Abstract
When a sperm and an oocyte unite upon fertilization, their cell membranes adhere and fuse, but little is known about the factors regulating sperm-oocyte adhesion. Here we explored the role of β-catenin in sperm-oocyte adhesion. Biochemical analysis revealed that E-cadherin and β-catenin formed a complex in oocytes and also in sperm. Sperm-oocyte adhesion was impaired when β-catenin-deficient oocytes were inseminated with sperm. Furthermore, expression of β-catenin decreased from the sperm head and the site of an oocyte to which a sperm adheres after completion of sperm-oocyte adhesion. UBE1-41, an inhibitor of ubiquitin-activating enzyme 1, inhibited the degradation of β-catenin, and reduced the fusing ability of wild-type (but not β-catenin-deficient) oocytes. These results indicate that β-catenin is not only involved in membrane adhesion, but also in the transition to membrane fusion upon fertilization.
An oocyte fuses to only one sperm at fertilization, which results in the creation of a single cell with two nuclei that undergoes a series of complex processes (Supplementary Fig. S1a)1. After the sperm detaches the cumulus cells, the somatic cells surrounding oocytes, from the oocytes by enzymatic activities, the sperm adheres to the zona pellucida (ZP), the oocyte extracellular matrix. The sperm then penetrates the ZP and adheres to the oocyte cell membrane. At this time, fusion occurs between sperm and oocyte.
CD92 and Izumo13 belong to the tetraspan protein family (tetraspanin) and immunoglobulin superfamily, respectively, and play a crucial role in sperm-oocyte fusion3,4,5. Both CD9-deficient oocytes and Izumo1-deficient sperm are unable to fuse to their wild-type partner's cells, but retain adhesive activity3,4. A couple of these findings suggest that the molecular event underlying membrane adhesion is different from that underlying membrane fusion. The mechanism of membrane fusion has been explored by us6 and others7,8, but little is known about the factors regulating the adhesion of a sperm to an oocyte membrane.
Three proteins, β-catenin, α-catenin and E-cadherin, are a well-known functional set mediating intercellular junctions, which are called adherens junctions and typically served as a lateral connector between epithelial cells9. Besides epithelial cells, these three proteins are co-expressed in non-epithelial female germ cells, such as immature oocytes and fully-grown oocytes (to which only one sperm can adhere upon fertilization)10. Typically, β-catenin directly binds to the cytoplasmic domain of E-cadherin and connects to the adherens junctional complex with actin, a major component of microfilaments11. The β-catenin bound to E-cadherin is involved in intercellular adhesion, while E-cadherin-free β-catenin functions as a transcriptional factor driving the Wnt signaling pathway that regulates embryonic morphogenesis12.
In mouse oocytes, the presence of both β-catenin and E-cadherin has been reported10, but it remains unclear whether these two proteins are essential during the process of fertilization. In this study, we explored the possible role of β-catenin in sperm-oocyte adhesion, one of the important steps leading to mammalian fertilization.
Results
Subcellular localization of actin and its possible function in sperm-oocyte adhesion
To identify candidate proteins involved in sperm-oocyte membrane adhesion, we first examined immunocytochemically whether two cytoskeletal proteins, actin and tubulin, would exhibit cortical localization in ovulated oocytes. These two monoclonal antibodies (mAbs) are raised against the conserved domain of actin isoforms or β-tubulin. Confocal microscopic observation demonstrated that actin was asymmetrically localized in the oocyte: namely, metaphase II-arrested chromosomes enclosed by the actin were observed in one side of the oocyte cytoplasm (Supplementary Fig. S1b, c). In addition, actin was found to be concentrated on the cortical surface of the oocyte and appeared to exist as orderly arranged spots beneath the oocyte cell membrane (Supplementary Fig. S1d). Since tetraspanin CD9 is known to be present on the microvilli that regularly line the cell surface of an oocyte8, oocytes were subjected to double staining with CD9 and actin mAbs. Neither protein was co-localized, and the actin-rich cortical area was clearly separated from the CD9-rich area (Supplementary Fig. S1d, e), implying the presence of at least two types of membranous structures in the oocyte cell membrane, as suggested previously13. Since CD9 plays an important role in sperm-oocyte fusion, but not sperm-oocyte adhesion4, it was hypothesized that the actin-rich membranous structure on the cell surface of an oocyte may be involved in sperm-oocyte membrane ‘adhesion'.
E-cadherin/β-catenin complex formation in both oocytes and sperm
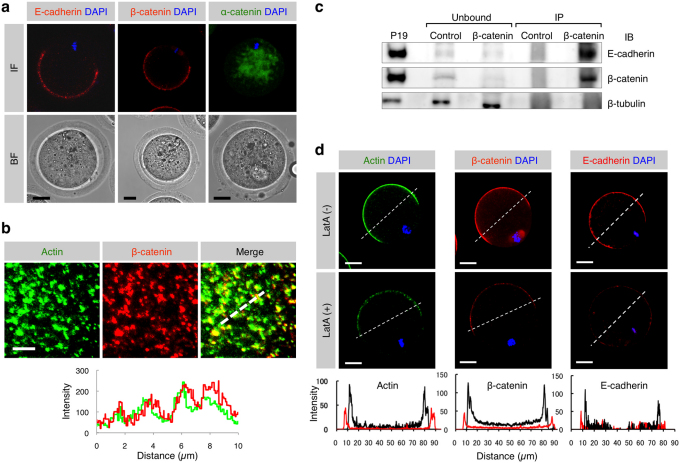
Since E-cadherin/β-catenin complex has been known to bind to actin, by which cell-cell membrane adhesion is regulated9, we considered that this E-cadherin/β-catenin complex may play a role as a regulator of sperm-oocyte membrane adhesion. To assess the problem, we first examined the possible interaction between E-cadherin/β-catenin complex and actin on an oocyte using immunocytochemical methods. In Fig. 1, subcellular localization of α- and β-catenins and E-cadherin is shown and data on the comparison between their localization and the localization of actin is also shown. Regarding the immunoreactivity of E-cadherin to an oocyte, a mAb that recognizes an N-terminal extracellular region of E-cadherin was used. Immunocytochemical staining demonstrated that E-cadherin was localized on the cell membrane (microvillar region) of an oocyte that has not been treated with permeabilization (Fig. 1a). β-catenin was detected beneath the oocyte cell membrane, and its localization pattern appeared to be similar to that of E-cadherin (Fig. 1a). In contrast, α-catenin was present in the oocyte cytoplasm (Fig. 1a). When the distribution of β-catenin on an oocyte was compared to that of actin, both proteins were found to be co-localized (Fig. 1b).
Figure 1. Expression of E-cadherin and β-catenin and localization of E-cadherin/β-catenin complex in oocytes.
(a) Localization of E-cadherin, β-catenin and α-catenin in ovulated oocytes. Similar distribution pattern of E-cadherin and β-catenin on an oocyte suggests complex formation between these two proteins. IF, immunofluorescence; BF, bright field. Scale bars: 20 µm. (b) Localization of β- and γ-actin isoforms and β-catenin beneath the oocyte cell membrane and their fluorescent intensities. The route scanned on the membrane was indicated as a dotted line. Fluorescence intensities for each protein were measured and graphed based on the 3D image, as described in the Experimental Procedures. Red and green lines in the lower panel indicate intensities of β-catenin and actin, respectively. Scale bar: 5 µm. (c) Biochemical evidence for the presence of E-cadherin/β-catenin complex in oocytes. The extract from 905 oocytes immunoprecipitated (IP) by anti-β-catenin mAb and mouse IgG purified from preimmune serum (Control) was subjected to immunoblotting with anti-E-cadherin, anti-β-catenin or anti-β-tubulin mAb. Extracts from mouse embryonic carcinoma cell line P1935 were also subjected to immunoprecipitation with anti-β-catenin mAb and the resulting immunoprecipitates were reacted with each mAb as a positive control. Note that the extract (IP) immunoprecipitated by anti-anti-β-catenin mAb was reactive with both anti-β-catenin and anti-E-cadherin mAbs, but the extract (Unbound) that was not immunoprecipitated by anti-β-catenin mAb failed to bind to both antibodies. On the other hand, the β-tubulin was detectable in the Ab-unbound (but not Ab-IP) fractions. (d) Disassembly of β-catenin, E-cadherin and actin induced by latrunculin A (latA) treatment. Oocytes were doubly immunostained with anti-β- and γ-actin isoforms mAbs and DAPI (shown as ‘Actin DAPI') or with anti-β-catenin mAb or with anti-E-cadherin mAb and DAPI (shown as ‘β-catenin DAPI' or ‘E-cadherin DAPI'). In the lower panels, the fluorescence intensities measured after being traced along dotted lines in the figures of the upper panels are shown. The intensities of latA-treated oocytes are indicated by red lines, while those of the latA-untreated oocytes are shown by black lines. Scale bar: 20 µm.
