Abstract
We report a new, suspended-microsphere diagnostic test to detect antibodies to West Nile (WN) virus in human serum and cerebrospinal fluid (CSF). The microsphere immunofluorescence assay can be performed in less than 3 h on specimens of ≤30 μl. A recombinant WN virus envelope (E) protein antigen is covalently coupled to fluorescent polystyrene microspheres. After incubation with diluted serum or CSF, antibodies bound to the E protein antigen are detected with fluorescently labeled anti-human immunoglobulin antibody and flow analysis in a dual-laser Luminex 100 instrument. Retrospective testing of 833 sera from New York patients with suspected viral encephalitis demonstrated concordance with results obtained with the traditional enzyme-linked immunosorbent assay for immunoglobulin G (IgG) antibodies to WN virus (kappa = 0.85). One hundred eighty-eight (22.4%) of the samples, which were collected from June to November 2002, tested positive for antibodies to WN virus in the microsphere assay. Specimens depleted of IgG with anti-IgG antibody were reassayed to measure anti-E protein IgM antibodies and to provide an indication of current or recent WN virus infection. The assay also detects antibodies to E proteins from related flaviviruses, including St. Louis encephalitis, Japanese encephalitis, and dengue viruses. The new microsphere immunoassay provides a sensitive and rapid alternative to traditional enzyme-linked immunosorbent assays that detect antibodies to flavivirus E proteins. This assay can aid physicians and public health workers in the management of outbreaks of WN virus and related flaviviruses.
West Nile (WN) virus made its unexpected initial North American appearance in Queens, New York, in 1999 (2, 20). Despite a prompt public health response, 59 patients were hospitalized with WN virus infection, and 7 patients died (26). During the next 3 years, WN virus rapidly spread across the continent (19, 28). In 2002, more than 3,700 human cases of WN virus infection were reported to the Centers for Disease Control and Prevention (CDC), including 201 fatal cases (10). Mosquito vectors and avian hosts amplify WN virus, with secondary transmission to humans and other mammals (8, 15). A surveillance network of federal, state, and local health departments monitors WN virus activity in wildlife hosts, vectors, and humans (16, 25). Vector control measures and mosquito avoidance are used to manage outbreaks. Currently, there are no approved human WN virus vaccines, and clinical options for treating WN virus meningioencephalitis are limited.
Serologic testing is the primary method of diagnosing WN virus infection. The recommended immunoassays are the immunoglobulin M (IgM) antibody capture enzyme-linked immunosorbent assay (ELISA) and the indirect IgG ELISA (13, 17, 24) developed by the CDC. The WN virus IgM capture and IgG ELISAs were successfully used to confirm WN encephalitis in the recent U.S. epidemic (16, 26) and also in the 1996 WN fever epidemic in Bucharest, Hungary (32). Most public health laboratories in the United States perform these ELISAs with reagents and protocols (16) developed by the CDC. Positive ELISA results are confirmed by flavivirus plaque reduction neutralization (PRN) tests (21) performed in biosafety level 3 facilities. This combination of assays is highly sensitive and specific, but performing a complete panel of ELISAs requires 2 to 3 working days to complete, as overnight incubations are deemed necessary to enhance sensitivity (17, 24). Indirect fluorescent-antibody tests may also be used for flavivirus diagnosis, but they are not suitable for high throughput of specimens and they are less sensitive than ELISA (16). Rapid and accurate WN virus serologic testing is a public health priority prompted by the dramatic increase in WN virus infections in the United States and by evidence that the virus can be transmitted by blood and organ donations (11).
Using fluorescent microsphere immunoassay (MIA) technology (22, 23), we have developed a new test to measure antibodies induced by flavivirus infection. This assay is based on a recombinant WN virus envelope (E) glycoprotein antigen (rWNV-E). The MIA is designed to evaluate encephalitis patients for serologic evidence of flavivirus infection, with virus-specific PRN assays as confirmatory tests. This first-generation MIA for serodiagnosis of flavivirus infection provides the basis for multiplex MIAs that simultaneously measure antibody reactivity with several recombinant flavivirus antigens.
MATERIALS AND METHODS
Human sera.
Patient sera previously tested for WN virus antibodies by standardized IgM capture and indirect IgG ELISAs (17, 24) were from frozen serum banks at the Wadsworth Center, New York State Department of Health, Albany, or from the Arbovirus Diseases Branch, Division of Vector-Borne Infectious Diseases, CDC, Ft. Collins, Colo. Sera were tested with all patient identifiers removed under conditions following National Institutes of Health guidelines and approved by the Institutional Review Board of the New York State Department of Health.
Sera from Wadsworth Center archives were chosen to establish normal MIA ranges for positive and negative samples. Ten sera were selected on the basis of positive results in standard WN virus ELISAs. WN and St. Louis encephalitis (SLE) virus PRN test results for paired acute- and convalescent-phase sera confirmed WN virus as the infecting agent. Ten sera that were negative for WN virus antibodies in IgM capture and IgG ELISAs were selected as negative control sera. For assay covariance studies, the 10 WN virus patient sera were combined into a positive control serum pool, and the 10 negative sera were combined into a negative control serum pool.
A coded panel of 19 sera provided by the CDC Arbovirus Diseases Branch included three sera from confirmed WN viral encephalitis patients, six sera from SLE patients, and three sera from dengue (DEN) fever patients. For 10 of 12 sera from infected patients, the infectious agent was confirmed by virus PRN tests with WN virus, SLE virus, or DEN virus. Cross-neutralization data classified the sera as specific for WN virus, DEN virus, or SLE virus infections. Seven negative control sera were from presumed healthy subjects lacking evidence of previous flavivirus infection. The CDC provided ELISA data for these sera when the samples were decoded.
A third serum panel was from eight individuals vaccinated with three doses of JE-VAX (Connaught Laboratories, Missisauga, Ontario, Canada). These sera were from the Wadsworth Center Arbovirus Laboratory. JE-VAX is a licensed, formalin-inactivated Japanese encephalitis (JE) virus vaccine. The vaccinated individuals had a history of occupational exposure to flaviviruses and, in some cases, prior vaccination against a flavivirus. The sera, which included pre- and postvaccination sera, were tested for neutralizing antibodies in JE virus PRN assays.
Another serum panel represented serial specimens from a patient with WN virus infection confirmed by PRN tests. Blood specimens were collected at 2, 18, 72, 260, and 430 days after disease onset and, by coincidence, 3 days prior to virus exposure.
