Abstract
Knowing the distribution of marine animals is central to understanding climatic and other environmental influences on population ecology. This information has proven difficult to gain through capture-based methods biased by capture location. Here we show that marine location can be inferred from animal tissues. As the carbon isotope composition of animal tissues varies with sea surface temperature, marine location can be identified by matching time series of carbon isotopes measured in tissues to sea surface temperature records. Applying this technique to populations of Atlantic salmon (Salmo salar L.) produces isotopically-derived maps of oceanic feeding grounds, consistent with the current understanding of salmon migrations, that additionally reveal geographic segregation in feeding grounds between individual philopatric populations and age-classes. Carbon isotope ratios can be used to identify the location of open ocean feeding grounds for any pelagic animals for which tissue archives and matching records of sea surface temperature are available.
Populations of most commercially exploited fish and their predators have declined significantly over the last century (e.g.1,2). Effective management of marine ecosystems is reliant on accurate assessments of population identity, location and mixing, but this information is often lacking, particularly in highly migratory pelagic animals3. Conventionally, marine animals are studied by tag-recapture methods, where animals tagged in a known location are subsequently caught either in research or commercial fisheries elsewhere. In pelagic fish, high levels of mortality and low recapture rates mean that very large numbers must be tagged to provide statistically meaningful results, and recapture is frequently dependent on the location of commercial fisheries. Consequently, tag-recapture studies are inherently biased towards the numerically dominant species or populations occurring within capture areas, and are not ideally suited to studying rare species or mixed stock populations. Increasingly, electronic data storage tags (e.g.4,5) and satellite tags (e.g.6) are deployed to investigate marine migrations, but data storage tags also rely on recapture, satellite tags are still too large to deploy on small or juvenile animals, and both are prohibitively expensive for large-scale studies. An urgent need remains for alternative methods to study spatial movements of pelagic animals on a population specific basis, independently of biases introduced by capture locations.
Stable isotopes have been used to identify population location in wide-ranging terrestrial species by relating the isotopic composition of animal tissues to known geographic variations in isotope distribution7, which are often termed ‘isoscapes'. The reference isoscape is derived either from measures of tissues of the study organism in known locations7, from other non-migrant animals8, or from inorganic materials such as rainwater9 or geology10. This method assumes that the regional isoscape is constant over the time period of the study, and the accuracy and precision of any isoscape-based method depends on the quality of the underlying isoscape. Application of the isoscape method to marine environments is complicated by the relatively homogenous spatial distributions of oxygen, hydrogen and strontium isotope ratios in seawater11. Latitudinal and offshore gradients in the isotopic composition of both carbon and nitrogen have previously been used to track movements of marine mammals12,13,14. Short-term temporal and spatial variations in the isotopic compositions of C and N at the base of the marine food web, however, restrict the geographic resolution available from fixed isoscape approaches to broad basin-scale differences12 or latitudinal effects14.
The isotopic composition of carbon (expressed here as δ13C values) in tissues of pelagic animals is strongly dependent on fractionation of carbon isotopes during photosynthesis by the phytoplankton community at the base of the marine food web15. The extent of fractionation of carbon isotopes during photosynthesis (Ep) varies inversely with the ratio of cell growth rate to dissolved carbonate concentration both in laboratory and natural ocean conditions (e.g.16,17,18,19,20), and the nature of this relationship is further influenced by the degree of light and nutrient limitation20,21. Both cell growth rates and dissolved carbonate concentrations are intrinsically related to temperature, and vary spatially and temporally across ocean basins (e.g.22), leading to spatio-temporal variations in the δ13C values of plankton in the North Atlantic in excess of 6‰14,23,24,25. By contrast, fractionation of carbon isotopes associated with an increase in trophic level is on the order of 0–2‰ (e.g.26,27,28). Thus, the carbon isotope composition of similarly sized pelagic predators varies largely according to feeding location (e.g.12,13,14,28,29,30).
Sea surface temperature (SST) is a useful proxy for phytoplankton growth rates22, phytoplankton community structure31 and total global primary production32,33, as it integrates many of the variables influencing phytoplankton growth. Dissolved carbonate concentrations also vary with SST; therefore, as all of the variables influencing Ep are either directly or indirectly associated with SST, SST should be a useful proxy for Ep and thus plankton δ13C values, as seen in the strong latitudinal δ13C gradients14. The algebraic relationship between SST and δ13C values is complex (e.g.21), and varies spatially with climatic and oceanographic conditions. In any single area, however, temporal variations in SST will result in temporal fluctuations in Ep and therefore will be coincident with variations in δ13C values of plankton. Thus the regions occupied by feeding animals are more likely to be regions where there is high co-variance between time series of tissue δ13C values and SST than those regions showing little or no covariance34,35. This approach assumes that animals return to the same area over the duration of the time series, but has advantages over the fixed isoscape approach in that no prior knowledge of baseline isotope values is needed, and the arithmetic relationship linking the controlling variable to the tissue isotope value is free to vary with location.
