Abstract
Serotype-cross-reactive memory T cells responding to secondary dengue virus (DENV) infection are thought to contribute to disease. However, epitope-specific T cell responses have not been thoroughly compared between subjects with primary versus secondary DENV infection. We studied CD8+ T cells specific for the HLA-A*1101-restricted NS3133 epitope in a cohort of A11+ DENV-infected patients throughout acute illness and convalescence. We compared the expansion, serotype-cross-reactivity, and activation of these cells in PBMC from patients experiencing primary or secondary infection and mild or severe disease by flow cytometry. Our results show expansion and activation of DENV-specific CD8+ T cells during acute infection, which are predominantly serotype-cross-reactive regardless of DENV infection history. These data confirm marked T cell activation and serotype-cross-reactivity during the febrile phase of dengue; however, A11-NS3133-specific responses did not correlate with prior antigenic exposure or current disease severity.
The four dengue virus serotypes (DENV 1–4) have a significant and growing impact on global health. They are responsible for over 38 million reported dengue cases each year with ∼21,000 deaths1. Dengue disease encompasses a wide range of symptoms, usually presenting as an uncomplicated acute febrile illness (dengue fever, DF); however, a small percentage of infections are associated with a plasma leakage syndrome (dengue hemorrhagic fever, DHF), which can be life-threatening. Plasma leakage in DHF coincides with defervescence and viral clearance2,3 suggesting that severe disease arises from the immune response rather than a direct viral effect. In support of this, epidemiological studies indicate that severe dengue disease most often occurs during secondary heterotypic DENV infection4,5,6.
Current hypotheses suggest that serotype-cross-reactive memory T cells reactivated in response to secondary DENV infection mediate a sub-optimal immune response, contributing to dengue disease pathology7. Various studies have explored the immunopathogenic role of cross-reactive memory T cells in DENV infection utilizing blood samples from infected patients. A high percentage of DENV-specific T cells recognize multiple DENV serotypes, as demonstrated by peptide-MHC (pMHC) tetramer binding and in vitro functional assays8,9,10,11,12,13,14,15,16,17. Furthermore, patients with DHF have been shown to have greater T cell activation in vivo than patients with DF, based on serum markers of activation18,19,20,21,22 or cell surface CD69 expression23. Few studies have reported a higher frequency of DENV epitope-specific T cells in patients with DHF13,14,24, but other studies have questioned the timing of T cell activation and its association with disease severity25,26. None of the previous reports have compared responses to multiple serotypes in cohorts of dengue patients, distinguishing between primary and secondary infection, in association with data on outcome of infection. In this study, we compared the expansion, serotype-cross-reactivity, and activation of DENV-specific CD8+ T cells in serial blood samples during acute infection and convalescence in patients experiencing primary or secondary infection and mild or severe disease.
Results
Dengue virus-specific CD8+ T cells expand during acute infection
We identified 44 HLA-A*1101+ children experiencing primary or secondary DENV infection (Table 1 and Supplementary Table S1). We prepared pMHC tetramers using three peptide variants of the previously defined A11-restricted NS3133–142 DENV epitope13, corresponding to different DENV serotypes, with different fluorochromes (Table 2). The specificity of each tetramer, and conditions for simultaneous staining of cells, was demonstrated using PBMC and epitope-specific T cell lines (Supplementary Figure S1; see also ref 10). We stained PBMC from all time points available for each subject with all three tetramers along with antibodies to activation and phenotypic markers. Each experiment included PBMC from a healthy, A11+ DENV-naïve subject as a negative control and healthy PBMC spiked with an epitope-specific T cell line as a tetramer-positive control. A positive cutoff value for tetramer frequencies was defined as any frequency greater than that measured for 11 of 12 A11+ DENV-naïve subjects (>0.14% of total CD8+ T cells equated to >92% specificity). Our flow cytometry gating strategy is presented in Fig. 1a.
Table 1. Summary of HLA-A11+ cohort information.
| Serology (no.) | ||||
|---|---|---|---|---|
| Serotypea | Diagnosisb | Primary | Secondary | Total (no.) |
| DENV-1 | DF | 6 | 5 | 11 |
| DHF | 1 | 6 | 7 | |
| DENV-2 | DF | 2 | 2 | 4 |
| DHF | - | 3 | 3 | |
| DENV-3 | DF | 5 | 4 | 9 |
| DHF | 3 | 6 | 9 | |
| DENV-4 | DF | - | 1 | 1 |
| DHF | - | - | - | |
| 44 | ||||
aOf current infection
bAccording to original WHO guidelines; DF = dengue fever, DHF = dengue hemorrhagic fever
Table 2. Summary of epitope sequence information.
