.04959.x. [DOI] [PubMed] [Google Scholar]
- Devaud JM. Experimental studies of adult Drosophila chemosensory behaviour. Behav Processes. 2003;64:177–196. doi: 10.1016/s0376-6357(03)00134-7. [DOI] [PubMed] [Google Scholar]
- Devaud JM, Acebes A, Ferrus A. Odor exposure causes central adaptation and morphological changes in selected olfactory glomeruli in Drosophila. J Neurosci. 2001;21:6274–6282. doi: 10.1523/JNEUROSCI.21-16-06274.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaud JM, Acebes A, Ramaswami M, Ferrus A. Structural and functional changes in the olfactory pathway of adult Drosophila take place at a critical age. J Neurobiol. 2003;56:13–23. doi: 10.1002/neu.10215. [DOI] [PubMed] [Google Scholar]
- Dubnau J, Chiang AS, Grady L, Barditch J, Gossweiler S, McNeil J, Smith P, Buldoc F, Scott R, Certa U, et al. The staufen/pumilio pathway is involved in Drosophila long-term memory. Curr Biol. 2003;13:286–296. doi: 10.1016/s0960-9822(03)00064-2. [DOI] [PubMed] [Google Scholar]
- Ebina Y, Takahara Y, Kishi F, Nakazawa A, Brent R. LexA protein is a repressor of the colicin E1 gene. J Biol Chem. 1983;258:13258–13261. [PubMed] [Google Scholar]
- Elmore T, Ignell R, Carlson JR, Smith DP. Targeted mutation of a Drosophila odor receptor defines receptor requirement in a novel class of sensillum. J Neurosci. 2003;23:9906–9912. doi: 10.1523/JNEUROSCI.23-30-09906.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishilevich E, Vosshall LB. Genetic and functional subdivision of the Drosophila antennal lobe. Curr Biol. 2005;15:1548–1553. doi: 10.1016/j.cub.2005.07.066. [DOI] [PubMed] [Google Scholar]
- Gao Q, Yuan B, Chess A. Convergent projections of Drosophila olfactory neurons to specific glomeruli in the antennal lobe. Nat Neurosci. 2000;3:780–785. doi: 10.1038/77680. [DOI] [PubMed] [Google Scholar]
- Ge X, Hannan F, Xie Z, Feng C, Tully T, Zhou H, Zhong Y. Notch signaling in Drosophila long-term memory formation. Proc Natl Acad Sci U S A. 2004;101:10172–10176. doi: 10.1073/pnas.0403497101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald I. LIN-12/Notch signaling: lessons from worms and flies. Genes Dev. 1998;12:1751–1762. doi: 10.1101/gad.12.12.1751. [DOI] [PubMed] [Google Scholar]
- Gyuris J, Golemis E, Chertkov H, Brent R. Cdi1, a human G1 and S phase protein phosphatase that associates with Cdk2. Cell. 1993;75:791–803. doi: 10.1016/0092-8674(93)90498-f. [DOI] [PubMed] [Google Scholar]
- Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125:143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- Hallem EA, Ho MG, Carlson JR. The molecular basis of odor coding in the Drosophila antenna. Cell. 2004;117:965–979. doi: 10.1016/j.cell.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai EC. Notch signaling: control of cell communication and cell fate. Development. 2004;131:965–973. doi: 10.1242/dev.01074. [DOI] [PubMed] [Google Scholar]
- Lecourtois M, Schweisguth F. Indirect evidence for Delta-dependent intracellular processing of notch in Drosophila embryos. Curr Biol. 1998;8:771–774. doi: 10.1016/s0960-9822(98)70300-8. [DOI] [PubMed] [Google Scholar]
- Lehmann R, Jimenez F, Dietrich U, Campos-Ortega JA. On the phenotype and development of mutants of early neurogenesis in Drosophila melanogaster. Rouxs Arch Dev Biol. 1983;192:62–74. doi: 10.1007/BF00848482. [DOI] [PubMed] [Google Scholar]
- Lei H, Vickers N. Central processing of natural odor mixtures in insects. J Chem Ecol. 2008;34:915–927. doi: 10.1007/s10886-008-9487-2. [DOI] [PubMed] [Google Scholar]
- Lieber T, Kidd S, Alcamo E, Corbin V, Young MW. Antineurogenic phenotypes induced by truncated Notch proteins indicate a role in signal transduction and may point to a novel function for Notch in nuclei. Genes Dev. 1993;7:1949–1965. doi: 10.1101/gad.7.10.1949. [DOI] [PubMed] [Google Scholar]
- Lin da Y, Shea SD, Katz LC. Representation of natural stimuli in the rodent main olfactory bulb. Neuron. 2006;50:937–949. doi: 10.1016/j.neuron.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7:93–102. doi: 10.1038/nrn1847. [DOI] [PubMed] [Google Scholar]