We illustrate the use of carbon isotope time series as a geolocation tool using the Atlantic salmon (Salmo salar L.) as a test species.
Atlantic salmon are economically, culturally and ecologically valuable anadromous fish, which return from the ocean to spawn in their natal rivers after spending either one winter at sea (1SW fish), or more than one winter at sea (MSW fish). Marine mortality in Atlantic salmon (Salmo salar L.) has increased from ∼70% in the 1970s to over 90% by 200536, coincident with a major decline in the global population. The current understanding of marine location in salmon is derived largely from tagging fish in home waters and recovering tagged fish in the high seas fisheries37,38,39,40,41. Almost 4 million fish have been tagged in English and Welsh rivers since the 1950s, but fewer than 3000 (<0.08%) have been recovered at sea, almost all from the two historic fishery areas west of Greenland and north of the Faroe Islands39. Based on these data, European fish from rivers above 62°N are believed to migrate to feeding grounds off the Faroe Islands and in the Norwegian and Barents Seas, while fish originating in European rivers below 62°N are thought to feed further west with some MSW-returning fish feeding in the Labrador Sea west of Greenland35,42,43,44,45,46,47. It is generally assumed that many river stocks and ages are mixed within marine feeding areas39, but identifying feeding locations used by specific river stocks or regional populations is challenging37, and stock-based management and conservation is hampered by a lack of information in the marine phase of life39. Given the above information, and the availability of long-term tissue archives, Atlantic salmon are an ideal test species for isotope-based investigations of migration and distribution. We use the relationship between time series of SST and measured tissue isotopes to identify likely feeding grounds at a stock- and cohort-specific level.
Results
The effect of trophic level on carbon isotope values
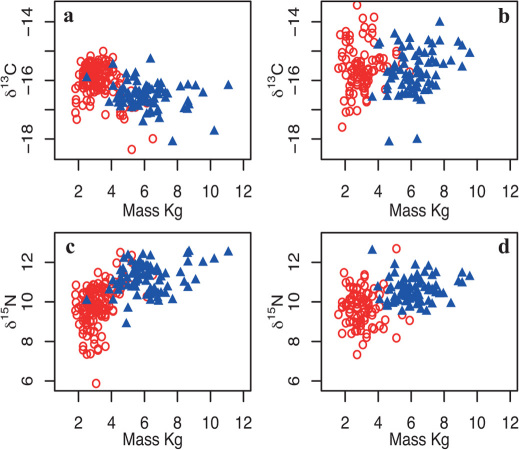
The potential influence of trophic level on measured δ13C values was tested by comparison between carbon and nitrogen isotope compositions in scale tissue from salmon populations returning to the UK River Frome (RF) and North East Coast (NEC). The isotopic composition of carbon and nitrogen in fish tissues is controlled to different degrees by variations in isotopic composition at the base of the food web and by trophic discrimination. Nitrogen is more strongly fractionated than carbon during dietary assimilation, with a mean trophic increase of c.3‰ compared to c.1‰ in carbon26,27,28. In our sampled scales, δ15N values correlate positively with size (RF salmon: n = 231, R2 = 0.41, p<0.001; NEC salmon: n = 162, R2 = 0.17, p<0.001), reflecting changes in trophic level (Fig. 1). Fish returning after more than one winter at sea clearly fed at higher trophic levels than the smaller 1SW returning fish. δ13C values also differ between 1SW and MSW returning fish, but show no positive relationship with either size (Fig. 1) or with δ15N values (Fig. 2), indicating that differences in the carbon isotope composition of scales cannot be explained by mass or trophic level. This strongly indicates that the carbon isotope composition of salmon collagen is dominated by variations in photosynthesis-related fractionation at the base of the food chain, and that salmon feeding in a common marine area are expected to have similar δ13C values45.
Figure 1. Relationship between fish mass and stable isotope composition in salmon returning to natal rivers either as 1SW (circle symbols) or MSW (triangle symbols) fish.
(a) River Frome δ13C values, (b) North East Coast δ13C values, (c) River Frome δ15N values, (d) North East Coast δ15N values.
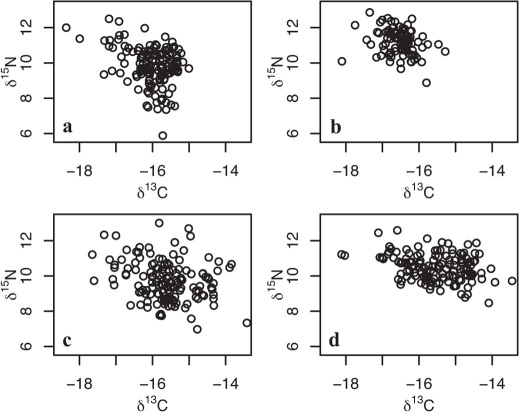
Figure 2. Relationship between the stable isotope composition of carbon and nitrogen in salmon returning to natal rivers either as 1SW or MSW fish.