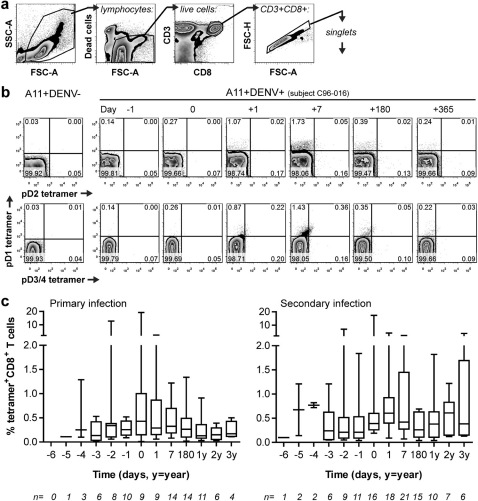
Figure 1. A11-NS3133-specific T cells expand in acute dengue virus infection.
a The gating strategy used to identify tetramer+CD8+ T cells selected cells within a generous lymphocyte gate as defined by forward and side scatter profiles. Live cells were next selected by exclusion of the viability marker LIVE/DEAD Aqua. T cells were identified by dual CD3 and CD8 expression followed by gating for singlet cells. b Representative flow plots of triple tetramer staining of PBMC from a healthy HLA-A11+ DENV-naïve control donor (A11+DENV-) as well as a HLA-A11+ DENV-infected (A11+DENV+) individual over the course of acute illness and convalescence. The two rows of plots are different views of live, CD3+CD8+ singlet lymphocytes. c Box and whisker plots show total tetramer-positive T cell frequencies in PBMC from subjects with primary (n = 16) or secondary (n = 23) DENV infection over time. Days are relative to the day of defervescence (d0).
A11-NS3133 tetramer-positive CD8+ T cells demonstrated clear expansion and contraction from acute infection into convalescence (Figure 1b and c and Supplementary Figure S2). Peak tetramer frequencies could be defined in 36 of the 44 subjects (see Supplementary Table S1). Of the remaining 8 subjects, 3 had data from one or no sample during acute infection and 3 were missing data for one of the tetramer variants; only 2 subjects with adequate data failed to show an epitope-specific T cell response. Peak frequencies ranged from 0.15% to 19% of total CD8+ T cells (median = 0.75%, mean = 2.33%). Dramatic changes in tetramer-positive T cell frequencies between consecutive daily blood samples were observed in a few subjects (Supplementary Figure S2); however, sample unavailability precluded confirmation of these findings and we therefore interpret these values with caution. Contraction of epitope-specific CD8+ T cells averaged 76% of the peak frequency at 1 year post-infection for 16 of 20 subjects with samples available. The remaining 4 subjects had tetramer-positive T cell frequencies at 1–3 years post-infection that were equal to or higher than the peak frequency during acute infection. These subjects showed lower frequencies at 6 months post-infection; although there was no serologic evidence of reinfection in these individuals we cannot exclude the possibility that these subjects experienced another DENV infection.
Antigen-specific CD8+ T cells are serotype-cross-reactive in both primary and secondary dengue virus infection
We compared the distribution of tetramer-positive T cells in subjects with primary versus secondary DENV infection and with mild (DF) versus severe (DHF) disease (Figure 2). The proportion of A11-NS3133-specific T cells that bound tetramers specific for heterologous DENV serotypes was similar in subjects with primary infection compared to those with secondary infection. For example, at 1 week post-defervescence median frequencies of 77% and 65% (p = 0.72) of epitope-specific T cells bound heterotypic tetramers (those specific for serotypes other than the currently infecting serotype) in primary and secondary infection, respectively. The pattern of tetramer staining showed great sample-to-sample variability, but, notably, it varied less across time points from the same individual than between individuals. Despite the lack of clear and consistent staining patterns according to the serotype of the infecting virus or clinical diagnosis, we did note some similarities among individuals within particular subject groups. For example, subjects with primary DENV-3 infections had more pD2+ cells (shown in yellow) than subjects with secondary DENV-3 infections (median = 15% versus 6% of epitope-specific T cells, respectively; p = 0.02) or any DENV-1 infection (median = 4%; p = 0.002) at 1 week post-infection. In addition, subjects infected with DENV-1 appeared to have a greater percentage of pD3/4+ cells (shown in red) than those infected with DENV-3 at fever day +1, although the difference did not reach statistical significance (median = 30% versus 13% of epitope-specific T cells; p = 0.16). In general, relatively few T cells bound to two or more tetramers at once (shown in green, purple, orange and brown) and were more often found in subjects with secondary infections (median = 4% of epitope-specific T cells for primary infections versus 12% for secondary infections at 1 week, p = 0.03).
Figure 2. Tetramer-positive T cells are highly serotype-cross-reactive in subjects with primary or secondary infection.