A fifth serum panel included human sera that previously tested positive in standard serological assays for antibodies to Epstein-Barr virus (EBV), cytomegalovirus (CMV), herpes simplex virus (HSV), human immunodeficiency virus (HIV), Treponema pallidum (the syphilis spirochete), Borrelia burgdorferi, Anaplasma phagocytophilum, autoimmune nuclear antigens (antinuclear antibodies), or rheumatoid factor. These sera were from frozen sera archived at the Wadsworth Center. The syphilis patient sera were negative in WN or SLE virus PRN tests performed at the Arbovirus Laboratory of the Wadsworth Center. Sixteen normal human sera were purchased from United States Biological (Swampscott, Mass.). Twelve additional sera from healthy individuals were from the Wadsworth Center or L2 Diagnostics (New Haven, Conn.).
The Wadsworth Center provided sera from 833 patients with suspected viral encephalitis. These sera were submitted to the New York State Department of Health between June and November 2002. These sera had previously been tested for antibodies to WN virus by using the IgM capture and IgG ELISAs.
IgG or IgM was selectively depleted from serum specimens with goat anti-human IgG or goat anti-human IgM, respectively. For IgG depletions, 5 μl of serum was mixed with 45 μl of goat anti-human IgG (GullSORB; Meridian Diagnostics, Cincinnati, Ohio). The mixtures were centrifuged at 14,000 × g to remove antibody-bound IgG. According to the manufacturer, this is sufficient to deplete IgG at concentrations of up to 15 mg/ml, the upper limit of normal human IgG concentration. Removal of detectable IgG antibodies to WN virus was confirmed by negative results in WN virus IgG ELISAs and indirect immunofluorescence assays with SLE virus antigen on arbovirus slides (Focus Technologies, Cypress, Calif.).
A similar pretreatment with anti-IgM antibody depleted serum samples of IgM. Ten microliters of serum was mixed with 10 μl of goat anti-human Mu chain (2.5 mg/ml) (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.) prior to addition of 20 μl of phosphate-buffered saline (PBS) and centrifugation for 4 min at 14,000 × g to remove antibody-bound IgM.
Human CSF specimens.
Cerebrospinal fluid (CSF) specimens were from frozen archives of the Wadsworth Center. When available, CSF and serum obtained from the same patient on the same day were assayed. CSF specimens were previously tested for antibodies to flaviviruses in IgM capture ELISAs. Paired sera were previously evaluated for antibodies to WN virus in IgM capture and IgG ELISAs. PRN tests identified infecting viruses as WN, SLE, or DEN virus. These specimens were provided with all patient identifiers removed and were tested with approval of the Institutional Review Board of the New York State Department of Health.
CSF was depleted of IgG by addition of 30 μl of CSF to 30 μl of anti-IgG (GullSORB) diluted 1:100 and centrifugation of the mixture at 14,000 × g for 4 min. The anti-human IgG concentration is sufficient to deplete CSF IgG, assuming that the spinal fluid IgG concentrations are 1,000-fold lower than serum IgG concentrations (7).
IgM capture and indirect IgG ELISAs.
Sera provided by the CDC Arbovirus Diseases Branch were tested by the CDC for antibodies to WN virus, SLE virus, and/or DEN virus in IgM capture and indirect IgG ELISAs (17, 24). The ELISA antigens included a WN virus noninfectious recombinant antigen preparation of recombinant E, prM, and M proteins (13); a sucrose acetone extract of SLE virus-infected suckling mouse brain; and acetone-extracted DEN virus from supernatants of infected C6/36 mosquito cell cultures. Control wells were coated with mock antigen prepared in a similar manner from uninfected cells or tissue.
The New York State Department of Health tested sera and CSF for antibodies to WN virus by using the WN virus noninfectious recombinant antigen and control mock antigen provided by the CDC in the IgM capture and indirect IgG ELISAs (13, 17, 24).
A specimen was considered positive if, at a patient/negative (P/N) ratio (see below) of ≥3.0, a twofold greater immunoreactivity was observed for viral antigen relative to control antigen (13). ELISA results were considered uninterpretable due to nonspecific binding if this criterion was not met (17, 24).
PRN tests.
Neutralizing antibodies to flaviviruses were detected in PRN tests with WN, SLE or JE virus according to the protocol recommended by the CDC (21, 33).
Coupling of rWNV-E antigen to polystyrene microspheres.
A two-step carbodiimide process (3, 18, 30) recommended by Luminex Corporation (Austin Tex.), was used to link 50 μg of purified rWNV-E (L2 Diagnostics) to the surface of 6.25 × 106 microspheres. Multianalyte microspheres with carboxylated surfaces were obtained from Luminex Corporation. Activation was initiated with 50 μl of N-hydroxysuccinimide (50 mg/ml) (Pierce, Rockford, Ill.), followed by treatment with 50 μl of 1-ethyl-3-(3-dimethylamino-propyl)carbodiimide-HCl (50 mg/ml) (Pierce) and a 20-min incubation at room temperature. Recombinant antigen was coupled to the activated microspheres during a 2-h room temperature incubation performed in darkness on a LabTech tube rotator (Barstead/Thermolyne, Dubuque, Iowa). The microspheres were then washed twice by centrifugation and resuspension in 1.0 ml of PBN buffer (PBS [pH 7.4] with 1% bovine serum albumin [Sigma Aldrich, St. Louis, Mo.] and 0.05% Ultra sodium azide [Sigma Aldrich]). The antigen-conjugated microspheres were stored in 1 ml of PBN at 4°C.
MIA.
A two-step suspension MIA was performed. Multiscreen 96-well filter plates with 1.2-μm-pore-size Durapore filters (Millipore, Bedford, Mass.) and a Multiscreen vacuum manifold (Millipore) facilitated microsphere washing. Briefly, filter plate wells were blocked for 2 min with 100 μl of PBN, washed once with 150 μl of PBS-T buffer (PBS [pH 7.4] with 0.05% Tween 20 [Sigma Aldrich]), and then wetted with 20 μl of PBN. Diluted serum samples (50 μl, diluted 1:100 in PBN unless otherwise noted) were added to test wells. IgG-depleted sera were diluted 10-fold during depletion and were diluted an additional 10-fold in PBN for analysis in the rWNV-E MIA with polyvalent secondary antibody conjugate. IgM-depleted sera were similarly diluted in PBN to a final serum dilution of 1:100. Antigen-conjugated microspheres (2,500 in 50 μl of PBN) were added to each well. The filter plates were incubated in darkness on a plate shaker for 30 min at 37°C and then washed three times with PBS-T by using the vacuum manifold. Diluted fluorochrome-labeled secondary antibody (50 μl of a 1:250 dilution in PBN) was added to each well. Unless otherwise noted, the secondary antibody was polyvalent goat F(ab′)2 anti-human immunoglobulins (IgG plus IgA plus IgM) conjugated to red-phycoerythrin (R-PE) (Bio-Source International, Camarillo, Calif.). Alternative secondary antibodies were goat F(ab′)2 anti-human IgG R-PE conjugate and goat F(ab′)2 anti-human IgM R-PE conjugate (Bio-Source International). After incubation for 30 min at 37°C in darkness with shaking, filter plates were washed twice with PBS-T by using the vacuum manifold. Microspheres were then resuspended in 125 μl of PBN per well. Seventy-five-microliter aliquots were transferred to opaque black EIA/RIA 96-well plates with breakaway strips (Costar, Corning, N.Y.) and evaluated for microsphere fluorescence intensity with a Luminex 100 instrument. This instrument is a dual-laser flow analyzer. The first laser excites the fluorochrome mixture intrinsic to the microspheres, enabling the bead identity to be determined as the beads pass single file through the laser path in the flow cell. The second laser excites the extrinsic fluorochrome (R-PE) that is covalently attached to the reporter antibodies (goat anti-human immunoglobulins). The dual lasers allow the operator to mix beads with different antigens together in a well of a filter plate, thus enabling multiplex analysis of different antibody specificities at one time.
