Abstract
Eyespot formation in butterfly wings has been explained by the concentration gradient model. However, this model has recently been questioned, and dynamic interactions between the black-inducing signal and its inhibitory signal have been proposed. Here, the validity of these models was examined using a nymphalid butterfly Junonia almana. Early focal damage to the major eyespots often made them smaller, whereas the late damage made the outer ring larger and the inner ring smaller in a single eyespot. Non-focal damage at the outer ring not only attracted the whole eyespot structure toward the damaged site but also reduced the overall size of the eyespot. Surprisingly, a reduction of the major eyespot was accompanied by an enlargement of the associated miniature eyespots. These results demonstrate limitations of the conventional gradient model and support a dynamic interactive nature of morphogenic signals for colour-pattern determination in butterfly wings.
Butterfly wing colour patterns are highly complex, but it is believed that these patterns are produced by simple rules that determine the fate of immature scale cells fixed on a two-dimensional plane. Concentric rings of a few colours called eyespots are simple and conspicuous elements, and partly for this reason, characterisation of eyespots by physical damage and transplantation has been intensively performed, mainly focusing on the forewing eyespots of the nymphalid butterflies, Junonia coenia1,2,3 and Bicyclus anynana4,5,6. The forewing eyespots of both J. coenia and B. anynana have similar colour-patterns: a white focus, black, yellow, and then black rings from the central to peripheral areas. Other nymphalid species, Junonia orithya and Ypthima argus, have been examined in a similar study7.
The experimental results obtained in these studies are interpreted as evidence for the concentration gradient model for positional information, which was originally proposed as a general mechanism of pattern formation8. The main reason for this interpretation is the focus-dependence of the eyespot formation on the dorsal forewings, which was revealed by the early focal damage that resulted in smaller eyespots1,2,3,4,5,6. Following this line of discussion, the putative morphogens, Wingless and TGF-β, have been shown to be expressed in eyespots9. These putative morphogens are believed to be secreted from the prospective eyespot foci and determine the eyespot ring colours10,11,12,13,14, although functional evidence for these candidate morphogens has been lacking.
In contrast, based on the actual colour-pattern diversity of nymphalid butterfly wings using inductive logic, the induction model has been proposed15,16, in which wave-like, black-inducing signals are successively released. These signals are self-propagating and self-enhancing, and they also induce their inhibitory signals for light rings. This model, largely described by reaction-diffusion equations16, can explain not only the diverse nymphalid colour-patterns but also the experimental results that the gradient model cannot explain15,16. However, the experimental evidence for this model has been lacking.
In the present study, I focused on the forewing eyespots of the peacock pansy butterfly Junonia almana, one of the nymphalid butterflies found in Southeast Asia, that has been used in previous studies17. The eyespots of this species have several notable features. First, the major eyespot has two large black rings separated by a narrow yellow (or orange) ring. The yellow ring appears to be “fine-tuned” in width during the eyespot formation. Second, the major eyespot is depicted against the plain orange background, whose colour is similar to the yellow ring. This eyespot is ideal for characterisation because there is no other pattern element around it, and the background is lightly coloured. Third, the major eyespot is accompanied by the miniature eyespots at the anterior or posterior positions, thus facilitating the examination of the interactions between eyespots. Fourth, the major eyespot is relatively large, spanning more than 5 mm in diameter in adults, which facilitates fine morphological analyses. These unique features prompted me to examine the forewing eyespots of this species systematically. Here, I provide experimental evidence for the dynamic interactions between morphogenic signals for colour-pattern determination in butterfly wings.
Results
Structural diversity of the miniature eyespots
A miniature eyespot was occasionally located at the anterior or posterior position of the major eyespot (Fig. 1). Among the 325 individuals examined, 25 individuals (7.7%) had miniature eyespots. These miniature eyespots had various sizes and structures, which appeared to reflect not only the various activity levels of organising centres but also the dynamics of eyespot signalling during the fate determination process.
Figure 1. Structural diversity of the miniature eyespots of the J. almana dorsal forewings.