The distribution of sub-populations within the whole population of A11-NS3133 tetramer+CD8+ T cells is depicted using pie charts. Tetramer-positive samples from subjects with a (a–c) primary or (d–g) secondary infection with (a, d) DENV-1, (b, e) DENV-2, (c, f) DENV-3 or (g) DENV-4 are shown. No subjects with primary DENV-4 infection were available. Each row represents data from a single donor over time. Time points shown are relative to the day of defervescence (d0), d = day, w = week, m = month, y = year.
We detected preferential binding to the pD1 tetramer when T cells were stained with the three A11-NS3133 tetramer variants. We observed a similar staining pattern in peptide-stimulated short term bulk cultures and epitope-specific T cell lines10. To evaluate the possibility that preferential binding to the pD1 tetramer was an artifact of our staining conditions, we compared staining with all three tetramer variants at once to staining with each variant individually (Supplementary Figure S3a). Relative staining with individual tetramer variants was consistent with staining detected when all three tetramers were added together. To determine whether there was competition for tetramer binding and if the concentration of tetramer influenced the staining patterns, we stained T cell lines with decreasing concentrations of the pD1 or pD3/4 tetramer while keeping the other tetramer concentrations constant. We observed no increase in the frequency of cells that bound to the tetramer variants kept constant (Supplementary Figure S3b). To determine whether tetramer binding reflected functional responses, selected PBMC samples obtained from several of the DENV-3 infected subjects in early convalescence (within 1 week of defervescence) were stimulated ex vivo with each of the A11-NS3133 epitope variants in intracellular cytokine staining assays. Cytokine production and/or degranulation by CD8+ T cells in response to heterologous epitope variants was detected (Supplementary Figure S3c), consistent with our prior findings of serotype-cross-reactive epitope-specific CD8+ T cell lines isolated from patients with primary DENV infection10.
We considered the possibility that the placement of gates could have been inexact in delineating positive and negative tetramer staining and, thereby, our interpretation of serotype-cross-reactivity. Quadrant gates were placed based on staining in positive and negative control specimens. For some subjects, pD1 tetramer-positive T cells seemed to stain weakly with the pD3/4 tetramer, but did not reach the threshold used to count as pD3/4 tetramer-positive (pD1+3/4+; refer to Figure 1b). To account for this, the intensity of pD3/4 tetramer staining of all pD1 tetramer-positive CD8+ T cells was compared across all time points for subjects with primary versus secondary DENV-3 infections (Supplementary Figure S3d). We found no statistically significant differences between these subject groups, further supporting our finding that the extent of serotype-cross-reactivity did not differ according to DENV infection history.
Epitope-specific T cell frequencies peak earlier in primary than in secondary dengue virus infection
We compared the kinetics of A11-NS3133-specific T cell expansion in primary versus secondary infection. We found that subjects undergoing primary infection reached their peak tetramer frequency earlier in the course of illness than those with secondary infection (p = 0.02; Table 3). Given the more rapid proliferation of memory T cells, and the more rapid clearance of viremia in secondary infection2, this result was not anticipated. However, epitope-specific T cells down-regulated CD45RA expression earlier in secondary infection than in primary infection (Supplementary Figure S4). CD45RA is expressed on some memory T cell populations27, as shown for A11-NS3133-specific T cells in our cohort (Supplementary Figure S4). Therefore, earlier down-regulation of CD45RA supports the reactivation of epitope-specific memory T cells during secondary DENV infection. We did not detect differences in the timing of peak tetramer frequency between DF and DHF patients (data not shown).
Table 3. Tetramer frequencies peak earlier in primary DENV infectiona.
aThis analysis only considers those subjects for whom we have blood samples from all three of these time points (n = 21)
bOne primary subject had peak tetramer frequency at fever day -2
cThe actual day post-defervescence differs for each donor (range = 4–12, mean = 7)
dThe difference between fever days 0 and +1 is significant by Fisher's exact test (p = 0.02)
The frequency of A11-NS3133-specific T cells does not correlate with clinical findings
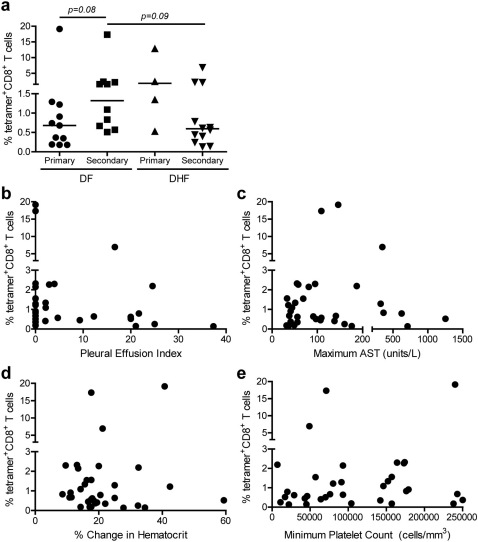
We tested whether the frequency of A11-NS3133-specific T cells correlated with clinical diagnosis at days 0, +1 and +7 and found no significant differences (data not shown). Therefore, we determined whether the peak frequency of epitope-specific T cells, regardless of timing, correlated with clinical findings. No statistically significant differences were found between subjects with DHF and those with DF (Figure 3a). However, among subjects with DF, peak tetramer frequencies were slightly higher in secondary infection than primary infection (p = 0.08), and among subjects with secondary DENV infections, peak tetramer frequencies were slightly higher in subjects with DF than those with DHF (p = 0.09). We also found no correlations between peak tetramer frequencies and other measures of disease severity, including pleural effusion index (Figure 3b), aspartate aminotransferase values (Figure 3c), change in hematocrit (Figure 3d), and platelet count (Figure 3e).
