Abstract
The current protocols for blocking background staining in immunohistochemistry are based on conflicting reports. Background staining is thought to occur as a result of either non-specific antibody (Ab) binding to endogenous Fc receptors (FcRs) or a combination of ionic and hydrophobic interactions. In this study, cell and tissue samples were processed according to routine protocols either with or without a blocking step (goat serum or BSA). Surprisingly, no Abs in samples processed without a blocking step showed any propensity for non-specific binding leading to background staining, implying that endogenous FcRs do not retain their ability to bind the Fc portion of Abs after standard fixation. Likewise, we did not find any non-specific Ab binding ascribable to either ionic or hydrophobic interactions. We determined that traditionally used protein blocking steps are unnecessary in the immunostaining of routinely fixed cell and tissue samples.
Although the causes of non-specific background immunostaining may differ, they all equally complicate the use of immunohistochemistry. Whereas unwanted background staining due to endogenous enzyme activities or endogenous biotin is no longer a problem in contemporary immunohistochemistry, nonspecific antibody (Ab) binding leading to unwanted background staining remains subject to considerable debate. Among the possible causes of non-specific binding of Abs, the attraction of primary and secondary Abs to endogenous Fc receptors (FcRs) is thought to be the main source of unwanted staining.
FcRs are structures on the surface of certain cells that bind the Fc region of Abs. Cross linking of Ab bound by FcRs provides an important link between the cellular and humoral branches of the immune system by inducing several responses, including phagocytosis, endocytosis, antibody-dependent cytotoxicity, release of inflammatory mediators, and enhancement of antigen presentation1. The nature of the response depends primarily on the cell type on which these FcRs are expressed. There are several types of FcR, which are classified on the basis of the type of immunoglobulins that they recognise2. FcRs for immunoglobulin G (IgG), the most common class of Ab used in immunohistochemistry, are designated Fc-gamma receptors (FcγRs). Other FcRs are expressed on multiple cell types and are similar in structure to MHC class I. Being involved in antigen presentation, these receptors can also bind IgG3.
It is theorised that FcRs bind the Fc region of Abs not only in vivo but also during immunohistochemical assays of cell and tissue samples. This concept has been mentioned in all publications regarding immunohistochemistry since its inception half a century ago4,5,6,7, but we have been unable to find the original source of the idea. It is thought that preincubation of a histological sample with 5–10% normal serum from the species that the secondary Ab is derived from will prevent non-specific binding of secondary Abs to endogenous FcRs. This makes little sense for the immunohistochemical staining of human cell and tissue samples, as the vast majority of secondary Abs used in human immunohistopathology are derived from goats, and goat serum has long been reported not to bind to FcRs on human cells8. Preincubation with solutions containing normal goat serum have also been assumed to prevent background staining that might result from ionic and hydrophobic interactions5. Blocking the non-specific background due to FcRs or ionic and hydrophobic interactions is considered an obligatory step prior to incubation with primary Ab. This can be observed in immunohistochemical protocols in all contemporary Ab manufacturers' catalogues (e.g., Dianova, ZytoMed and Jackson ImmunoResearch Laboratories Inc.), as well as on the popular IHC WORLD homepage and the homepages of the Ab manufacturers. All Ab manufacturers offer their own ready-to-use blocking solutions, and their formulations are trade secrets in many cases.
In spite of the fact that goat serum does not bind to FcRs on human cells8, goat serum remains the most popular blocking agent in human immunohistopathology. Some histochemists prefer FcR blocking with normal swine or rabbit serum9, but do not provide any experimental support for their preference. Additionally, more complicated blocking strategies have been reported, such as employing papain-digested fragments of unlabeled secondary Ab enriched with Fc fragments of the same IgG10. In theory, the most rational approach to prevent the possible non-specific background due to FcR binding would be the use of F(ab′)2 fragments of Ab instead of the whole IgG molecule11, provided that the endogenous FcRs do retain their ability to bind the Fc portion of IgG Ab after proper fixation.
Other blocking solutions based on bovine serum albumin (BSA), coldwater fish gelatine, tryptone casein peptone, non-fat dry milk or casein are thought to prevent non-specific background by blocking hydrophobic interaction between proteins and ionic or electrostatic interactions9,12,13. Casein is thought to be more effective than normal serum for blocking hydrophobic background staining7. However, casein, BSA, and dry milk can all contain bovine IgG14. Many secondary Abs, such as anti-bovine Ig Ab, anti-goat Ig Ab, and anti-sheep Ig Ab, will react strongly with bovine IgG. Therefore, the use of BSA, dry milk or casein as a blocking agent may actually increase background and reduce antibody titer. Somewhat more complicated methods thought to reduce Ab binding to tissue proteins include using diluent buffers with low ionic strength, and adding non-ionic detergents (such as Tween 20 or Triton X) or ethylene glycol to the diluent15,16. Other techniques thought to decrease background include co-incubation of primary antibodies with reduced glutathione, L-cysteine, iodoacetic acid, Ellman's reagent and other thiophilic reagents17,18.