The instrument was calibrated with CL1/CL2 and RP1 calibration microspheres from Luminex Corp. according to the manufacturer's directions. The median fluorescence intensity (MFI) of fluorochrome-conjugated secondary antibody bound to individual microspheres was derived from flow analysis of 100 microspheres per well. Results for each assay were expressed both as MFI and as a P/N MFI ratio, i.e., the MFI for the patient's specimen divided by the MFI obtained from a pool of 10 negative control sera. The negative control sera contained no detectable antibodies to WN virus in IgM capture and IgG ELISAs. Serum MIA P/N values of ≥4.0 were considered positive for antibodies to WN virus E protein.
The rWNV-E MIA was performed on CSF as described for serum specimens, except that the CSF was tested at a 1:2 dilution, prepared by addition of 30 μl of CSF to 30 μl of PBS. IgG-depleted CSF specimens, diluted 1:2 during the IgG removal procedure, were assayed without further dilution. CSF results were reported as MFI values. CSF specimens with MFI values of >426 were considered positive for antibodies to WN virus E protein.
Statistical analysis.
Microsoft Excel software was used for statistical analysis. Data from different groups were compared by use of two-tailed Student t tests. Relationships between paired variables were evaluated with Pearson r correlation. Two-way contingency table analysis with distributed JavaStat software provided the kappa statistic, sensitivity, specificity, and predictive values.
RESULTS
MIA parameters.
rWNV-E conjugated to fluorescent microspheres provided the basis for a novel immunoassay to detect antibodies induced by flavivirus infection. The MIA quantitatively measures anti-E protein antibody binding over a broad range of antibody concentrations (Fig. 1). A standardized, 2.5-h MIA procedure was developed to detect antibodies to WN virus E protein in ≤30 μl of human serum or CSF, diluted 1:100 and 1:2, respectively. Performance of the suspension assay at 37°C with continual shaking enhanced assay kinetics. Antigen-conjugated microspheres exhibited long-term stability when stored at 4°C. Conjugated microspheres were held at 4, 25, 37, or 50°C. Reactivities of the rWNV-E microspheres with a positive control serum were tested at several intervals during a 35-day storage period (12). The thermal stability of this key assay component (expressed as time to 90% potency) was observed to be <1 day at 37 and 50°C, 3.1 days at 25°C, and >35 days at 4°C. The immunoreactivity of antigen-conjugated microspheres is stable for >4 months when used in serial MIAs (data not shown).
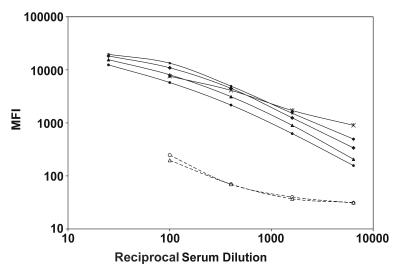
FIG. 1.
rWNV-E MIA analysis of serially diluted serum specimens. Sera from patients with WN virus infection (closed symbols), and negative control human sera (open symbols) were serially diluted and evaluated in the rWNV-E MIA with a polyvalent detector antibody. Results are reported as MFI per 100 microspheres.
MIA ranges for positive and negative control sera were established by evaluation of 20 human sera. Ten negative control sera had no detectable virus-specific antibodies in WN virus IgM capture and IgG ELISAs. The mean microsphere MFI for these sera was 247 ± 74, establishing an MFI of ≥988 (P/N ratio of ≥4.0) as a cutoff for a positive result. The 10 sera from WN viral encephalitis patients all tested positive for antibodies to WN virus E protein. The mean MFI for the patient sera was 7,626 ± 4,312 (P < 0.001; range, 2,763 to 17,188), corresponding to a mean P/N ratio of 30.8 ± 17.4 (range, 11.2 to 69.4). MIA results from repeated experiments were compared to determine interoperator reproducibility. For intra-assay imprecision studies, 10 aliquots of a WN virus encephalitis patient serum pool and 10 aliquots of a negative control serum pool were tested separately in the rWNV-E MIA. The intra-assay imprecision of the positive pool provided a coefficient of variation (CV) of 7%. The intra-assay imprecision of the negative serum pool provided a CV of 11%. These same negative and positive serum pools were analyzed on several days for estimates of interassay imprecision. The interassay CVs for the positive and negative serum pools were 17 and 32%, respectively. MIA results for 91 positive and negative sera independently analyzed by two individuals demonstrated interoperator assay reproducibility (kappa = 0.85; P/N r2 = 0.99; slope = 1.12).
The MIA detects anti-E protein antibodies induced by related flaviviruses.
E proteins are structurally related among flaviviruses, and anti-E protein antibodies are cross-reactive (4-7, 24). Testing of a coded serum panel revealed that the rWNV-E MIA also detects human antibodies elicited by SLE and DEN viruses (Table 1). The serum panel, provided by the CDC Arbovirus Diseases Branch, included sera from patients infected with WN, SLE, or DEN virus. Ten of 19 sera in the panel were positive in the rWNV-E MIA (mean P/N ratio, 25.75 ± 20.26; range, 4.28 to 55.23), using a P/N ratio of >4.0 as an MIA positive cutoff value. Decoding of the serum panel revealed that the rWNV-E MIA detected 10 of 12 sera from flavivirus-infected individuals (kappa = 0.79). All six sera from WN viral encephalitis or DEN fever patients (Table 1) were positive. The MIA also identified four sera from patients infected with SLE virus (Table 1). Two sera from patients infected with SLE virus were negative in the rWNV-E MIA. These two sera were obtained within 1 day after disease onset, when significant anti-SLE virus antibody titers may not be present (8, 16, 24). The MIA produced no false-positive results with seven sera negative for neutralizing flavivirus antibodies in PRN assays (Table 1). One negative control specimen consistently tested falsely positive in IgM capture ELISAs. Comparison of WN virus ELISA results for negative control sera and sera containing anti-WN virus antibodies (Table 1) indicated interlaboratory agreement for IgG ELISA (kappa = 1.00; P/N r2 = 0.98) and IgM capture ELISA (kappa = 0.80; P/N r2 = 0.94) results.