Figure 3. The magnitude of epitope-specific T cells does not correlate with disease severity.
Peak A11-NS3133 tetramer+CD8+ T cell frequencies are plotted versus a clinical diagnosis (DF versus DHF), b pleural effusion index, c maximum aspartate aminotransferase (AST) value, d % change in hematocrit, and e minimum platelet count. No significant correlations were detected by Mann-Whitney (a) or Spearman's test (b–e).
Antigen-specific CD8+ T cells are highly activated during acute dengue virus infection
We compared CD38 expression on antigen-specific T cells in primary versus secondary infection according to disease severity. High CD38 expression was seen in total CD8+ T cells and even higher expression in epitope-specific T cells was seen during acute infection and shortly after defervescence (Figure 4a). While levels of CD38 expression varied across individuals, the averaged fluorescence intensity of tetramer-positive T cells in PBMC from the four subject groups (primary DF, primary DHF, secondary DF and secondary DHF) showed similar patterns over time (Figure 4b).
Figure 4. Antigen-specific T cells are highly activated during acute DENV infection and early convalescence.
a Representative histograms show the expression of CD38 over time in total CD8+ T cells from a healthy A11+ donor (shaded) and an A11+ DENV-infected donor (dark blue line) as well as A11-NS3133 tetramer+CD8+ T cells from the same DENV-infected donor (light blue line). b The fold change in geometric mean fluorescence intensity (gMFI) of CD38 staining in A11-NS3133 tetramer+CD8+ T cells from subjects with primary (solid lines) and secondary (dotted lines) DENV infections with either DF (closed circles) or DHF (open squares) over time. Data are presented as means and are relative to the gMFI of CD38 of total CD8+ T cells from a healthy control donor included in each experiment.
T cell expansion in acute dengue virus infection is antigen-specific
We considered that conclusions based on the analysis of a single DENV epitope might not be generalizable or could reflect non-specific rather than antigen-specific T cell expansion. Taking advantage of previously identified epitopes for which we had tetramers available, we identified a subset of A11+ subjects that were also HLA-A2+ or HLA-B7+. We stained samples from these subjects with the pD1 tetramer together with a tetramer specific for either the B7-restricted DENV NS3222–231 epitope (B7-DENV) or the A201-restricted influenza virus M158–66 epitope (A2-Flu; refer to Table 2).
Similar to pD1-specific T cells, B7-NS3222-specific CD8+ T cells expanded during acute infection, followed by a contraction phase in late convalescence (Figure 5a). Frequencies of B7-DENV cells were consistently higher than pD1-specific T cells in 4 of the 5 subjects studied. A2-Flu M158-specific T cells were also present in a subset of our patients, but their frequencies remained stable or decreased during acute DENV infection. Although CD38 expression on A2-Flu M158-specific T cells was slightly increased during acute DENV infection, the average CD38 staining intensity on these cells was lower than on DENV-specific T cells detected by either the A11-NS3133 or B7-NS3222 tetramers (Figure 5b). These data demonstrate selective activation and expansion of antigen-specific T cells during acute DENV infection and imply that there was minimal contribution of bystander cells to the T cell response to acute infection.
Figure 5. T cell expansion and activation during dengue virus infection is antigen specific.
a Box and whisker plots show the frequency of B7-NS3222 DENV epitope-specific or A2-M158 Flu epitope-specific T cells over the course of acute infection and convalescence in HLA-A11+B7+ (n = 5) or HLA-A2+A11+ subjects (n = 6), respectively. b The mean fold change in gMFI of CD38 expression in pD1+CD8+ T cells (blue), total CD8+ T cells (purple), and B7-DENV+CD8+ T cells (green) or A2-Flu+CD8+ T cells (orange) relative to total CD8+ T cells from healthy control donor PBMC (same donor used in all experiments). Bars indicate standard deviation at each time point.
