Abstract
Increasing evidence suggests that the loss of functional stem cells may be important in the aging process. Our experiments were originally aimed at testing the idea that, in the specific case of age-related osteoporosis, declining function of osteogenic precursor cells might be at least partially responsible. To test this, aging female mice were transplanted with mesenchymal stem cells from aged or young male donors. We find that transplantation of young mesenchymal stem cells significantly slows the loss of bone density and, surprisingly, prolongs the life span of old mice. These observations lend further support to the idea that age-related diminution of stem cell number or function may play a critical role in age-related loss of bone density in aging animals and may be one determinant of overall longevity.
Osteoporosis is a disease characterized by low bone mass and deteriorating bone structure leading to increased bone fragility and risk of fractures1,2. This disease, primarily affecting older population, has placed a tremendous burden on the health care system3,4. Although advanced age is the best predictor of osteoporosis1,3, the link between aging and osteoporosis is not fully understood. Our investigations, involving the simple strategy of introducing young bone marrow mesenchymal stem cells (BMSCs) into old recipients, were intended to test the possibility that age-related loss of functional stem cells might be at least partially responsible for this disorder.
Extensive evidence suggests that osteoporosis is caused by complex interactions between endogenous (genetic and hormonal) and exogenous (nutritional and physical activity) factors regulating bone tissue remodeling1,5. It is therefore thought that osteoporosis may be prevented by increased daily intake of calcium and vitamin D, appropriate and persistent exercise, hormone replacement and many other alternative therapies2,6. However, none of these has been shown to be completely effective in preventing age-related osteoporosis.
It has recently been proposed that a decrease of osteogenic progenitor cells within the bone marrow with advancing age might be related to osteoporosis7,8. In this regard, a relative increase in the proportion of osteoclasts in aged bone may tilt the delicate balance between the bone-forming activity of osteoblasts and bone resorbing activity of osteoclasts7,8. A related proposal suggests that aging bone marrow cells may shift from pro-osteoblastogenesis to pro-adipogenesis phenotypes, reflected by an accumulation of bone marrow fat with aging9. In partial support of this possibility, stem cells isolated from older donors have been shown to have defective functions, including impaired capacity of proliferation and differentiation in vitro, compared with those isolated from young individuals10,11,12.
Despite of the latter observations, it remains uncertain whether aging of stem cells is responsible for the pathogenesis of age-related osteoporosis. To test the possible impact of aging on stem cell function, we have carried out a series of studies to compare the function of BMSCs isolated from young (1–2 months old) or old (20–24 months old) mice. To test whether defective stem cells function might be responsible for the progression of osteoporosis, old female mice were irradiated then transplanted with BMSCs from young or old males. The fate and function of transplanted BMSCs and their effect on bone mineral density of elderly recipient mice were then evaluated.
Results
Bone marrow stem cell isolation and characterization
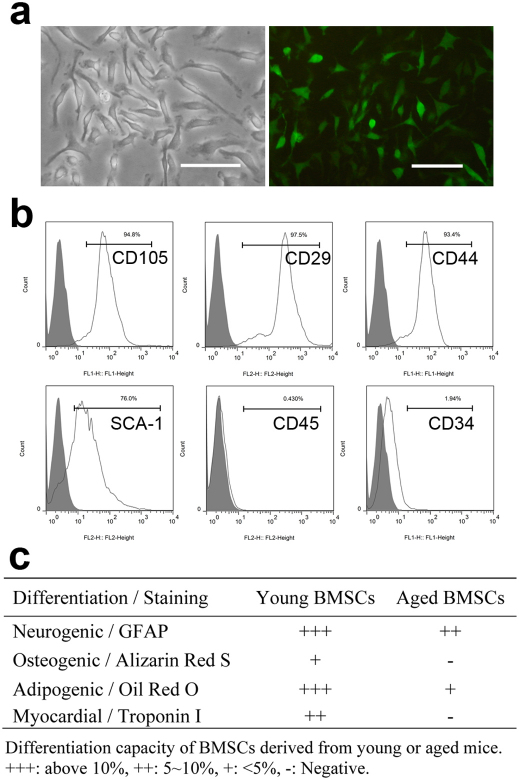
Femoral BMSCs were isolated from 1–2 months old and 20–24 months old male EGFP transgenic C57BL/6 mice and Balb/c mice. BMSCs from young and old EGFP donors showed similar fibroblast-like spindle shape and green fluorescence (Figure 1A) following in vitro culture with similar doubling times of ∼80 hours. Flow cytometry analyses showed that second passage cells from Balb/c mice were positive for CD44, CD29, CD105, SCA-1, and negative for CD34, CD45 (Figure 1B). After culture in the presence of various differentiation agents, BMSCs isolated from younger animals were easily differentiated into osteogenic, adipogenic, neurogenic and myogenic cell types. However, under similar culture conditions, we found that BMSCs isolated from old animals had significantly less capability to undergo osteogenic, adipogenic, neurogenic and myogenic differentiation (Figure 1C). These results suggested that BMSCs lose the ability to differentiate into certain lineages during the aging process.
Figure 1. Characterization of BMSCs.
