Abstract
It was our objective to study the role of mechanical stimulation on fibronectin (FN) reorganization and recruitment by exposing fibroblasts to shear fluid flow and equibiaxial stretch. Mechanical stimulation was also combined with a Rho inhibitor to probe their coupled effects on FN. Mechanically stimulated cells revealed a localization of FN around the cell periphery as well as an increase in FN fibril formation. Mechanical stimulation coupled with chemical stimulation also revealed an increase in FN fibrils around the cell periphery. Complimentary to this, fibroblasts exposed to fluid shear stress structurally rearranged pre-coated surface FN, but unstimulated and stretched cells did not. These results show that mechanical stimulation directly affected FN reorganization and recruitment, despite perturbation by chemical stimulation. Our findings will help elucidate the mechanisms of FN biosynthesis and organization by furthering the link of the role of mechanics with FN.
Fibronectin (FN) is an abundant ECM protein secreted by cells as a soluble dimer and assembled into an insoluble fibrillar network at the cell surface, forming a FN matrix1,2. The complex, dynamic process of FN matrix assembly is a cell-mediated process that involves interactions between receptors on the cell surface and FN2,3. FN initially exists as a compact, folded structure that contains sets of binding domains for a variety of extracellular and cell surface molecules such as collagen, glycosaminoglycans, fibrin, integrins, and FN itself2,4,5. Although FN contains binding domains for many proteins, FN matrix assembly is believed to be induced by a conformational change through binding of FN to α5β1integrins on the cell surface2,4,6,7. This conformational change induces focal adhesion-integrin co-localization and subsequent integrin clustering and syndecan co-localization, which recruits signaling and cytoskeletal proteins4,5,8,9. Integrin – cytoskeleton interactions have proved to be requisite for matrix assembly and mediated by the Rho family of GTPases, specifically Rho, Rac, and Cdc4210,11,12. For example lysophosphatidic acid (LPA)-induced Rho activation has been shown to induce actin stress fiber formation and enhance FN matrix assembly13,14 while Rho inhibition has been shown to inhibit LPA-induced stress fiber formation and matrix assembly10,13. Therefore FN matrix assembly depends on the precise coordination and synergy between extracellular events and intracellular pathways.
Recent studies have demonstrated the FN's ability to serve a variety of functions for cells, beyond just serving as the supporting scaffold7,15,16,17. FN matrices have been demonstrated to also function as storage sites for various growth factors and regulate important cellular processes such as cellular proliferation10,18, differentiation17,19, and branching morphogenesis16. In addition, FN has been shown to inhibit apoptosis via various intracellular pathways20. Though most of these studies have probed these questions through chemical stimulation, mechanical stimulation has been shown to be extremely important. In general, the study of physical forces induced on cells by mechanical stimulation has lead to advances in the field linking biochemistry and mechanics in cells (i.e., mechanotransduction)21,22,23,24,25,26. Mechanical stimulation has also been shown to regulate ECM gene expression and protein synthesis27,28. Cells have transmembrane proteins and focal adhesions that link their intracellular and extracellular environment29,30, therefore examining the structure and mechanics is a logical step, but one that needs more research, especially in examining the ECM. FN is especially important as FN is constantly exposed to chemical and mechanical stimulations7,28. Thus a thorough understanding of both mechanical and chemical stimulation separately, as well as coupled together, is integral to gain insight into FN synthesis, function, and the effects of various pathology-induced abnormalities that can perturb this multi-functional protein. Understanding of the effects of mechanics as well as linking mechanics and chemistry to normal FN behavior has many implications as various FN abnormalities can cause a range of issues especially in cells found in various connective tissues, such as fibroblasts. For example, abnormal FN synthesis in fibroblasts has been observed to cause impaired cell migration and reduced VEGF production31. Due to the significance of fibroblasts and their ability to maintain and structurally support various connective tissues within the body as well as synthesize and maintain the ECM, impaired fibroblast behavior has many implications. A better understanding of the behavior of various cell types such as fibroblasts and the synthesis of FN at the cellular level in a more physiologically-relevant environment that provides for mechano-physical control will enable a vital understanding of FN and comparisons between FN in its native versus perturbed state.