Secondly, we assessed the possible formation of β-catenin and E-cadherin complex using an immunoprecipitation method. A cell extract of mouse oocytes (n = 905) was immunoprecipitated with anti-E-cadherin mAb, and the resulting precipitate was then reacted with anti-β-catenin mAb. As a result, the cell extract immunoprecipitated with anti-E-cadherin mAb reacted with the anti-β-catenin mAb (Fig. 1c), indicating the presence of β-catenin and E-cadherin complex in an oocyte.
Thirdly, we assessed the effect of latrunculin A (latA), an inhibitor of actin polymerization, on the formation of β-catenin and E-cadherin complex. When oocytes were treated with 10 μM latA for 1 h at 37°C, actin immunoreactivity was reduced along with decreased immunoreactivity to β-catenin and E-cadherin (Fig. 1d). These results suggest that the β-catenin/E-cadherin complex formed in the oocyte cell membrane is closely associated with actin.
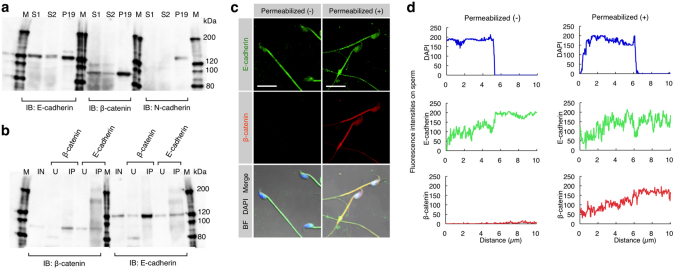
Since the β-catenin/E-cadherin complex is known to play a role in cell-cell adhesion via its homophilic interaction with E-cadherin14, we predicted that this protein complex would also be produced in sperm. To test this hypothesis, epididymal capacitated sperm were collected from 10-week-old males and subjected to Western blotting (Fig. 2a) and immunoprecipitation (Fig. 2b) analyses. Western blotting revealed that both E-cadherin and β-catenin were detected in the sperm collected (Fig. 2a); however, N-cadherin was not detectable in those samples (Fig. 2a), although its expression has been reported in mouse oocytes15. Immunoprecipitation analysis also revealed the presence of the E-cadherin/β-catenin complex in sperm. The sperm extracts immunoprecipitated with anti-β-catenin mAb were reactive with anti-E-cadherin mAb, and those immunoprecipitated with anti-E-cadherin mAb were reactive with anti-β-catenin mAb (Fig. 2b). To confirm this further, immunocytochemical staining was performed for the isolated sperm. Staining of unpermeabilized sperm with anti-E-cadherin mAb demonstrated that E-cadherin was broadly expressed on the cell membrane of sperm from the head region to the mid-piece as well as part of the tail (Fig. 2c, d). Staining of permeabilized sperm with anti-β-catenin mAb revealed that the expression of β-catenin was localized beneath the sperm cell membrane and, notably, its localization pattern was similar to that of E-cadherin (Fig. 2c, d).
Figure 2. Expression of E-cadherin and β-catenin and their interaction in sperm.
(a) Expression of E-cadherin and β-catenin in epididymal sperm. E-cadherin and β-catenin, but not N-cadherin, in the sperm were detected by immunoblotting (IB). Sperm (S1 and S2) were collected from the epididymis of two males and used for IB. Extracts from P19 cells were used as a positive control. IB was performed using anti-E-cadherin, anti-β-catenin or anti-N-cadherin mAbs. M, molecular weight markers. (b) Interaction between E-cadherin and β-catenin in sperm. Extracts from the sperm (used as input sample (IN)) were immunoprecipitated by anti-E-cadherin or anti-β-catenin mAb. The precipitates (IP) and unbound extracts (U) were immunoblotted (IB) with anti-E-cadherin or anti-β-catenin mAb. M, molecular weight markers. (c) Localization of E-cadherin and β-catenin in sperm. Unpermeabilized or permeabilized sperm were doubly immunostained with anti-E-cadherin (ECCD-2) and anti-β-catenin mAbs, and their nuclei were stained with DAPI. ECCD-2, which recognizes an epitope in the N-terminal extracellular region of E-cadherin, bound to E-cadherin without permeabilization pretreatment. Scale bar: 5 µm. (d) The fluorescence intensity profiles of E-cadherin and β-catenin in sperm shown in (c). Fluorescence intensities were measured after being traced on the sperm along dotted lines shown at the bottom of the panels in (c).
In mammals, both sperm-oocyte fusion and adhesion have been believed to occur in a specific region of the sperm head, called an equatorial segment (ES)1. Therefore, it is reasonable to consider that factor(s) regulating sperm-oocyte adhesion should exist in this segment. Since in the sperm head of the Asian musk shrew, Suncus murinus, ES is recessed within the waist of the sperm nucleus16, it is easy to detect proteins localized in this segment. When immunocytochemical staining of the permeabilized shrew capacitated sperm was performed using anti-E-cadherin mAb, E-cadherin was expressed on the ES, the mid-piece and part of the tail (Supplementary Fig. S2a). Staining with anti-β-catenin mAb revealed the expression of β-catenin specifically localized on the ES of the sperm head (Supplementary Fig. S2b). Notably, its localization pattern was similar to that of E-cadherin in the shrew sperm (Supplementary Fig. S2a vs. Fig. S2b) and also to that of E-cadherin in the mouse sperm (Fig. 2c, d). Furthermore, some β-catenin molecules were released from the acrosomes of the shrew sperm (Supplementary Fig. S2b). Since the ES is recessed in the acrosome of the shrew sperm16, β-catenin may have accumulated specifically in the ES upon completion of ES formation. These collected results led to a conclusion that mammalian epididymal sperm always form E-cadherin/β-catenin complex.
Generation of β-catenin-, α-catenin- and E-cadherin-deficient oocytes
To determine which genes are involved in sperm-oocyte adhesion among β-catenin, α-catenin and E-cadherin genes, three strains (E-cadherinfloxed/floxed 17, β-cateninfloxed/floxed 18, and α-cateninfloxed/floxed 19) with loxP-flanked genes were inter-crossed with the transgenic mouse strain (TgZP3-cre/+) expressing cre-recombinase in an oocyte-specific manner. Offspring (F2) lacking each type of gene (E-cadherinfloxed/floxedTgZP3-cre/+, β-cateninfloxed/floxedTgZP3-cre/+ and α-cateninfloxed/floxedTgZP3-cre/+) were successfully obtained according to the Menderian inheritance rule (see Methods; Supplementary Fig. S3a), and were all viable and normal in size without displaying any overt physical or behavioral abnormalities. When the number of ovulated oocytes from these superovulated offspring was counted and compared with that of oocytes from the control floxed mice, there were no clear differences in the number of ovulated oocytes between the two groups: 11.7 ± 1.4 (n = 25) for E-cadherinfloxed/floxedTgZP3-cre/+and 13.7 ± 1.9 (n = 22) for E-cadherinfloxed/floxed; 23.2 ± 1.5 (n = 21) for β-cateninfloxed/floxedTgZP3-cre/+ and 23.2 ± 1.5 (n = 19) for β-cateninfloxed/floxed; 15.6 ± 2.4 (n = 9) for α-cateninfloxed/floxedTgZP3-cre/+ and 12.4 ± 2.3 (n = 9) for α-cateninfloxed/floxed. Oocytes isolated from each line carrying the cre-recombinase gene were not morphologically distinguishable from those from each control floxed line (Supplementary Fig. S3b). To confirm whether oocytes from these gene-disrupted mice exhibit loss of target protein expression, oocytes were subjected to immunocytochemical staining together with oocytes from control floxed mice. E-cadherin, β-catenin and α-catenin were indeed absent from oocytes of E-cadherinfloxed/floxedTgZP3-cre/+, β-cateninfloxed/floxedTgZP3-cre/+ and α-cateninfloxed/floxedTgZP3-cre/+, respectively (Supplementary Fig. S3b). These results suggest that these three genes are not essential for the maturation and ovulation of mouse oocytes.