It has not been explicitly documented whether the non-specific binding of Fc fragments of Ab is equally problematic for frozen tissue sections and paraffin-embedded tissue sections. It is thought that the non-specific staining due to the attraction of Fc fragments to FcRs is more common in frozen sections than in routinely aldehyde-fixed, paraffin-embedded tissue sections19,20. Others have stated that the increased hydrophobicity of proteins after aldehyde fixation and paraffin embedding increases the non-specific binding of the Fc portion of IgG Ab7,9. Non-specific staining in paraffin sections has also been presumed to occur because of attraction of the Fc portion of IgG Ab to basic groups present in collagen fibres21.
The reports of possible background immunohistochemical staining due to non-specific Ab binding in frozen and paraffin-embedded tissue sections and in cytological preparations are conflicting, and most of these reports are outdated and lack clear-cut experimental support. This prompted us to explore whether commercially available Abs have a propensity for random non-specific binding in the immunolabelling of routinely fixed cell and tissue samples.
Results
Immunostaining on two immediately adjacent sections with and without the blocking step was evaluated by three microscopists in a researcher-blinded manner. We observed no differences in immunostaining between cell and tissue samples that were processed either with or without protein blocking. Contrary to the belief that nonspecific background staining is more common for frozen sections and cell smears than for paraffin-embedded tissue sections19,20, background staining did not appear to be a problem with frozen tissue sections fixed either with formaldehyde (Fig. 1a–c) or with acetone. We also observed no difference between preparations with blood cell smears, cell culture monolayers and cytospins processed either with or without protein blocking and fixed in methanol (not shown).
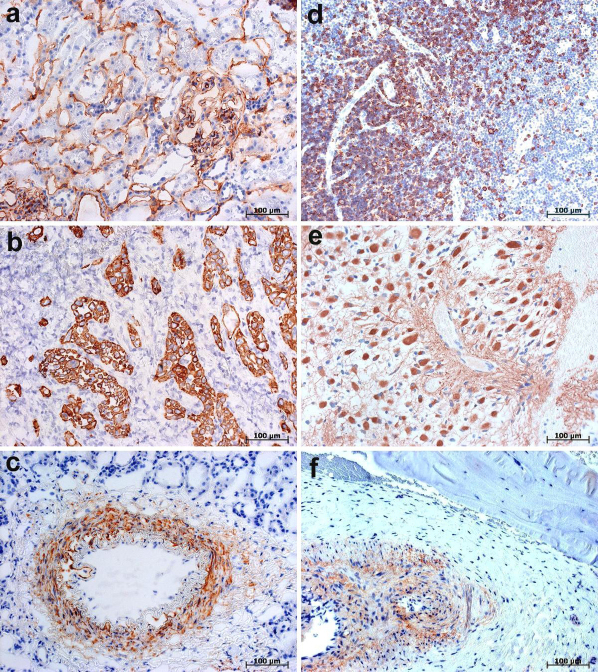
Figure 1. Immunohistochemical staining of human tissue samples processed without the protein blocking step prior to incubation with primary Abs.
Bound primary Abs were detected using DAKO EnVision+ System-HRP (DAKO Corporation, Hamburg, Germany) or AmpliStain™ HRP conjugate (SDT GmbH, Baesweiler, Germany) with NovaRed substrate kit. Nuclei counterstained with Ehrlich hematoxylin. (a–c) Immunostaining of human tissue cryosections after routine formaldehyde fixation (3 min by room temperature). (d–f) Immunostaining of routinely formaldehyde-fixed paraffin-embedded human tissue sections. (a) Immunolabelling of CD34 in capillary endothelium of human kidney. (b) Immunolabelling of cytokeratins 8/18/19 in human pancreas carcinoma. (c) Immunolocalization of α Smooth Muscle Actin in arterial cell wall in human kidney. (d) Immunolabelling of CD20 in B lymphocytes in human tonsil. (e) Immunolabelling of Glial Fibrillary Acidic Protein in human brain tumor astrocytoma. (f) Specific immunolabelling of collagen IV in blood vessel adventitia in inflammatory bone tissue. Note that collagens in connective tissue (collagen I) and in bone (collagen I and V) do not demonstrate any affinity to Fc fragments of either primary or secondary Ab.