Fibroblasts are a unique cell type that help form the various connective tissues of the body such as tendons, skin, and corneas. In addition, they also produce various supportive components to organs including the liver, lungs, nerves, uterus, and kidneys reflecting this robust cell type's physiological diversity27,32. The diversity of induced responses in these cells is also related directly to mechanical stimulation, which is critical in many connective tissues and organs. For example, fibroblasts exposed to equibiaxial stretch have been shown to experience altered gene expression of various growth factors including VEGF27 and increased cellular proliferation33. In addition to stretch-based experiments, flow-induced shear stresses also influence fibroblast activity in areas such as calcium transients and oscillations34 as well as magnitude-dependent migration35. The effects of shear stress and stretching on fibroblasts are both physiologically relevant since tendon fibroblasts experience stretching forces from normal extension of the tendon while advential fibroblasts experience transvascular interstitial fluid flow during capillary arterializaiton and intimal hyperplasia35. While previous studies have examined the biosynthesis of ECM production and maintenance related to biochemical modifications36,37,38, few studies have probed the link between mechanics and biochemical processes in the area of ECM synthesis. The goal of our present study was to probe the response of fibroblasts to mechanical stimulation. With respect to the organization and recruitment of FN, we exposed fibroblasts to separate trials of equibiaxial stretch and shear stress to understand how mechanics induces changes in the ECM. This ability to study the same cells with two distinct modes of mechanical stimulation is critical, as mechanical stimulation is not simply a binary addition of an external stimulation, but is highly sensitive to the parameters of stimulations that are imposed. Immunofluorescence was then used to visualize how our various modes of mechanical stimulation affected FN matrix formation, which provided insight into how fibroblasts modify their environment in response to mechanical stimulation. In addition, we also investigated the effect of cross-talk between mechanical stimulation and chemical modulation on FN matrix formation through Rho inhibition. The Rho family of GTPases is an important component in mechanotransduction, and is an ideal candidate for investigating the chemically linked intracellular signaling effects in this pathway. The results from this work, we believe, will help gain a better understanding of the relationship between mechanics and the ECM, specifically FN.
Results
Fibroblasts are robust cells abundant in various connective tissues in the body. These cells have regulatory functions with FN, which has been linked to numerous cellular processes. As a result, any perturbation of FN's normal interactions, and thus fibroblast function, has a diversity of implications due to the importance of the ECM16,39,40. The link to mechanical stimulation in cellular behavior such as in fibroblasts is important due to the intertwined ECM and mechanics behaviors that have been shown. To probe the complex links between mechanical stimulation and FN response, we examined the effects of mechanical and chemical stimulation on FN. NIH-3T3 fibroblasts were exposed to equibiaxial stretch and shear fluid flow for a period of 24 hours, after previously being in culture for 24 hours, using a custom-fabricated device that imparts shear or stretching41,42 forces (Fig. 1). We first examined the cells in a mechanically and chemically unstimulated state. Unstimulated cells were maintained in culture concurrently for 24 hours with cultures of cells exposed to mechanical and chemical stimulation. Phase contrast and epifluorescent images of unstimulated cells appeared to have thin FN fibrils clustered and distributed throughout the entire basal surface of the cell (Fig. 2A), while cells exposed to 24 hours of 10% equibiaxial stretching (Fig. 2B) appeared to form thicker FN fibrils and to have an observable increase in FN fluorescence intensity. Cells exposed to shear fluid flow (Fig. 2C), also appeared to demonstrate similar behavior in terms of having thicker FN fibrils as well as an observable increase in FN fibril intensity around the basal surface of cells relative to unstimulated cells.
Figure 1. Schematic of mechanical stimulation system used to exert shear stress and equibiaxial stretch on living cells.
