Abstract
It is well known that adult humans detect snakes as targets more quickly than flowers as the targets and that how rapidly they detect a snake picture does not differ whether the images are in color or gray-scale, whereas they find a flower picture more rapidly when the images are in color than when the images are gray-scale. In the present study, a total of 111 children were presented with 3-by-3 matrices of images of snakes and flowers in either color or gray-scale displays. Unlike the adults reported on previously, the present participants responded to the target faster when it was in color than when it was gray-scale, whether the target was a snake or a flower, regardless of their age. When detecting snakes, human children appear to selectively attend to their color, which would contribute to the detection being more rapidly at the expense of its precision.
Humans are extremely sensitive to biologically threatening stimuli1. This is typically the case for their response to poisonous snakes2. Indeed, a recent hypothesis3,4 stresses the importance of predator avoidance for the evolution of the present vision of humans. It reasons that some of the basic properties of the human visual system might have evolved precisely because they facilitated the detection of snakes. In fact, snake-phobia is regarded as a phenomenon that has been widespread throughout human evolution1,2. Recent investigations have shown the fact that human adults have an attentional bias for the detection of fear-relevant stimuli like snakes compared to neutral stimuli like flowers5,6,7. In those studies, typically, the researchers presented adults with 3-by-3 matrices of images of the fear-relevant stimuli and the neutral stimuli. The images were presented either in black and white or in color. When reaction times (RTs) were measured, they were found to be significantly shorter for fear-relevant targets than for neutral targets whether the images were in color or gray-scale. Nevertheless, the participants detected flowers more quickly when they were in color than when they were in gray-scale. In addition, one of the studies reported that the difference between RTs recorded for fear-relevant targets and RTs recorded for fear-irrelevant/neutral targets was most pronounced for the participants presented with a gray-scale version of the search arrays7.
More recent studies have documented that preschool children, 8- to 14-month-old infants, and even non-human primates also detect snakes more quickly than flowers in gray-scale8,9,10,11. On the basis of those findings, the authors of the studies argued the possible evolution of a fear module in primates that enables them to experience fear of snakes. However, little is known yet about how this putative module develops in each individual, and that developmental process might be a complex intertwining at the ‘nature' and ‘nurture' levels. As a step toward disentangling this process, here we attempted to compare the perception of snakes as fear-relevant stimuli in human children when the stimuli were in gray-scale and when they were in color, an issue that has not been investigated previously.
It is well known that among placental mammals, only higher primates have evolved unique trichromatic color vision distinct from the dichromatic vision of their ancestors4,12,13,14. That means that trichromatic primates are provided, exceptionally among mammals, with a ‘red-green' chromatic channel in addition to luminance and ‘blue-yellow' channels. While the evolutionary advantage of the trichromatic vision is still unclear, there have been a growing number of cognitive and neurophysiological studies that reveal how color exerts its benefits on object recognition processes during the encoding phase as well as the time of retrieval15,16,17,18,19,20. They have shown that, like shape, color acts at high semantic processing in object recognition, with color acting independently of shape processing, but doing so only when object shape is uninformative or degraded. Here we conducted experiments to investigate the manner by which color affects the detection of a target among many distractors in human children, and we attempt to explain the results with reference to notions proposed in the recent literature.