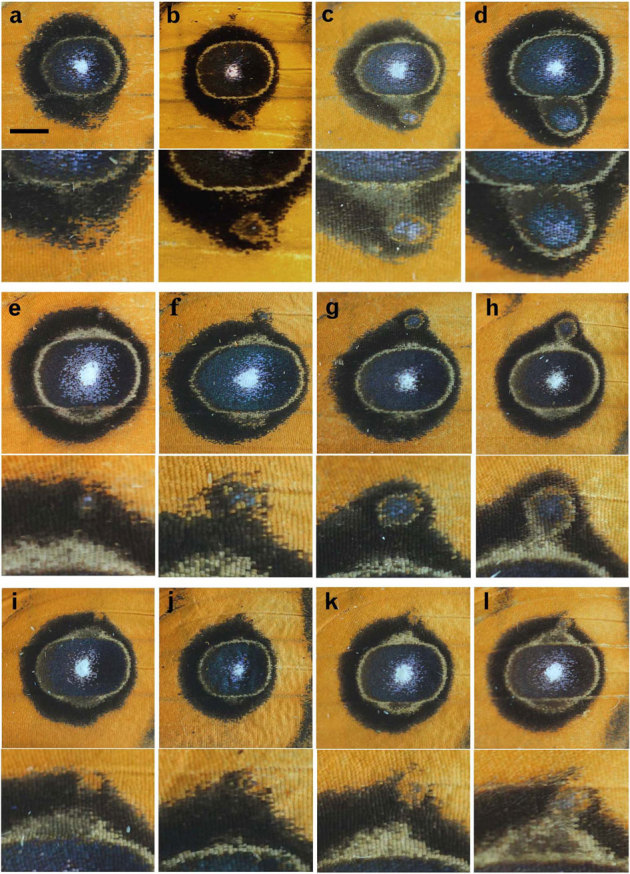
In each panel, the miniature eyespot is also shown in higher magnification. (a–d) Miniature eyespot at the posterior side of the major eyespot. Scale bar, 2.0 mm, which applies to all panels in this figure. (e–l) Miniature eyespot at the anterior side of the major eyespot.
Some miniature eyespots were simple orange circles embedded in the black outer ring of the major eyespot (Fig. 1a, i). Others had a black inner core ring with blue scales in it, and thus were structurally very similar to the major eyespot despite its small size (Fig. 1b–h). Yet others had a blurred inner core ring at the centre (Fig. 1j–l). It is possible to align these various miniature eyespots from simple to complex and from small to large ones (Fig. 1a–d, e–h, i–l). These aligned structures possibly represent either a developmental time sequence or different activity levels of organising centres.
The black outer rings of the miniature and major eyespots often fused together (Fig. 1). The narrow outer ring of the miniature eyespot was likely encircled by the wider black ring provided by the major eyespot. Nevertheless, the narrow yellow ring of the miniature eyespot was not erased. Furthermore, the yellow rings of the miniature eyespots, when enlarged enough, appeared to fuse with those of the major eyespots (Fig. 1c, d, h, l).
Focal damage: reduction and uncoupling
Early focal damage that was performed within 18 hours after pupation produced smaller eyespots in most individuals (Figs. 2a–d, 3a). In the most severe cases, small and narrow black rings were observed, having a relatively wide yellow (or orange) ring in between (Fig. 2a). In the less severe cases, a small and relatively similitude eyespot was produced (Fig. 2b–d).
Figure 2. Focal damage to the major eyespots.
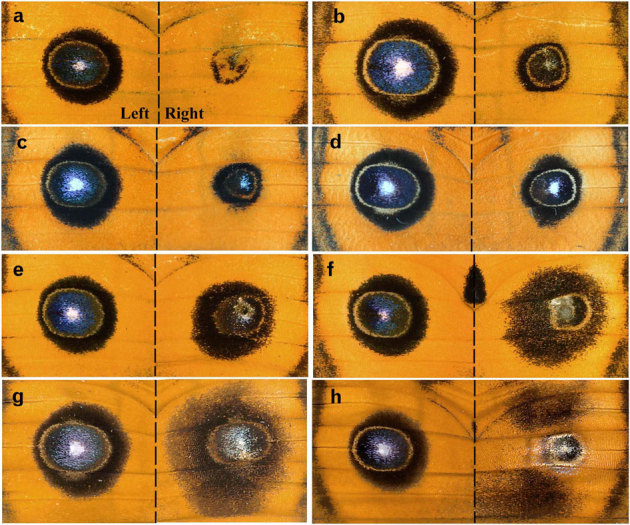
Damage was performed only on the right wing. (a, b) Damage was performed 3 hours post-pupation, (c) 6 hours post-pupation, (d) 12 hours post-pupation, and (e–h) 1 day post-pupation. In h, scales in the central area of the major eyespot were lost on the dorsal side, and the colour pattern of the ventral side was seen through.