- Marin EC, Watts RJ, Tanaka NK, Ito K, Luo L. Developmentally programmed remodeling of the Drosophila olfactory circuit. Development. 2005;132:725–737. doi: 10.1242/dev.01614. [DOI] [PubMed] [Google Scholar]
- Matsuno M, Horiuchi J, Tully T, Saitoe M. The Drosophila cell adhesion molecule klingon is required for long-term memory formation and is regulated by Notch. Proc Natl Acad Sci U S A. 2009;106:310–315. doi: 10.1073/pnas.0807665106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal Rescue of Memory Dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- O’Neill EM, Rebay I, Tjian R, Rubin GM. The activities of two Ets-related transcription factors required for Drosophila eye development are modulated by the Ras/MAPK pathway. Cell. 1994;78:137–147. doi: 10.1016/0092-8674(94)90580-0. [DOI] [PubMed] [Google Scholar]
- Okada R, Awasaki T, Ito K. Gamma-aminobuyric acid (GABA)-mediated neural connections in the Drosophila antennal lobe. J Comp Neurol. 2009;514:74–91. doi: 10.1002/cne.21971. [DOI] [PubMed] [Google Scholar]
- Olsen SR, Bhandawat V, Wilson RI. Divisive normalization in olfactory population codes. Neuron. 2010;66:287–299. doi: 10.1016/j.neuron.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks AL, Muskavitch MA. Delta function is required for bristle organ determination and morphogenesis in Drosophila. Dev Biol. 1993;157:484–496. doi: 10.1006/dbio.1993.1151. [DOI] [PubMed] [Google Scholar]
- Parody TR, Muskavitch MA. The pleiotropic function of Delta during postembryonic development of Drosophila melanogaster. Genetics. 1993;135:527–539. doi: 10.1093/genetics/135.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presente A, Boyles RS, Serway CN, de Belle JS, Andres AJ. Notch is required for long-term memory in Drosophila. Proc Natl Acad Sci U S A. 2004;101:1764–1768. doi: 10.1073/pnas.0308259100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H, Rand MD, Wu X, Sestan N, Wang W, Rakic P, Xu T, Artavanis-Tsakonas S. Processing of the notch ligand delta by the metalloprotease Kuzbanian. Science. 1999;283:91–94. doi: 10.1126/science.283.5398.91. [DOI] [PubMed] [Google Scholar]
- Rasband WS. ImageJ. U.S. National Institures of Health; Bethesda, Maryland, USA: 1997–2008. [Google Scholar]
- Riffell JA, Lei H, Christensen TA, Hildebrand JG. Characterization and Coding of Behaviorally Significant Odor Mixtures. Curr Biol. 2009 doi: 10.1016/j.cub.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root CM, Masuyama K, Green DS, Enell LE, Nassel DR, Lee CH, Wang JW. A presynaptic gain control mechanism fine-tunes olfactory behavior. Neuron. 2008;59:311–321. doi: 10.1016/j.neuron.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachse S, Rueckert E, Keller A, Okada R, Tanaka NK, Ito K, Vosshall LB. Activity-dependent plasticity in an olfactory circuit. Neuron. 2007;56:838–850. doi: 10.1016/j.neuron.2007.10.035. [DOI] [PubMed] [Google Scholar]
- Schlief ML, Wilson RI. Olfactory processing and behavior downstream from highly selective receptor neurons. Nat Neurosci. 2007;10:623–630. doi: 10.1038/nn1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott K, Brady R, Jr, Cravchik A, Morozov P, Rzhetsky A, Zuker C, Axel R. A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell. 2001;104:661–673. doi: 10.1016/s0092-8674(01)00263-x. [DOI] [PubMed] [Google Scholar]
- Semmelhack JL, Wang JW. Select Drosophila glomeruli mediate innate olfactory attraction and aversion. Nature. 2009;459:218–223. doi: 10.1038/nature07983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A, Shetty C, Jhaveri D, Rodrigues V. Distinct types of glial cells populate the Drosophila antenna. BMC Dev Biol. 2005;5:25. doi: 10.1186/1471-213X-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestan N, Artavanis-Tsakonas S, Rakic P. Contact-dependent inhibition of cortical neurite growth mediated by Notch signaling. Science. 1999;286:741–746. doi: 10.1126/science.286.5440.741. [DOI] [PubMed] [Google Scholar]