(A) BMSCs isolated from EGFP transgenic C57BL/6 mice have typical fibroblast like morphology and exhibit green fluorescence (×20; bar = 100µm). (B) Flow cytometry analysis on BMSCs isolated from Balb/C mice shows the majority of cells are CD105+, CD29+, CD44+, SCA-1+, CD45−, CD34−. (C) Differentiation capacity of BMSCs isolated from young (1–2 months old) and old (20–24 months old) Balb/C mice. The average ratio of positive cells to total cells in three random ×20 views was calculated as the differentiation rate. N = 5 for each group.
Cellular senescence-related markers in BMSCs
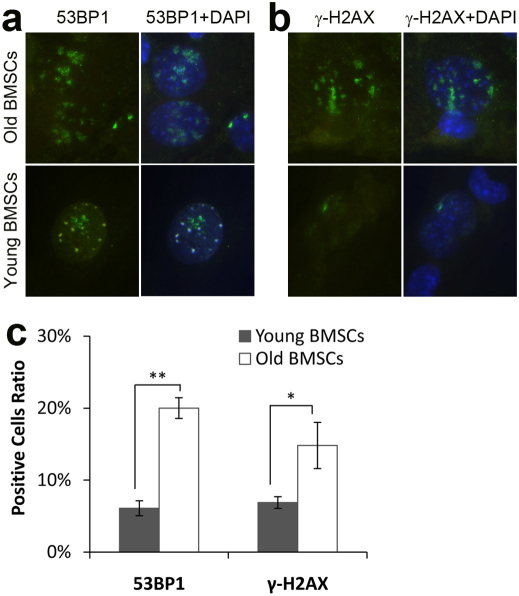
Further studies were carried out to determine whether BMSCs retrieved from young or old mice might differ in terms of the presence of certain cellular senescence markers, including DNA double-strand break marker phosphorylated H2AX (γ-H2AX), DNA damage checkpoint response mediator 53BP1 (p53 gene binding protein 1). These were analyzed in cultured BMSCs in vitro. Interestingly, we found the DNA damage checkpoint response mediators 53BP1 and γ-H2AX were much higher in BMSCs from old mice than those from young mice (p = 0.014 and p = 1.71E−4, respectively. Figure 2A–C).
Figure 2. Expression of cellular senescence-related markers 53BP1 and γ-H2AX in BMSCs isolated from young mice (1–2 months old, n = 3) and aged female Balb/C mice (20–24 months old, n = 3).
(A) 53BP1 expression (green) in BMSCs nuclei overlapping with DAPI staining (blue). (B) γ-H2AX expression (green) in BMSCs nuclei overlapping with DAPI staining (blue). (C) Percentage of 53BP1 and γ-H2AX positive cells in BMSCs. *: p = 0.014, **: p = 1.71E-4.
BMSCs transplantation and migration in host mice
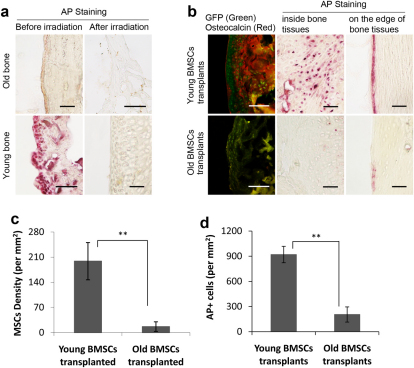
If a defect in differentiation of aged BMSCs is responsible for osteoporosis, we thought it likely that osteoporosis would be delayed with the transplantation of BMSCs from young donors. To test this, we irradiated 18–24 months old female Balb/C mice and then transplanted them with 1 × 106 BMSCs isolated from either young or old male EGFP C57BL/6 mice. To assess the effect of irradiation on osteoblast activities, we evaluated alkaline phosphatase (AP) activity in bone tissues. Interestingly, bone AP activities were consistently lower in aged mice than in young mice (Figure 3A). Immediately following irradiation, AP activities were substantially reduced in both groups of mice indicating that radiation treatment was effective in depleting resident osteoblast function in bones of treated animals (Figure 3A).
Figure 3. Osteogenic activities and BMSCs migration in bone (n = 7 for each groups).
(A) Prior to radiation, there were few AP+ (pink) cells in bone from old mice while there were significant numbers of AP+ cells in young ones. Immediately following irradiation treatment, few AP+ cells were found in both groups, bar = 100µm. (B) Six months following BMSCs transplantation, the presence of transplanted BMSCs and osteogenic activities were visualized based on anti-GFP (green) and osteocalcin immunohistochemistry staining (red). AP+ cells were also imaged inside or on the edge of bone, bar = 100µm. The density of (C) GFP+ BMSCs and (D) AP+ cells in the bones from mice with either old BMSCs or young BMSCs transplants was determined. **: p<0.01.
Six months following transplantation, we analyzed femoral bone from mice transplanted with either young or old BMSCs. Surprisingly, we found significantly higher densities of GFP positive BMSCs in the bone of mice transplanted with young BMSCs compared to those given old BMSCs (p = 8.43E−06; Figures 3B & 3C). Furthermore, we found higher expression of osteocalcin (Figure 3B) and higher numbers of alkaline phosphatase positive (AP+) cells (Figure 3B) present in femurs of mice transplanted with young BMSCs than those with old BMSCs (p = 3.83E−5; Figure 3D). These observations suggest that transplanted old BMSCs are relatively unable to populate, replicate or differentiate into osteoblasts within bones of aged recipients.