Discussion
Our results demonstrate similar frequencies of serotype-cross-reactive T cells ex vivo in naturally-infected patients with primary and secondary DENV infections. T cell functional responses to heterologous DENV epitopes were observed previously in some subjects following primary DENV infections8,28, but this is the first demonstration of serotype-cross-reactivity in primary infection based on binding of heterotypic tetramers ex vivo, and illustrates the breadth of the phenomenon. Mongkolsapaya et al. found that A11-NS3133-specific T cells in patients with secondary DENV infections preferentially stained ex vivo with pMHC tetramers corresponding to DENV serotypes heterologous to the infecting serotype13. We used a similar approach, but extended our analysis to primary DENV infections, an important comparison group absent in the earlier study. Preferential binding to the pD1 tetramer (followed by the pD3/4 tetramer and, finally, the pD2 tetramer) was also seen in our previous study of T cell lines, which we postulated could be explained by their predicted MHC binding (pD3/4 ≥ pD1 ≫ pD2)10. Heterotypic tetramer binding for this epitope thus does not reflect the history of prior infection with other DENV serotypes; other possible explanations for this phenomenon are heterologous immunity from prior unrelated infections29, characteristics of the T cell repertoire30, or inherent immunogenicity of different epitope variants8,31. It is possible that preferential binding to the pD1 tetramer was a reflection of it being the most stable of the three tetramers. However, this preferential binding would apply equally to the entire dataset, which would not affect our ability to detect differences in the overall pattern of tetramer staining between various subject groups. Despite donor-to-donor variability, we did note some similarities in patterns of tetramer staining among individuals with the same infection status (DENV serotype, primary versus secondary). This suggests that the sequence of infection does play a role in shaping the DENV-specific memory T cell repertoire, although other factors are also important.
Mongkolsapaya et al. reported low but detectable frequencies of A11-NS3133-specific T cells during acute secondary DENV infection in Thai children which peaked in early convalescence13. In contrast, Dung et al. reported that A11-NS3133-specific T cells were undetectable until after the development of plasma leakage among infected Vietnamese children25. Our data (for both the A11-NS3133 and B7-NS3222 epitopes) are clearly more consistent with the earlier report, and, in fact, several of our subjects had very high frequencies of (activated) epitope-specific T cells during acute infection. These immune responses occurring prior to the onset of plasma leakage therefore have the potential (i.e. are available) to contribute to disease pathogenesis. The finding of antigen-specific T cell expansion and activation during acute illness is also more consistent with observations in other viral diseases27,32,33 and with other evidence of T cell activation during acute DENV infection20,23,26. The divergent findings of Dung et al. may reflect differences in experimental technique; a more intriguing possibility is that the expansion of A11-NS3133-specific T cells might differ between Thai and Vietnamese patients. In this regard, it is of interest that an association with dengue disease severity has been observed for HLA-A11 in our Thai cohort34 but was not noted in a study in Vietnam35 despite the similar genetic backgrounds of these two populations.
We found that the frequency of epitope-specific T cells peaked earlier in subjects with primary infection than in those with secondary infection. Prior studies in which DENV-specific T cells were tracked over time had not differentiated between subjects with primary and secondary infections. Although this result was not anticipated, it is consistent with findings in DENV infection of BALB/c mice31. More rapid activation of memory T cells would not necessarily correspond to an earlier peak T cell frequency, since sustained proliferation signals balanced by apoptosis13,36 would affect the timing of peak responses to a greater extent. Future studies need to incorporate analysis of these mechanisms as well as other immunomodulatory signals such as regulatory T cells37.
The magnitude of A11-NS3133-specific T cells did not correlate with disease severity in this cohort. This held true whether the data were analyzed according to clinical diagnosis (DF versus DHF) or continuous measures of disease severity such as pleural effusion index, hemoconcentration, or platelet nadir. A similar lack of association was reported in a study in Vietnam25. Other studies had reported higher frequencies of DENV-specific T cells in patients with DHF, but these associations were found at 2 weeks13,14 or 6 months24 post-infection.
The interpretation of our data is subject to several limitations. Although our patient cohort was relatively large, the small number of HLA-A11+ subjects with the same DENV serotype, serologic response (primary/secondary infection), and clinical outcome (DF/DHF) limits the statistical power for important subgroup analyses, and data from HLA association studies suggest that the influence of T cell responses may not be the same for all four serotypes34. Additionally, although subjects were followed daily during acute illness, adequate specimens were not available for all of these flow cytometry studies from all time points for all subjects. Given that rapid changes were observed in tetramer-positive T cell frequencies during acute infection, it is likely that peak frequencies were missed in some subjects. Furthermore, few of the DHF patients in our study experienced shock (DHF grades 3 or 4). As Mongkolsapaya et al. found the highest frequencies of tetramer-positive T cells in patients with shock13,14, it is possible that the milder disease in our study cohort concealed a relationship to the most severe disease. Alternatively, we cannot exclude the possibility that the A11-NS3133 epitope is not representative of the global DENV-specific CD8+ T cell response. Duangchinda et al. recently showed higher cytokine responses in children with DHF compared to DF when overlapping peptides covering the entire NS3 protein were used38. However, that study only analyzed PBMC collected several weeks post-infection and the same association was not detected by Simmons et al.39. Finally, human studies are limited to analysis of blood samples and we may have missed pathogenic T cells that were bound to the endothelium or located in other tissues.