(a) River Frome 1SW, (b) River Frome MSW, (c) North East Coast 1SW, (d) North East Coast ΜSW. Despite the large spread in δ15N values, there is no positive covariance between δ13C and δ15N values, indicating limited effects of trophic level on δ13C values.
Despite the large variation in plankton δ13C values across the North Atlantic Ocean14,23,48, salmon returning to the River Frome maintain consistently different δ13C values to those returning to the NE Coast throughout the 18 year sampling period (ANOVA, with geographic origin as sole co-factor; n = 523, df = 1, F = 116.9, p<0.001). In addition, year and sea age both significantly influence δ13C values (Year, n = 523, df = 17, F = 9.1, p<0.001; sea age df = 1, F = 16, p<0.001), with a significant interaction between all factors. These data strongly suggest that, in each sampled year, returning salmon from different natal origins fed in distinct locations. Stable isotope evidence for stock separation in feeding grounds is further explored in the supplementary information.
Locating marine feeding grounds from stable isotope time series
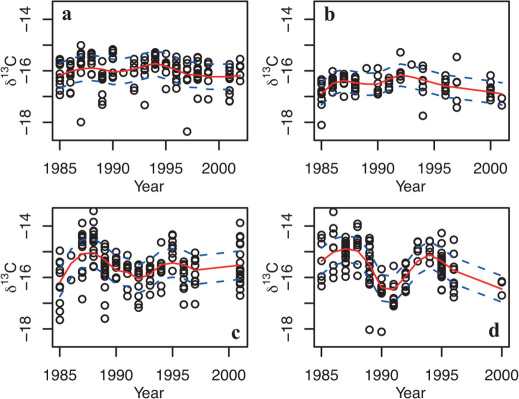
To determine the likely location of open ocean feeding areas for our test salmon populations, we assessed the temporal covariance between scale δ13C values and SST in each one-degree grid square between 45–75°N latitude and 65°W–20°E longitude for the period 1985 to 200249,50. Time series of tissue isotopes and SST are autocorrelated, violating the assumptions of serial independence demanded by most classical inference tests51. Autocorrelated time series are frequently de-trended to remove underlying low-frequency variations, however we are primarily interested in low frequency variations that are common to both time series, thus de-trending would be counter-productive51. Instead, the influence of high frequency fluctuations in the time series was reduced by applying LOESS smoothing with a span of 0.5 and polynomial order 2 (Fig. 3). Low frequency variations in the smoothed time series were correlated, and the influence of autocorrelation on significance tests was accounted for by adjusting the effective degrees of freedom for each time series pair using the modified Chelton method51. Areas with the highest correspondence between temporal variations in SST and measured δ13C values are suggested as the most likely marine feeding areas during deposition of scale collagen (Fig. 4).
Figure 3. Temporal trends in δ13C values (‰) in salmon scale collagen.
(a) River Frome 1SW, (b) River Frome MSW, (c) North East Coast 1SW, (d) North East Coast ΜSW. Temporal scale represents the last year of marine feeding. Solid (red) line is a first-order LOESS fit with a span width of 0.5; dashed (blue) lines show +/− standard error of the residuals of the smoothed fit.
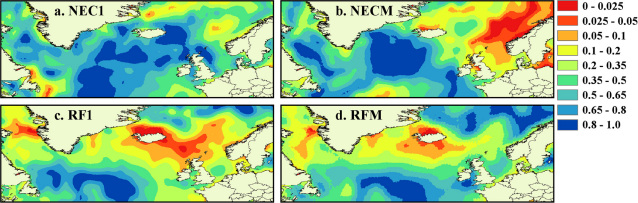
Figure 4. Proposed feeding areas for two Atlantic salmon populations indicated by the strength of correlation between temporal records of sea surface temperature and scale collagen δ13C values.
Eight month summer SST (March to October) records were extracted from the HADISST dataset49,50 at the native one-degree grid square resolution between 45–75°N latitude and 65°W–20°E longitude for the period 1985 to 2002. Yearly median summer temperatures were calculated for each degree square throughout the period studied. Correlations are based on LOESS fits to temporal data (span = 0.5). Colours indicate the significance of the correlation (p value) after adjustment of effective degrees of freedom to account for autocorrelation in both time series51. Each map represents feeding areas for a specific population and cohort: (a) North East Coast 1SW, (b) North East Coast MSW, (c) River Frome 1SW, (d) River Frome MSW.
The correlation approach taken here is based on the LOESS smoothed fit, and is sensitive to within-year variance, i.e. the assumption that all fish sampled return from the same location at sea. We tested the effect of within-year variance in δ13C values by removing all years where within-year δ13C standard deviations exceeded 5% of the mean value for that year. Two years were removed from the River Frome 1SW data, one from the River Frome MSW data, four from the NE Coast 1SW data and three from the NE Coast MSW data. Removing years of high variance did not have a dramatic effect on the locations of the proposed feeding grounds, but increased the strength of correlations.