Figure 3. Response profile of the damage-induced colour-pattern changes.
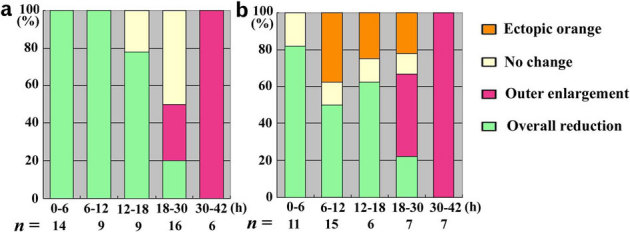
The treatment time is shown on the x-axis. The number of individuals treated is shown at the bottom of each bar. (a) Focal damage. (b) Semi-focal damage.
Late focal damage that was performed 1 day post-pupation produced intriguing changes (Figs. 2e–h, 3a). There was a tendency that the inner core ring and the yellow ring were reduced in size, whereas the black outer ring was enlarged (Fig. 2e–g). In these eyespots, the density of the black scale in the enlarged outer ring appeared to be reduced, but the inner core ring was as dark as that of the non-treated eyespot. A similar but more severe case is shown in Fig. 2h, in which the outer black ring was more diffusely dissipated as if the tethering interactions were lost.
Semi-focal damage: ectopic orange area inside the major eyespots
Damage at the inner core ring (called semi-focal damage) produced similar results to the focal damage; i.e., the overall size reduction (Figs. 3b, 4a, b). In addition, it uniquely induced an ectopic orange area, occasionally with a white “focal” area on the inside. The normal focal white area of the major eyespot and its surrounding blue scales near the damaged site were partly erased, although the induced orange area did not completely touch the normal white focal area and its surrounding blue scales. This erasing can be explained by assuming that the ectopic black ring was induced around the induced orange area, which was also suggested by the proximal extension of the black area (Fig. 4a, b).
Figure 4. Semi-focal, non-focal, and background damage.
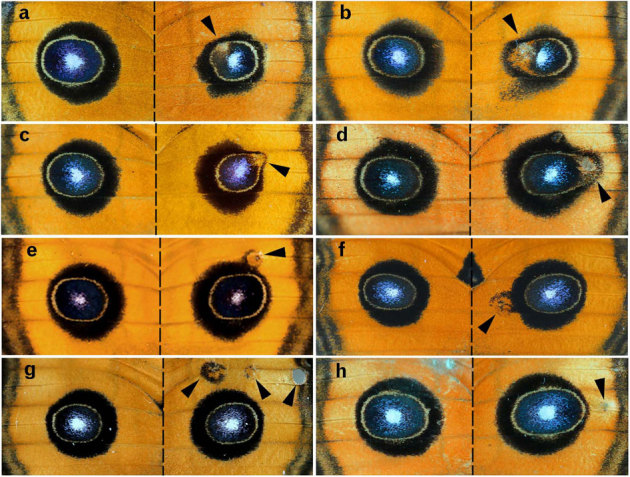
Arrowheads indicate the damaged sites on the right wings. (a) Semi-focal damage performed 9 hours post-pupation. (b) Semi-focal damage performed 7 hours post-pupation. (c, d) Non-focal damage performed 6 hours post-pupation. (e–h) Background damage performed 1–3 hours post-pupation.
Non-focal and background damage: attraction of the major eyespots
Non-focal damage at the outer black ring induced the orange area and the black ring around it in all individuals treated within 6 hours post-pupation (n = 19; Fig. 4c, d), as in the semi-focal damage. The damage deformed the overall shape of the major eyespot by distorting the whole major eyespot toward the damaged site. When treated at 6–12 hours post-pupation, the orange area was similarly induced in 6 individuals out of 9, but all individuals showed distortion of the eyespot towards the damaged site.
The damage that was performed near the eyespot (but still in the background area) induced a black ring around the damaged orange area, which fused with the black outer ring of the eyespot with or without the inner core (Fig. 4e, f). These ectopic eyespots had relatively large orange areas, which were reminiscent of the natural miniature eyespots.