In epithelial cells, β-catenin is required for localization of E-cadherin on the cell surface, and endocytosis of E-cadherin into the cytoplasm occurs in the absence of β-catenin20. In addition, a model was proposed: α-catenin participates to bind to the E-cadherin/β-catenin complex to connect with actin microfilaments under certain specific conditions9. In analogy to this, it is possible that cellular localization of E-cadherin, β-catenin and α-catenin is mutually regulated in oocytes. Such a possibility is already depicted in Fig. 1a, in which E-cadherin was co-localized with β-catenin, but not with α-catenin on a wild-type oocyte. To examine whether the formation of E-cadherin/β-catenin complex (possibly E-cadherin/β-catenin/α-catenin complex) is impaired when either one of these composite proteins is deficient, oocytes collected from all of the gene-ablated strains were immunocytochemically assessed for localization of these three proteins (Supplementary Fig. S4a–c). Expression of E-cadherin was strongly reduced on the cell membrane of β-catenin-deficient oocytes, but not α-catenin-deficient oocytes (Supplementary Fig. S4a vs. Fig. S4b). On the other hand, loss of E-cadherin did not affect the localization pattern of β-catenin and α-catenin (Supplementary Fig. S4c). Similar results were also obtained when α-catenin-deficient oocytes were examined (Supplementary Fig. S4a). These results indicate that β-catenin regulates the membrane localization of E-cadherin in mouse oocytes.
Sperm-oocyte adhesion or fusion assay
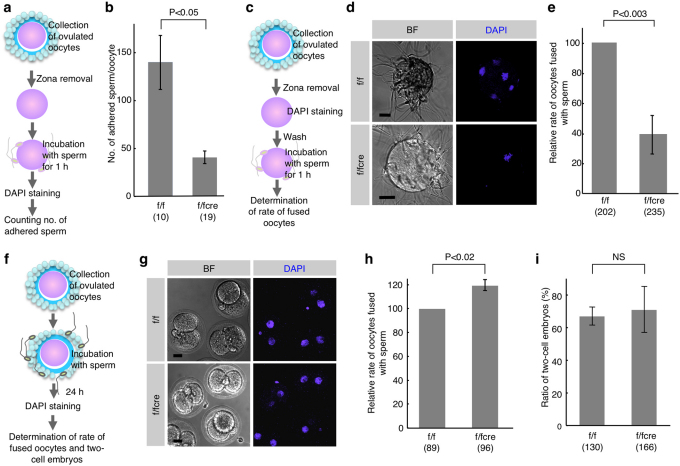
Membrane interaction between oocytes and sperm occurs after the penetration of sperm into ZP (Supplementary Fig. S1a). To monitor such interaction directly, ‘ZP-free' β-catenin-deficient oocytes after enzymatic digestion of ZP were inseminated with wild-type epididymal sperm (Fig. 3a, b for adhesion assay; Fig. 3c–e for fusion assay). ‘ZP-free' oocytes from β-cateninfloxed/floxed mice were used as a control. When the oocytes were inspected 1 h after insemination and stained with 4',6-diamidino-2-phenylindole (DAPI) after fixation, as depicted in Fig. 3a, the number of sperm adhered to ‘ZP-free' β-catenin-deficient oocytes was significantly reduced (Fig. 3b) compared to sperm bound to control oocytes. Similarly, when DAPI-preloaded oocytes were inspected 1 h after insemination, as depicted in Fig. 3c, the relative rate of ‘ZP-free' β-catenin-deficient oocytes fused with sperm was also significantly reduced (39.2 ± 12.7 vs. 100.0 for control oocytes; P<0.003; Fig. 3d, e). We next examined the expression pattern of CD9, an essential protein for fusion4, in β-catenin-deficient oocytes immunocytochemically and immunobiochemically to assess the ability of wild-type C57BL/6N sperm to fuse with their membrane. CD9 was expressed on the plasma membrane of ‘ZP-free' β-catenin-deficient oocytes at a level comparable to that of ‘ZP-free' control oocytes (Supplementary Fig. S5a). The total amount of CD9 quantified by immunoblotting in β-catenin-deficient oocytes was comparable to that of control oocytes (Supplementary Fig. S5b). These findings suggest that β-catenin is involved in sperm-oocyte adhesion.
Figure 3. In vitro fertilizing ability of β-catenin-deficient oocytes.
(a) Experimental flow for testing sperm-oocyte membrane ‘adhesion', and comparison of the number of wild-type sperm adhered to an ‘zona-free' oocyte between f/fcre and f/f oocytes. After ZP removal, ‘zona-free' oocytes were mixed with sperm for 1 h. (b) The number of sperm adhered to an oocyte was counted by DAPI-derived fluorescence in sperm heads on the surface of an oocyte. Parentheses indicate the number of oocytes examined. Values are the mean ± standard error (SE). (c) Experimental flow for testing sperm-oocyte membrane ‘fusion' and fused sperm (shown as DAPI-positive sperm) in ‘zona-free' β-catenin-deficient (f/fcre) and control (f/f) oocytes. After ZP removal, subsequent preincubation for 20 min in the presence of DAPI and washing, ‘zona-free' oocytes were mixed with the wild-type sperm for 1 h. (d) Comparison of oocytes fused with sperm between f/fcre and f/f oocytes. BF, bright field. Bars: 20 µm. (e) Comparison of the relative rate of oocytes fused with sperm between f/fcre and f/f oocytes. Only oocytes having at least one fused sperm were counted. The comparative values relative to the control (f/f oocytes; set to 100.0) were displayed as the relative rate of fused oocytes. Parentheses indicate the number of oocytes examined in triplicate experiments. Values are the mean ± SE. (f) Experimental flow for testing sperm-oocyte membrane interaction and fused sperm in two-cell embryos developing from ‘cumulus-intact' β-catenin-deficient (f/fcre) and control (f/f) oocytes. DAPI staining was performed to detect fused sperm on the developing two-cell embryos. (g) Comparison of oocytes fused with sperm between “cumulus-intact” f/fcre and f/f oocytes. Bars: 20 µm. (h) Comparison of the relative rate of oocytes fused with sperm between “cumulus-intact” f/fcre and f/f oocytes. Parentheses indicate the number of oocytes examined in triplicate experiments. Values are the mean ± SE. (i) Comparison of the ratio of oocytes developing to two-cell stage 24 h after fertilization between “cumulus-intact” f/fcre and f/f oocytes, according to the procedure described in (f). Parentheses indicate the total number of oocytes examined in triplicate experiments. NS, not significant. Values are the mean ± SE.