- Shanbhag SR, Muller B, Steinbrecht RA. Atlas of olfactory organs of Drosophila melanogaster 2. Internal organization and cellular architecture of olfactory sensilla. Arthropod Struct Dev. 2000;29:211–229. doi: 10.1016/s1467-8039(00)00028-1. [DOI] [PubMed] [Google Scholar]
- Shang Y, Claridge-Chang A, Sjulson L, Pypaert M, Miesenbock G. Excitatory local circuits and their implications for olfactory processing in the fly antennal lobe. Cell. 2007;128:601–612. doi: 10.1016/j.cell.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbering AF, Galizia CG. Processing of odor mixtures in the Drosophila antennal lobe reveals both global inhibition and glomerulus-specific interactions. J Neurosci. 2007;27:11966–11977. doi: 10.1523/JNEUROSCI.3099-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivan-Loukianova E, Eberl DF. Synaptic ultrastructure of Drosophila Johnston’s organ axon terminals as revealed by an enhancer trap. J Comp Neurol. 2005;491:46–55. doi: 10.1002/cne.20687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker RF, Lienhard MC, Borst A, Fischbach KF. Neuronal architecture of the antennal lobe in Drosophila melanogaster. Cell Tissue Res. 1990;262:9–34. doi: 10.1007/BF00327741. [DOI] [PubMed] [Google Scholar]
- Stortkuhl KF, Hovemann BT, Carlson JR. Olfactory adaptation depends on the Trp Ca2+ channel in Drosophila. J Neurosci. 1999;19:4839–4846. doi: 10.1523/JNEUROSCI.19-12-04839.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G, Adachi A. Nuclear access and action of notch in vivo. Cell. 1998;93:649–660. doi: 10.1016/s0092-8674(00)81193-9. [DOI] [PubMed] [Google Scholar]
- Struhl G, Adachi A. Requirements for presenilin-dependent cleavage of notch and other transmembrane proteins. Mol Cell. 2000;6:625–636. doi: 10.1016/s1097-2765(00)00061-7. [DOI] [PubMed] [Google Scholar]
- Suh GS, Wong AM, Hergarden AC, Wang JW, Simon AF, Benzer S, Axel R, Anderson DJ. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- Sweeney ST, Broadie K, Keane J, Niemann H, O’Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- Tabor R, Yaksi E, Weislogel JM, Friedrich RW. Processing of odor mixtures in the zebrafish olfactory bulb. J Neurosci. 2004;24:6611–6620. doi: 10.1523/JNEUROSCI.1834-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol [A] 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Wong AM, Axel R. An olfactory sensory map in the fly brain. Cell. 2000;102:147–159. doi: 10.1016/s0092-8674(00)00021-0. [DOI] [PubMed] [Google Scholar]
- Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, Durrbeck H, Buchner S, Dabauvalle MC, Schmidt M, Qin G, et al. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron. 2006;49:833–844. doi: 10.1016/j.neuron.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell. 2003;112:271–282. doi: 10.1016/s0092-8674(03)00004-7. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chan SL, Miele L, Yao PJ, Mackes J, Ingram DK, Mattson MP, Furukawa K. Involvement of Notch signaling in hippocampal synaptic plasticity. Proc Natl Acad Sci U S A. 2004a;101:9458–9462. doi: 10.1073/pnas.0308126101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004b;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Wilson RI. Neural and behavioral mechanisms of olfactory perception. Curr Opin Neurobiol. 2008;18:408–412. doi: 10.1016/j.conb.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Caron LA, Fehon RG, Artavanis-Tsakonas S. The involvement of the Notch locus in Drosophila oogenesis. Development. 1992;115:913–922. doi: 10.1242/dev.115.4.913. [DOI] [PubMed] [Google Scholar]
- Xu T, Rebay I, Fleming RJ, Scottgale TN, Artavanis-Tsakonas S. The Notch locus and the genetic circuitry involved in early Drosophila neurogenesis. Genes Dev. 1990;4:464–475. doi: 10.1101/gad.4.3.464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.