Correlations between time series of scale δ13C values and SST vary spatially across the possible range for Atlantic salmon, reaching p values below 0.02 in MSW fish from both populations, with significant covariance (p values lower than 0.05) seen for all populations and sea ages. The spatial distribution of correlation coefficients is highly structured, and provides estimates of location that are consistent with known salmon feeding grounds and migration patterns35,38,47,52,53. The largest contiguous areas of highest correlations, and thus most likely feeding areas, for both the 1SW and MSW components of the NE Coast mixed stock are found in the Norwegian Sea. Correlations for the 1SW component of the NE Coast stock are relatively low, perhaps reflecting a greater range of feeding locations within this population, but a region of high correlation is located within the suspected feeding ground north of the Faroe Islands. Two relatively small areas of high correlation are seen in the Grand Banks and Davis Strait areas. Based on information from tagging and likely swimming capabilities of 1SW returning fish41,52,53, these can be identified as spurious correlations, and as with all such studies, the results of isotopic analyses should be viewed in the context of other available data to aid interpretation. Tissue isotope records for the MSW component of the NE Coast stock are strongly correlated with SST in the Norwegian Sea. These results are highly consistent with tag-recapture and return rate datasets35,41,47,52,53, and suggest that salmon originating from the North East Coast of the UK generally follow the easterly branch of the North Atlantic current into the Norwegian Sea37,47. Our results suggest that fish from the River Frome, in contrast, feed in more westerly regions, with 1SW returning fish occupying an area centred around the Faroe Islands and east Iceland (Fig. 4). The isotopic composition of MSW fish returning to the River Frome strongly implies marine feeding around the Icelandic shelf. Tagging databases show that southern European origin fish are caught more frequently in the west Greenland than the Faroese fisheries53, and limited fishing surveys suggest a salmon feeding ground east of Greenland, possibly in the Irminger Sea46,53, both indicating an overall westerly migration route for salmon originating from southern European rivers. Our results support this, suggesting that fish originating from the River Frome follow the western branch of the North Atlantic current. The confidence associated with any location identified through correlation analyses will depend on the length of the time series and the degree of variability in both SST and measured δ13C values. The strong match between isotope derived feeding grounds, and known feeding areas strongly suggests that in this case the 18 year archive is sufficient to identity feeding areas at least to the same resolution as is possible through traditional tagging and capture fisheries.
Large multi-year variations in δ13C scale values are seen in the NE Coast salmon (Fig. 3), suggesting that their feeding areas are subject to higher inter-annual variability in oceanic conditions than those of the River Frome fish. This implies that variations in ocean climate have greater potential to influence growth or return rates for the NE Coast origin fish than for the Frome stock. A significant negative relationship has indeed been found between the numbers of fish returning to rivers in north east Scotland and SST in the Norwegian Sea35. Differences in marine feeding location may thus underpin the variation in relationships between growth indices, return rates and ocean climate indices observed between salmon populations35,38.
Where autocorrelation adjusted correlations are significant at the 95% level, variations in SST explain between 43 and 97% of the observed variation in δ13C scale values. The unexplained variation may be attributed to a combination of variations in the isotopic composition of salmon diet (e.g. variations in trophic level, trophic fractionation, and plankton community composition), violations of the assumption of site fidelity within and between years, and possible contributions of later-formed collagen. The spatially constrained nature of the areas of high correlation, however, demonstrates that unexplained sources of isotopic variation are relatively minor compared to the effect of SST.
Implications for models of salmon migration
Our hypotheses of feeding ground location proposed from isotopic data are consistent with previous salmon migration models, In both the North East Coast and River Frome populations, proposed feeding areas for fish successfully returning after one winter at sea are closer to the river of origin than those proposed for MSW fish, and logical migration routes following the prevailing currents connect the proposed 1SW and MSW fish feeding areas. Our interpretations are based exclusively on fish that returned successfully to the home waters, and therefore we cannot rule out the possibility that fish from individual river stocks feed in a wider range of regions, but with differential rates of return. Tagged fish of UK origin (including one fish originating in the River Frome) have been recovered in the west Greenland fishery, but our isotopic data suggest that this is either a rare occurrence, or that River Frome origin fish feeding off west Greenland are relatively unlikely to return successfully.