In summary, our data points to a complex picture of T cell involvement in DENV infection. We found a selective activation and expansion of DENV-specific T cells during acute infection and a diverse pattern of serotype-cross-reactivity in both primary and secondary infections. These findings will need to be taken into account in future studies of T cell responses to natural DENV infection and/or vaccines.
Methods
Study subjects and blood samples
The clinical study design and collection of blood samples have been reported elsewhere3,40. Briefly, the study enrolled Thai children 6 months to 14 years of age with acute febrile illnesses. Acute DENV infections were confirmed by serology and virus isolation/detection, and primary and secondary infections were distinguished based on IgM∶IgG ratio and hemagglutination-inhibition antibody titer. Blood samples were obtained daily during acute illness, once in early convalescence (∼10 days after study entry) and at intervals during late convalescence (6 months – 3 years after study entry), and PBMC were cryopreserved. Time points are reported relative to the day of defervescence (i.e. the day at which fever dissipated and the patient subsequently maintained a temperature below 38°C), which was termed fever day 0 (d0). Fever days -1, -2, etc. occurred before defervescence, and day +1, etc. occurred after defervescence. Written informed consent was obtained from each subject and/or his/her parent or guardian. The study protocol was approved by the Institutional Review Boards of the Thai Ministry of Public Health, the Office of the U.S. Army Surgeon General and the University of Massachusetts Medical School. HLA typing was performed at the University of Massachusetts Medical School or the Department of Transfusion Medicine, Siriraj Hospital, as described34,41.
Peptide-MHC multimers
Peptides were purchased at >90% purity from AnaSpec, Inc. (San Jose, CA, USA). Sequences are shown in Table 2. pMHC multimers (all referred to as tetramers) were generated at the University of Massachusetts Medical School Tetramer Core as described42. The different pMHC monomers were mixed with their respective fluorochrome conjugates at molar ratios of 4∶1 (A11-NS3133 epitope variants), 25∶1 (Flu M158 epitope) and 50∶1 (B7-NS3222 epitope). Tetramers were conjugated to distinct fluorochromes to allow for staining with multiple epitopes/variants simultaneously (APC-A11-NS3133-pD1, PE-Cy7-A11-NS3133-pD2, PE-A11-NS3133-pD3/4, Qdot-605-A2-Flu M158, Qdot-605-B7-NS3222).
Staining and flow cytometry
Cryopreserved PBMC were thawed and rested in RPMI/10% FBS at 37°C for approximately 2 hours. Cells were washed in PBS and stained with LIVE/DEAD Aqua (Molecular Probes, Invitrogen Corp.) according to the manufacturer's instructions. Cells were washed and incubated with 0.5–2μL tetramer for 20 minutes at 4°C. Monoclonal antibodies specific for CD3 (clone UCHT1, Alexa Fluor 700 or clone SK7, PerCP-Cy5.5; BD Biosciences, San Jose, CA, USA), CD8 (clone SK1, PerCP-Cy5.5 from BD Biosciences, clone 3B5, Qdot-655 from Invitrogen Corp., Carlsbad, CA, USA, or clone RAP-T8, Alexa Fluor 700 from BD Biosciences), CD45RA (clone HI100, FITC; BD Biosciences), and CD38 (clone HIT2 conjugated to Qdot-655 from Invitrogen Corp. or to PerCP-Cy5.5 from BD Biosciences) were then added to the cells to incubate at 4°C for 30 minutes. Cells were washed and fixed with BD Stabilizing Fixative (BD Biosciences). Data were collected on a BD FACSAria and analyzed using FlowJo version 7.5.5.
Statistical analysis
For variables with a normal distribution, t-tests were used to compare continuous outcomes at a single time point between two groups and random intercept models were used to make comparisons between multiple groups across time points with the assumption that each individual within a group follows the same slope. For these models, missing values in the CD38 and CD45RA data sets were imputed with the means of all subjects at each time point. For variables that were not normally distributed, the Mann-Whitney rank sum test was used to make comparisons between two groups and Friedman's test was used to make comparisons between three or more groups. Correlations between skewed variables were determined using Spearman's rank correlation. Fischer's exact test was used to compare categorical data. All statistical analysis was performed using Stata Intercooled version 9 (Stata Corporation, College Station, TX, USA) and GraphPad Prism (La Jolla, CA, USA) software packages.
Author Contributions
HF, AM and ALR conceived and designed the experiments and wrote the manuscript text. HF, HB and TTM performed the experiments. HF and JAP analyzed the data. HF prepared the figures. RVG, AN, AS, SG and SK enrolled patients and collected samples. TG contributed reagents and HAFS provided HLA typing data. All authors reviewed the manuscript and agree with the results and conclusions.