In paraffin sections of formaldehyde-fixed human tissue samples processed without the protein blocking step (Fig. 1d–f), we did not observe any background staining that could be ascribed to the increased hydrophobicity of proteins after aldehyde fixation and paraffin embedding7,9. Contrary to the belief that non-specific staining might take place in paraffin sections because of attraction of the Fc portion of IgG Ab to basic groups present in collagen fibres21, we did not observe any nonspecific background immunostaining in paraffin sections of various collagen-rich tissues, such as media of blood vessels (Fig. 1c), inflammatory bone tissue (Fig. 1f) or bone tissue seen in bone marrow preparations (Fig. 2a).
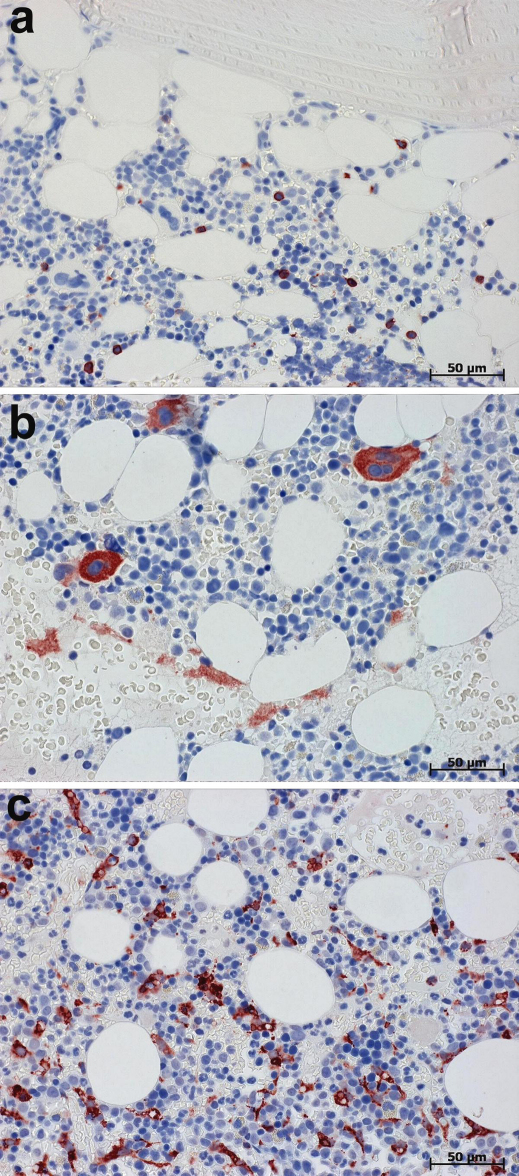
Figure 2. Immunodetection of markers of Clusters of Differentiation (CD) in bone marrow preparations processed without the protein blocking step prior to incubation with primary Abs.
(a) Immunolabelling of CD20 in B lymphocytes. (b) Immunolabelling of CD61 in megakaryocytes and in thrombocytes. (c) Immunolabelling of CD68 in fibroblastic dendritic cell and in monocytes. Bound primary Abs were visualized using AmpliStain™ HRP conjugate (SDT GmbH, Baesweiler, Germany) with NovaRed substrate kit. Nuclei counterstained with Ehrlich hematoxylin. Immunohistochemical staining was performed without protein block prior to incubation with primary Ab. Note in (a) the absence of unspecific Ab binding to bone tissue and to hematopoetic cells, in (b) the absence of unspecific Ab binding to granulocytes and monocytes and in (c) the absence of unspecific Ab binding to granulocytes and megakaryocytes.
In view of the fact that FcRs are expressed primarily on monocytes, macrophages, B cells, dendritic cells, neutrophils and platelets2, we paid special attention to cell and tissue samples where these cells are abundant, specifically bone marrow, spleen, tonsils and blood cell smears. As demonstrated by CD20 immunolabelling of human tonsils (Fig. 1d) and with bone marrow preparations immunostained for CD20, CD61 and CD68 (Fig. 2), no unwanted background was observed in tissue samples processed with the omission of protein blocking prior to incubation with primary Ab. Likewise, no unwanted background was found in the corresponding negative controls with the omission of incubation with primary Abs. Because of this, we concluded that endogenous FcRs do not retain their ability to bind the Fc portion of IgG Ab after fixation routinely used in immunohistochemistry.