The similar temporal trends, and thus relatively close geographic association between feeding grounds for 1SW and MSW fish for both test populations imply that stocks are spatially differentiated during their early outward migration. Hypotheses regarding stock origin within marine feeding grounds may be tested by genetically identifying the natal river origin of salmon caught in these feeding areas. Our interpretations are not biased by historic fishery areas, and identify a high degree of spatial segregation in marine feeding areas between regional populations and potentially, individual river stocks. This is similar to the strong coherence in regional populations noted for Pacific salmonids54, and supported by long term temporal records of catch and return rates in Norwegian and Scottish salmon38. Conservation of dwindling salmon stocks may require identification and management of open seas feeding grounds on a region- or river stock-specific basis, reflecting the different ocean conditions experienced by each stock.
Discussion
The isotopic composition of carbon varies significantly both spatially and temporally across ocean basins, especially in the temperate ocean, and these variations are transferred up food webs. Fractionation of carbon isotopes during photosynthesis varies with sea surface temperature, and consequently stable isotope analyses may be used to identify location in migratory marine animals by correlating coeval temporal records in tissue isotopes and SST over the possible spatial range of that species. This novel approach provides higher spatial resolution at a population-scale than is possible with traditional tag-recovery studies, and crucially is not restricted by historical fishing or tagging effort. Unlike the isoscape method for geolocation, our approach does not require a priori knowledge of distribution of baseline isotope values, and accommodates temporal variation in these baseline isotope values.
Critically, our approach can be applied retrospectively, and the influence of long-term climate variation on the spatial distribution of populations can be studied. Stable isotope-based techniques can be applied to any marine pelagic species (e.g. tuna, herring, turtles, seabirds etc.) for which long-term tissue archives are available or could be initiated, and would be particularly valuable in identifying regions suitable for marine no-take zones or protected areas.
Methods
Our samples come from archived collections of scales taken from wild Atlantic salmon returning to two distinct regions of the UK: the North East Coast drift net fishery, which samples a mixed stock originating in rivers from the North East coast of the UK55, around 55°N and 1°W, and the Dorset River Frome stock from the south coast of England, around 50.5°N and 2°W. Both sampled stocks belong to the proposed southern European population. For both archives, we determined δ13C and δ15N values of scales from approximately 10 1SW and 10 MSW fish for each available year between 1985 and 2002 (see supplementary information for a statistical justification of the sample numbers).
Scales are composite bioapatite-collagen structures. The mineralised component contains a minor inorganic carbonate component, but acid pre-treatment of scales results in biologically insignificant changes in the bulk isotopic composition56. δ13C values in the collagen component of fish scales are consistently enriched in δ13C relative to muscle values reflecting the high proportion of glycine in collagen57. Scale δ13C values are, however, linearly related to the isotopic composition of other body tissues across multiple genera, under differing diet conditions and growth rates56,58,59, indicating a consistent relationship between scale collagen and diet δ13C values.
Scales are incremental tissues growing allometrically with body size increase. Scale collagen is deposited only during growth, and is not turned over metabolically. Occasionally, scales are resorbed, but this is easily identified, and any scales showing resorption are not used for analyses. We therefore target collagen formed during spring-summer growth. Scales were briefly (c. 2–5 minutes) soaked in de-ionised water, manually cleaned using forceps and a scalpel to remove adherents such as lipids and guanine, and dissected under a transmitted light microscope. The last summer of growth at sea (indicated by widely spaced circuli) was excised to obtain a temporally distinct sample60. In 1SW fish, the summer section from the edge of the scale was sampled, while in MSW fish the summer immediately before the final winter at sea was sampled. Because of the three dimensional nature of growth of collagen in scales60 both 1SW and, to a lesser extent, 2SW samples will include a minor contribution of collagen laid down during the return migration. This additional collagen may contribute to the relatively high within year variance in seen in δ13C values in 1SW fish. The measured values represent the isotopic composition of fish tissues averaged over the last full season of marine growth (approximately 8 months, March – October). Samples were weighed to ∼0.60mg, and the isotope ratios determined by elemental analysis isotope ratio mass spectrometry (EA-IRMS), using L-glutamic acid as an in-house calibration standard. Measurement precision assessed as 2× standard deviation of 16 replicate analyses of USGS40 glutamic acid for δ15N and δ13C is, respectively, 0.7‰ and 0.1‰. Comparisons of carbon isotope values between populations were performed using ANOVA with geographic origin, year and sea age as factors. All statistical analyses were performed using the R statistical language61.
Author Contributions
KMM prepared and analysed samples. Data were interpreted by KMM, CNT and PMRP with contribution from all authors, AM, ATI and WRCB provided access to samples and expertise on salmon ecology, DJSP provided SST temperature data and aided data processing. The project was designed by CNT, PMRP and AM. The manuscript was written by KMM, CNT and PMRP with contributions from all authors.
Supplementary Material
Supplementary Information
Acknowledgments
This research was funded by Defra projects SF0250 and SF0246 to CNT. Cefas provided help, advice and samples from the North East Coast Driftnet Archive, with particular thanks to Barry Bendall and William Riley, and to CEH for help and samples from the River Frome Archive. Thanks also to Catherine Cole for help with sample preparation, to Mike Bolshaw for mass-spectrometry assistance and to Mark Moore and Elizabeth Kent for advice, all of the University of Southampton.