When the damaged site was located far from the major eyespot, it induced an ectopic eyespot in 4 of 21 individuals (Fig. 4g), although 14 individuals showed no colour-pattern change. In 5 cases where it did not induce any colour-pattern around the damaged site, the major eyespot was still distorted towards the damaged site and enlarged (Fig. 4h). The eyespot was enlarged along the proximal-distal axis and along the anterior-posterior axis despite the fact that the damaged site was relatively distant from the major eyespot. In these 5 individuals, the ratios of the eyespot sizes between the treated and non-treated sides were 1.055 ± 0.035 (mean ± SD) along the proximal-distal axis and 1.062 ± 0.049 (mean ± SD) along the anterior-posterior axis. In the non-treated individuals, the right-to-left ratios were 1.005 ± 0.015 (mean ± SD) along the proximal-distal axis and 1.003 ± 0.015 (mean ± SD) along the anterior-posterior axis (n = 9). The differences were scored at the level of p = 0.019 along the proximal-distal axis and p = 0.012 along the anterior-posterior axis.
Emergence or enlargement of the miniature eyespots
When the major eyespot was reduced in size by either focal or non-focal damage performed within an eyespot area as discussed above, the associated miniature eyespot was enlarged or newly appeared (n = 15), which was independent of the damaged position (Fig. 5a–f).
Figure 5. Enlargement of the miniature eyespot in response to the size reduction of the major eyespot.
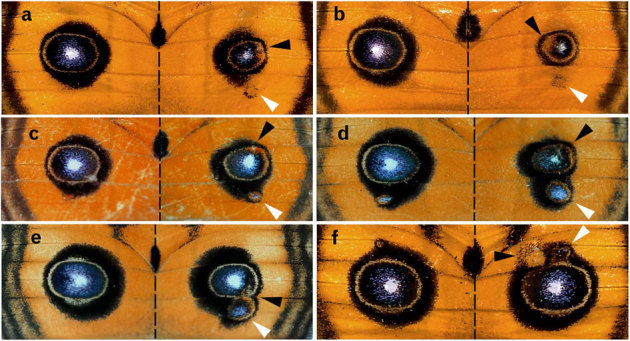
Black arrowheads indicate the damaged sites, and white arrowheads indicate the enlarged miniature eyespots. (a) Damage was performed 6 hours post-pupation, (b) 24 hours post-pupation, (c) 9 hours post-pupation, (d) 15 hours post-pupation, (e) 3 hours post-pupation, and (f) 12 hours post-pupation.
Discussion
Here, I examined the damage-induced pattern changes of the dorsal forewing eyespots of a nymphalid butterfly Junonia almana. The results that were obtained in the present study not only confirmed the previous surgical experiments performed in other species but also added new unexpected findings that have not been predicted by the conventional gradient model for positional information. The reasons for these new findings are probably largely based on the use of J. almana, as its forewing colouration and simple patterns resulted in higher resolution of detecting eyespot changes and helped to infer the signal dynamics that could underlie the fundamental mechanism for colour-pattern determination.
At first sight, the result of the focal damage that was obtained in this study, i.e., the size reduction of the damaged eyespot seems to support the concentration gradient model, in which the signal is dependent on a continuous focal activity from the focal organising centre. In contrast, if the signal is wave-like, it is independent of the focal area once the signal is released, because the signal propagates autonomously. In that case, focal damage would not cause any size reduction of eyespots.
However, even if focal damage causes a size reduction, the signal could be wave-like if a few independently released signals established an equilibrium state that mutually depend upon one another, as assumed at the beginning of the colour-pattern analysis that led to the induction model15. Alternatively (but not mutually exclusively), if the period of the signal release begins relatively late or if the signal is released continuously for a relatively long period, focal damage can reduce the number of functional cells at the focal area before the complete release of wave-like signals16, resulting in a size reduction after the focal damage. This explanation has been employed to logically solve the hindwing paradox16 and the PFE (parafocal element) paradox15,16,17,18,19.
Based on the structural diversity of natural miniature eyespots, I propose a possible time sequence of signalling dynamics as follows. The black-inducing signal originates from the inside and expands to the outside as the whole eyespot becomes larger. The signal for the outer ring is emitted first, and the signal for the inner core ring is then released. The gap between these signals is expressed as a yellow ring. The yellow ring is relatively wide at first and becomes narrower as the whole eyespot becomes larger. This yellow area is self-enhanced later, serving as an inhibitory area against black rings. These signal dynamics are consistent with the induction model that is fundamentally described by reaction-diffusion equations15,16.