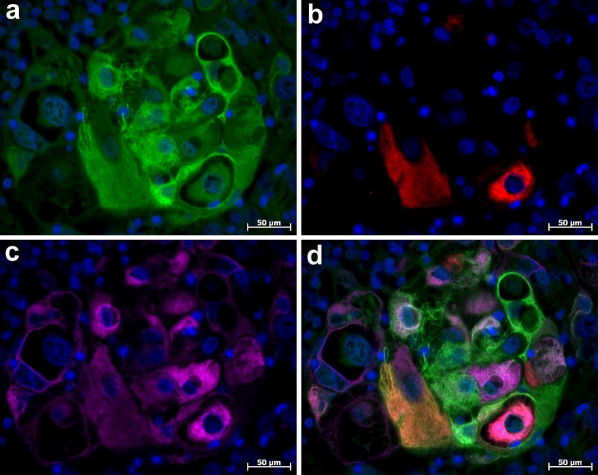
After performing immunostaining using fluorophore-conjugated Abs, we also found that the omission of the protein blocking step did not lead to non-specific background staining in single or multiple fluorescence immunolabelling with the use of either fluorophore-conjugated Ab or streptavidin. This is demonstrated with the immunofluorescent triple staining of cytokeratin 5, cytokeratin 10 and cytokeratin 14 in adeno-squamous carcinoma of the human mammary gland (Fig. 3).
Figure 3. Immunofluorescent triple staining of cytokeratin 5, cytokeratin 10 and cytokeratin 14 in adeno-squamouse carcinoma of human mammary gland.
Immunolabelling was performed without the protein blocking step prior to incubation with primary Abs. (a) Immunolocalization of cytokeratin 14 (Alexa 488, green channel). (b) Immunolocalization of cytokeratins 10 (Cy3, red channel). (c) Immunolocalization of cytokeratin 5 (Alexa 647, pink channel). (d) Composite image. Nuclei are counterstained with DAPI.
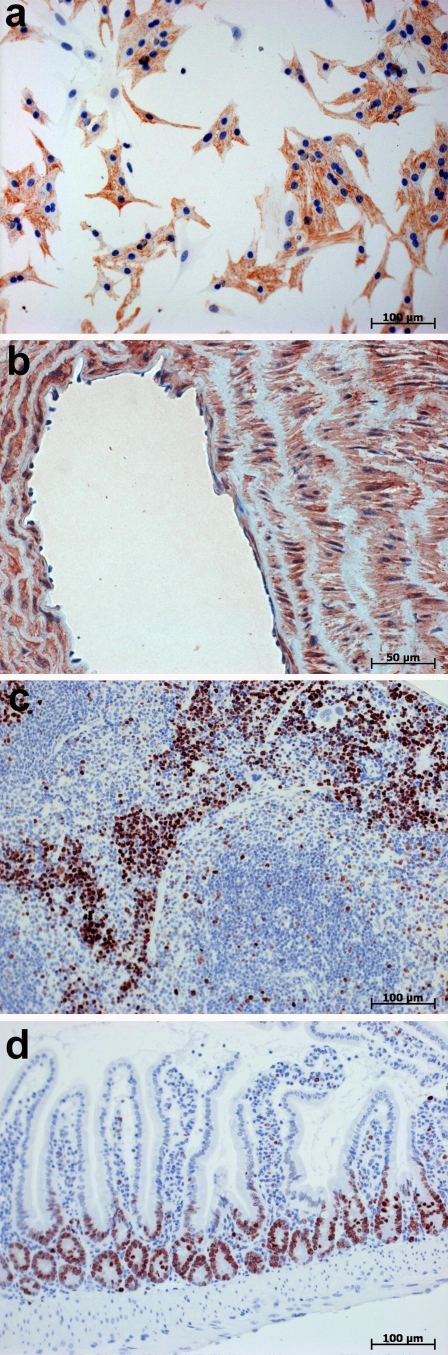
This was a clinically oriented study focused on human patient tissue samples. However, many researchers perform immunohistochemistry on tissues from experimental animals, particularly rodent tissues. Because of this, we performed immunostaining also on neonatal-rat-cultured cardiomyocytes, mouse and rat tissue samples (Fig. 4), as well as on tissue samples taken from cow and swine (not shown), processed either with or without the protein blocking step. Similar to immunostaining of human cell and tissue samples, omission of the protein blocking step did not lead to unwanted background staining in histological samples from experimental and farmed animals.
Figure 4. Immunohistochemical staining of cell and tissues samples from experimental animals.
Immunolabelling was performed without the protein blocking step prior to incubation with primary Abs. Bound primary Abs were detected using AmpliStain™ HRP conjugate (SDT GmbH, Baesweiler, Germany) with NovaRed substrate kit. Nuclei counterstained with Ehrlich hematoxylin. (a) Immunostaining of desmin in neonatal-rat-cultured cardiomyocytes. (b) Immunostaining of α Smooth Muscle Actin in the rat aorta cell wall. Note that collagens in elastic laminae do not demonstrate any affinity to Fc fragments of either primary or secondary Ab. (c) Immunostaining of Ki67 in the mouse spleen. (d) Immunostaining of Ki67 in the mouse gut.