Supplementary Material
Supplementary Information
Acknowledgments
This work was funded by the National Institutes of Health, grants P01 AI34533 and U19 AI57319 and core support from NIH P30 DK032520. We would like to thank the donors who generously provided PBMC for use in our studies. We thank Drs. Suchitra Nimmannitya and David Vaughn, as well as the staffs of the Queen Sirikit National Institute for Child Health, the Department of Virology, Armed Forces Research Institute of Medical Sciences and the Department of Transfusion Medicine, Siriraj Hospital, for patient recruitment, blood collection and clinical, virology and HLA information. We would also like to thank Joyce Pepe, Jim Coderre and the UMass Tetramer Core for their time and effort to generate the pMHC multimers, Marcia Woda for sharing her flow cytometry expertise and help with data collection, and Lynne Burns for her technical support.
References
- Beatty M. Pediatric Dengue Vaccine Initiative. Global Burden of Dengue.<http://www.pdvi.org/about_dengue/GBD.asp> (accessed 29 March 2011)
- Vaughn D. W. et al.. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. The Journal of infectious diseases 181, 2–9 (2000). [DOI] [PubMed] [Google Scholar]
- Vaughn D. W. et al.. Dengue in the early febrile phase: viremia and antibody responses. The Journal of infectious diseases 176, 322–330 (1997). [DOI] [PubMed] [Google Scholar]
- Burke D. S., Nisalak A., Johnson D. E. & Scott R. M. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg 38, 172–180 (1988). [DOI] [PubMed] [Google Scholar]
- Guzman M. G. et al.. Dengue hemorrhagic fever in Cuba, 1981: a retrospective seroepidemiologic study. Am J Trop Med Hyg 42, 179–184 (1990). [DOI] [PubMed] [Google Scholar]
- Thein S. et al.. Risk factors in dengue shock syndrome. Am J Trop Med Hyg 56, 566–572 (1997). [DOI] [PubMed] [Google Scholar]
- Rothman A. L. Cellular immunology of sequential dengue virus infection and its role in disease pathogenesis. Current topics in microbiology and immunology 338, 83–98, 10.1007/978-3-642-02215-9_7 (2010). [DOI] [PubMed] [Google Scholar]
- Bashyam H. S., Green S. & Rothman A. L. Dengue virus-reactive CD8+ T cells display quantitative and qualitative differences in their response to variant epitopes of heterologous viral serotypes. J Immunol 176, 2817–2824 (2006). [DOI] [PubMed] [Google Scholar]
- Dong T. et al.. High pro-inflammatory cytokine secretion and loss of high avidity cross-reactive cytotoxic T-cells during the course of secondary dengue virus infection. PLoS One 2, e1192 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friberg H. et al.. Memory CD8(+) T cells from naturally acquired primary dengue virus infection are highly cross-reactive. Immunol Cell Biol 89, 122–129 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imrie A. et al.. Differential functional avidity of dengue virus-specific T-cell clones for variant peptides representing heterologous and previously encountered serotypes. Journal of virology 81, 10081–10091 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangada M. M. & Rothman A. L. Altered cytokine responses of dengue-specific CD4+ T cells to heterologous serotypes. J Immunol 175, 2676–2683 (2005). [DOI] [PubMed] [Google Scholar]
- Mongkolsapaya J. et al.. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat Med 9, 921–927 (2003). [DOI] [PubMed] [Google Scholar]
- Mongkolsapaya J. et al.. T cell responses in dengue hemorrhagic fever: are cross-reactive T cells suboptimal? J Immunol 176, 3821–3829 (2006). [DOI] [PubMed] [Google Scholar]
- Moran E. et al.. Preservation of a critical epitope core region is associated with the high degree of flaviviral cross-reactivity exhibited by a dengue-specific CD4+ T cell clone. European journal of immunology 38, 1050–1057 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra B. et al.. Long-term memory cellular immune response to dengue virus after a natural primary infection. Int J Infect Dis 6, 125–128 (2002). [DOI] [PubMed] [Google Scholar]
- Zivny J. et al.. Partial agonist effect influences the CTL response to a heterologous dengue virus serotype. J Immunol 163, 2754–2760 (1999). [PubMed] [Google Scholar]
- Green S. et al.. Early immune activation in acute dengue illness is related to development of plasma leakage and disease severity. The Journal of infectious diseases 179, 755–762 (1999). [DOI] [PubMed] [Google Scholar]
- Green S. et al.. Elevated plasma interleukin-10 levels in acute dengue correlate with disease severity. Journal of medical virology 59, 329–334 (1999). [PubMed] [Google Scholar]
- Kurane I. et al.. Activation of T lymphocytes in dengue virus infections. High levels of soluble interleukin 2 receptor, soluble CD4, soluble CD8, interleukin 2, and interferon-gamma in sera of children with dengue. J Clin Invest 88, 1473–1480, 10.1172/JCI115457 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libraty D. H. et al.. Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infections. The Journal of infectious diseases 185, 1213–1221 (2002). [DOI] [PubMed] [Google Scholar]