BMSCs transplantation affects the longevity of recipient mice
Without further treatment, we noticed that most of the mice transplanted with young BMSCs had a significantly longer life span than other groups of mice (Figure 4A). The mean life span of control mice was 765 days (Figure 4B). However, the mean life span for mice that received young BMSCs transplants was 890 days (vs. control group, p = 0.009). The increase of life span is probably unrelated to radiation, since the mean life span of mice transplanted with old BMSCs was 789 days (vs. young BMSCs transplants, p = 0.002) (Figure 4B). In addition, there was no significant difference between control animals and mice transplanted with old BMSCs (p = 0.846). Overall, these results suggest that transplantation of BMSCs derived from young animals extends life span. However, it is not clear whether the prolonged lifespan may be associated with improved tissue regeneration.
Figure 4. Effect of BMSCs transplantation on the life span of Balb/c mice.
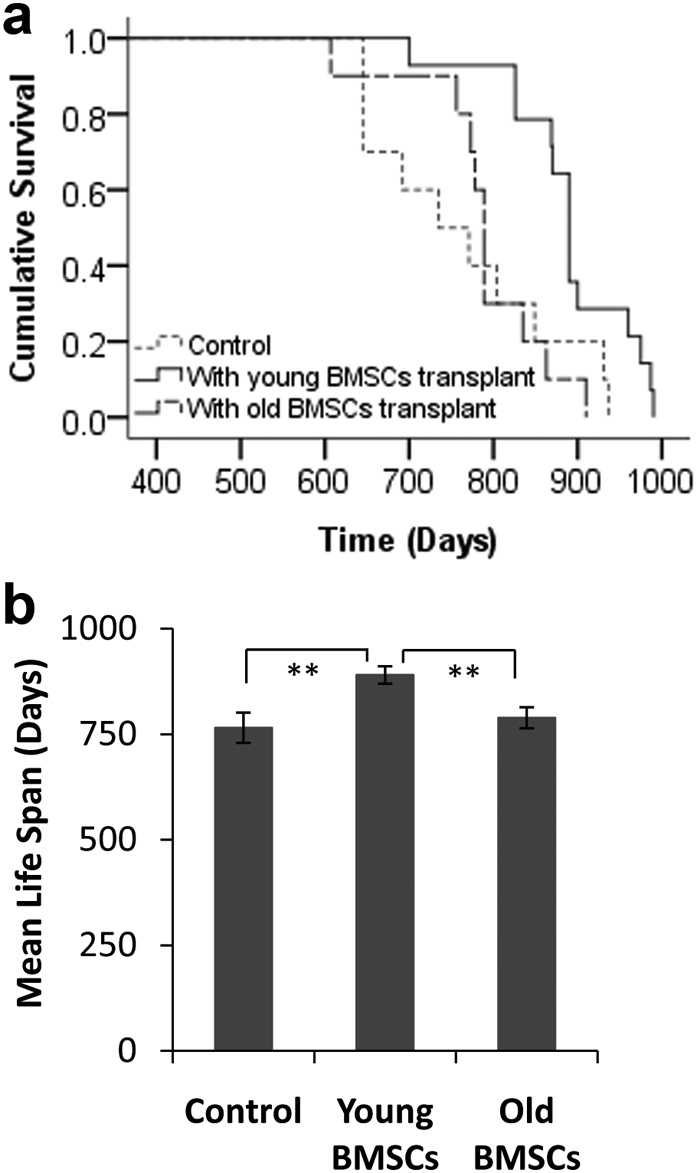
(A) The lifespan of mice without (control, n = 10) and with BMSCs transplants from either young (n = 14) or old mice (n = 10). (B) The mean survival time of control animals vs. BMSCs transplanted mice. Kaplan-Meier Log Rank pairwise comparison statistics revealed significant differences of lifespan between the control and young BMSCs transplantation groups (p = 0.009), and between young BMSC transplantation and old BMSCs transplantation groups (p = 0.002). There was no significant difference between the control and old BMSCs transplantation groups (p = 0.846). **: p<0.01.
Effects of BMSCs osteogenic activities on bone mineral density
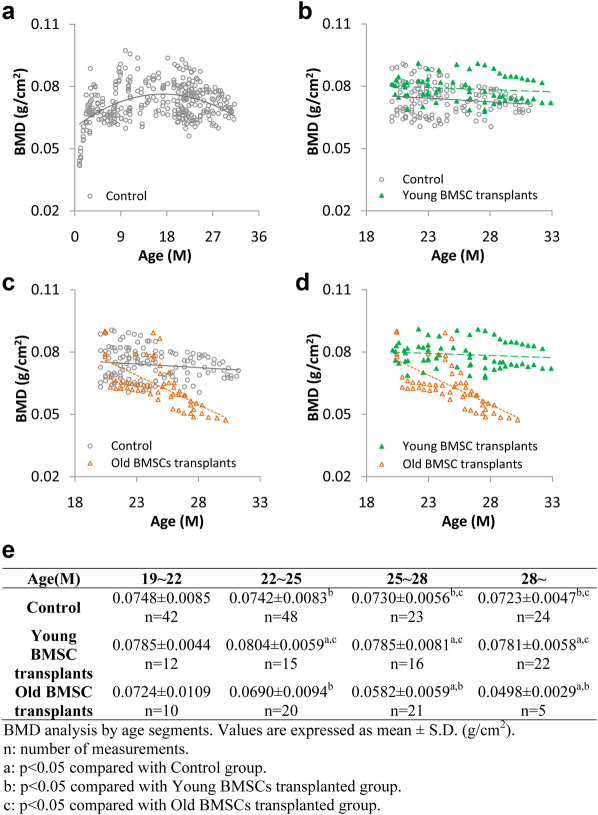
To determine the effect of the osteogenic activities of transplanted BMSCs on bone strength we measured the change of bone mineral density (BMD) in the knee joint area of treated mice over time. Untreated control Balb/C mice showed substantial bone density increase with age from 1 month to 10 months (Figure 5A). The bone density reached a plateau between 10 months to 12 months of age. After 12 months, bone density started to decrease and the decrease accelerated after 18 months (Figure 5A). Compared to control mice, the mice transplanted with young BMSCs showed substantially higher BMD distribution following transplantation at 18–24 months (and assessed at 20–32 months) (Figure 5B). On the other hand, the BMD distribution of mice transplanted with old BMSCs were lower than those of the control group or the young BMSCs transplanted group (Figure 5C and D). By comparing the mean bone mineral densities at different age ranges (Figure 5E), our results clearly demonstrate that transplantation of young BMSCs significantly delays the decrease of bone mineral densities while old BMSCs transplantation may actually accelerate the decline of bone density.