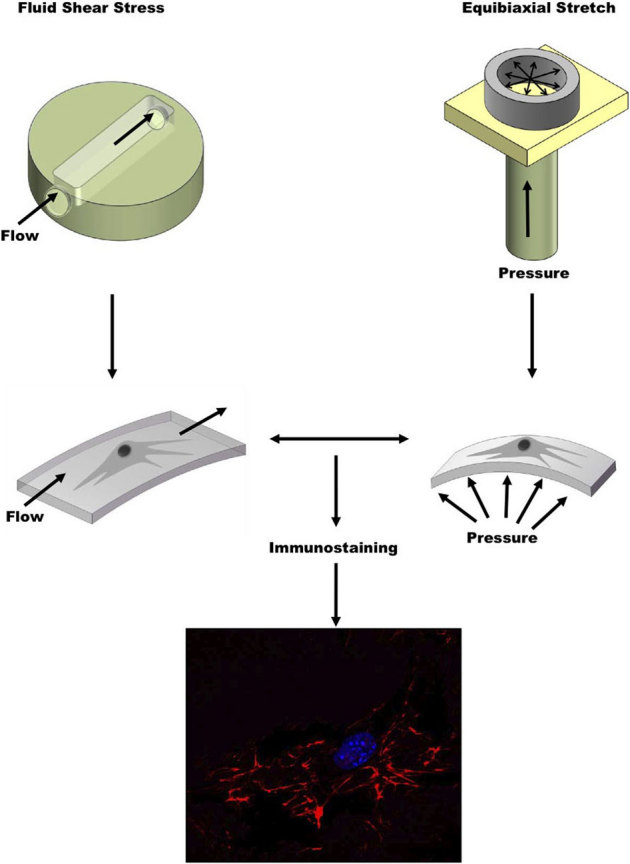
Our system uses a peristaltic flow pump to exert a shear stress across the apical surface of cells cultured on PDMS and a pressure regulator to apply a hydrostatic pressure-induced equibiaxial strain on the basal surface of cells. After mechanical stimulation, immunostaining is used to visualize FN.
Figure 2. Examining the effects of mechanical stretching on FN.
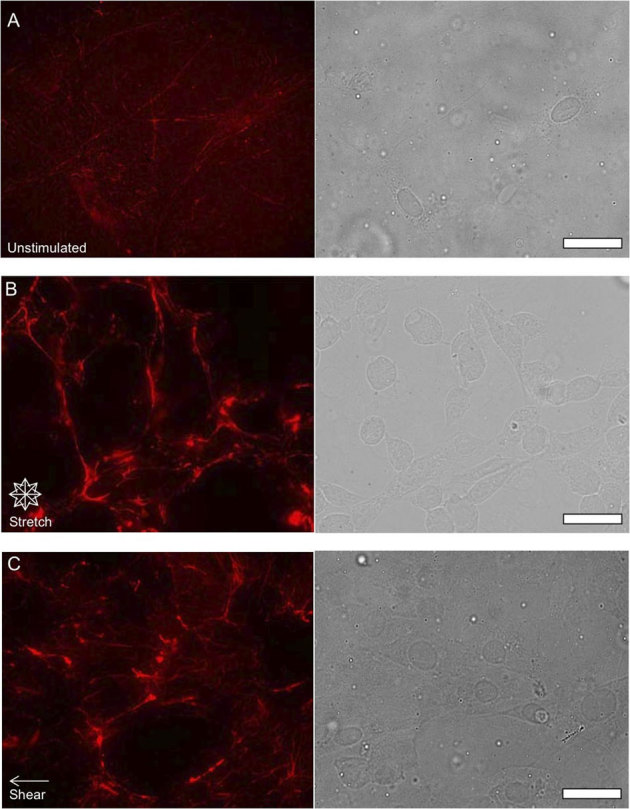
Epifluorescent images of FN (red pseudo color) and phase contrast images of NIH 3T3 fibroblast morphology unstimulated (A) after 24 hours of 10% equibiaxial strain (B) and after 24 hours of 6±3 dynes/cm2 of fluid shear stress (C) Cells exposed to mechanical stimulation appear to exhibit an increase in fibril formation as well as form thicker FN bundles. (scale bars = 10 μm).