Discussion
During the past few decades, improvements in the reagents and protocols used for immunohistopathology have led to increased sensitivity of detection systems, widely contributing to the elimination of non-specific background immunostaining. However, current protocols for blocking unwanted background signals are based on conflicting and outdated reports. The concepts of the attraction of Fc portions of Ab to FcRs and of non-specific Ab binding due to hydrophobic interaction between proteins and ionic and electrostatic interactions seem to reflect desperate attempts of immunohistochemists half a century ago to find a plausible explanation for poor immunostaining with home-made Abs that were not always of the best quality. During this time period, antisera were occasionally collected and stored in inappropriate ways, and primary Abs were sometimes applied in supra-optimal concentrations. Non-specific background immunostaining may have also resulted from other factors, such as inappropriate immunohistochemical detection methods, protracted time of chromogen application, improper fixation, protracted fixation time and interval before fixation. Maintenance of the specimen's morphology during fixation is the most important prerequisite for good immunostaining22. If the specimen's morphology is poor, one cannot expect a quality result.
This study was performed on cryosections, cell culture monolayers, blood cell smears and cytospins fixed for no longer than 15 min in either acetone or alcohol. For paraffin embedding, cell and tissue samples were fixed in 4% buffered formaldehyde for 18–48 hrs at room temperature. Evaluation of the possible undesirable effects of either protracted fixation or the use of stronger fixation media was beyond the scope of this study because the influence of fixation strength and duration on the availability and conformation of antigen epitopes and on specimen morphology is unknown and often unpredictable. To allow proper evaluation and replication of our immunohistochemical experiments, Abs in this study were applied strictly according to manufacturers' recommendations. Using these standardised conditions, we did not observe any differences in immunostaining in any samples processed either with or without protein blocking. This means that the protein blocking step traditionally used in immunohistochemistry does not influence the quality of immunostaining and that omission of the protein blocking step does not lead to unwanted background staining.
Our data allowed us to conclude that endogenous FcRs do not retain the ability to bind the Fc portion of IgG Ab after fixation in formaldehyde, acetone or alcohol. Likewise, non-specific Ab binding to tissue proteins due to hydrophobic interaction between proteins or ionic and electrostatic interactions does not take place in routinely fixed cell and tissue samples. In contrast to the commonly accepted view, we found that the protein blocking step traditionally used in immunohistochemistry is unnecessary in the immunostaining of routinely fixed cell and tissue samples taken both from human patients and from nonhuman species. Omission of the traditional protein blocking step may save substantial reagent costs and preparation time both in research and immunohistopathology.
Methods
We performed comparative immunostaining procedures with and without the protein blocking step on frozen and paraffin-embedded tissue sections, as well as on cell culture monolayers and cytospins. For paraffin embedding, tissue samples were fixed with 4% formaldehyde in PBS. Four-micrometre-thick paraffin tissue sections were deparaffinised with xylene and graded ethanol, and antigen retrieval was performed by heating the sections in 10 mM sodium citrate buffer, pH 6.0, at 95° C for 30 min in a domestic vegetable steamer23. Frozen tissue sections, cell monolayers, blood cell smears and cytospins were immunostained after fixation with 4% formaldehyde, methanol or acetone. The blocking step prior to incubation with the primary Ab was performed with either 5–10% normal goat serum or 1% BSA in PBS.
All Abs were applied according to manufacturers' recommendations. The final concentration of primary Ab was between 1 and 5 µg/ml PBS. For immunostaining, we used 45 mouse monoclonal and rabbit polyclonal primary Abs (Table 1). For bright-field microscopy, bound primary Abs were detected with a EnVision Horse Radish Peroxidase (HRP) System (DAKO Corporation, Hamburg, Germany) or with an AmpliStain™ HRP conjugate (SDT GmbH, Baesweiler, Germany) according to the manufacturers' instructions. HRP labelling was visualised using a NovaRed substrate kit (Vector Laboratories, Burlingame, CA, USA). For fluorescence microscopy, we used a goat secondary Ab conjugated with Cy3, Alexa Fluor-488, Alexa Fluor-647 or biotin. The latter was visualised using fluorophore-labelled streptavidin. Secondary Abs and other reagents used in this study are listed in Table 2. The final concentration of secondary Ab was between 5 and 10 µg/ml PBS. Single and multiple immunofluorescence labelling were performed according to standard protocols23. Immunostained sections were examined on a Zeiss Axio Imager Z1 microscope. Microscopy images were captured using AxioCam digital microscope cameras and AxioVision image processing (Carl Zeiss Vision, Germany). The images were acquired at 96 DPI and submitted with the final revision of the manuscript at 300 DPI.