TABLE 1.
Detection of flavivirus antibodies by the WNV-E MIA and by ELISA in a blinded serum panel
| Serum no. | Etiologic virus, PRN titer | P/N ratioa
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| rWNV-E MIA, polyvalent | WN virus ELISA, IgG, NYSb | WN virus ELISA, IgG, CDC | WN virus ELISA, IgM, NYS | WN virus ELISA, IgM, CDC | DEN virus ELISA, IgG, NYS | SLE virus ELISA, IgG, CDC | SLE virus ELISA, IgM, CDC | ||
| 1 | NEGc | 1.31 | 2.01 | 1.20 | 1.51 | 1.59 | 0.12 | NDd | ND |
| 2 | NEG | 0.79 | 0.78 | ND | 0.93 | 1.17 | 0.08 | ND | ND |
| 3 | NEG | 0.81 | 0.62 | ND | 0.96 | 0.95 | 0.10 | ND | ND |
| 4 | NEG | 1.90 | 0.48 | 0.82 | 11.79 | 5.04 | 0.16 | ND | ND |
| 5 | NEG | 0.86 | 0.96 | 0.89 | 0.46 | 1.26 | 0.14 | ND | ND |
| 6 | NEG | 2.62 | 1.06 | 0.97 | 5.89 | 2.23 | 0.31 | ND | ND |
| 7 | NEG | 1.48 | 0.88 | 0.90 | 1.34 | 1.25 | 0.30 | ND | ND |
| 8 | WN virus, 160 | 5.89 | 8.96 | 4.40 | 5.76 | 4.02 | 0.49 | ND | ND |
| 9 | WN virus, 160 | 45.15 | 13.96 | 5.28 | 16.25 | 8.90 | 2.58 | ND | ND |
| 10 | WN virus, 320 | 42.99 | 12.77 | 5.80 | 13.73 | 6.16 | 3.04 | ND | ND |
| 11 | SLE virus, 2,560 | 4.28 | 2.56 | ND | 7.57 | 3.26 | 0.57 | 1.68 | 8.89 |
| 12 | SLE virus, 40 | 0.90 | 1.44 | 1.05 | 1.98 | 1.52 | 0.10 | 1.39 | 4.48 |
| 13 | SLE virus, 1,280 | 18.88 | 7.26 | 7.05 | 10.06 | 3.67 | 1.34 | 7.69 | 8.53 |
| 14 | SLE virus, 1,280 | 14.34 | 3.17 | 3.63 | 14.33 | 7.00 | 0.86 | 5.45 | 8.69 |
| 15 | SLE virus, 80 | 4.80 | 1.43 | 1.45 | 8.44 | 3.65 | 0.19 | ND | 8.43 |
| 16 | SLE virus, 10 | 0.80 | 0.98 | 0.77 | 3.52 | 1.74 | 0.20 | 0.80 | 2.76 |
| 17 | DEN virus, ND | 49.90 | 20.06 | ND | 12.90 | 1.62 | 10.39 | ND | ND |
| 18 | DEN virus, ND | 15.99 | 3.22 | ND | 13.59 | 1.72 | 1.59 | ND | ND |
| 19 | DEN virus, 160 | 55.23 | 15.85 | ND | 3.85 | ND | 8.28 | ND | ND |
Boldface indicates positive result (P/N ratio was greater than the cutoff value).
NYS, tests were performed at the New York State Department of Health, Wadsworth Center, Albany, N.Y.
NEG, specimen was negative to neutralizing flavivirus antibodies.
ND, test was not performed on specimen.
The rWNV-E MIA detects antibodies elicited by JE-VAX, the licensed JE virus vaccine. Sera from eight individuals with occupational exposure to flaviviruses were collected before and after vaccination with JE-VAX. Mean polyvalent rWNV-E MIA values were 4.2 ± 4.5 for prevaccination sera and 13.3 ± 12.7 for postvaccination sera (P < 0.05). The vaccination induced JE virus-neutralizing antibodies in six vaccinees that were detectable in JE virus PRN tests.
Specificity of the polyvalent rWNV-E MIA.
Sera from patients with various viral infections, bacterial infections, or autoimmune diseases were tested in the rWNV-E MIA. Twenty-four sera from presumed healthy subjects were also tested; four (17%) were borderline positive, with P/N values of <4.9. Only sera from patients with syphilis had a high frequency of false-positive results (Table 2). Sera in the first syphilis panel were all treponemal antibody positive. In the second syphilis serum panel, sera were rapid plasma reagin negative and less cross-reactive. Cross-reactive antibodies in syphilis patient sera were also detected by the WN virus IgG ELISA (data not shown). However, the syphilitic sera did not contain virus-specific neutralizing antibodies detectable in WN virus or SLE virus PRN tests (data not shown). A BLAST (1) computer search of the T. pallidum genomic database (14) failed to reveal possible cross-reactive epitopes.
TABLE 2.
Human specificity control sera tested by polyvalent rWNV-E MIA
| Specimen type | n | Mean P/N ratio ± SD (range) | No. (%) of specimens with a P/N ratio of:
|
|
|---|---|---|---|---|
| >4.0 | >5.0 | |||
| HSV infection | 5 | 1.77 ± 1.00 (0.64-2.83) | 0 | 0 |
| EBV infection | 5 | 1.44 ± 0.52 (0.92-2.31) | 0 | 0 |
| Syphilis panel 1a | 10 | 21.22 ± 15.9 (1.15-41.1) | 8 (80) | 7 (70) |
| Syphilis panel 2b | 10 | 5.62 ± 10.7 (0.35-32.3) | 2 (20) | 2 (20) |
| CMV infection | 5 | 3.58 ± 2.80 (0.89-7.64) | 2 (40) | 2 (40) |
| HIV infection | 10 | 3.36 ± 5.83 (0.25-19.7) | 1 (10) | 1 (10) |
| B. burgdorferi infection | 10 | 1.77 ± 0.56 (1.09-3.08) | 0 | 0 |
| A. phagocytophila infection | 10 | 1.72 ± 1.05 (0.45-3.78) | 0 | 0 |
| Antinuclear antibody positive | 10 | 0.86 ± 0.41 (0.37-1.63) | 0 | 0 |
| Rheumatoid factor positive | 6 | 0.62 ± 0.34 (0.17-1.11) | 0 | 0 |
| Normal serum | 24 | 2.34 ± 1.26 (0.96-4.82) | 4 (17) | 0 |
| Total | 105 | 17 (16) | 12 (11) | |
Rapid plasma reagin positive.
T. pallidum particle agglutination positive and rapid plasma reagin RPR negative.
MIA assessment of the humoral response to WN virus infection.