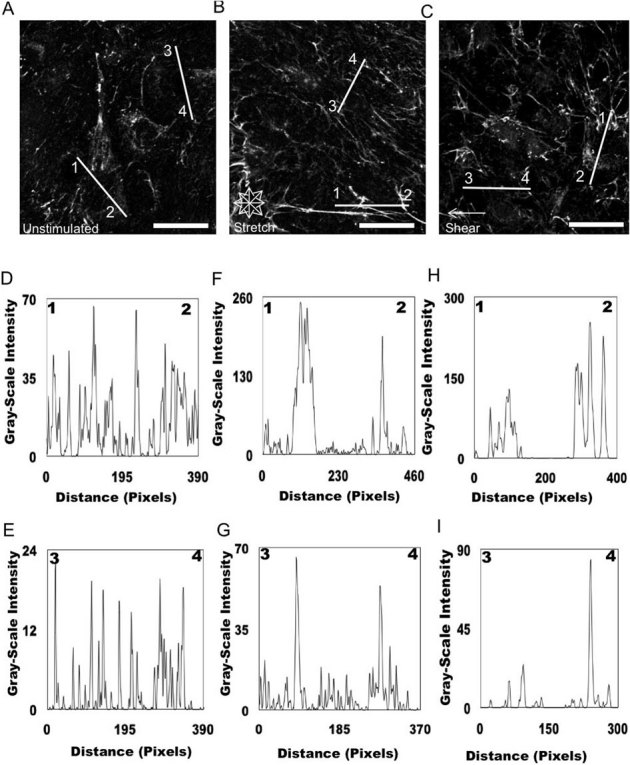
We next quantified this FN response under mechanical stimulation. Our approach was to deconvolve and analyze using ImageJ the immunofluorescent images of FN that were unstimulated (Fig. 3A), exposed to equibiaxial stretch (Fig. 3B), and exposed to shear fluid flow (Fig. 3C). Analysis of the distribution and density of unstimulated FN further validated our observations revealing randomly distributed FN fibrils, which are represented as a function of intensity in gray-scale values due to the FN staining (Figs. 3D and 3E). FN secreted by fibroblasts exposed to equibiaxial stretching displayed an increase in the density of FN fibrils. These fibrils were also observed to localize at the cell periphery (Figs. 3F and 3G). In comparison, unstimulated cells displayed a lower fibril density along with the FN being distributed throughout the entire cell. We then wanted to determine whether this observed behavior was unique to a specific mode of mechanical stimulation (equibiaxial stretch) by applying a separate mode of mechanical stimulation (fluid shear flow) to the cells. These two modes of stimulation have previously been shown to produce distinct cellular responses34,43. FN distribution in cells exposed to shear fluid flow was also found to localize at the cell periphery and exhibit an observable increase in FN fibrils (Figs. 3H and 3I). The response of the cells to fluid shear was similar to the cells exposed to equibiaxial stretch and not unstimulated cells.
Figure 3. Analysis of FN organization of cells unstimulated and exposed to mechanical stimulation.
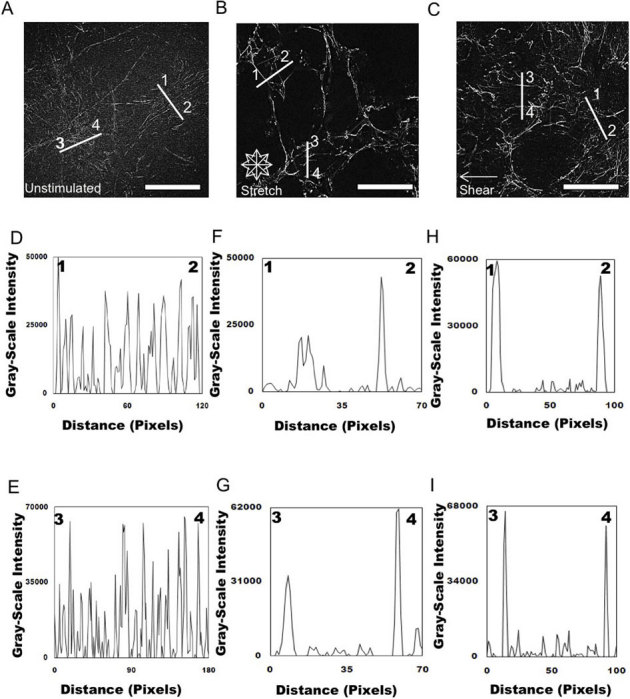
Epifluorescent images were imported into ImageJ software and deconvolved to analyze the distribution of unstimulated (A) and mechanically stimulated (equibiaxial stretch (B) shear fluid flow (C) FN across the cell. Representative plots of intensity profiles plotted as a function of distance (measured in pixels) versus intensity (measured in gray-scale values) showed FN distribution for unstimulated cells (D) line 1–2 and (E) line 3–4. The multiple fluorescence intensity peaks in a diversity of areas revealed that FN did not seem to be localized in specific areas within the cell. Plots of intensity profiles of FN distribution after 24 hours of 10% equibiaxial stretch (F) line 1–2 and (G) line 3–4 revealed FN redistributed around the cell periphery, which was reflected in the distinct fluorescence intensity peaks located at the ends of the lines, indicating the periphery of the cell. Analysis of FN distribution after 24 hours of 6±3 dynes/cm2 of fluid flow shear stress, (H) line 1–2 and (I) line 3–4 revealed that the FN was distributed around the cell periphery, which was reflected in the distinct fluorescence intensity peaks located at the ends of the lines indicating the periphery of the cell. (scale bar = 10 μm). On average 100 cells (20 – 30 lines/cell) were analyzed for all of the conditions; these are representative responses.