The miniature eyespot that was enlarged because of the reduction of the major eyespot can be compared with the natural miniature eyespot on the non-treated wing of an identical individual. Organising centres for these two miniature eyespots would have the same signal intensity, but they were subjected to different surrounding signals from the major eyespots when one focus was damaged. Structural differences between the right and left miniature eyespots seen in Fig. 5 are consistent with the results of the colour-pattern analysis of natural miniature eyespots shown in Fig. 1.
It appeared that the two black rings (i.e., the outer ring and the inner core ring) in an eyespot were independent and could be uncoupled by physical damage. At the time of the late damage, the signal for the outer ring may be independent of the focal activity, but the signal for the inner ring is still dependent on it. The expansion of the outer ring by the late damage is likely to be caused by a damage-induced wave-like signal that is different from the activity change of organising centre. This is, an artificial extra-wave is added to the existing and expanding signal for the outer black ring.
It is likely that homophilic and heterophobic interactions may operate during the colour-pattern determination process. This notion was first suggested by the interactions between the major and miniature eyespots; the black rings of the two eyespots tended to fuse together, and similarly, the yellow rings of the two eyespots tended to fuse together. The similarly coloured rings seemed to attract each other. The ectopic orange area also fused with the yellow ring. Furthermore, the narrow width of the yellow ring between the two black rings in an eyespot is probably maintained by the repulsion between the two rings of different colours.
The conventional gradient model states that the morphogen concentration of the yellow (or orange) area is lower than that of the inner core ring, and thus, the induced orange area within the inner core ring of the major eyespot as shown in the present study is supposed to have a lower concentration of morphogen than its surroundings. If the conventional model is correct, the orange area inside the core ring is supposed to be overwritten and erased completely, although one could still argue that the damage-induced signals determine the fate of the scale cells around the damaged site before the arrival of the major eyespot signal.
Another result that requires a non-conventional model for its explanation is obtained after the semi-focal damage. The semi-focal damage occasionally reduced the size of the major eyespot. This may be partly because the results of the damage also nullified some organising cells due to the close proximity of the damaged site to the focal area. However, the damage at the outer ring that was farther from the focal area was still able to induce a size reduction. In most cases, the modified colour patterns in the hindwing surfaces confirmed that an accurate operation was performed at a single position. I propose that the overall reduction of the major eyespot by non-focal damage is mainly because an artificially induced signal suppressed the expansion of the major eyespot signal through the homophilic and heterophobic interactions between the ectopic and natural eyespot signals.
To interpret the enlargement of the miniature eyespot in response to the size reduction of the major eyespot, it is necessary to reject the conventional model, which assumes relatively static signals, and to propose dynamic interactions between eyespot signals. In a non-treated wing, the major eyespot signal may have interfered with the miniature eyespot signal, making the miniature eyespot smaller than its potential size. This idea is consistent with the somewhat perplexing fact that the wing compartments located anterior and posterior to the major eyespot harbour relatively large pupal cuticle spots7. When the major eyespot signal was diminished or delayed due to damage, this interference did not work well, and as a result, the miniature eyespot enlarged. This result together with the other results discussed previously demonstrated that dynamic interactions between eyespot signals determine the final colour patterns.
The dynamic interactions between the major and miniature eyespots may be similar, if not identical, to the interactions between the major eyespot and the damaged site. The ectopic emergence of a damage-induced signal could simply inhibit the enlargement of the major eyespot as a whole, which in turn made the miniature eyespot larger.
Such dynamic inter-eyespot interactions may be seen in an individual where the anterior damage just next to the anterior miniature eyespot did not reduce the major eyespot, but it still enlarged the miniature eyespot (Fig. 5f). The damage probably delayed the arrival of the major eyespot signal to the position of the miniature eyespot. As a result, the anterior miniature eyespot enlarged because it had enough time to enlarge before the arrival of the signal from the major eyespot. The damage did not interfere with the enlargement of the miniature eyespots, suggesting that the miniature eyespot signals were released after the damage-induced signal subsided. Therefore, in a normal wing, the major eyespot signal is up-regulated first, and then the miniature eyespot signal is up-regulated later.