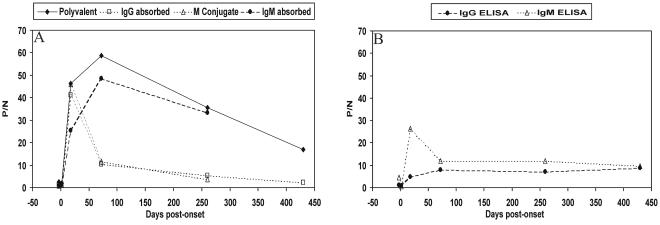
Several WN virus antibody detection methods were compared by using serial serum samples from a patient with WN virus infection (Fig. 2). The polyvalent MIA, which detects both IgM and IgG (Fig. 2A) and the ELISAs (Fig. 2B) were first used to evaluate the specimens. The MIA procedure was then modified to measure IgM antibodies to WN virus E protein. Sera were depleted of IgG with goat anti-human IgG and analyzed with polyvalent R-PE-conjugated detector antibody or with IgM-specific conjugate. These two detection systems yielded equivalent P/N results (Fig. 2A) (r2 = 0.998; slope = 1.14) that correlated with P/N values obtained with the IgM capture ELISA (r2 = 0.85). Each method detected maximal IgM reactivity in the serum specimen obtained 18 days after disease onset.
FIG. 2.
rWNV-E MIA and ELISA analyses of anti-WN virus antibodies in sequential serum specimens from a patient infected with WN virus. (A) Unadsorbed sera were evaluated in the rWNV-E MIA with a polyvalent detector antibody. Sera adsorbed with anti-IgG (IgG adsorbed) or anti-IgM (IgM adsorbed) were evaluated in the rWNV-E MIA with a polyvalent detector antibody. The IgM-adsorbed sera were also analyzed in the rWNV-E MIA with an anti-IgM detector antibody (M conjugate). (B) Comparison of results with the MAC-ELISA and indirect IgG ELISA with sequential sera.
IgG antibodies to WN virus E protein in the serial serum samples were also evaluated by MIA(Fig. 2A). Removal of IgM with anti-human IgM enhanced the correlation between the MIA and IgG ELISA P/N values (r2 = 0.45 and 0.98 before and after IgM depletion, respectively). The MIA detected maximal IgG reactivity 72 days after disease onset, whereas the IgG ELISA P/N value was highest at 430 days after onset. In convalescent-phase specimens obtained at days 72 and 260 after disease onset, anti-IgG treatment depleted >80% of rWNV-E MIA reactivity. Anti-IgG treatment of the day 18 specimen depleted only 11% of the MIA reactivity. These data indicate that this patient's IgM response to WN virus infection predominated only during acute infection.
rWNV-E MIA analysis of sera from patients with suspected viral encephalitis.
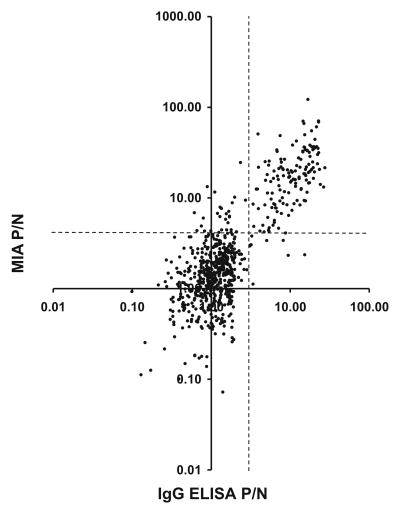
New York State Department of Health serum archives provided an opportunity to evaluate 833 sera from patients with suspected viral encephalitis in the rWNV-E MIA. With a P/N ratio of >4.0 used as a positive cutoff, 188 sera (23%) were positive in the MIA (mean P/N ratio, 18.3 ± 15.8; range, 4.07 to 122). A polyvalent (IgM plus IgG) MIA result was obtained for each of the 833 sera. In contrast, IgG ELISA results for 131 sera (16%) were reported as uninterpretable due to high nonspecific background in negative control ELISAs (17, 24). One hundred fifty-one (18%) of the 833 sera were positive in the IgG ELISA (mean P/N ratio, 11.51 ± 5.96; range, 3.08 to 27.4). The MIA had high sensitivity (0.94) and specificity (0.95) for sera with anti-WN virus IgG antibodies detected by the IgG ELISA (positive predictive value = 0.829; negative predictive value = 0.98). Positive and negative test results for the two assays were concordant (kappa = 0.85). Figure 3 compares MIA and IgG ELISA P/N results for sera with interpretable IgG ELISA results.
FIG. 3.
Retrospective parallel rWNV-E MIA and WN virus IgG ELISA analyses of sera from patients with suspected viral encephalitis. Dashed lines indicate P/N cutoff values for a positive result (n = 702, r2 = 0.60, and slope = 1.68).
IgM capture ELISA data were available for 806 of the 833 sera from patients with suspected viral encephalitis. Ninety-six sera (12%) were positive in this assay (mean P/N ratio, 12.49 ± 4.13; range, 7.18 to 25.9). Seven hundred sera (87%) were negative. Ten sera (1%) provided nonspecific results. The polyvalent MIA detected 80 (83%) of the 96 sera that were positive in the IgM capture ELISA. Sera positive in the standard polyvalent MIA (n = 172) were reassayed after depletion of IgG to measure IgM antibodies to WN virus E protein. Eighty sera (46%) were positive after removing IgG (mean P/N ratio, 19.5 ± 29.9; range, 4.00 to 178). The IgG-depleted MIA sensitivity (0.61) and specificity (0.64) for sera positive in the IgM capture ELISA (kappa = 0.25) suggested that capturing IgM antibodies may enhance anti-WN virus IgM assay sensitivity. However, 23 (36%) of the 64 discordant samples were positive in the IgG-depleted MIA, positive in the IgG ELISA, but negative in the IgM capture ELISA. These samples apparently have IgM antibodies to WN virus E protein not detected by the IgM capture ELISA. Forty-three (5%) of the 833 sera from patients with suspected flavivirus infection tested positive (mean P/N ratio, 7.28 ± 5.74; range, 4.07 to 39.5) in the polyvalent rWNV-E MIA but were negative or uninterpretable in IgM capture and IgG ELISAs. These sera were initially identified as nonreactive or nonspecific in the ELISAs, and no diagnostic or clinical follow-up that could rule out WN virus infection was performed. The combined data indicate that the rWNV-E MIA has ≥95% specificity in detecting flavivirus antibodies in sera from patients with suspected viral encephalitis.
The MIA detects anti-flavivirus antibodies in CSF.