Results
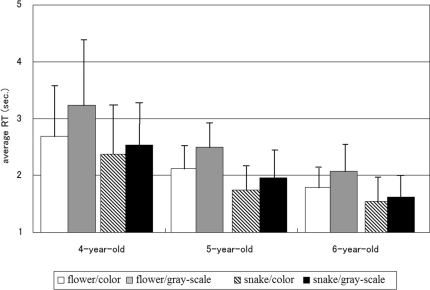
When the collected data were analyzed by a 2 (type of the target picture, TARGET) × 2 (color of the stimulus, COLOR) × 3 (age of the participant, AGE) ANOVA (analysis of variance), all of the three main effects were statistically significant (F(1, 108) = 81.1, P < 0.001, η2p = 0.429 for TARGET, F(1, 108) = 122.8, P < 0.001, η2p = 0.532 for COLOR, F(2, 108) = 29.75, P < 0.001, η2p = 0.355 for AGE). Interactions between TARGET and COLOR, and between COLOR and AGE were also significant (F(1, 108) = 33.9, P< 0.001, η2p = 0.239 for TARGET and COLOR, and F(1, 108) = 4.74, P< 0.05, η2p = 0.081 for COLOR and AGE). However the interaction between TARGET and AGE was not significant (F(1, 108) = 0.827, P = 0.44, η2p = 0.015). The interaction among TARGET, COLOR and AGE was not significant, either (F(2, 108) = 2.01, P = 0.14, η2p = 0.036) (Figure 1).
Figure 1. Experiments.
Mean reaction time (RT) of the participants to detect snake or flower targets. Error bars represent SDs.
Subsequent analyses of simple main effects, which were performed because of the significant interactions between TARGET and COLOR and between COLOR and AGE, revealed that the effects of COLOR were statistically significant whether TARGET was flower (F(1,108) = 134.73, P < 0.001) or snake (F(1,108) = 21.46, P < 0.001), and that those of TARGET were statistically significant whether COLOR was color (F(1,108) = 34.47, P < 0.001) or gray-scale (F(1,108) = 110.28, P < 0.001). Also, the simple effects of COLOR were statistically significant in each age group of the participants (F(1,108) = 68.11, 47.68 and 16.17 for 4-year-olds, 5-year-olds and 6-year-olds, respectively, Ps < 0.001) and those of AGE were statistically significant whether the target was color (F(1,108) = 28.09, P < 0.001) or gray-scale (F(1,108) = 31.28, P < 0.001). Post-hoc analyses, using the least significant difference method, revealed that 6-year-olds responded to the target more rapidly than 4- and 5-year-olds (mean RT ± SD = 1758.14 ± 453.73 ms, 2078.38± 514.86 ms, 2709.28 ± 961.57 ms for 6-year-olds, 5-year-olds, and 4-year-olds, respectively, Ps < 0.05) and 5-year-olds also responded to the target more rapidly than 4-year-olds (P < 0.05).
Discussion
In accordance with previous reports5,6,7, the present results showed that all participants detected snakes as the targets more quickly than flowers as the targets whether the images were in color or gray-scale. As the age of the participants increased, moreover, they responded to the target more rapidly. These results confirm the proposal of the snake detection theory that the propensity for particularly rapid visual detection of snakes is shared between human children and adults, and that an evolved bias for the detection of evolutionarily relevant threatening stimuli exists in humans2,5,6. As far as these results are concerned, the shape of snakes should no doubt be more crucial than their color among the precise stimulus attributes that underlie this bias, as has also been noted previously7,8. However, the participants in our study responded to a snake picture as well as a flower picture as the target faster when it was in color than when it was gray-scale regardless of their age. These results are in contrast to the previous findings that the degree of rapidity with which adults detect a snake picture among many flower pictures does not differ whether the images are in color or gray-scale.
Several studies of object recognition have been conducted in humans to investigate how the color and shape of visual stimuli are processed when the stimuli are conjointly presented and represent real and familiar entities about which individuals presumably have specific ‘object color knowledge' (including animals and plants)15,16,17. Neurophysiological studies have shown that the brain areas devoted to the selective processing of color are anatomically quite separate from those devoted to the selective processing of shape, and that color is processed faster than shape18,19. Moreover, the processing of shape depends on color relevance, whereas the processing of color occurs independently of whether shape is relevant or not20,21.