Table 1. Primary antibodies used in this study.
| Antibodies | Source | Dilution | Tissues/Cells* |
|---|---|---|---|
| IgA (alpha), (rabbit Ab) | DAKO | 1/2000 | 1, 2, 3 |
| IgG (gamma), (rabbit Ab) | DAKO | 1/1000 | 1, 2, 3 |
| IgM (my, µ), (rabbit Ab) | DAKO | 1/1000 | 1, 2, 3 |
| Bcl2 (mouse Ab) | DAKO | 1/100 | 2, 4, 5, 6, 7 |
| α Smooth Muscle Actin (mouse Ab) | DAKO | 1/50 | 4, 5, 6, 7, 8, 9 |
| α Smooth Muscle Actin (rabbit Ab) | AbCam | 1/200 | 4, 5, 6, 7, 8, 9 |
| ApoE (rabbit Ab) | Santa Cruz | 1/100 | 10 |
| CD3 (mouse Ab | Novocastra | 1/200 | 2, 23 |
| CD10 (mouse Ab) | Novocastra | 1/50 | 5 |
| CD20 (mouse Ab | DAKO | 1/500 | 2, 3, 10 |
| CD32 (mouse Ab) | AbCam | 1/1000 | 3, 23 |
| CD34 (mouse Ab) | Novocastra | 1/50 | 1, 9, 25 |
| CD61 (mouse Ab) | Novocastra | 1/100 | 10 |
| CD68 (mouse Ab) | DAKO | 1/200 | 2, 3, 10 |
| CD117, c-Kit (rabbit Ab) | DAKO | 1/100 | 4, 5 |
| Cytokeratins 5 (rabbit Ab) | Medac | 1/100 | 4, 5, 6, 7, 8, 24, 25 |
| Cytokeratin 5/6 (mouse Ab) | DAKO | 1/50 | 4, 5, 6, 7, 8, 24, 25 |
| Cytokeratin 7 (mouse Ab) | DAKO | 1/200 | 4, 5, 6, 7, 8, 24, 25 |
| Cytokeratin 10 (mouse Ab) | DAKO | 1/50 | 4, 5, 6, 7, 13 |
| Cytokeratin 14 (mouse Ab) | Jackson ImmunoRes | 1/500 | 4, 5, 6, 7, 8, 24, 25 |
| Cytokeratin 18 (mouse Ab) | Sigma | 1/50 | 4, 5, 6, 7, 8, 24 |
| Cytokeratin 8/18 (mouse Ab) | Zytomed | 1/50 | 4, 5, 6, 7, 8, 24, 25 |
| Cytokeratin AE1/AE3 (mouse Ab) | DAKO | 1/50 | 2, 4, 5, 6, 7, 13 |
| Cytokeratins 8/18/19 (mouse Ab) | Immunotech | 1/100 | 4, 5, 6, 7, 8, 25 |
| Collagen IV (mouse Ab) | DAKO | 1/20 | 5, 11 |
| Desmin (mouse Ab) | DAKO | 1/200 | 12, 14 |
| E-Cadherin (mouse Ab) | DAKO | 1/50 | 1, 4, 5 |
| Calcitonin (rabbit Ab) | DAKO | 1∶500 | 15 |
| Calponin (mouse Ab) | DAKO | 1/50 | 9, 16, 17 |
| EMA (mouse Ab) | DAKO | 1/50 | 1 |
| Estrogen Receptors (rabbit Ab) | Thermo | 1/200 | 4, 5 |
| GFAP (mouse Ab) | DAKO | 1/100 | 18 |
| GFP (rabbit Ab) | AbCam | 1/500 | 19 |
| HMB45 (mouse Ab) | DAKO | 1/50 | 13 |
| Kappa Light Chains (rabbit Ab) | DAKO | 1/8000 | 2, 10 |
| Lambda Light Chains (rabbit Ab) | DAKO | 1/8000 | 2, 10 |
| Ki67 (rabbit Ab) | Thermo | 1/200 | 2, 4, 5, 6 , 7, 24 |
| MIB1 (mouse Ab) | DAKO | 1/20 | 2, 4, 5, 6 , 7, 24 |
| Myf-4 (mouse Ab) | Zytomed | 1∶50 | 20 |
| nNOS (rabbit Ab) | Transduction Lab. | 16, 17, 21, 22 | |
| eNOS (rabbit Ab) | Transduction Lab. | 9, 16, 17, 21, 22 | |
| P53 (mouse Ab) | DAKO | 1/50 | 5, 7 |
| p63 (mouse Ab) | DAKO | 1/200 | 4, 5, 6, 7 |
| S100 (rabbit Ab) | DAKO | 1/2000 | 2, 5, 7, 10 |
| Vimentin (mouse Ab) | DAKO | 1/200 | 15 |
| Vimentin (rabbit Ab) | AbCam | 1/1000 | 5, 9 |
*Cell and tissue samples immunostained in this study: Human kidney (1), Human tonsil (2), Human lymph nodes (3), Human mammary gland (4), human breast tumors (5), Human salivary gland (6), Human salivary gland tumors (7), Human lacrimary gland (8), Human aorta (9), Human bone marrow (10), Human bone tissue (11), Human gastrointestinal tissue (12), Human skin (13), neonatal-rat-cultured cardiomyocytes (14), Human thyroid gland (15), Human muscle tissue (16), Rat muscle tissue (17), Human brain astrocytoma (18), Mouse heart, spleen and kidney (19), Human rabdomyosarcoma (20), Human pancreas (21), Rat pancreas (22), Human blood cell smears and cytospins (23), Cell cultures of human adenoid cystic carcinoma (24), Cow and pig mammary gland (25)
Table 2. Secondary antibodies and other reagents.