Thirty-five CSF specimens were evaluated in the rWNV-E MIA after depletion of IgG from the specimens. Twenty negative control CSF specimens (mean MFI, 69 ± 119; range, 5 to 540) were evaluated, establishing an MFI of >426 (3 standard deviations above the mean) as a cutoff for a positive CSF result. The MIA was then used to evaluate CSF specimens from 15 encephalitis patients with flavivirus infection confirmed by serum PRN tests (Table 3). Twelve specimens (80%) were positive in the MIA before and after depletion of IgG, including eight CSF specimens from patients infected with WN virus, two CSF specimens from patients infected with DEN virus, and two CSF specimens from patients infected with an unidentified flavivirus. For most of these CSF specimens, depletion of IgG minimally reduced MFI values (Table 3), indicating that the anti-E protein antibodies were predominately IgM. MIA-negative CSF specimens 9 and 10 were from patients with WN virus-specific serum antibodies. PRN tests of acute- and convalescent-phase sera indicated that these two patients did not have active flavivirus infection.
TABLE 3.
Detection of anti-flavivirus antibodies in CSF
| Specimen no. | MFI of CSF diluted 1:2 in:
|
Viral etiology by PRN assaysc | |
|---|---|---|---|
| PBSa (IgG + IgA + IgM) | GullSORBb (IgM) | ||
| 1 | 909 | 932 | WN virus, UT |
| 2 | 1,632 | 1,050 | WN virus, C or R |
| 3 | 3,838 | 3,783 | WN virus, UT |
| 4 | 1,629 | 634 | WN virus, UT |
| 5 | 2,778 | 2,114 | WN virus, UT |
| 6 | 15,746 | 7,308 | WN virus, UT |
| 7 | 4,496 | 4,879 | WN virus, C or R |
| 8 | 1,240 | 1,488 | WN virus, C or R |
| 9 | 390 | 39 | WN virus, UT |
| 10 | 196 | 217 | WN virus, UT |
| 11 | 1,142 | 913 | DEN virus, UT |
| 12 | 4,066 | 3,150 | DEN virus, UT |
| 13 | 4,421 | 3,287 | FLAVI, UT |
| 14 | 589 | 217 | FLAVI, UT |
| 15 | 9,244 | 9,040 | FLAVI, UT |
For 100 beads with polyvalent conjugate
For 100 beads following IgG depletion
UT, undetermined time of infection; C or R, current or recent infection; FLAVI, indeterminate flavivirus.
Paired CSF and serum specimens obtained on the same day were available for patients 1 to 6. These patients had WN virus infection confirmed by serum ELISA and PRN tests. All six CSF specimens were positive in MIAs (Table 3). In contrast, only one of these CSF specimens, that from patient 2, was positive in IgM capture ELISAs (data not shown).
DISCUSSION
In this report, we describe a new immunoassay to aid in the diagnosis of patients with suspected flavivirus infections. The MIA measures antibodies that bind to rWNV-E protein. A broad dynamic range, high P/N ratios, speed, convenience, and small volumes of specimen required are advantages offered by this MIA. Fifty micrograms of rWNV-E conjugated to microspheres is sufficient for 2,500 assays. Stability of the antigen-coated beads contributes to low interassay variance. A standardized, 2.5-h MIA procedure measures anti-E protein antibodies in human serum and CSF.
Assessment of 10 sera from healthy individuals established a P/N ratio of ≥4.0 as a positive MIA cutoff value. With this cutoff, the MIA specificity was 90% with sera from normal, healthy individuals or from patients with autoimmune diseases. The MIA specificity was 93% with sera from patients infected with HIV, EBV, HSV, CMV, A. phagocytophilum, or B. burgdorferi. An unexpected result was that 50% of syphilis patient sera tested had cross-reactive antibodies. WN and SLE virus PRN assays ruled out infections with these flaviviruses as the source of cross-reactive antibodies in the syphilis patient specimens. For the 85 nonsyphilitic sera in the specificity panel, the overall MIA specificity was 92%. If a P/N ratio of between 4 and 5 is considered an indeterminate range for these sera, the specificity with nonsyphilitic sera increases to 96%.
The availability of archived sera at the New York State Department of Health during the WN virus outbreak of 1999 to 2002 provided an opportunity to compare the MIA, IgM capture ELISA, and IgG ELISA methods of detecting virus-specific antibodies. MIA P/N values correlated well with IgG ELISA P/N values, indicating that virus-specific antibodies in this serum panel are predominately IgG antibodies to WN virus E protein. The MIA was also positive with specimens containing primarily IgM antibodies to WN virus. Discordant MIA and ELISA results were typically positive in the MIA and negative or nonspecific in ELISAs. These specimens may represent cases of WN virus infection that were not detected by conventional WN virus ELISAs. However, in the absence of follow-up serology or clinical data for these coded specimens, we were unable to determine whether these sera were from WN virus-infected individuals. CSF results also revealed MIA sensitivity. Five of six WN virus encephalitis patient CSF specimens were positive in MIAs and negative in the IgM capture ELISA.
Several assay parameters contribute to WNV-E MIA sensitivity. The MIA is based on a single protein that represents the immunodominant E protein of flaviviruses. The recombinant protein is covalently coupled to fluorescent microspheres. Antigen purity eliminates problems with sera that give high nonspecific binding in ELISAs. Low background binding and linear assay results across a broad dynamic range of antibody concentrations allow MIAs to be performed at a serum dilution of 1:100. In contrast, WN virus ELISAs use serum diluted 1:400 in order to maintain accurate P/N ratios relative to end points obtained by titer determination (17, 24). A polyvalent, fluorochrome-conjugated secondary antibody that detects both IgG and IgM also enhances MIA sensitivity. Furthermore, steric hindrance by irrelevant IgM antibodies, which may affect IgM capture assays, will not occur in the suspension MIA assay format. These factors contribute to P/N results that are usually higher in MIAs than in ELISAs.
WN virus infection typically induces virus-specific IgM serum antibodies 2 to 8 days after disease onset (29). WN virus-specific IgG antibodies generally appear a few days after virus-specific IgM antibodies are detected (29). Serum IgM antibodies may persist for over 1 year (8, 28, 30). This reduces the ability of IgM capture ELISAs to discriminate acute infection from prior exposure to WN virus. In serial specimens from a WN virus encephalitis patient, peak IgM antibody levels were detected 18 days after disease onset. In convalescent-phase sera, a seroconversion to virus-specific IgG was evident with MIA assays. Seroconversion was less evident with ELISAs. IgM capture ELISA P/N values were persistently higher than IgG ELISA P/N values throughout the convalescent phase. The data suggest that MIA analysis of serum specimens before and after IgG depletion may be useful in serodiagnosis of acute infection.