Given this evidence, one can reason that as the cognitive strategy for snake detection, attending selectively to their shape should have been more efficient than attending to their color for the human adults investigated in the previous research, because the snake detection theory regards snakes, in principle, as animals that have evolved to be cryptic, their coloration being designed to break up the detection of their distinctive shape5,6,7. When a person recognizes an object with a snake-like shape, the adaptive response to it should be to withdraw as rapidly as possible no matter what color it is. In accordance with this strategy, the rapidity with which adults detect a snake picture as the target does not differ whether the stimuli are presented in color or gray-scale. Flowers, on the other hand, may be a variety of shapes (at least much more variable than snakes) but are colorful in order to attract pollinators. In order to detect the flowers, therefore, attending selectively to their color should be more efficient than attending to their shape. This strategy would result in more rapid detection of a flower picture as the target when the stimuli are presented in color than when the stimuli are presented in gray-scale. These predictions were also confirmed in human children in the present study.
On the other hand, the present findings of the more rapid response by the participants to a snake picture as the target when the stimuli were presented in color than when they were presented in gray-scale strongly indicate the possibility that the children also selectively attended to the color even when a snake picture was presented as the target. Seemingly, this strategy is not consistent with the reasoning of the snake detection theory, which proposes that color vision is not useful and can be a hindrance for superior detection of cryptic snakes for which higher primates including humans are adapted, as evidenced by the superior detection of camouflaged objects by dichromat humans22,23. Indeed, such a possibility has been argued4.
Nevertheless, the fact cannot be neglected that there are many venomous snake species whose appearance have been referred to as a ‘warning pattern' (e.g., a banded pattern). The consensus among herpetologists is that snakes with this pattern can be detected more distinctively and be perceived as more threatening24. Recognition of such snakes could be facilitated by selectively attending to their color25,26. In addition and more importantly, it must also be noted that even though humans as young children are able to detect snakes more rapidly than other objects, their ability to visually search is still under development and is obviously inferior to that of adults and that, as a result, the rapidity with which the children detect snakes is also much slower than that of adults8,11. Children are more likely than adults to be exposed to the risk of predation, which could be compensated to some extent by the increased rapidity with which snakes as the target are detected, if color is selectively attended to. Apparently there is a trade-off between the rapidity and the precision of snake detection in humans, who would show enhanced rapidity at the expense of precision by selectively attending to the color of snakes during their childhood.
Methods
Participants. The participants who were originally included in the present study were 34 four-year-old (M ± SD = 53.9 ± 3.45 months, range = 48 – 59), 40 five-year-old (M = 65.9 ± 3.14 months, range = 60 – 71), and 43 six-year-old (M = 75.5 ± 2.48 months, range = 72 – 81) children. None of them had visual or hearing impairments. However, data collected from 6 children (3 four-year-olds, 1 five-year-old and 2 six-year-olds) who failed to follow our instructions: in experimental trials were excluded from the subsequent analysis.
Materials. For each experiment, we selected 24 photographs for each stimulus category. In a given trial, 9 of these photographs were displayed in a 3-by-3 matrix. Each matrix contained 1 target picture from one category and 8 distracter pictures from another category: A flower matrix would contain a snake target, and a snake matrix would contain a flower target. This yielded two combinations: a snake among flowers, and a flower among snakes (Figure 2). A RDT151TU (MITSUBISHI) color touch-screen monitor was used to present each picture matrix on a 38.1cm (15-in.) screen. Each of the 24 pictures in the target category served as the target once. Each of the 24 pictures in the distracter category appeared multiple times; the different distracters were presented approximately the same number of times across trials. Twenty-four trials consisted of 12 colored arrays and 12 gray-scale arrays, and the stimulus order was created by randomly arranging the matrices. An outline of a child's handprints was located on the table immediately in front of the monitor.
Figure 2. Examples of a matrix with 9 pictures.
Left 2 panels show the color-scale matrix and right 2 panels show the gray-scale matrix. Each display consisted of 1 target (a snake or a flower) and 8 distracter (the other) pictures.