Figure 5. BMD measurements of Balb/c mice.
(A) The distribution of bone density in untreated mice from 1–33 months (open circles with polynomial treadline, order = 2, n = 99). (B) The distribution of BMD in mice transplanted with BMSCs isolated from young mice (green solid triangles with green dash linear trendline, n = 15) compared with untreated controls (gray open circles with linear trendline, n = 9). (C) The distribution of BMD in mice transplanted with BMSCs isolated from old mice (orange open triangles with orange dash linear trendline, n = 10) compared with untreated controls (gray open circles with linear trendline, n = 9). (D) Direct comparisons of BMD distribution between mice transplanted with young BMSCs (green solid triangles with green dash linear trendline, n = 15) and those with old BMSCs (orange open triangles with orange dash linear trendline, n = 10). (E) Comparison of the averages of BMD at different age ranges.
Micro-CT imaging of femurs from BMSCs transplanted animals
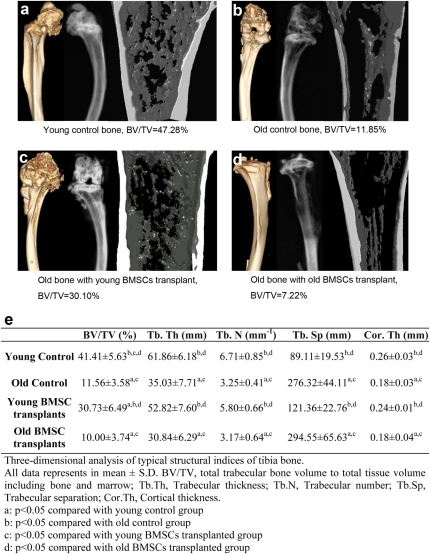
To visualize the bone micro structure of the BMSCs transplanted animals, distal tibiae were isolated and examined with a Siemens Inveon CT/PET Multimodality system (Siemens Medical Solutions Inc., Knoxville, TN, USA) operating in the cone-beam mode. Imaging was obtained at 80 kV and 500 CT images were reconstructed with a down sample factor of 1 using Cobra Reconstruction software. As shown in Figure 6, there were clear differences in the X-ray and micro-CT reconstruction images of tibiae isolated from different groups of animals. Specifically, tibiae from young animals showed thicker compact bone with denser trabecular network (white/gray), small marrow space (black) and a rod and plate aspect in the 3D microarchitecture (Figure 6A). As expected, a more alveolar structure with a thinner trabecular bone and less distinct plate structure was seen in the tibiae of old animals (Figure 6B). Very interestingly, the transplantation of young BMSCs substantially increased the trabecular network and bone density of old mice (Figure 6C). Such changes of bone structure were not found in the old mice transplanted with old BMSCs which retained typical thinner trabecular bone and lesser plate feature (Figure 6D). The differences of bone structure among various treatment groups were also reflected in 3D analyses of tibia bone. Figure 6E shows that the volume ratios of bone to total tissue of young mice and old mice transplanted with young BMSCs were significantly greater than old mice and old mice transplanted with old BMSCs. Similar differences were also seen in the trabecular separation, cortical thickness, trabecular thickness and trabecular number among the test groups (Figure 6E).
Figure 6. Micro structure of distal tibia bones from controls and young/old BMSCs transplanted mice 6 months after transplant.
Left images are the three-dimensional (3D) images of mouse tibias, middle images are the X-ray images taken from a KODAK in vivo FX machine, and the right images are the 3D microCT reconstruction images of samples of mouse tibias. Typical bone images from (A) young control mouse, (B) old control mouse, (C) mouse transplanted with young BMSCs, (D) mouse transplanted with old BMSCs, (E) Tabulated parameters and statistical analyses of bone microstructure based on micro-CT analyses, n = 5 for each group.