The amino acid sequences of JE, SLE, and DEN virus E proteins are 40 to 77% identical to the WN virus E protein amino acid sequence in BLAST database searches (1). The rWNV-E MIA detects cross-reactive E protein antibodies elicited by SLE, JE, and DEN viruses. WN virus ELISAs also detect these cross-reactive antibodies (29, 30, 32). Follow-up PRN tests are required to identify the infecting virus. ELISA sensitivity for cross-reactive antibodies, particularly IgM antibodies (30, 32), is insufficient to use a single ELISA to screen for antibodies to WN, SLE, and DEN viruses. As a result, public health laboratories in the United States typically perform IgG and IgM capture ELISAs with SLE, WN, and DEN virus antigens. The antigens are produced in different culture systems and purified by different methods (13, 17). Several sera from patients exposed to DEN or SLE virus had P/N values that were higher in the MIA than in ELISAs with homologous antigen. Extensive antibody cross-reactivity with rWNV-E suggests that the MIA may provide a standardized alternative to performance of a panel of ELISAs.
Guidelines for serodiagnosis of WN virus infection currently recommend a series of ELISAs and viral PRN tests. The ELISA procedures require 3 days to complete. In contrast, an initial MIA could identify, within 2.5 h, patients with no serologic evidence of infection by these flaviviruses. Repeat analysis after depletion of IgG would detect specimens with a high proportion of IgM anti-E protein antibodies, indicating recent or current infection. MIA analysis of positive sera could be completed within 5 working hours. This MIA screening protocol can also evaluate CSF for virus-specific antibodies. This MIA testing strategy, where all specimens are screened with one basic screening assay, followed by repeat tests of positive sera after depletion of IgG, could potentially replace six ELISAs (IgM capture and IgG ELISAs for antibodies to WN virus, SLE virus, and DEN virus).
The rWNV-E MIA is a first-generation fluorescent MIA to detect antibodies to flaviviruses. Second-generation tests can incorporate other recombinant flavivirus antigens into a multiplex microsphere immunoassay. The Luminex flow analyzer is designed to analyze microspheres with up to 100 different fluorescent microsphere codes (22). Multiplex microsphere assays are performed with several antigens linked to uniquely coded fluorescent microspheres (27). Serologic studies of JE virus antigens suggest that a multiplex assay with recombinant WN virus E, premembrane, and nonstructural-1 proteins may prove to be specific for WN virus infection. Immunoassays based on JE virus prM can distinguish antibodies induced by JE virus and DEN virus (9). Nonstructural-1 protein immunoassays can discriminate infection from vaccination (31). We found that adding recombinant flavivirus NS5 antigens to the rWNV-E MIA provides a multiplex test that can distinguish antibodies induced by WN virus from antibodies elicited by SLE or DEN virus infection, JE vaccination, or yellow fever vaccination (34).
The new flavivirus antibody assay described in this study can aid physicians and public health workers in the management of epidemics caused by WN virus or related flaviviruses. The rWNV-E MIA provides a rapid and reliable alternative to traditional ELISAs used to evaluate sera and CSF specimens from patients with suspected viral encephalitis. This new WN virus antibody assay format, with specificity enhanced by multiplexing with additional antigens (34), can also be used in seroepidemiologic studies and in surveillance studies and to evaluate blood and organ donors. The MIA detects antibodies induced by SLE, JE, and DEN viruses and may aid in the management of outbreaks of these viruses, which cause significant morbidity and mortality in many parts of the world. The rWNV-E MIA can be multiplexed with additional recombinant flavivirus antigens to develop second-generation MIAs capable of identifying the infecting flavivirus and discriminating between current and past infections.
Acknowledgments
We thank Jane Johnson, Robert Lanciotti, and John Roehrig of the CDC for kindly providing a blinded panel of sera to evaluate the rWNV-E MIA. We are indebted to Alan Dupuis of the Wadsworth Center for conducting PRN assays and to Cinnia Huang of the Wadsworth Center for spinal fluids from the New York State WN virus surveillance program. We are grateful to Karen Kulas, Carol Franchell, and technologists of the Diagnostic Immunology Laboratory of the Wadsworth Center for technical assistance and to Benjamin Koski for assistance with preparation of the manuscript. We thank Ronald Bellisario of the Wadsworth Center and Katherine Kellar of the CDC for advice on microsphere immunoassays.
CDC grants and cooperative agreements U50/CCU 212415 and U50/CCU 213698 supported this study. Support was also provided by Public Health Service grant R43 AI49646 from the National Institute of Allergy and Infectious Diseases. E. Fikrig is L2 Diagnostics partner and consultant and is the recipient of a Clinical-Scientist Award in Translational Research from the Burroughs Welcome Fund.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, J. F., T. G. Andreadis, C. R. Vossbrinck, S. Tirrell, E. M. Waken, R. A. French, A. E. Garmendig, and H. J. Van Kruiningen. 1999. Isolation of WN virus from mosquitoes, crows, and a Cooper's hawk in Connecticut. Science 286:2331-2333. [DOI] [PubMed] [Google Scholar]
- 3.Bellisario, R., R. J. Colinas, and K. A. Pass. 2002. Simultaneous measurement of thyroxine(T4) and thyrotropin (TSH) from newborn dried blood-spot specimens using a multiplexed fluorescent microsphere immunoassay. Clin. Chem. 46:1422-1424. [PubMed] [Google Scholar]
- 4.Brinton, M. A. 2002. The molecular biology of West Nile virus: a new invader of the Western Hemisphere. Annu. Rev. Microbiol. 56:371-402. [DOI] [PubMed] [Google Scholar]
- 5.Brinton, M. A., I. Kurane, A. Mathew, L. Zeng, P. Y. Shi, A. Rothman, and F. A. Ennis. 1998. Immune mediated and inherited defences against flaviviruses. Clin. Diagn. Virol. 10:129-139. [DOI] [PubMed] [Google Scholar]
- 6.Burke, D. S., and A. Nisalak. 1982. Detection of Japanese encephalitis virus immunoglobulin M antibodies in serum by antibody capture radioimmunoassay. J. Clin. Microbiol. 16:353-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burke, D. S., A. Nisalak, and M. A. Ussery. 1982. Antibody capture immunoassay detection of Japanese encephalitis virus immunoglobulin M and G antibodies in cerebrospinal fluid. J. Clin. Microbiol. 16:1034-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell, G. L., A. A. Marfin, R. S. Lanciotti, and D. L. Gubler. 2002. West Nile virus. Lancet Infect. Dis. 2:519-529. [DOI] [PubMed] [Google Scholar]