- Perez A. B. et al.. IL-10 levels in Dengue patients: some findings from the exceptional epidemiological conditions in Cuba. Journal of medical virology 73, 230–234 (2004). [DOI] [PubMed] [Google Scholar]
- Green S. et al.. Early CD69 expression on peripheral blood lymphocytes from children with dengue hemorrhagic fever. The Journal of infectious diseases 180, 1429–1435 (1999). [DOI] [PubMed] [Google Scholar]
- Zivna I. et al.. T cell responses to an HLA-B*07-restricted epitope on the dengue NS3 protein correlate with disease severity. J Immunol 168, 5959–5965 (2002). [DOI] [PubMed] [Google Scholar]
- Dung N. T. et al.. Timing of CD8+ T cell responses in relation to commencement of capillary leakage in children with dengue. J Immunol 184, 7281–7287 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau T. N. et al.. Dengue in Vietnamese infants–results of infection-enhancement assays correlate with age-related disease epidemiology, and cellular immune responses correlate with disease severity. The Journal of infectious diseases 198, 516–524 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akondy R. S. et al.. The yellow fever virus vaccine induces a broad and polyfunctional human memory CD8+ T cell response. J Immunol 183, 7919–7930 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friberg H. et al.. Memory CD8(+) T cells from naturally acquired primary dengue virus infection are highly cross-reactive. Immunology and cell biology (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R. M., Che J. W., Brehm M. A. & Selin L. K. Heterologous immunity between viruses. Immunological reviews 235, 244–266 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R. M. et al.. The privacy of T cell memory to viruses. Current topics in microbiology and immunology 311, 117–153 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumier C. M., Mathew A., Bashyam H. S. & Rothman A. L. Cross-reactive memory CD8(+) T cells alter the immune response to heterologous secondary dengue virus infections in mice in a sequence-specific manner. The Journal of infectious diseases 197, 608–617 (2008). [DOI] [PubMed] [Google Scholar]
- Miller J. D. et al.. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity 28, 710–722 (2008). [DOI] [PubMed] [Google Scholar]
- Wiesel M., Walton S., Richter K. & Oxenius A. Virus-specific CD8 T cells: activation, differentiation and memory formation. Apmis 117, 356–381 (2009). [DOI] [PubMed] [Google Scholar]
- Stephens H. A. et al.. HLA-A and -B allele associations with secondary dengue virus infections correlate with disease severity and the infecting viral serotype in ethnic Thais. Tissue Antigens 60, 309–318 (2002). [DOI] [PubMed] [Google Scholar]
- Nguyen T. P. et al.. Protective and enhancing HLA alleles, HLA-DRB1*0901 and HLA-A*24, for severe forms of dengue virus infection, dengue hemorrhagic fever and dengue shock syndrome. PLoS neglected tropical diseases 2, e304 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myint K. S. et al.. Cellular immune activation in children with acute dengue virus infections is modulated by apoptosis. The Journal of infectious diseases 194, 600–607 (2006). [DOI] [PubMed] [Google Scholar]
- Luhn K. et al.. Increased frequencies of CD4+ CD25(high) regulatory T cells in acute dengue infection. The Journal of experimental medicine 204, 979–985 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duangchinda T. et al.. Immunodominant T-cell responses to dengue virus NS3 are associated with DHF. Proceedings of the National Academy of Sciences of the United States of America 107, 16922–16927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons C. P. et al.. Early T-cell responses to dengue virus epitopes in Vietnamese adults with secondary dengue virus infections. Journal of virology 79, 5665–5675 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalayanarooj S. et al.. Early clinical and laboratory indicators of acute dengue illness. The Journal of infectious diseases 176, 313–321 (1997). [DOI] [PubMed] [Google Scholar]
- Mathew A. et al.. Predominance of HLA-restricted cytotoxic T-lymphocyte responses to serotype-cross-reactive epitopes on nonstructural proteins following natural secondary dengue virus infection. Journal of virology 72, 3999–4004 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzuriaga K. et al.. Early therapy of vertical human immunodeficiency virus type 1 (HIV-1) infection: control of viral replication and absence of persistent HIV-1-specific immune responses. Journal of virology 74, 6984–6991 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek M. A. et al.. The minimum peptide epitope from the influenza virus matrix protein. Extra and intracellular loading of the HLA-A2. J Immunol 147, 4047–4053 (1991). [PubMed] [Google Scholar]
- Morrison J. et al.. Identification of the nonamer peptide from influenza A matrix protein and the role of pockets of HLA-A2 in its recognition by cytotoxic T lymphocytes. Eur J Immunol 22, 903–907 (1992). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information