Discussion
Aging is the best predictor of osteoporosis1,3. Aging-associated changes in metabolism and hormonal balance have been shown to play important roles in the progression of osteoporosis13,14. Indeed, supplemental intake of calcium (1000–1200 mg) and vitamin D (700–800 IU) is currently recommended to prevent and, perhaps, treat osteoporosis5,6. In addition, appropriate and persistent exercise has been shown to increase bone density and reduce the risk of hip fracture in older women15. Pharmacologic interventions2,5,6, including anabolic agents such as hormone replacement, bisphosphonates, calcitonin, raloxifene, teriparatide and alternative therapies are currently being used in osteoporosis prevention and treatment. Although these strategies are at least partially effective, recent evidence indicates that the loss of functional osteogenic progenitor cells with age might be responsible for age-related osteoporosis. Specially, the proliferative capacity of osteogenic progenitor cells derived from the bone marrow of old mice is substantially lower than those from young ones7. A significant age-dependent decline of cells with osteogenic potential has also been observed in human bone marrow16,17. Additionally, alkaline phosphatase-positive osteogenic progenitor colonies (CFU-ALP) and total fibroblastic colonies (CFU-F) of mice, rats and human BMSCs have also shown declined osteogenic and growth potential with age18,19,20.
To determine whether the senescence of BMSCs contributes to the accelerated bone mass loss, we compared the in vitro differentiation capacity of BMSCs isolated from young (1–2 months old) vs. old mice (20–24 months old). Our results indicate that the differentiation capability of young BMSCs is significantly higher than those of old BMSCs. This is in agreement with recent findings that mesenchymal stem cells derived from mice, Wistar rats, rhesus monkeys and humans show declined viability and capacity for differentiation with age.10,11,12,17,19. The molecular mechanism(s) underlying BMSCs aging are unclear. Cumulative DNA damage has been reported to affect cell aging21. Upon DNA damage (i.e., double strand breaks), phosphorylated H2AX accumulates at sites of DNA lesions, which triggers a DNA damage checkpoint response22. 53BP1 is a DNA damage checkpoint response mediator and plays an important role in DNA damage checkpoint response and damage repair23. Our results show that there is a good agreement between BMSCs aging and 53BP1 and γ-H2AX up-regulation. However, we cannot conclude yet whether cumulative DNA damage is the cause of BMSCs aging and loss of function.
By analyzing bone sections from transplanted mice, we have found that more young BMSCs than old BMSCs appeared in the tissue sections, suggesting that the migratory ability of BMSCs may decline with age. This is supported by parallel observations that the migration of endothelial progenitor cells is significantly reduced in older humans and mice24,25. It has also been shown that the migratory ability of human BMSCs significantly declines with age26. Such aged-related impaired migration has also been shown in human lung, skin and dermal fibroblasts27,28. The inability of BMSCs from aged individuals to migrate throughout the body may impede the distribution of the BMSCs and later regeneration of various tissues and organs which might, in the present case, lead to osteoporosis.
It is well established that, compared with young individuals, old animals have significant reductions of osteogenic activity in bone, including the expression and production osteocalcin29. Our results showed that transplantation of BMSCs from young mice leads to a dramatic increase of AP and osteocalcin activities in bone tissue. On the other hand, the transplantation of BMSCs from old animals had no such effect. These results suggest that young BMSCs can actively migrate, and differentiate into a variety of cells, including osteoblasts. However, old BMSCs may somehow lose such capability. These observations are in agreement with several recent findings. First, with increasing age, murine bone marrow derived stem cells lose their ability to adhere, proliferate, and undergo chondrogenic and osteogenic differentiation10. Second, stem cells isolated from old animals were found to engender less bone formation when subcutaneously implanted in porous hydroxyapatite scaffolds than stem cells recovered from young ones30. Finally, a negative correlation has been reported between in vitro osteogenic activity of CD105+ human MSCs and cell donor age31.
Using Micro-CT imaging it has been established that, during the development of age-related osteoporosis, there are substantial decreases of bone to total tissue ratio, trabecular thickness, trabecular number, and cortical thickness while the values for trabecular separation are increased significantly32,33. In fact, these bone physical characteristics have been used as indicators for diagnosis and treatment of osteoporosis34,35. Trabecular architecture parameter assessments using CT or MRI as a bone mineral density-independent determinant of bone strength are able to separate patients with and without osteoporotic fractures better than with BMD alone, and parameters of trabecular architecture are also more sensitive to treatment effects than BMD36. Equally important, trabecular architecture parameters and microstructure measurements using micro-CT have proven to be a very good tool to evaluate osteogenic activity and new bone formation37,38. We find that, simply by transplantation of BMSCs from young animals, it is possible to restore almost all aspects of bone microstructure in aged animals. However, transplantation of BMSCs from old animals had little or no effect on bone microstructure in similar animals. Similar morphological changes have been shown to reflect osteogenic activities39.
Surprisingly, we observed that recipients of BMSCs from young animals had significantly increased life spans. It is not clear how administration of young BMSCs leads to increased longevity but it is possible that the transplanted young BMSCs may enhance cell/tissue regeneration and thereby slow down the aging process. This potential influence is based on the following evidence. First, EGFP positive cells were found in many vital organs, including skin, heart, spleen, lung, liver, bone marrow, etc. Second, studies have shown that transplants of young mesenchymal stem cells were able to restore cardiac angiogenic function and also improve functional outcomes in old mice after a myocardial infarction40. Third, similar transplants of young mesenchymal stem cells in old female mice were also reported to postpone age-related reproductive failure and improve offspring survival41. In addition, bone marrow transplantation from young donors was also found to be able to rejuvenate the B-cell lineage in aged mice42. Finally, myofiber-associated satellite cell transplantation has been found to be effective in the prevention of age-related sarcopenia43. Overall, these observations support the idea that young BMSCs transplantation might extend life span by improving the functions of multiple vital organs, including lung, heart, and immune system, albeit through presently unknown mechanisms.