References
- Pauly D., Christensen V., Dalsgaard J., Froese R. & Torres F. Fishing down marine food webs. Science 279, 860–863 (1998). [DOI] [PubMed] [Google Scholar]
- Myers R. A. & Worm B. Rapid worldwide depletion of predatory fish communities. Nature 423, 280–283 (2003). [DOI] [PubMed] [Google Scholar]
- Begg G. A. Friedland K. D. & Pearce J. B. Stock identification and its role in stock assessment and fisheries management: an overview. Fish. Res. 43, 1–8 (1999). [Google Scholar]
- Hunter E., Metcalfe J. D. & Reynolds J. D. Migration route and spawning area fidelity by North Sea plaice. Proc. R. Soc. B, 270, 2097–2103 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright P. J., Neat F. C., Gibb F. M. & Thordarson H. Evidence for metapopulation structuring in cod from the west of Scotland and North Sea. J. Fish Biol. 69, 181–199 (2006). [Google Scholar]
- Wilson S. G., Lutcavage M. E., Brill R. W., Genovese M. P. Cooper A. B. & Everly A. W. Movements of bluefin tuna (Thunnus thynnus) in the northwestern Atlantic Ocean recorded by pop-up satellite archival tags. Mar. Biol. 146, 409–423 (2005). [Google Scholar]
- Hobson K. A. Tracing origins and migration of wildlife using stable isotopes: a review. Oecologia 120, 314–326 (1999). [DOI] [PubMed] [Google Scholar]
- Jennings S. & Warr K. J. Environmental correlates of large-scale spatial variation in the delta N-15 of marine animals. Mar. Biol. 142, 1131–1140 (2003). [Google Scholar]
- Bowen G. J. Isoscapes: Spatial pattern in isotopic biogeochemistry. Ann. Rev. Earth Planet. Sci. 38, 161–187 (2010). [Google Scholar]
- Beard B. L. & Johnson C. M. Strontium isotope composition of skeletal material can determine the birth place and geographic mobility of humans and animals. J. Foren. Sci. 45, 1049–1061 (2002). [PubMed] [Google Scholar]
- Faure G. Principles of Isotope Geology. 2nd Edition. (John Wiley and Sons Ltd., 1986). [Google Scholar]
- Schell D. M. Saupe S. M. & Haubenstock N. Bowhead whale (Balaena mysticetus) growth and feeding as estimated by δ13C techniques. Mar Biol. 103, 433–443 (1989). [Google Scholar]
- Cherel Y. & Hobson K. A. Geographical variation in carbon stable isotope signatures of marine predators: a tool to investigate their foraging areas in the Southern Ocean. Mar. Ecol. Prog. Ser. 329, 281–287 (2007). [Google Scholar]
- Graham B. S., Koch P. L., Newsome S. D., McMahon K. W. & Aurioles D. Using isoscapes to trace the movements and foraging behaviour of top predators in oceanic ecosystems. In: Isoscapes: Understanding Movement, Pattern and Process on Earth Trough Isotope Mapping (eds.West J. B., et al.), pp. 299–317 (Springer, 2010). [Google Scholar]
- Goericke R. & Fry B. Variations of marine plankton δ13C with latitude, temperature, and dissolved CO2 in the world ocean. Glob. Biogeoch. Cycles 8, 85–90 (1994). [Google Scholar]
- Laws E. A., Popp B. N., Bidigare R. R., Kennicutt M. C. & Macko S. A. Dependence of phytoplankton carbon isotope composition on growth rate and [CO2(aq)]: Theoretical considerations and experimental results. Geochim. Cosmochim. Acta 59, 1131–1138 (1995). [Google Scholar]
- Laws E. A., Bidigare R. R. & Popp B. N. Effect of growth rate and CO2 concentration on carbon isotopic fractionation by the marine diatom Phaeodactylum tricornutum. Limnol. Oceanog. 42, 1552–1560 (1997). [Google Scholar]
- Popp B. N. et al. Controls on the carbon isotopic composition of Southern Ocean phytoplankton. Glob. Biogeoch. Cycles 13, 827–843 (1999). [Google Scholar]
- Tagliabue A. & Bopp L. Towards understanding global variability in carbon-13. Glob. Biogeoch. Cycles 22, 1025 (2008). [Google Scholar]
- Reibesell U., Burkhardt S., Dauelsberg A. & Kroon B. Carbon isotope fractionation by a marine diatom: dependence on the growth-rate-limiting resource. Mar. Ecol. Prog. Ser. 193, 295–303 (2000). [Google Scholar]
- Cassar N., Laws E. A. & Popp B. N. Carbon isotopic fractionation by the by the marine diatom Phaeodactylum tricornutum under nutrient- and light-limited growth conditions. Geochim. Cosmochim. Acta 70, 5323–5335 (2006). [Google Scholar]