In vitro fertilizing ability of β-catenin-deficient oocytes
To know how fertilization is influenced by the dysfunction of sperm-oocyte adhesion, we determined the fertilization rate of β-catenin-deficient oocytes. The β-catenin-deficient oocytes surrounded by cumulus cells (herein referred to as ‘cumulus-intact' oocytes) were isolated from oviducts and directly subjected to IVF with wild-type sperm, as depicted in Fig. 3f. ‘Cumulus-intact' oocytes from β-cateninfloxed/floxed mice were used as a control. When the oocytes were inspected 24 h after insemination, the relative rate of β-catenin-deficient oocytes fused with sperm was not reduced (Fig. 3g). Quantitative analysis revealed that the rate of β-catenin-deficient oocytes fused with sperm was rather enhanced (119.6 ± 4.6 vs. 100.0 for control oocytes; P<0.02; Fig. 3h), in contrast with the results of the previous adhesion/fusion assay (Fig. 3a–e). This is probably due to the occasional presence of the oocytes fused to sperm, which failed to develop to the two-cell stage; however, the fact that certain embryos developed to the two-cell stage would not exclude the possibility of pathogenetic activation of oocytes. On the other hand, the IVF rate (which is evaluated by the development of fertilized oocytes to the two-cell stage) was comparable between the two groups (Fig. 3i).
We further confirmed the above point by counting litters obtained through mating between β-cateninfloxed/floxedTgZP3-cre/+ females and β-cateninfloxed/floxed males. The control β-cateninfloxed/floxed females were similarly mated. The litter size of β-cateninfloxed/floxedTgZP3-cre/+ females was 5.3 ± 0.4, which was comparable with that of control females (5.8 ± 0.4) (Supplementary Fig. S6). These results indicate that oocytes lacking β-catenin expression reduce the ability to adhere with sperm, but sustain the ability to fuse with sperm as well as the total reproductive ability needed for delivering pups.
Possible involvement of β-catenin in transition of membrane adhesion to fusion
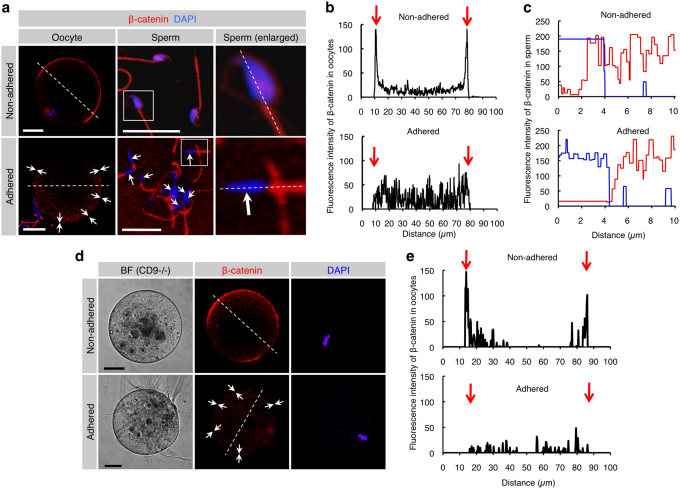
To examine the dynamics of β-catenin at sperm-oocyte membrane adhesion, alteration in the localization pattern of β-catenin at the sperm attachment sites of the in vitro fertilized “zona-free” oocyte was monitored (Fig. 4a–c). Before sperm attachment, β-catenin-rich patches (as shown in Fig. 1b) were clearly detected on the surface of an oocyte (upper left panel of Fig. 4a); however, these patches became undetectable 30 min after sperm attachment (arrows in the lower left panel; Fig. 4a). Furthermore, β-catenin was abundantly present in the capacitated sperm head (upper middle and right panels of Fig. 4a) before sperm attachment, but the amount of β-catenin in sperm heads was also greatly reduced after insemination (arrows in the lower middle and right panels; Fig. 4a). In addition, β-catenin was localized in the sperm head, although its localization pattern was slightly different in each sperm. Notably, β-catenin tended to be concentrated at ES (Fig. 2c; Supplementary Fig. S2; Fig. 4a).
Figure 4. Reduced levels of β-catenin localized beneath the cell membrane of both oocytes and sperm after membrane adhesion.
(a) β-catenin disassembly induced by membrane adhesion in oocytes and sperm. In the ‘Non-adhered' group (upper panels), ZP-denuded C57BL/6N oocytes were stained with DAPI and subsequently reacted with anti-β-catenin mAb. Also, epididymal sperm were stained with DAPI and anti-β-catenin mAb. In the ‘Adhered' group (lower panels), ZP-denuded C57BL/6N oocytes were stained with DAPI and then subjected to IVF for 30 min prior to incubation with anti-β-catenin mAb. Arrows indicate areas where fluorescent intensities of β-catenin are reduced. In each panel, boxes in middle sets of panels were enlarged and shown on the right. Scale bars: 20 and 10 µm in left and middle panels, respectively. (b) Fluorescence intensities of oocytes before and after sperm-oocyte adhesion. Fluorescence intensities were measured after being traced along dotted lines in the left panels of (a). Arrows indicate both sides of the oocyte cell membranes. (c) Fluorescence intensities of sperm before and after sperm-oocyte adhesion. Fluorescence intensities were measured after being traced along dotted lines in the right panels of a. Red and blue lines indicate fluorescent intensities for β-catenin and DAPI, respectively. (d) β-Catenin disassembly induced by membrane adhesion in oocytes and sperm. In the ‘Non-adhered' group (upper panels), ZP-denuded CD9-/- oocytes were stained with DAPI and subsequently reacted with anti-β-catenin mAb. In the ‘Adhered' group (lower panels), ZP-denuded CD9-/- oocytes were preloaded with DAPI as depicted in Fig. 3c and then subjected to IVF for 30 min prior to incubation with anti-β-catenin mAb. Arrows indicate areas where fluorescent intensities of β-catenin are reduced. In each panel, Scale bars: 20 µm. (e) Fluorescence intensities of CD9-/- oocytes before and after sperm-oocyte adhesion. Fluorescence intensities were measured after being traced along dotted lines in the left panels of d. Arrows indicate both sides of the oocyte cell membranes.
These findings could also be supported by measurement of fluorescent intensities of β-catenin (Fig. 4b, c). When fluorescence intensity at the equator of an oocyte (dotted line in the upper image of the left panels; Fig. 4a) was compared with that in the region of an oocyte adhered to sperm (dotted line in the lower image of the left panels; Fig. 4a), intense localization of β-catenin in the oocyte exhibiting no sperm attachment was observed beneath the oocyte membrane (arrows in the upper graph; Fig. 4b); however, 30 min after sperm attachment, the fluorescent intensity of β-catenin beneath the oocyte membrane was markedly reduced (arrows in the lower graph; Fig. 4b). Concomitantly, fluorescence intensity throughout the entire sperm (dotted lines in the right panels; Fig. 4a) was quantitatively compared before and after sperm adhesion to the oocyte membrane (Fig. 4c). Before attachment to the oocyte membrane, β-catenin was broadly localized in the sperm head (corresponding to the DAPI-stained region), mid-piece and part of the tail (Fig. 2c, d; upper graph of Fig. 4c); however, after attachment to the oocyte membrane, the intensity of β-catenin in the sperm head (but not in a mid-piece and tail) was markedly decreased (arrows in the lower middle and right panels of Fig. 4a; lower graph of Fig. 4c). Moreover, to confirm that the amount of β-catenin is reduced at steps between sperm-oocyte adhesion and fusion, localization pattern of β-catenin at the sperm attachment sites of the in vitro fertilized “zona-free” CD9-deficient oocyte was monitored (Fig. 4d, e). Before sperm attachment, intense localization of β-catenin (as shown in the upper left panel of Fig. 4a) was clearly seen on the surface of an oocyte (upper middle panel of Fig. 4d); however, these patches became undetectable 30 min after sperm attachment (arrows in the lower middle panel; Fig. 4d). These findings were also confirmed by measurement of fluorescent intensities of β-catenin (Fig. 4e). When fluorescence intensity at the equator of an oocyte (dotted line in the upper image of the middle panel; Fig. 4d) was compared with that in the region of an oocyte adhered to sperm (dotted line in the lower image of the middle panel; Fig. 4d), intense localization of β-catenin in the oocyte exhibiting no sperm attachment was observed beneath the oocyte membrane (arrows in the upper graph; Fig. 4e); however, 30 min after sperm attachment, the fluorescent intensity of β-catenin beneath the oocyte membrane was markedly reduced (arrows in the lower graph; Fig. 4e). These collective data imply that alteration in the localization of β-catenin in both sperm and oocyte membranes may contribute to the transition of cell adhesion to fusion.