In conclusion, the transplantation of BMSCs from young animals effectively restores bone microstructure and density in aged animals. As an unexpected consequence, old mice receiving BMSCs from young animals also have longer life spans compared to control mice or mice with transplanted BMSCs from old mice. These findings provide a potential link between stem cell activities and aging. However, further studies are needed to determine mechanisms through which BMSCs transplantation restores bone function and prolongs life.
Methods
Materials
Dulbecco's Modified Eagle's Medium (DMEM), Fetal Bovine Serum (FBS) and Pen-Strep were purchased from Invitrogen Corporation (Carlsbad, California), trypsin, ascorbic acid-2-phosphate, dexamethasone and β-glycerolphosphate were purchased from Sigma-Aldrich (St. Louis, MO). BMP-2 was from R&D Systems (Minneapolis, MN). Primary antibodies CD34, CD44, CD105, CD29, SCA-1, CD45, osteocalcin, 53BP1, γ-H2AX, were purchased from Santa Cruz Biotechnology Inc (Santa Cruz, CA) and BD Bioscience (San Jose, CA). FITC and Texas Red conjugated secondary antibodies were from Jackson ImmunoResearch laboratories, Inc. (West Grove, PA).
BMSCs Isolation, Proliferation, and Characterization
BMSC isolations were carried out on EGFP transgenic male C57Bl/6 mice and Balb/c mice (Taconic Farm, Hudson, NY) following standard bone marrow aspirate procedure44. Young and old BMSCs were recovered from 1–2 months old and 20–24 months mice, respectively. CD105, CD29, CD44, CD34 and CD45 expression on the 2nd passage of BMSCs from Balb/c mice were analyzed using flow cytometry following standard manufacturing protocols. To determine their differentiation ability, the 2nd passage of BMSCs underwent osteogenic, neurogenic, adipogenic, and myogenic differentiation by culture the cells in respective induction medium45,46,47,48. Immunohistological staining of aging related markers 53BP1 and γ-H2AX, were carried out to compare the expression profiles between different sources of BMSCs.
BMSCs transplantation in vivo
For BMSCs transplantation study, we used female Balb/C mice (female, 18–24 month old). All mice underwent whole body X-irradiation (500 cGy) using a Varian Clinac Linear accelerator (2100C) to eradicate host bone marrow stem cells using established procedures49. Twenty four hours following the irradiation, mice were intravenously transplanted with EGFP-transgenic BMSCs (1×106 cells/ animal) from either young or old mice. More than 95% of the host mice survived after radiation and subsequent BMSCs transplantation. GFP and osteocalcin immunohistochemistry were applied to evaluate in vivo BMSCs population and osteogenic activities. The extent of BMSC migration was estimated by measuring the densities of GFP positive cells in femur tissue sections. Bone alkaline phosphatase activities were also determined to reflect the degree of osteogenic activities based on the established procedure50. The animal use protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Texas at Arlington.
Measurement of bone mineral density
All treated and control animals were monitored every 2–3 weeks for their BMD using Lunar PIXImus machine (Lunar PIXImus Co., Madison, WI). BMD of the femur joint was analyzed using Lunar PIXImus 2.0 software.
Micro-CT imaging of femurs microarchitecture
At the end of the experiments, tibia bone samples were examined with a Siemens Inveon CT/PET Multimodality system (Siemens Medical Solutions Inc., Knoxville, TN, USA) operating in the cone-beam method. Imaging was obtained at 80 kV and 500 mA with a focal spot of 58 μm. The total rotation of the gantry was 360° with 1080 rotation steps obtained at an exposure time of approximately 715 ms/frame. The images were attained using a bin factor of 1 and an average frame of 3. Under high magnification the effective pixel size was 11.34 μm. CT images were reconstructed with a down sample factor of 1 using Cobra Reconstruction software. Three-dimensional reconstruction of the bone was analyzed with Inveon Research Workplace software. For each bone sample, the trabecular region was defined as the epiphysis of the distal femur excluding the growth plate. Regions of interest (ROI) were drawn approximately 180 μm from the growth plate and extended approximately 200 pixels along the cortical wall. Fewer contours needed to be drawn since a routine facility calculated all the intermediary masks by interpolation. The ROI's were interpolated yielding a final ROI encompassing approximately 65 slices. The trabecular bone was separated from the bone marrow and analyzed to determine the following parameters using the provided algorithm software. For trabecular bone measurement, the volume of interest (VOI) was comprised only of cancellous bone, and the cortices were excluded. The following parameters were determined: bone volume fraction (BV/TV) is the ratio of total trabecular bone volume (BV) to total tissue volume (TV, including bone and marrow). Trabecular thickness (Tb.Th.) is derived from the average thickness of the trabecular bone structures within VOI. Mean trabecular separation (Tb.Sp) is the average distance between trabecular structures within the VOI. Trabecular number (Tb.N) was derived according to the number of trabecular structures intersected per unit length within the VOI. The cortical wall thickness (Cor.Th) was measured in the center of the bone shaft. Four measurements were taken perpendicular to the cortical wall on either side of the shaft and averaged together.