- Richardson A. J. & Schoeman D. S. Climate impact on plankton ecosystems in the northeast Atlantic. Science 305, 1609–1612 (2004). [DOI] [PubMed] [Google Scholar]
- Barnes C., Jennings S. & Barry J. T. Environmental correlates of large scale spatial variation in the δ13C of marine animals. Est. Coast. Shelf Sci. 81, 368–374 (2009). [Google Scholar]
- Zohary T., Erez J., Gophen M., Bermanfrank I. & Stiller M. Seasonality of stable carbon isotopes within the pelagic food-web of Lake Kinneret. Limnol. Oceanog. 39, 1030–1043 (1994). [Google Scholar]
- Rolff C. Seasonal variation in delta C-13 and delta N-15 of size-fractionated plankton at a coastal station in the northern Baltic proper. Mar. Ecol. Prog. Ser. 203, 47–65 (2000). [Google Scholar]
- Vander Zanden M. J. & Rasmussen J. B. Primary consumer delta C-13 and delta N-15 and the trophic position of aquatic consumers. Ecology 80, 1395–1404 (1999). [Google Scholar]
- McCutchan J. H., Lewis W. M., Kendall C. & McGrath C. C. Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102, 378–390 (2003). [Google Scholar]
- Newsome S. D., Clementz M. T. & Koch P. L. Using stable isotope biogeochemistry to study marine mammal ecology. Mar. Mam. Sci. 26, 509–572 (2010). [Google Scholar]
- Jaeger A., Lecomte V. J., Weimerskirch H., Richard P. & Cherel Y. Seabird satellite tracking validates the use of latitudinal isoscapes to depict predators' foraging areas in the Southern Ocean. Rap. Comm. Mass Spec. 24, 3456–3460 (2010). [DOI] [PubMed] [Google Scholar]
- Phillips R. A., Bearhop S., Mcgill R. A. R. & Dawson D. A. Stable isotopes reveal individual variation in migration strategies and habitat preferences in a suite of seabirds during the breeding period. Oecologia 160, 795–806 (2009). [DOI] [PubMed] [Google Scholar]
- Barnes C., Irigoien X., De Oliveira J. A. A., Maxwell D. & Jennings S. Predicting marine phytoplankton community size structure from empirical relationships with remotely sensed variables. J. Plank. Res. 33, 13–24 (2011). [Google Scholar]
- Behrenfeld M. J. et al. Climate-driven trends in contemporary ocean productivity. Nature 444, 752–755 (2006). [DOI] [PubMed] [Google Scholar]
- Boyce D. G., Lewis M. R. & Worm B. Global phytoplankton decline over the past century. Nature 466, 591–596 (2010). [DOI] [PubMed] [Google Scholar]
- Szép T., Møller A. P., Piper S., Nuttall R., Szabó Z. D. & Pap P. L. Searching for potential wintering and migration areas of a Danish Barn Swallow population in South Africa by correlating NDVI with survival estimates. J. Ornithol. 147, 245–253 (2006). [Google Scholar]
- Friedland K. D. et al. The recruitment of Atlantic salmon in Europe. ICES J. Mar. Sci. 66, 289–304 (2009). [Google Scholar]
- International Council for the Exploration of the Sea. Report of the Working Group on North Atlantic Salmon. (www.ices.dk/reports/ACFM/2005/WGNAS/wgnas05sec1.pdf, 2005). [Google Scholar]
- Dadswell M. J., Spares A. D., Reader J. M. & Stokesbury M. J. W. The North Atlantic subpolar gyre and the marine migration of Atlantic salmon Salmo salar: the ‘Merry-Go-Round' hypothesis. J. Fish Biol. 77, 435–467 (2010). [DOI] [PubMed] [Google Scholar]
- Vøllestad L. A. et al. Divergent trends in anadromous salmonid populations in Norwegian and Scottish rivers. Proc. Roy. Soc. B. 276, 1021–1027 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozier W. W., Schon P. J., Chaput G., Potter E. C. E., O'Maoileidigh N. & MacLean J. C. Managing Atlantic salmon (Salmo salar L.) in the mixed stock environment: challenges and considerations. ICES J. Mar. Sci. 61, 1344–1358 (2004). [Google Scholar]
- Reddin D. G. Stansbury D. E. & Short P. B. Continent of origin of Atlantic salmon (Salmo salar L.) at West Greenland. ICES J. Mar. Sci. 44, 180–188 (1998). [Google Scholar]
- International Council for the Exploration of the Sea. Report of the workshop on salmon historical information – new investigations from old tagging data (WKSHINI), 18–20 September 2008, Halifax, Canada. ICES CM 2008/DFC:02. 55 (2008). [Google Scholar]
- Went A. E. Movements of salmon Salmo salar (L.) to and from Irish waters. J. Fish. Biol. 5, 659–671 (1973). [Google Scholar]