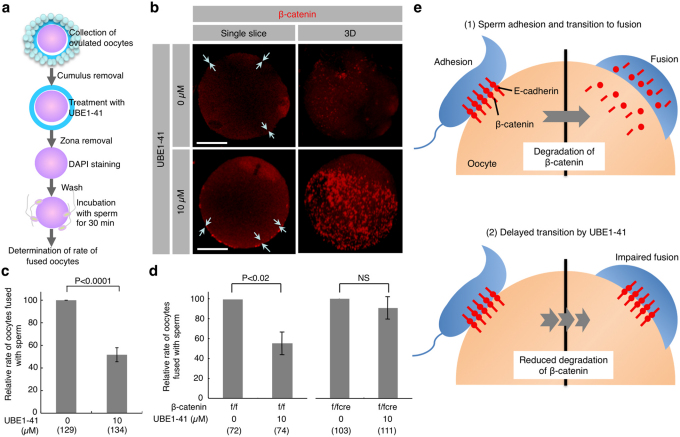
Generally, in the absence of Wnt signal, E-cadherin-free cytoplasmic β-catenin is rapidly degraded due to “ubiquitination”, while only membrane-anchored β-catenin, which is associated with E-cadherin, is resistant to such degradation21. Since the fluorescence intensity of β-catenin beneath cell membranes was greatly reduced in both sperm and oocytes after membrane adhesion, ubiquitination may be involved in such reduction. In other words, degradation of β-catenin may be involved in the transition from adhesion to fusion upon sperm-oocyte interaction. To test this possibility, we employed an inhibitor of the ubiquitination pathway to investigate whether it can disturb sperm-oocyte fusion. UBE1-41, a specific inhibitor of ubiquitin-activating enzyme 1 (UBE1), is known to impair antigen-induced FcεRI ubiquitination and internalization22 and to inhibit melanocortin-4 receptor internalization via ubiquitination23. To determine the optimal concentration of UBE1-41, we first assessed sperm-oocyte interaction by co-incubation of sperm and oocytes in TYH medium containing various amounts of UBE1-41 (0, 1, 5, 10, 20 or 50 µM). We finally decided to use 10 µM UBE1-41, because treatment with more than 20 µM UBE1-41 caused deleterious effects on oocytes. Notably, this concentration (10 µM) appears to be lower than that (50 µM) reported previously22. “Zona-free” oocytes treated with 10 μM UBE1-41 for 30 min were incubated with sperm and the ratio of fused oocytes was measured (Fig. 5a). As depicted in Fig. 4a, c, the fluorescence intensity of β-catenin beneath cell membranes was greatly reduced in the untreated oocyte after membrane adhesion with sperm (arrows in the upper left panel; Fig. 5b); however, intense localization of β-catenin was observed beneath the oocyte membrane in the oocyte treated with UBE1-41 even after membrane adhesion with sperm (arrows in the lower left panel; Fig. 5b), and β-catenin-rich patches (oocyte before membrane adhesion; Fig. 1b) were clearly detected on the surface of the oocyte (lower right panel; Fig. 5b). The rate of oocyte fusion with sperm was inversely correlated with the intense localization of β-catenin beneath the cell membrane: treatment with UBE1-41 lowered the rate of fused oocytes in contrast with that of untreated oocytes (51.7 ± 6.2 for UBE1-41-treated oocytes vs. 100.0 for untreated oocytes; P<0.0001; Fig. 5c; Supplementary Fig. S7). This result suggests that β-catenin ubiquitination leading to degradation is involved in transition from membrane adhesion to fusion. Given this background, it seems likely that the loss of β-catenin facilitates sperm-oocyte fusion. In other words, the fusing ability of β-catenin-deficient oocytes should be unaffected by treatment with UBE1-41. In fact, β-catenin-deficient oocytes exhibited fusing ability, although they were unable to adhere to sperm (Fig. 3). Furthermore, treatment of β-cateninfloxed/floxedTgZP3-cre/+ oocytes with UBE1-41 did not affect the rate of fused oocytes (90.6 ± 11.3 for UBE1-4-treated oocytes vs. 100.0 for untreated oocytes; Fig. 5d). By contrast, similar treatment of β-cateninfloxed/floxed oocytes resulted in reduction of their fusing ability, as expected (55.3 ± 11.5 for UBE1-4-treated oocytes vs. 100.0 for untreated oocytes; P<0.02; Fig. 5d). These results led us to consider that β-catenin may be accumulated around sperm attachment sites through the formation of a protein complex with E-cadherin, but immediately diminished when sperm-oocyte fusion occurred. This transient formation of such complex encouraged us to suppose that the E-cadherin/β-catenin complex plays an important role in the transition from membrane adhesion to membrane fusion during sperm-oocyte interaction (Fig. 5e).
Figure 5. Reduced fusing ability of oocytes treated with UBE1-41, an inhibitor of ubiquitination.
(a) Experimental flow. Cumulus cells attached to the oocytes collected from oviducts were removed. The oocytes were then treated with UBE1-41 for 30 min, followed by ZP removal. After 20-min incubation with DAPI-containing medium and subsequent washing, ‘zona-free' oocytes were mixed with the sperm and incubated for 30 min. Only oocytes having at least one fused sperm were counted as fused oocytes. (b) Sustained expression of β-catenin on the surface of “zona-free” wild-type oocytes treated with UBE1-41 after membrane adhesion with sperm. Arrows indicate oocyte cell membranes where β-catenin deposition is noted. One sperm fused with the untreated oocyte (upper panel), but not with the UBE1-41-treated oocyte (lower panel). To focus on the β-catenin expression, only fluorescent images are shown. Single slice, Image captured when the diameter of an oocyte was longest; 3D, 3-dimensional image reconstructed from serial scanned images. Bars: 20 µm. (c) Decreased number of fused sperm on “zona-free” wild-type oocytes after treatment with UBE1-41. The relative rate of oocytes carrying fused sperm was compared between UBE1-41-treated and -untreated wild-type oocytes. The comparative values relative to the control (set to 100.0) are displayed as the relative rate of fused oocytes. As described in the legend of Fig. 3c, oocytes fused with sperm are defined as those with at least one DAPI-positive sperm. (d) Comparison of the relative rate of oocytes carrying fused sperm between UBE1-41-treated and untreated oocytes (β-catenin-deficient (f/fcre) vs. β-catenin-intact (f/f) oocytes). Comparison was made as described in (c). NS, not significant. Parentheses indicate the total number of oocytes examined in triplicate experiments. Values are the mean ± SE. (e) A model of the possible role of β-catenin in transition from membrane adhesion to fusion. At fertilization (as described in (1)), adhesion of sperm to the surface of an oocyte is mediated by E-cadherin/β-catenin complex; however, subsequent fusion requires rapid degradation of β-catenin. On the other hand, in the presence of UBE1-41 (as described in (2)), adhesion occurs normally, but fusion is impaired since UBE1-41 inhibits degradation of β-catenin.
Discussion
Membrane fusion occurs after membrane adhesion. Such a process is also true for sperm-oocyte interaction1. Our present results indicate that both E-cadherin and β-catenin are involved in sperm-oocyte membrane adhesion. This is demonstrated by the fact that β-catenin-deficient oocytes can fuse to sperm, and develop normally to term.
E-cadherin is involved in homophilic adhesion between epithelial cells, and transduces external signaling via α-catenin and β-catenin9. In contrast, E-cadherin remains essential for basic cell-cell adhesion, even in the absence of α-catenin, in human prostate carcinoma PC3 cells24, suggesting that the formation of E-cadherin/β-catenin/actin microfilament complex is proceeded by other molecule(s) in the absence of α-catenin. In analogy, at sperm-oocyte adhesion, association between the E-cadherin/β-catenin complex and actin microfilaments may be mediated by molecule(s) other than α-catenin or the E-cadherin/β-catenin complex itself can directly bind to actin microfilaments, although evidence for this remains to be provided.