Statistical analyses
Statistic was analyzed using IBM SPSS Statistics 19. All parameters were expressed as means ± SD, except mean life span was express as means ± SE. Kaplan-Meier Log Rank pairwise test was used to compare life spans between different groups. One-way ANOVA and Post Hoc Scheffe test was used for multiple comparisons of BMD between groups at different age periods. Two tailed independent samples t-test was used in other parameters comparison between groups. p<0.05 was considered significant.
Author Contributions
J.S. performed BMSCs isolation and characterization, cellular senescence markers staining, BMSCs osteogenic activity assay, life span analysis, and BMD data analysis. J.S. and L.T. performed mice irradiation and BMSCs transplantation. J.S. and Y.T. measured and analyzed mice BMD. N.D. oversaw BMD analysis. Y.T., M.L., and X.S. performed micro-CT analysis. J.S, Y.T. and L.T. prepared the manuscript. L.T. supervised the project.
Acknowledgments
This work was supported by NIH grant RO1 EB007271. We thank Drs. Sheng Zhang and Chengcheng Zhang for their help on BMSCs differentiation work and flow cytometry analyses. The authors would like to acknowledge Professor John W. Eaton for his critical review of this work.
References
- Raisz L. G. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest 115, 3318–3325 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauck K. F. & Clarke B. L. Diagnosis, screening, prevention, and treatment of osteoporosis. Mayo Clin Proc 81, 662–672 (2006). [DOI] [PubMed] [Google Scholar]
- Reginster J. Y. & Burlet N. Osteoporosis: a still increasing prevalence. Bone 38, S4–9 (2006). [DOI] [PubMed] [Google Scholar]
- Harvey N., Dennison E. & Cooper C. Osteoporosis: impact on health and economics. Nat Rev Rheumatol 6, 99–105 (2010). [DOI] [PubMed] [Google Scholar]
- Llorens R. A review of osteoporosis: diagnosis and treatment. Mo Med 103, 612–615; quiz 615–616 (2006). [PubMed] [Google Scholar]
- Sweet M. G., Sweet J. M., Jeremiah M. P. & Galazka S. S. Diagnosis and treatment of osteoporosis. Am Fam Physician 79, 193–200 (2009). [PubMed] [Google Scholar]
- Bergman R. J., et al.. Age-related changes in osteogenic stem cells in mice. J Bone Miner Res 11, 568–577 (1996). [DOI] [PubMed] [Google Scholar]
- Jilka R. L., Weinstein R. S., Takahashi K., Parfitt A. M. & Manolagas S. C. Linkage of decreased bone mass with impaired osteoblastogenesis in a murine model of accelerated senescence. J Clin Invest 97, 1732–1740 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moerman E. J., Teng K., Lipschitz D. A. & Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell 3, 379–389 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretlow J. D., et al.. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol 9, 60 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S. C., et al.. Mechanical strain modulates age-related changes in the proliferation and differentiation of mouse adipose-derived stromal cells. BMC Cell Biol 11, 18 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J. M., et al.. Age-related changes in mesenchymal stem cells derived from rhesus macaque bone marrow. Aging Cell (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi H. Molecular backgrounds of age-related osteoporosis from mouse genetics approaches. Rev Endocr Metab Disord 7, 17–22 (2006). [DOI] [PubMed] [Google Scholar]
- Riggs B. L. Endocrine causes of age-related bone loss and osteoporosis. Novartis Found Symp 242, 247–259; discussion 260–244 (2002). [PubMed] [Google Scholar]
- Feskanich D., Willett W. & Colditz G. Walking and leisure-time activity and risk of hip fracture in postmenopausal women. JAMA 288, 2300–2306 (2002). [DOI] [PubMed] [Google Scholar]
- D'Ippolito G., Schiller P. C., Ricordi C., Roos B. A. & Howard G. A. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res 14, 1115–1122 (1999). [DOI] [PubMed] [Google Scholar]
- Zhou S., et al.. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell 7, 335–343 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. L. Inhibition of growth and differentiation of osteoprogenitors in mouse bone marrow stromal cell cultures by increased donor age and glucocorticoid treatment. Bone 35, 83–95 (2004). [DOI] [PubMed] [Google Scholar]
- Stolzing A. & Scutt A. Age-related impairment of mesenchymal progenitor cell function. Aging Cell 5, 213–224 (2006). [DOI] [PubMed] [Google Scholar]
- Stolzing A., Jones E., McGonagle D. & Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev 129, 163–173 (2008). [DOI] [PubMed] [Google Scholar]
- Beausejour C. Bone marrow-derived cells: the influence of aging and cellular senescence. Handb Exp Pharmacol, 67–88 (2007). [DOI] [PubMed] [Google Scholar]
- Liu B., et al.. Genomic instability in laminopathy-based premature aging. Nat Med 11, 780–785 (2005). [DOI] [PubMed] [Google Scholar]
- d'Adda di Fagagna F., et al.. A DNA damage checkpoint response in telomere-initiated senescence. Nature 426, 194–198 (2003). [DOI] [PubMed] [Google Scholar]
- Hoetzer G. L., et al.. Aging, exercise, and endothelial progenitor cell clonogenic and migratory capacity in men. J Appl Physiol 102, 847–852 (2007). [DOI] [PubMed] [Google Scholar]