After establishing the effects of mechanical stimulation on FN matrix formation and distribution, our subsequent goal was to use mechanical stimulation to probe a chemical pathway known to be related to FN function. We examined this by coupling mechanical stimulation with the disruption of Rho activity using C3 transferase. Rho disruption by C3 transferase was done by initially incubating cells with C3 transferase in serum-free medium for 24 hours and replenishing medium with serum media prior to testing. Figure 4 reveals an observable visible decrease in FN density and intensity with respect to cells that were not treated with C3 transferase. Epifluorescent images of FN secreted by fibroblasts that were unstimulated (Fig. 5A), exposed to equibiaxial stretch (Fig. 5B), or were stimulated with fluid shear stress (Fig. 5C) after being treated with C3 transferase were analyzed. Analysis of deconvolved, epifluorescent FN images of unstimulated (Figs. 5D and 5E) cells indicated a similar distinct response relative to cells lacking the C3 transferase treatment (Figs. 3D and 3E). FN of unstimulated cells exposed to C3 transferase was found to exist as fibrils that occur at localized areas in the cell. However FN from unstimulated cells (which served as an additional control) that were incubated in serum-free medium without C3 transferase for 24 hours and subsequently placed in serum medium prior to testing was observed to exist as small, punctate structures localized around the nuclear periphery (Supp. Fig. 1). FN in cells exposed to equibiaxial stretch (Figs. 5F and 5G) and shear fluid flow (Figs. 5H and 5I) still exhibited FN fibril localization at the cell periphery, despite treatment by C3 transferase, although there was an observable decrease in FN density and intensity when compared with the unstimulated cells.
Figure 4. Examining the effects of chemical and mechanical stimulation on FN.
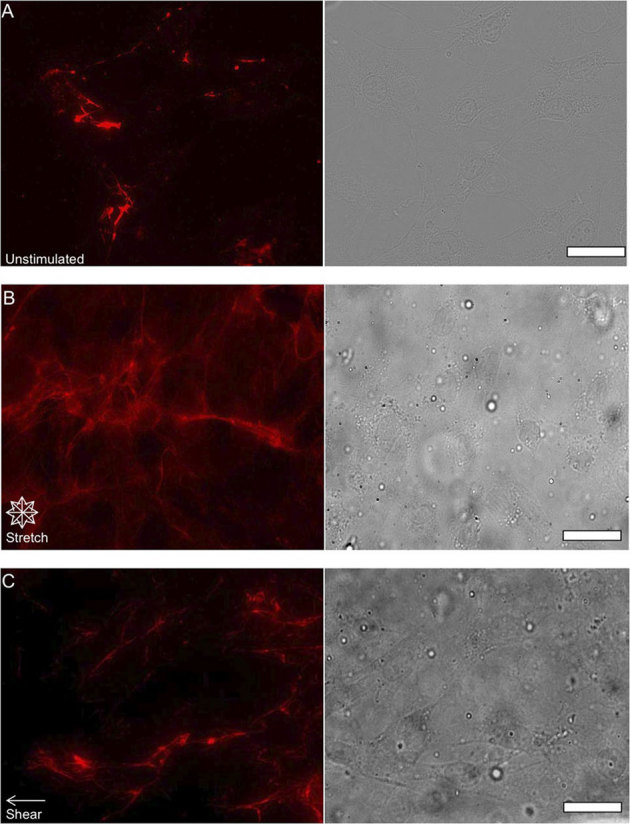
Epifluorescent images of FN (red pseudo color) and phase contrast images of NIH 3T3 fibroblast morphology after inhibition of Rho activity with C3 transferase that were unstimulated (A) after 24 hours of 10% equibiaxial strain (B) and after 24 hours of 6±3 dynes/cm2 of fluid shear stress (C) Cells exposed to mechanical stimulation appear to exhibit an increase in fibril formation as well as forming thicker FN bundles, but at lower densities relative to cells not treated with C3 transferase. (scale bars = 10 μm).