Rapid reduction in the level of β-catenin occurs after increased ubiquitination and degradation through a proteosomal pathway21. N-Acetyl-Leu-Leu-Nle-CHO (ALLN), a specific inhibitor of the proteolytic activity of proteasomes is reported to inhibit sperm-oocyte fusion upon fertilization25, implying that the ubiquitination-proteasome pathway may play a role in sperm-oocyte interaction by regulating the quantity of β-catenin on sperm and oocyte membranes. Giving that β-catenin is present on the sperm head and oocyte surface, and that its elimination impairs sperm-oocyte adhesion (see Fig. 3b), it is conceivable that the ubiquitination-proteasome pathway is a key that mediates phase transition from membrane adhesion to fusion, as genetic studies in C. elegans have identified multiple roles for the ubiquitin system in early development26.
Taken together, our results propose a model regarding the transition from membrane adhesion to fusion upon sperm-oocyte interaction (Fig. 5e). Before sperm-oocyte adhesion, both sperm and oocyte retain the β-catenin/E-cadherin complex, a complex important for sperm-oocyte adhesion. Once sperm-oocyte adhesion occurs, β-catenin is immediately ubiquitinated and probably degraded in both the sperm and oocyte, thereby initiating membrane fusion between these two cells; however, in oocytes treated with UBE1-41, ubiquitination of β-catenin associated with sperm-oocyte adhesion is suppressed, which will cause impaired fusion between sperm and oocyte. This sperm-oocyte adhesion and subsequent fusion appears to be each independent phenomena, since the absence of β-catenin results in a reduction in the ability of sperm to adhere to an oocyte, but sperm-oocyte membrane fusion occurs normally.
Similarly, importance of interchange between stabilization and degradation of β-catenin has been described at several developmental aspects, such as mesenchymal cell proliferation27 and primordial germ cell development28. Forced expression of a mutated β-catenin that is resistant to degradation causes developmental arrest at specific sites and time27,28. However, its deficiency has no impact on embryogenesis, probably due to compensation of other molecules that play roles similar to β-catenin27,28. Probably, degradation of β-catenin that occurs at appropriate stage and place is needed for normal development of an embryo/fetus and therefore β-catenin may be an important molecule that mediates as a molecular switch in embryogenesis. Our present results showed that although the cell membrane of β-catenin-deficient oocytes exhibit reduced ability to adhere sperm (Fig. 3b), these oocytes could be successfully fertilized, indicating that β-catenin contributes partly to sperm-oocyte membrane adhesion, but does not play an essential role in this event. It will be claimed that ZP removal by acidic Tyrode's solution can change cell surface protein composition or carbohydrate structure, which may affect sperm-oocyte interaction to some extents. Our present data, however, clearly suggest that β-catenin degradation is associated with transition from adhesion to fusion upon interaction between sperm and oocyte.
In human trophoblastic cells, an interrelationship between cell differentiation/ fusion and reduced expression of E-cadherin has been pointed out29. When the isolated mononuclear cytotrophoblasts are cultured, they tend to aggregate and then fuse to form syncytia. During this process, E-cadherin is detectable at the cell-cell contact sites of an aggregate. However, the fusing cytotrophoblasts (but not non-fusing cytotrophoblasts) exhibit marked reduction in the level of E-cadherin. Notably, exposure of the non-fusing cells to 8-bromo cyclic AMP causes reduced expression of E-cadherin, and induces their cellular fusion and syncytium formation. These results suggest that down-regulation of E-cadherin gene expression coincides with cell fusion, and evoke us to suppose that remodeling of the adhesion complex on the cell surface would induce subsequent cell fusion. Beside E-cadherin, junctional proteins found in tight and adherens junctions such as integral membrane, adaptor, regulator and signaling proteins are recently thought to be important as epithelial and endothelial barriers30. They can reversibly increase paracellular transport and drug delivery with less toxicity, indicating that alteration in lipid composition at cell surface membrane, as exemplified by alteration of cholesterol efflux, results in modulation of cellular junctions. Based on these results, we consider that remodeling or degradation of adhesion complex may change lipid composition in a cell membrane, which will then provide microenvironments where cell fusion occurs.
When the fusion step is genetically defective, sperm never fuses with the partner oocyte, as previously shown by using CD9-deficient oocytes and Izumo1-deficient sperm3,5,6. Furthermore, we showed that the presence or absence of β-catenin in oocytes does not affect the expression and localization of CD9 (see Supplementary Fig. S5). In addition, we also observed that the presence or absence of CD9 does not affect the change of β-catenin localization in oocytes before and after adhesion to sperm (see Fig. 4d, e). A couple of these results suggest that β-catenin is independent from CD9 tetraspanin network. These findings evoked us to suppose that the adhesion step may be distinguishable from the fusion step in mammalian fertilization. Interestingly, Jégou et al. (2011) recently demonstrated that CD9 is indeed involved in the sperm-egg binding step. This suggests a possible role of CD9 in sperm-oocyte adhesion, but does not exclude the previous finding that CD9 is involved in fusion31. Although further investigation on the role of CD9 molecule in sperm-oocyte adhesion and subsequent fusion is needed, it seems likely at present that CD9 may be involved in maintaining the strength of adhesion force on the oocyte cell membrane. Its absence would cause unstable adhesion force, resulting in decreased fertilizing ability of sperm.
In conclusion, we have shown that 1) β-catenin plays a role in sperm-oocyte membrane adhesion upon fertilization; and 2) β-catenin is also involved in the transition of membrane adhesion to fusion, a phenomenon essential for fertilization, and proteasome-mediated regulation of β-catenin is important in such sperm-oocyte fusion.
Methods
Antibodies
Two mAbs against mouse E-cadherin used for immunostaining and immunoblotting (No. ECCD-2; Takara-Bio) and immunoprecipitation (No. 36; BD). Two mAbs against mouse β-catenin were used for immunoprecipitation (No. C2206; Sigma) and immunostaining (No. 15B8; Sigma). A mAb against β-catenin (No. 14; BD) was used for immunoblotting. A polyclonal antibody against mouse α-catenin (No. C2081; Sigma), Cy3-conjugated mAb against C-terminal peptide conserved in β- and γ-actin isoforms (No. AC-40; Sigma), and FITC-conjugated mAb against β-tubulin (No. TUB2.1; Sigma) were used. A mAb against N-cadherin was used for immunoblotting (No. 32; BD). ECCD-2, which recognizes an epitope in the extracellular region of E-cadherin, bind to E-cadherin on the cell membrane without permeabilization32.
Immunostaining
Mouse oocytes were collected from oviducts of 8- to 12-week-old C57BL/6N superovulated mice (Japan SLC Inc.). The oocytes were fixed for 20 min at room temperature in a solution (termed PFA-GLA-PVP) containing 2% paraformaldehyde (PFA), 0.1% glutaraldehyde (GLA) and 0.1% polyvinylpyrolidone (PVP). After washing in phosphate-buffered saline (PBS), they were permeabilized with 1% Triton X-100 in PBS, and washed 3 times in PBS. The oocytes were then incubated with the primary antibodies (Abs) (2.5 µg/ml) in HEPES-buffered saline (HBS) containing 10 mM HEPES (pH 8.0), 0.15 M NaCl and 3% fetal bovine serum (FBS) for 2 h at 4°C. These oocytes were next treated with the secondary Abs (1.25 µg/ml), Alexa488- or Alexa546-conjugated IgG (Molecular Probes), and washed 3 times in HBS. Mouse sperm were also isolated from the cauda epididymides of 8- to 12-week-old C57BL/6N male mice by teasing them in TYH medium33, and immunostained as described above. In addition, sperm were collected from 24-week-old KAT strain16 males of the Suncus murinus, kindly provided by Dr. Senichi Oda and immunostained as described above. These immunostained sperm were then counterstained with DAPI (WAKO) at the final concentration of 10 µg/ml in HBS for 30 min at 4°C, and washed 3 times by transfer to HBS.