- Swain A. Tagging of smolts in European rivers with special reference to recaptures off west Greenland in 1972 and earlier years. Rapp. P.-V. Reun. CIESM 176, 93–113 (1980). [Google Scholar]
- Hansen L. P. & Jacobsen J. A. Origin and migration of wild and escaped farmed Atlantic salmon, Salmo salar L., in oceanic areas north of the Faroe Islands. ICES J. Mar. Sci. 60, 110–119 (2003). [Google Scholar]
- Jacobsen J. A., Lund R. A., Hansen L. P. & O Maoileidigh N. Seasonal differences in the origin of Atlantic salmon (Salmo salar, L.) in the Norwegian Sea based on estimates of age structures and tag recaptures. Fish. Res. 52, 1659–1677 (2001). [Google Scholar]
- Reddin D. G. Ocean life of Atlantic salmon (Salmo salar, L.) in the northwest Atlantic. In Atlantic salmon: Planning for the future (eds Mills, D. & Piggins, D.), pp. 483–511 ( Croom Helm, 1988). [Google Scholar]
- Todd C. T., Hughes S. L., Marshall C. T., MacLean J. C., Lonergan M. E. & Biuw M. Detrimental effects of recent ocean surface warming on growth condition of Atlantic salmon. Glob. Change Biol. 14, 958–907 (2008). [Google Scholar]
- Dempson J. B., Braithwaite V. A., Doherty D. & Power M. Stable isotope analysis of marine feeding signatures of Atlantic salmon in the North Atlantic. ICES J. Mar. Sci. 67, 52–61 (2010). [Google Scholar]
- Rayner N. A. et al. Global analyses of sea surface temperature, sea ice, and night marine air temperature since the late nineteenth century. J. Geophys. Res. 108, 4407–4410 (2003). [Google Scholar]
- UK Meteorological Office Hadley Centre, British Atmospheric Data Centre. HadISST 1.1 - Global sea-Ice coverage and SST (1870-Present), (http://badc.nerc.ac.uk/data/hadisst/, 2006)
- Pyper B. J. & Peterman R. M. Comparison of methods to account for autocorrelation in correlation analyses of fish data. Can J. Fish. Aquat. Sci. 55, 2127–2140 (1998). [Google Scholar]
- Holst J. C., Shelton R. G. J., Holm M. & Hansen L. P. Distribution and Possible Migration Routes of Post-smolt Atlantic Salmon in the North-east Atlantic. In The Ocean Life of Atlantic Salmon: Environmental and Biological Factors Influencing Survival. (ed. D. Mills), pp. 65–74, (Fishing News Books, Blackwell Science, 1999). [Google Scholar]
- International Council for the Exploration of the Sea. Report of the workshop on learning from salmon tagging records (WKLUSTRE), 16–18 September 2009, London, UK. ICES CM 2009/DFC:05. 39 (2009). [Google Scholar]
- Pyper B. J., Mueter F. J., Peterman R. M., Blackbourn D. J. & Wood C. C. Spatial covariation in survival rates of Northeast Pacific pink salmon (Oncorhynchus gorbuscha). Can. J. Fish. Aquat. Sci. 58, 1501–1515 (2001). [Google Scholar]
- Potter E. C. E. & Swain A. Effects of the English north-east coast salmon fisheries on Scottish salmon catches. Fish. Res. Tech. Rep. 67 (MAFF Dir. Fish. Res., Lowestoft, 1982). [Google Scholar]
- Sinnatamby R. N. et al. Spatial and Temporal Variability in the Trophic Ecology of Atlantic Salmon in the North Atlantic Inferred from Analyses of Stable Isotope Signatures. In Challenges for Diadromous Fishes in a Dynamic Global Environment, (eds. Haro, A. et al.) pp. 447–463 (American Fisheries Society, 2009). [Google Scholar]
- Koch P. L. Isotopic study of the biology of modern and fossil vertebrates. In: Stable Isotopes in Ecology and Environmental Science, 2nd edition, (eds Michener, R. & Lajtha, K.) pp 99–154 (Blackwell Scientific Publications, 2007). [Google Scholar]
- DeNiro M. J. & Epstein S. Influence of Diet on Distribution of Carbon Isotopes in Animals. Geochim. Cosmochim. Acta 42, 495–506 (1978). [Google Scholar]
- Satterfield F. R. & Finney B. P. Stable isotope analysis of Pacific salmon: insight into trophic status and oceanographic conditions over the last 30 years. Prog. Oceanog. 53, 231–246 (2002). [Google Scholar]
- Hutchinson J. J. & Trueman C. N. Stable isotope analyses of collagen in fish scales: limitations set by scale architecture. J. Fish Biol. 69, 1874–1880 (2006). [Google Scholar]
- R Development Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria, (http://www.R-project.org, 2008).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information