Four cases of possible long-range effects of damage were observed: (1) attraction or fusion of rings that have the same colour between the major and miniature eyespots, (2) enlargement of the outer ring caused by the late focal damage, (3) enlargement and reduction of the whole eyespots caused by the semi-focal and non-focal damage, and (4) enlargement of the major eyespot caused by the background damage. In the latter two cases, the damaged site appeared to attract the major eyespots. Depending on the locations of the damaged site, the attracted eyespot signal either halts its expansion or further elongates. In the case of the background damage, no direct physical contact seems to be required between the damaged site and the eyespot, although unseen interactions between signals may exist. Importantly, the eyespot was not only elongated toward the damaged site but also enlarged in both the anterior-posterior and proximal-distal axes. The interaction between the damaged site and its nearest site of the eyespot appears to affect the whole eyespot signals. Thus, there is a plastic ability of the morphogenic signals to self-reconstruct the entire morphology in response to surrounding signals. This flexibility is a general feature of a reaction-diffusion system.
The long-range effect occasionally spanned a millimetre on the adult wings. In all four cases of the possible long-range effects above, it is likely that the homophilic interactions (i.e., attraction between two black-inducing signals or between two yellow-inducing signals) and heterophobic interactions (i.e., repulsion between black-inducing and yellow-inducing signals) play important roles in long-range signalling dynamics for the colour-pattern determination. Similar interactions probably occur between rings in a given eyespot. The incorporation of physiological results into the present study20,21,22 may be expected in the future. Furthermore, comparison with mechanisms of other animal skin colours may be fruitful23,24,25.
Methods
All individuals of the peacock pansy butterfly J. almana examined in the present study were reared in our laboratory as previously described17. Briefly, eggs were collected from field-caught females in the Ishigaki-jima Island or Okinawa-jima Island, the Ryukyu Archipelago, Japan. Larvae were fed the natural host plants and were maintained at 27 ± 2 °C. At various time points, the right pupal wings were damaged with a needle (0.18 μm in diameter). Both the major and miniature eyespots have corresponding pupal cuticle spots on the surface of the pupal wing7, which were used as landmarks for the damage procedures. Only one side of the wings was damaged, leaving the other side as an internal control. Upon eclosion, adults were readily frozen to minimize scale detachment. Eyespot size ratios between the two wings in individuals that received the background damage were compared to those of non-treated individuals by Mann-Whitney U test using JSTAT 10.0 (2006).
Author Contributions
J.M.O. conceived the study, performed experiments, analyzed the data, and wrote the paper.
Acknowledgments
We thank Seira Kinjo for performing a part of the damage experiment, and Yoshikazu Ono, Wataru Taira, and other members of the BCPH Unit of Molecular Physiology for discussion. This work was partly supported by Research Foundation for Opto-Science and Technology, Hamamatsu, Japan.
References
- Nijhout H. F. Pattern formation on lepidopteran wings: Determination of an eyespot. Dev. Biol. 80, 267–274 (1980). [DOI] [PubMed] [Google Scholar]
- Nijhout H. F. Cautery-induced colour patterns in Precis coenia (Lepidoptera: Nymphalidae). J. Embryol. Exp. Morphol. 86, 191–203 (1985). [PubMed] [Google Scholar]
- Nijhout H. F. The Development and Evolution of Butterfly Wing Patterns. (Smithsonian Institution Press, 1991). [Google Scholar]
- French V. & Brakefield P. M. The development of eyespot patterns on butterfly wings: morphogen sources or sinks? Development 116, 103–109 (1992). [Google Scholar]
- French V. & Brakefield P. M. Eyespot development on butterfly wings: the focal signal. Dev. Biol. 168, 112–123 (1995). [DOI] [PubMed] [Google Scholar]