Procedure and analyses. The child was seated in front of the touch-screen monitor (approximately 40 cm from the base of the screen) and was told to place his or her hands on the handprints (Figure 3). This ensured that the child's hands were in the same place at the start of each trial, making it possible to collect reliable latency data. An experimenter was seated alongside to monitor and instruct the child throughout the procedure.
Figure 3. A participant.
A photo of a preschool child identifying the single snake target among 8 flower distracters by touching the snake image on a touch-screen monitor.
First, a set of nine practice trials was given to instruct the child how to use the touch screen. In the first three trials, a display of 1 target (a puppet of an animation well known to the children) and 8 distracter (another puppet also well known) pictures was presented. The child was asked to touch the target among distracters as quickly as possible, and then return his or her hands to the handprints. In the next six trials, the display consisted of 1 target (a snake or a flower) and 8 distracter (the other) pictures, and the child was asked to touch only the target picture. All pictures used in the practice trials were chosen randomly from the original sets of 24.
When children had learned the procedure, a series of test trials followed. The task comprised 48 trials in total ordered in 2 blocks of 24 trials. In each trial, a different picture matrix containing 1 target (snake or flower) and 8 distracters (the other) was presented. Between trials, a picture of a stuffed animal or a popular character appeared on the screen to keep the children's attention on the screen. The experimenter initiated a trial when she judged that the child was looking at the picture, causing the next matrix to appear in order to ensure that the child's full attention was on the screen before each matrix appeared. When the first block was over, another block began. If the first block target was snakes, the next target was flowers, or vice versa. Each child was randomly assigned to one of 2 block orders.
In each trial, the RT of the child was automatically recorded from the onset of the matrix to when the child touched one of the pictures on the screen. The following results were solely based upon analyses on the RT data collected in this manner (RTs of incorrect responses as well as extreme RT scores—defined as values more than 2 standard deviations above or below the mean relative to each participant's mean RT—were excluded from the analyses).
Author Contributions
NM conceived of the study, and participated in its design and coordination and drafted the manuscript. SH conducted the experiments. All authors participated in the data analysis and interpretation. All authors read and approved the final manuscript.
Acknowledgments
The research was supported by a grant (#20243034) as well as by the Global COE (Center for Excellence) Research Program from the Ministry of Education, Science, Sports and Culture, Japanese Government (A06 to Kyoto University). We are grateful to Naoko Watanabe for assistance when conducting experimentation and Masahiro Shibasaki for making comments on an earlier version of this manuscript.
References
- Seligman M. E. P. Phobias and preparedness. Behavioral Therapy 2, 307–320 (1971). [Google Scholar]
- Öhman A. & Mineka S. Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychological Review 108, 483–522 (2001). [DOI] [PubMed] [Google Scholar]
- Isbell L. A. Snakes as agents of evolutionary change in primate brains. Journal of Human Evolution 51, 1–35 (2006). [DOI] [PubMed] [Google Scholar]
- Isbell L. A. The Fruit, the Tree, and the Serpent: Why We See So Well. Cambridge, MA: Harvard University Press; 2009. [Google Scholar]
- Öhman A. & Soares J. J. F. On the automatic nature of phobic fear – Conditioned electrodermal responses to masked fear-relevant stimuli. Journal of Abnormal Psychology 102, 121–132 (1993). [DOI] [PubMed] [Google Scholar]