| Antibodies | Source | Dilution | Label |
|---|---|---|---|
| Goat Normal Serum | Jackson ImmunoRes | 1/100 | w/o |
| Mouse Normal Serum | Jackson ImmunoRes | 1/100 | w/o |
| Bovine serum albumin | Biomol | 1% | w/o |
| Goat anti–mouse IgG Ab | Invitrogen | 1/200 | Alexa Fluor 488 |
| Goat anti–mouse IgG Ab | Invitrogen | 1/200 | Alexa Fluor 555 |
| Goat anti–mouse IgG Ab | Invitrogen | 1/100 | Alexa Fluor 647 |
| Goat anti–rabbit IgG Ab | Invitrogen | 1/100 | Alexa Fluor 647 |
| Biotin-SP-AffiniPure Fab Fragment Goat Anti-Mouse | Jackson ImmunoRes | 2–10 µg/ml | Biotin |
| Biotin-conjugated anti-mouse IgG3 | BD Pharmingen | 1/25 | Biotin |
| Streptavidin | Jackson ImmunoRes | 1/200 | Cy3 |
| Anti-mouse EnVision+ System-HRP | DAKO Corporation | ready-to-use | HRP |
| Anti-rabbit EnVision+ System-HRP | DAKO Corporation | ready-to-use | HRP |
| AmpliStain™ anti-Mouse 1-Step HRP | SDT GmbH, Baesweiler, Germany | ready-to-use | HRP |
| AmpliStain™ anti-Rabbit 1-Step HRP | SDT GmbH, Baesweiler, Germany | ready-to-use | HRP |
| 4′,6-diamidino-2-phenylindole (DAPI, nuclear counterstaining) | Sigma | 5 µg/ml | w/o |
| Vector® NovaRED™ Substrate Kit | Vector Laboratories, Burlingame, CA, USA | ready-to-use | w/o |
| VECTASHIELD® Mounting Medium | Vector Laboratories, Burlingame, CA, USA | ready-to-use | w/o |
Author Contributions
I.B. designed experiments, analyzed and interpreted results, and wrote the manuscript, whereas all the authors contributed to its revision; V.S. did immunohistochemistry; W.B. analyzed and interpreted results; M.T. directed the study and contributed to financially support the research.
Acknowledgments
We thank colleagues from our Institute for sharing reagents and cell and tissue samples (frozen and paraffin-embedded tissue sections, bone marrow preparations, cell smears and cytospins), Dr. Gergs (Institute for Pharmacology and Toxicology, Halle/Saale, Germany) for providing monolayers of neonatal-rat-cultured cardiomyocytes, Prof. Stenman (Lundberg Laboratory for Cancer Research, Sahlgrenska Academy at University of Gothenburg, Sweden) for providing cultured adenoid cystic carcinoma cells and Prof. Emoto (Gunma University School of Health Sciences, Japan) for critical reading of the manuscript.