Sectioned fluorescent images were captured by a confocal microscope (LSM 510 model; Carl Zeiss), and transformed into three-dimensional (3D) images by LSM Image Brower Version 4.2.0.121. The fluorescence intensities of target proteins were then measured based on the 3D images (Supplementary Fig. S1d, e; Fig. 1b), and compared between latA-untreated and -treated oocytes (Fig. 1d), unpermeabilized and permeabilized sperm (Fig. 2c, d), and oocytes and sperm before and after membrane adhesion (Fig. 4a–c). All animal experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee at the National Institute for Child Health and Development.
Immunoblotting and immunoprecipitation
The sperm suspension (ca. 3.0 × 106 cells) prepared from C57BL/6N males was collected by centrifugation, as described by Inoue et al.3 A total of 200 oocytes were collected and then lysed in Laemmli's SDS sample buffer, boiled, resolved in SDS-PAGE on an 8% acrylamide gel, and immunoblotted as described previously34. Mouse embryonic carcinoma P19 cells35 were were used as a positive control. For immunoprecipitation, Sakakibara et al.34 describe this procedure in more detail citing references36. Proteins from sperm (ca. 3.0 × 106 cells) (Fig. 2b) or 905 oocytes (Fig. 1c) were immunoprecipitated with Abs (2.5 µg/ml) for 6 h at 4°C. The presence of β-tubulin was detected and used as an internal loading control.
Actin disassembly induced by latA treatment
Mouse oocytes collected from oviducts of superovulated mice were incubated in TYH medium containing 10 μM latA (Molecular Probes) for 1 h at 37°C, and fixed in PFA-GLA-PVP solution. After permeabilization with 1% Triton X-100, oocytes were doubly immunostained by mAbs against actin and β-catenin as mentioned in the ‘Immunostaining' section. The fluorescence intensities at the equator, as indicated by dotted lines, were then analyzed as described in the ‘Immunostaining' section.
Generation of mice with gene-ablated oocytes
To produce oocytes with a single gene deleted, floxed mutant mice for E-cadherin17, β-catenin18 or α-catenin gene19 were cross-mated with transgenic (Tg) mice expressing cre-recombinase in an oocyte-specific manner under the control of oocyte-specific ZP protein 3 (ZP3) promoter (TgZP3-cre/+), kindly provided by Dr. Barbara B. Knowles37. The F1 offspring, so-called E-cadherinfloxed/floxedTgZP3-cre/+, β-cateninfloxed/floxedTgZP3-cre/+ and α-cateninfloxed/floxedTgZP3-cre/+, were propagated through brother-sister mating. The presence of the cre-recombinase gene in these offspring was detected by PCR analysis using the following set of primers: Cre-S (5'-TGATGAGGTTCGCAAGAACC-3'; nucleotide no. 170 to 189 (GenBank Accession no. AB449974.1)) and Cre-A (5'-CCATGAGTGAACGAACCTGG-3'; nucleotide no. 539 to 558 (GenBank Accession no. AB449974.1)); this primer set yielded a band of 389 bp.
Determination of litter size and in vitro fertilization (IVF)
To determine the litter size, the number of pups delivered from an 8- to 12-week-old female (offspring of β-catenin floxed/floxedTgZP3-cre/+ mice) was recorded after mating for two months by placing an 8- to 12-week-old C57BL/6N male in the cage.
For IVF, oocytes were collected from the oviductal ampulla region of superovulated β-catenin floxed/floxedTgZP3-cre/+ females (8 to 12 weeks old) 14 to 16 h after hCG injection, and placed in a 30-µl drop of TYH medium covered with paraffin oil (Nacalai) equilibrated with 5% CO2 in air at 37°C. Sperm collected from the epididymides of 8- to 12-week-old C57BL/6N males were induced to capacitate by incubating in TYH medium for 90 min in an atmosphere of 5% CO2 in air at 37°C before insemination. The final concentration of sperm added to the oocytes was 1.5 × 105 sperm/ml. The oocytes collected from floxed/floxed mice were also inseminated with C57BL/6N sperm as a control.
To count the number of sperm fused to an oocyte, cumulus cells were dispersed from oocytes by incubating them for 10 min at 37°C in TYH medium containing hyaluronidase (300 µg/ml; Merk4Biosciences), and then the oocytes were denuded of the ZP by brief incubation in acid Tyrode's solution (Sigma). The ‘zona-free' oocytes were preincubated with DAPI at the final concentration of 10 µg/ml in TYH medium for 20 min at 37°C, and washed 3 times by being transferred to separate drops of TYH medium. DAPI is a fluorescent dye that can slowly permeate the living cell membrane (semi-permeable) and hardly leaks out of cells after washing, relative to Hoechst33342 (permeable), as shown in Invitrogen's instructions. This preincubation procedure with DAPI enables the staining of only fused sperm nuclei, probably through a mechanism in which the dye present within an oocyte is transferred to fused sperm upon membranous fusion. C57BL/6N sperm (ca. 1.5 × 105 sperm/ml) were added to a 30-µl drop of TYH medium containing 30 DAPI-treated ‘zona-free' oocytes and then the dish was incubated for 1 h at 37°C. After incubation, the oocytes were fixed with PFA-GLA-PVP solution for 20 min at room temperature. The rate of oocytes fused with sperm was determined by counting DAPI-transferred sperm on an oocyte under a fluorescence microscope. In this case, oocytes fused with sperm were defined as those with at least one DAPI-positive sperm. Moreover, in a separate group in which ‘zona-intact' oocytes were incubated with sperm for 24 h at 37°C and then stained by DAPI, the rate of those oocytes to develop to the two-cell stage was determined under a stereoscopic microscope without fixation.
To count the number of sperm adhered to an oocyte, cumulus cells were dispersed from oocytes in TYH medium containing hyaluronidase (Merk4Biosciences), and the oocytes were denuded of the ZP by incubation in acid Tyrode's solution (Sigma). The C57BL/6N sperm (ca. 1.5 × 105 sperm/ml) were added to a 30-µl drop of TYH medium containing 30 ‘zona-free' oocytes and then the dish was incubated for 1 h at 37°C. After incubation, the oocytes were fixed with a PFA-GLA-PVP solution and stained with DAPI. The number of sperm adhered to an oocyte was determined by counting DAPI-positive sperm on an oocyte.
Membrane localization of β-catenin before and after membrane adhesion
To observe the localization of β-catenin before and after membrane adhesion, oocytes were collected as mentioned in the ‘Determination of litter size and IVF' section. After ZP removal, ‘zona-free' oocytes were incubated with C57BL/6N epididymal sperm (ca. 1.5 × 105 sperm/ml) in a 30-µl drop of TYH medium for 30 min at 37°C. The oocytes adhered to sperm, oocytes before sperm adhesion, and epididymal sperm were fixed by placing them in PFA-GLA-PVP solution, washed, and immunostained with a mAb against β-catenin and DAPI, as described in the ‘Immunostaining' section.
IVF of oocytes treated with UBE1-41
To study the effect of UBE1-41 on sperm-oocyte fusion, oocytes were collected as mentioned in the ‘Determination of litter size and IVF' section, incubated in a 30-µl drop of TYH medium containing UBE1-41 (Biogenova) and DAPI for 1 h at 37°C, and washed with TYH medium. After ZP removal, ‘zona-free' oocytes (30 oocytes) were incubated with C57BL/6N epididymal sperm (ca. 1.5 × 105 sperm/ml) in a 30-µl drop of TYH medium for 30 min at 37°C. These oocytes were then fixed by placing them in PFA-GLA-PVP solution, washed, and immunostained with a mAb against β-catenin, as described in the ‘Immunostaining' section. The ‘zona-free' oocytes were similarly treated in a medium without UBE1-41 and used as the control. The relative rate of oocytes fused with sperm was compared between UBE1-41-treated and untreated oocytes.