- Öhman A., Flykt A. & Esteves F. Emotion drives attention: Detecting the snake in the grass. Journal of Experimental Psychology, General 130, 466–478 (2001). [DOI] [PubMed] [Google Scholar]
- Flykt A. Visual search with biological threat stimuli: Accuracy, reaction times, and heart rate changes. Emotion 5, 349–353 (2005). [DOI] [PubMed] [Google Scholar]
- LoBue V. & DeLoache J. S. Detecting the snake in the grass - Attention to fear-relevant stimuli by adults and young children. Psychological Science 19, 284–289 (2008). [DOI] [PubMed] [Google Scholar]
- Shibasaki M. & Kawai N. Rapid detection of snakes by Japanese monkeys (Macaca fuscata): An evolutionarily predisposed visual system. Journal of Comparative Psychology 123, 131–135 (2009). [DOI] [PubMed] [Google Scholar]
- LoBue V. & DeLoache J. S. Superior detection of threat-relevant stimuli in infancy. Developmental Science 13, 221–228 (2010). [DOI] [PubMed] [Google Scholar]
- Masataka N., Hayakawa S. & Kawai N. Human young children as well as adults demonstrate ‘superior' rapid snake detection when typical striking posture is displayed by the snake. PLoS One 5, e15222 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegenfurtner K. R. & Rieger J. Sensory and cognitive contributions of color to the recognition of natural scenes. Current Biology 10, 805–808 (2000). [DOI] [PubMed] [Google Scholar]
- LeDoux J. Synaptic Self: How Our Brains Becomes Who We Are. New York: Penguin; 2002. [Google Scholar]
- Masataka N. The Onset of Language. Cambridge: Cambridge University Press; 2003. [Google Scholar]
- Luzzatti C. & Davidoff J. Impaired retrieval of object-color knowledge with perceived color naming. Neuropsychology 32, 933–950 (1994). [DOI] [PubMed] [Google Scholar]
- Tanaka J. W. & Pressnell L. M. Color diagnosticity in object recognition. Perception and Psychophysics 61, 1140–1153 (1999). [DOI] [PubMed] [Google Scholar]
- Turatto M. & Galfano G. Color, form and luminance capture attention in visual search. Vision Research 40, 1639–1643 (2000). [DOI] [PubMed] [Google Scholar]
- Miceli G., Fouch E., Capasso R., Miceli G., Fouch E., Capasso R., Shelton R., Tomaiuolo F. & Caramazza A. The dissociation of color from form and function knowledge. Nature Neuroscience 4, 662–667 (2001). [DOI] [PubMed] [Google Scholar]
- Proverbio A. M., Burco F., del Zotto M. & Zoni A. Blue piglets? Electrophysiological evidence for the primacy of shape over color in object recognition. Cognitive Brain Research 18, 288–300 (2004). [DOI] [PubMed] [Google Scholar]
- Liebe S., Fischer E., Logothesis N. K. & Rainer G. Color and shape interaction in the recognition of natural scenes by human and monkey observers. Journal of Vision 9, 1–16 (2009). [DOI] [PubMed] [Google Scholar]
- Tanaka J., Weiskopf J. & Williams P. The role of color in high-level vision. Trends in Cognitive Sciences 5, 211–215 (2001). [DOI] [PubMed] [Google Scholar]
- Morgan M. J., Adam A. & Millon L. D. Dichromates detect color-camouflaged objects that are not detected by trichromates. Proceedings of the Royal Society of London Series B 248, 291–295 (1992). [DOI] [PubMed] [Google Scholar]
- Saito A., Mikami A., Hosokawa T. & Hasegawa T. Advantage of dichromats over trichromats in discrimination of color-camouflaged stimuli in humans. Perceptual and Motor Skills, 102, 3–12 (2006). [DOI] [PubMed] [Google Scholar]
- O'Shea M. Venomous Snakes in the World. Princeton, PA: Princeton University Press; 2006. [Google Scholar]
- Xu V. Limitations of object-based feature encoding in visual short-term memory. Journal of Experimental Psychology, Human Perception and Performances 28, 458–468 (2002). [DOI] [PubMed] [Google Scholar]
- Wurm L. H., Legge G. F., Isenberg L. M. & Luebker A. Color improves object recognition in normal and low vision. Journal of Experimental Psychology, Human Perception and Performances 19, 899–911 (1993). [DOI] [PubMed] [Google Scholar]