- Brakefield P. M. & French V. Eyespot development on butterfly wings: the epidermal response to damage. Dev. Biol. 168, 98–111 (1995). [DOI] [PubMed] [Google Scholar]
- Otaki J. M., Ogasawara T. & Yamamoto H. Morphological comparison of pupal wing cuticle patterns in butterflies. Zool. Sci. 22, 21–34 (2005). [DOI] [PubMed] [Google Scholar]
- Wolpert L. Positional information and the spatial pattern of cellular differentiation. J. Theor. Biol. 25, 1–47 (1969). [DOI] [PubMed] [Google Scholar]
- Monteiro A., Glaser G., Stockslager S., Glansdorp N. & Ramos D. Comparative insights into questions of lepidopteran wing pattern homology. BMC Dev. Biol. 6, 52 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S. B., Gates J., Keys D. N., Paddock S. W., Panganiban G. E. F., Selegue J. E. & Williams J. A. Pattern formation and eyespot determination in butterfly wings. Science 265, 109–114 (1994). [DOI] [PubMed] [Google Scholar]
- Brakefield P. M., Gates J., Keys D., Kesbeke F., Wijngaarden P. J., Monteiro A., French V. & Carroll S. B. Development, plasticity and evolution of butterfly eyespot patterns. Nature 384, 236–242 (1996). [DOI] [PubMed] [Google Scholar]
- Keys D. N., Lewis D. L., Selegue J. E., Pearson B. J., Goodrich L. V., Johnson R. L., Gates J., Scott M. P. & Carroll S. B. Recruitment of a hedgehog regulatory circuit in butterfly eyespot evolution. Science 283, 532–534 (1999). [DOI] [PubMed] [Google Scholar]
- Brunetti C. R., Selegue J. E., Brunetti C. R., Selegue J. E., Monteiro A., French V., Brakefield P. M. & Carroll S. B. The generation and diversification of butterfly eyespot color patterns. Curr. Biol. 11, 1578–1585 (2001). [DOI] [PubMed] [Google Scholar]
- Montéiro A., French V., Montéiro A., French V., Smit G., Brakefield P. M. & Metz A. J. Butterfly eyespot patterns: evidence for specification by a morphogen diffusion gradient. Acta Biotheor. 49, 77–88 (2001). [DOI] [PubMed] [Google Scholar]
- Otaki J. M. Color-pattern analysis of eyespots in butterfly wings: a critical examination of morphogen gradient models. Zool. Sci. 28, 403–413 (2011). [DOI] [PubMed] [Google Scholar]
- Otaki J. M. Generation of butterfly wing eyespot patterns: a model for morphological determination of eyespot and parafocal element. Zool. Sci. 28, 817–827 (2011). [DOI] [PubMed] [Google Scholar]
- Otaki J. M. Reversed type of color-pattern modifications of butterfly wings: A physiological mechanism of wing-wide color-pattern determination. J. Insect Physiol. 53, 526–537 (2007). [DOI] [PubMed] [Google Scholar]
- Dhungel B. & Otaki J. M. Local pharmacological effects of tungstate on the color-pattern determination of butterfly wings: a possible relationship between the eyespot and parafocal element. Zool. Sci. 26, 758–764 (2009). [DOI] [PubMed] [Google Scholar]
- Otaki J. M. Color-pattern analysis of parafocal elements in butterfly wings. Entomol. Sci. 12, 74–83 (2009). [Google Scholar]
- Otaki J. M. Physiologically-induced color-pattern changes in butterfly wings: mechanistic and evolutionary implications. J. Insect Physiol. 54, 1099–1112 (2008). [DOI] [PubMed] [Google Scholar]
- Kusaba K. & Otaki J. M. Positional dependence of scale size and shape in butterfly wings: wing-wide phenotypic coordination of color-pattern elements and background. J. Insect Physiol. 55, 174–182 (2009). [DOI] [PubMed] [Google Scholar]
- Mahdi S. H., Gima S., Tomita Y., Yamasaki H. & Otaki J. M. Physiological characterization of the cold-shock-induced humoral factor for wing color-pattern changes in butterflies. J. Insect Physiol. 56, 1022–1031 (2010). [DOI] [PubMed] [Google Scholar]
- Kondo S., Iwashita M. & Yamaguchi M. How animals get their skin patterns: fish pigment pattern as a live Turing wave. Int. J. Dev. Biol. 53, 851–856 (2009). [DOI] [PubMed] [Google Scholar]
- Kondo S. & Miura T. Reaction-diffusion model as a framework for understanding biological pattern formation. Science 329, 1616–1620 (2010). [DOI] [PubMed] [Google Scholar]
- Miyazawa S., Okamoto M. & Kondo S. . Blending of animal colour pattern by hybridization. Nat. Commun. 1, 66 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]