- Zhang W., Zhang G., Jin H. & Hu R. Characteristics of bone marrow-derived endothelial progenitor cells in aged mice. Biochem Biophys Res Commun 348, 1018–1023 (2006). [DOI] [PubMed] [Google Scholar]
- Xin Y., et al.. Aging adversely impacts biological properties of human bone marrow-derived mesenchymal stem cells: implications for tissue engineering heart valve construction. Artif Organs 34, 215–222 (2010). [DOI] [PubMed] [Google Scholar]
- Kondo H. & Yonezawa Y. Changes in the migratory ability of human lung and skin fibroblasts during in vitro aging and in vivo cellular senescence. Mech Ageing Dev 63, 223–233 (1992). [DOI] [PubMed] [Google Scholar]
- Mogford J. E., et al.. Effect of age and hypoxia on TGFbeta1 receptor expression and signal transduction in human dermal fibroblasts: impact on cell migration. J Cell Physiol 190, 259–265 (2002). [DOI] [PubMed] [Google Scholar]
- Lu C., et al.. Cellular basis for age-related changes in fracture repair. J Orthop Res 23, 1300–1307 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K., et al.. The effect of aging on bone formation in porous hydroxyapatite: biochemical and histological analysis. J Bone Miner Res 12, 989–994 (1997). [DOI] [PubMed] [Google Scholar]
- Roura S., et al.. Effect of aging on the pluripotential capacity of human CD105+ mesenchymal stem cells. Eur J Heart Fail 8, 555–563 (2006). [DOI] [PubMed] [Google Scholar]
- Duque G., et al.. Age-related bone loss in the LOU/c rat model of healthy ageing. Exp Gerontol 44, 183–189 (2009). [DOI] [PubMed] [Google Scholar]
- Halloran B. P., et al.. Changes in bone structure and mass with advancing age in the male C57BL/6J mouse. J Bone Miner Res 17, 1044–1050 (2002). [DOI] [PubMed] [Google Scholar]
- Wachter N. J., et al.. Predictive value of bone mineral density and morphology determined by peripheral quantitative computed tomography for cancellous bone strength of the proximal femur. Bone 28, 133–139 (2001). [DOI] [PubMed] [Google Scholar]
- Mehrotra M., Rosol M., Ogawa M. & Larue A. C. Amelioration of a mouse model of osteogenesis imperfecta with hematopoietic stem cell transplantation: microcomputed tomography studies. Exp Hematol 38, 593–602 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J. S. & Link T. M. Advances in osteoporosis imaging. Eur J Radiol 71, 440–449 (2009). [DOI] [PubMed] [Google Scholar]
- Xiang A., et al.. Changes in micro-CT 3D bone parameters reflect effects of a potent cathepsin K inhibitor (SB-553484) on bone resorption and cortical bone formation in ovariectomized mice. Bone 40, 1231–1237 (2007). [DOI] [PubMed] [Google Scholar]
- Cottrell J. A., et al.. Osteogenic activity of locally applied small molecule drugs in a rat femur defect model. J Biomed Biotechnol 2010, 597641 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day J. S., et al.. Bisphosphonate treatment affects trabecular bone apparent modulus through micro-architecture rather than matrix properties. J Orthop Res 22, 465–471 (2004). [DOI] [PubMed] [Google Scholar]
- Edelberg J. M., Tang L., Hattori K., Lyden D. & Rafii S. Young adult bone marrow-derived endothelial precursor cells restore aging-impaired cardiac angiogenic function. Circ Res 90, E89–93 (2002). [DOI] [PubMed] [Google Scholar]
- Selesniemi K., Lee H. J., Niikura T. & Tilly J. L. Young adult donor bone marrow infusions into female mice postpone age-related reproductive failure and improve offspring survival. Aging (Albany NY) 1, 49–57 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hida D., et al.. Intra-bone marrow bone marrow transplantation rejuvenates the B-cell lineage in aged mice. Immunol Cell Biol 88, 87–94 (2010). [DOI] [PubMed] [Google Scholar]
- Hall J. K., Banks G. B., Chamberlain J. S. & Olwin B. B. Prevention of muscle aging by myofiber-associated satellite cell transplantation. Sci Transl Med 2, 57ra83 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleimani M. & Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc 4, 102–106 (2009). [DOI] [PubMed] [Google Scholar]
- Tomita S., et al.. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation 100, II247–256 (1999). [DOI] [PubMed] [Google Scholar]
- Woodbury D., Schwarz E. J., Prockop D. J. & Black I. B. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res 61, 364–370 (2000). [DOI] [PubMed] [Google Scholar]
- Covas D. T., Siufi J. L., Silva A. R. & Orellana M. D. Isolation and culture of umbilical vein mesenchymal stem cells. Braz J Med Biol Res 36, 1179–1183 (2003). [DOI] [PubMed] [Google Scholar]
- Suva D., et al.. Non-hematopoietic human bone marrow contains long-lasting, pluripotential mesenchymal stem cells. J Cell Physiol 198, 110–118 (2004). [DOI] [PubMed] [Google Scholar]
- Awasthi S., et al.. RLIP76 is a major determinant of radiation sensitivity. Cancer Res 65, 6022–6028 (2005). [DOI] [PubMed] [Google Scholar]
- Miao D. & Scutt A. Histochemical localization of alkaline phosphatase activity in decalcified bone and cartilage. J Histochem Cytochem 50, 333–340 (2002). [DOI] [PubMed] [Google Scholar]