References
- Ravetch J. V. & Kinet J. P. Fc Receptors. Annu. Rev. Immunol. 9, 457–492 (1991). [DOI] [PubMed] [Google Scholar]
- Cruse J. M. & Lewis R. E. Atlas of Immunology (Springer, Heidelberg, 2000). [Google Scholar]
- Zhu X. et al. MHC class I-related neonatal Fc receptor for IgG is functionally expressed in monocytes, intestinal macrophages, and dendritic cells. J. Immunol. 166, 3266–3276 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussolati G. & Leonardo E. Technical pitfalls potentially affecting diagnoses in immunohistochemistry. J. Clin. Pathol. 61, 1184–1192 (2008). [DOI] [PubMed] [Google Scholar]
- Daneshtalab N., Dore J. J. & Smeda J. S. Troubleshooting tissue specificity and antibody selection: Procedures in immunohistochemical studies. J. Pharmacol. Toxicol. Methods 61, 127–135 (2010). [DOI] [PubMed] [Google Scholar]
- Fritschy J. M. Is my antibody-staining specific? How to deal with pitfalls of immunohistochemistry. Eur. J. Neurosci. 28, 2365–2370 (2008). [DOI] [PubMed] [Google Scholar]
- Ramos-Vara J. A. Technical aspects of immunohistochemistry. Vet. Pathol. 42, 405–426 (2005). [DOI] [PubMed] [Google Scholar]
- Alexander E. L. & Sanders S. K. F(ab′)2 reagents are not required if goat, rather than rabbit, antibodies are used to detect human surface immunoglobulin. J. Immunol. 119, 1084–1088 (1977). [PubMed] [Google Scholar]
- Boenisch T. Handbook on Immunohistochemical Staining Methods (DAKO Corporation, Carpinteria, CA., 2001). [Google Scholar]
- Lu Q. L. & Partridge T. A. A new blocking method for application of murine monoclonal antibody to mouse tissue sections. J. Histochem. Cytochem. 46, 977–984 (1998). [DOI] [PubMed] [Google Scholar]
- Brandon C. Improved immunocytochemical staining through the use of Fab fragments of primary antibody, Fab-specific second antibody, and Fab-horseradish peroxidase. J. Histochem. Cytochem. 33, 715–719 (1985). [DOI] [PubMed] [Google Scholar]
- Kim S. H. et al. An improved protocol of biotinylated tyramine-based immunohistochemistry minimizing nonspecific background staining. J. Histochem. Cytochem. 51, 129–132 (2003). [DOI] [PubMed] [Google Scholar]
- Renshaw S. Immunohistochemistry (Scion Publishing Ltd, Cambridge, 2007). [Google Scholar]
- Hurley I. P., Coleman R. C., Ireland H. E., & Williams J. H. Measurement of bovine IgG by indirect competitive ELISA as a means of detecting milk adulteration. J. Dairy Sci. 87, 543–549 (2004). [DOI] [PubMed] [Google Scholar]
- Pino R. M. Binding of Fab-horseradish peroxidase conjugates by charge and not by immunospecificity. J. Histochem. Cytochem. 33, 55–58 (1985). [DOI] [PubMed] [Google Scholar]
- Juhl B. R., Norgaard T. & Bjerrum O. J. The effect of Tween 20 on indirect immunoperoxidase staining of blood group antigen A in human urothelium. J. Histochem. Cytochem. 32, 935–941 (1984). [DOI] [PubMed] [Google Scholar]
- Richards J. M. & Witkin S. S. Non-immune IgG binding to the surface of spermatozoa by disulphide rearrangement. Clin. Exp. Immunol. 58, 493–501 (1984). [PMC free article] [PubMed] [Google Scholar]
- Rogers A. B., Cormier K. S. & Fox J. G. Thiol-reactive compounds prevent nonspecific antibody binding in immunohistochemistry. Lab. Invest. 86, 526–533 (2006). [DOI] [PubMed] [Google Scholar]
- Elias J. M. Immunohistochemical methods in Immunohistopathology. in A Practical Approach to Diagnosis (ed. Elias, J. M.) 1–110 (ASCP Press, Chicago, IL, 2003). [Google Scholar]
- Larsson L. I. Immunocytochemistry: Theory and Practice (CRC Press, Boca Raton, FL, 1988). [Google Scholar]
- Weston P. D. & Poole A. R. Antibodies to enzymes and their use, with specific reference to cathepsin D and other lysosomal enzymes in Lysosomes in Biology and Pathology. (ed. Dingle, J. T.) 425–464 (North Holland Publishing, Amsterdam, 1973). [Google Scholar]
- Miller R. T. Technical Immunohistochemistry: Achieving Reliability and Reproducibility of Immunostains. Society for Applied Immunohistochemistry, 2001 Annual Meeting.1–56 (2011). Full text available on the Website <http://www.ihcworld.com/_books/Miller_handout.pdf>
- Buchwalow I. B. & Boecker W. Immunohistochemistry: Basics and Methods (Springer, Heidelberg, Dordrecht, London, New York, 2010). [Google Scholar]