- 9.Cardosa, M. J., S. M. Wang, M. S. H. Sum, and P. H. Tio. 2002. Antibodies against prM protein distinguish between previous infection with dengue and Japanese encephalitis viruses. BMC Mol. Biol. 2:9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. 2002. West Nile virus activity—United States, November 21-26, 2002. Morb. Mortal. Wkly. Rep. 51:1072-1073. [Google Scholar]
- 11.Centers for Disease Control and Prevention. 2002. West Nile virus activity—United States, September 26-October 2, 2002, and investigations of West Nile virus infections in recipients of blood transfusions and organ transplantations. Morb. Mortal. Wkly. Rep. 51:884-895. [PubMed] [Google Scholar]
- 12.Crowther, J. R. 2001. Validation of diagnostic tests for infectious diseases. Methods Mol. Biol. 149:301-345. [DOI] [PubMed] [Google Scholar]
- 13.Davis, B. S., G.-J. J. Chang, B. Cropp, J. T. Roehrig, D. A. Martin, C. J. Mitchell, R. Bowen, and M. L. Bunning. 2001. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J. Virol. 75:4040-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraser, C. M., S. J. Norris, G. M. Weinstock, O. White, G. G. Sutton, et al. 1998. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science 281:3389-3402. [DOI] [PubMed] [Google Scholar]
- 15.Gubler, D. J. 2002. The global emergence/resurgence of arboviral diseases as public health problems. Arch. Med. Res. 33:330-342. [DOI] [PubMed] [Google Scholar]
- 16.Gubler, D. J., G. L. Campbell, R. Nasci, N. Komar, L. Petersen, and J. T. Roehrig. 2000. West Nile virus in the United States: guidelines for detection, prevention, and control. Viral Immunol. 13:469-475. [DOI] [PubMed] [Google Scholar]
- 17.Johnson, A. J., D. A. Martin, N. Karabatsos, and J. T. Roehrig. 2000. Detection of anti-arboviral immunoglobulin G by using a monoclonal antibody-based capture enzyme-linked immunosorbent assay. J. Clin. Microbiol. 38:1827-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kellar, K. L., R. R. Kalwar, K. A. Dubous, D. Crouse, W. D. Chafin and B.-E. Kane. 2001. Multiplexed fluorescent bead-based immunoassays for quantitation of human cytokines in serum and culture supernatants. Cytometry 45:27-36. [DOI] [PubMed] [Google Scholar]
- 19.Kramer, L. D., and K. A. Bernard. 2001. West Nile virus in the western hemisphere. Curr. Opin. Infect. Dis. 14:519-525. [DOI] [PubMed] [Google Scholar]
- 20.Lanciotti, R. S., J. T. Roehrig, V. Deubel, J. Smith, M. Parker, K. Steele, B Crise, K. E. Volpe, M. B. Crabtree, K. H. Scherret, et al. 1999. Origin of the WN virus responsible for an outbreak of encephalitis in the north eastern United States. Science 286:2333-2337. [DOI] [PubMed] [Google Scholar]
- 21.Lindsey, H. S., C. H. Calisher, and J. H. Mathews. 1976. Serum dilution neutralization test for California serogroup virus identification and serology. J. Clin. Microbiol. 4:503-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mandy, F. F., T. Nakamura, M. Bergeron, and K. Sekiguchi. 2001. Overview and application of suspension array technology. Clin. Lab. Med. 21:713-729. [PubMed] [Google Scholar]
- 23.Mariella, R., Jr. 2002. MEMS for bioassays. Biomed. Microdevices 4:77-87. [Google Scholar]
- 24.Martin, D. A., D. A. Muth, T. Brown, A. J. Johnson, N. Karabatsos, and J. T. Rochrig. 2000. Standardization of immunoglobulin M capture enzyme-linked immunosorbent assays for routine diagnosis of arboviral infections. J. Clin. Microbiol. 38:1823-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McLean, R. G., S. R. Ubico, D. Bourne, and N. Omar. 2002. West Nile virus in livestock and wildlife. Curr. Top. Microbiol. Immunol. 267:271-308. [DOI] [PubMed] [Google Scholar]
- 26.Nash, D., F. Mostashari, A. Fine, J. Miller, D. O'Leary, K. Murray, A. Huang, A. Rosenberg, A. Greenberg, M. Sherman, S. Wong, and M. Layton. 2001. The outbreak of West Nile virus infection in the New York City area in 1999. N. Engl. J. Med. 344:1807-1814. [DOI] [PubMed] [Google Scholar]
- 27.Pickering, J. W., T. B. Martins, R. W. Greer, M. C. Schroeder, M. E. Astill, C. M. Litwin, S. W. Hildreth, and H. R. Hill. 2002. A multiplexed fluorescent microsphere immunoassay for antibodies to pneumococcal capsular polysaccharides. Am. J. Clin. Pathol. 117:589-596. [DOI] [PubMed] [Google Scholar]
- 28.Roehrig, J. T., D. Nash, B. Malden, A. Labowitz, D. A. Martin, R. S. Lanciotti, and G. L. Campbell. 2003. Persistence of virus-reactive serum immunoglobulin M antibody in confirmed West Nile virus encephalitis cases. Emerg. Infect. Dis. 9:376-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roehrig J. T., M. Layton, P Smith, G. L. Campbell, R. Nasci, and R. S. Lanciotti. 2002. The emergence of West Nile virus in North America: ecology, epidemiology, and surveillance. Curr. Top. Immunol. 267:223-240. [DOI] [PubMed] [Google Scholar]
- 30.Roehrig, J. T., L. A. Staudinger, A. R. Hunt, J. H. Mathews, and C. D. Blair. 2001. Antibody prophylaxis and therapy for flavivirus encephalitis infections. Ann. N.Y. Acad. Sci. 951:286-297. [DOI] [PubMed] [Google Scholar]
- 31.Shu, P.-Y., L.-K. Chen., S.-F. Chang, et al. 2001. Antibody to nonstructural protein NS1 of Japanese encephalitis virus: potential application of mAb-based indirect ELISA to discriminate infection from vaccination. Vaccine 19:1753-1763. [DOI] [PubMed] [Google Scholar]
- 32.Tardei, G., S. Ruta, V. Chitu, C. Rossi, T. F. Tsai, and C. Cernescu. 2000. Evaluation of immunoglobulin M (IgM) and IgG enzyme immunoassays in serologic diagnosis of West Nile virus infection, J. Clin. Microbiol. 38:2232-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, T., J. F. Anderson, L. A. Magnarelli, S. J. Wong, R. A. Koski, and E. Fikrig. 2001. Immunization of mice against WN virus with recombinant envelope protein. J. Immunol. 167:5273-5277. [DOI] [PubMed] [Google Scholar]
- 34.Wong, S. J., R. H. Boyle, V. L. Demarest, A. N. Woodmansee, L. D. Kramer, H. Li, M. Drebot, R. A. Koski, E. Fikrig, D. A. Martin, and P.-Y. Shi. An immunoassay targeting nonstructural protein 5 to differentiate West Nile virus infection from dengue and St. Louis encephalitis virus infections and flavivirus vaccination. J. Clin. Microbiol. 41:4217-4223. [DOI] [PMC free article] [PubMed]