Author Contributions
KM conceived and designed the experiments. YT, KY, MS, AN, NK, KS, TK, YH, NO, SK, and MM performed the experiments. HS, YT, HA, and AU analyzed the data. KM wrote the main manuscript text and prepared figures. All authors reviewed the manuscript.
Supplementary Material
Movie 1
Movie 2
Supplementary Information
Acknowledgments
We thank W.N. de Vries and B.B. Knowles for the Zp3-Cre transgenic mice. This work was supported by a grant from The Ministry of Health, Labor and Welfare, and grant-in-aid for Scientific Research, The Ministry of Education, Culture, Sports, and Technology of Japan.
References
- Ikawa M., Inoue N., Benham A. M., and Okabe M. Fertilization: a sperm's journey to and interaction with the oocyte. J Clin Invest 120, 984–994 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitamura T. et al.. The 27-kD diphtheria toxin receptor-associated protein (DRAP27) from vero cells is the monkey homologue of human CD9 antigen: expression of DRAP27 elevates the number of diphtheria toxin receptors on toxin-sensitive cells. J Cell Biol 118, 1389–1399 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue N., Ikawa M., Isotani A., and Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature 434, 234–238 (2005). [DOI] [PubMed] [Google Scholar]
- Miyado K. et al.. Requirement of CD9 on the egg plasma membrane for fertilization. Science 287, 321–324 (2000). [DOI] [PubMed] [Google Scholar]
- Le Naour F. et al.., Severely reduced female fertility in CD9-deficient mice. Science 287, 319–321 (2000). [DOI] [PubMed] [Google Scholar]
- Miyado K. et al.. The fusing ability of sperm is bestowed by CD9-containing vesicles released from eggs in mice. Proc Natl Acad Sci USA 105, 12921–12926 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraud-Lange V. et al.. Transfer of oocyte membrane fragments to fertilizing spermatozoa. FASEB J 21, 3446–3449 (2007). [DOI] [PubMed] [Google Scholar]
- Runge K. E. et al.. Oocyte CD9 is enriched on the microvillar membrane and required for normal microvillar shape and distribution. Dev Biol 304, 317–325 (2007). [DOI] [PubMed] [Google Scholar]
- Yamada S. et al.. Deconstructing the cadherin-catenin-actin complex. Cell 123, 889–901 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries W. N. et al.. Maternal beta-catenin and E-cadherin in mouse development. Development 131, 4435–4445 (2004). [DOI] [PubMed] [Google Scholar]
- Nagafuchi A., Ishihara S., and Tsukita S. The roles of catenins in the cadherin-mediated cell adhesion: functional analysis of E-cadherin-alpha catenin fusion molecules. J Cell Biol 127, 235–245 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J. et al.. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 382, 638–642 (1996). [DOI] [PubMed] [Google Scholar]
- Fulka J. Jr, Flechon B., and Flechon J. E. Fusion of mammalian oocytes: SEM observations of surface changes. Reprod Nutr Dev 29, 551–557 (1989). [DOI] [PubMed] [Google Scholar]
- Takeichi M. The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development 102, 639–655 (1988). [DOI] [PubMed] [Google Scholar]
- Ziv S., Rufas O., and Shalgi R. Cadherins expression during gamete maturation and fertilization in the rat. Mol Reprod Dev 62, 547–556 (2002). [DOI] [PubMed] [Google Scholar]
- Bedford J. M., Mori T., and Oda S. The unusual state of the cumulus oophorus and of sperm behaviour within it, in the musk shrew, Suncus murinus. J Reprod Fertil 110, 127–134 (1997). [DOI] [PubMed] [Google Scholar]
- Boussadia O. et al.. E-cadherin is a survival factor for the lactating mouse mammary gland. Mech Dev 115, 53–62 (2002). [DOI] [PubMed] [Google Scholar]
- Brault V. et al.. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128, 1253–1264 (2001). [DOI] [PubMed] [Google Scholar]
- Vasioukhin V. et al.. Hyperproliferation and defects in epithelial polarity upon conditional ablation of alpha-catenin in skin. Cell 104, 605–617 (2001). [DOI] [PubMed] [Google Scholar]
- Le T. L., Yap A. S., and Stow J. L. Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics. J Cell Biol 146, 219–232 (1999). [PMC free article] [PubMed] [Google Scholar]
- Aberle H. et al.. Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J 16, 3797–3804 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molfetta R. et al.. Lipid raft-dependent FcepsilonRI ubiquitination regulates receptor endocytosis through the action of ubiquitin binding adaptors. PLoS One 4, e5604 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granell S., Mohammad S., Ramanagoudr-Bhojappa R., and Baldini G. Obesity-linked variants of melanocortin-4 receptor are misfolded in the endoplasmic reticulum and can be rescued to the cell surface by a chemical chaperone. Mol Endocrinol 24, 1805–1821 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel J. M. and Reynolds A. B. The tyrosine kinase substrate p120cas binds directly to E-cadherin but not to the adenomatous polyposis coli protein or alpha-catenin.. Mol Cell Biol 15, 4819–4824 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brind S., Swann K., and Carroll J. Inositol 1,4,5-trisphosphate receptors are downregulated in mouse oocytes in response to sperm or adenophostin A but not to increases in intracellular Ca(2+) or egg activation.. Dev Biol 223, 251–265 (2000). [DOI] [PubMed] [Google Scholar]
- Bowerman B. and Kurz T. Degrade to create: developmental requirements for ubiquitin-mediated proteolysis during early C. elegans embryogenesis. Development 133, 773–784 (2006). [DOI] [PubMed] [Google Scholar]
- Cheon S. S. et al.. beta-Catenin stabilization dysregulates mesenchymal cell proliferation, motility, and invasiveness and causes aggressive fibromatosis and hyperplastic cutaneous wounds. Proc Natl Acad Sci U S A 99, 6973–6978 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T. et al.. The stabilization of beta-catenin leads to impaired primordial germ cell development via aberrant cell cycle progression. Dev Biol 300, 545–553 (2006). [DOI] [PubMed] [Google Scholar]
- Coutifaris C. et al.. E-cadherin expression during the differentiation of human trophoblasts. Development 113, 767–777 (1991). [DOI] [PubMed] [Google Scholar]
- Deli M. A. Potential use of tight junction modulators to reversibly open membranous barriers and improve drug delivery.. Biochim Biophys Acta 1788, 892–910 (2009). [DOI] [PubMed] [Google Scholar]
- Jegou A. et al.. CD9 tetraspanin generates fusion competent sites on the egg membrane for mammalian fertilization. Proc Natl Acad Sci U S A 108, 10946–10951 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamallo C. et al.. Correlation of E-cadherin expression with differentiation grade and histological type in breast carcinoma. Am J Pathol 142, 987–993 (1993). [PMC free article] [PubMed] [Google Scholar]
- Choi Y. H. and Toyoda Y. Cyclodextrin removes cholesterol from mouse sperm and induces capacitation in a protein-free medium. Biol Reprod 59, 1328–1333 (1998). [DOI] [PubMed] [Google Scholar]
- Sakakibara K. et al.. Molecular identification and characterization of Xenopus egg uroplakin III, an egg raft-associated transmembrane protein that is tyrosine-phosphorylated upon fertilization. J Biol Chem 280, 15029–15037 (2005). [DOI] [PubMed] [Google Scholar]
- Jones-Villeneuve E. M., McBurney M. W., Rogers K. A., and Kalnins V. I. Retinoic acid induces embryonal carcinoma cells to differentiate into neurons and glial cells.. J Cell Biol 94, 253–262 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K. et al.. Low density detergent-insoluble membrane of Xenopus eggs: subcellular microdomain for tyrosine kinase signaling in fertilization. Development 129, 885–896 (2002). [DOI] [PubMed] [Google Scholar]
- de Vries W. N. et al.. Expression of Cre recombinase in mouse oocytes: a means to study maternal effect genes. Genesis 26, 110–112 (2000). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie 1
Movie 2
Supplementary Information