Figure 5. Analysis of FN organization after disruption of Rho activity via addition of C3 transferase and exposure to mechanical stimulation.
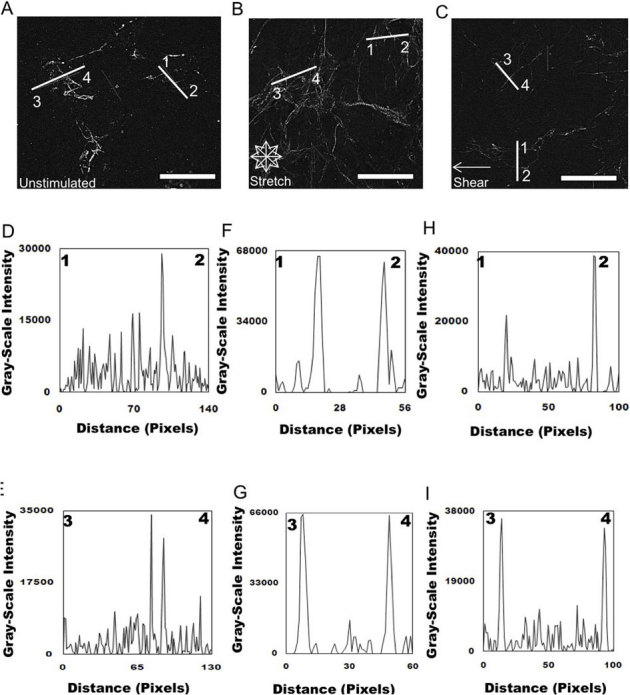
Epifluorescent images were imported into ImageJ software and deconvolved to analyze the distribution of unstimulated (A) and mechanically stimulated (equibiaxial stretch (B) shear fluid flow (C) FN across the cell. Representative plots of intensity profiles plotted as a function of distance (measured in pixels) versus intensity (measured in gray-scale values) showed FN distribution for unstimulated cells (D) line 1–2 and (E) line 3–4. FN distribution of unstimulated cells exposed to C3 transferase was found to occur at only localized areas in the cell. Plots of intensity profiles of FN distribution after 24 hours of 10% equibiaxial stretch (F) line 1–2 and (G) line 3–4 revealed FN redistributed around the cell periphery, which was reflected in the distinct fluorescence intensity peaks located at the ends of the lines, indicating the periphery of the cell. Analysis of FN distribution after 24 hours of 6±3 dynes/cm2 of fluid flow shear stress, (H) line 1–2 and (I) line 3–4 revealed that the FN was distributed around the cell periphery, which was reflected in the distinct fluorescence intensity peaks located at the ends of the lines indicating the periphery of the cell. (scale bar = 10 μm).
Next, we examined the cell-substrate interactions by mechanically stimulating cells on rhodamine labeled FN thus allowing us to track the structural reorganization of substrate coated as well as cell secreted FN in response to mechanical stimulation. Since the FN matrix assembles into a 3-D matrix over time, we used confocal microscopy to acquire high-resolution Z-stacks to further characterize matrix reorganization and localization. Volume rendering of unstimulated cells (Figure 6 & Supp. Movie 1) revealed results similar to epifluorescence images where FN fibrils were found to localize throughout the basal surface of the cell. Also substrate coated rhodamine-FN was observed to remain structurally unmodified. However FN matrices of the mechanically stimulated cells revealed distinct structural organization patterns. Fibroblasts stimulated by equibiaxial stretch (Figure 7 & Supp. Movie 2) were observed to display highly dense fibrils mainly located around the cellular periphery (Figure 6). Once again though, surface labeled FN remained structurally unperturbed. Cells exposed to shear stress (Figure 8 and Supp. Movie 3) displayed results similar to cells stretched equibiaxially where a more structurally dense fibrillar matrix was observed, but surface coated FN was observed to be structurally rearranged in response to mechanical stimulation.
Figure 6. Probing cell-substrate interactions of unstimulated cells through high-resolution confocal imaging.
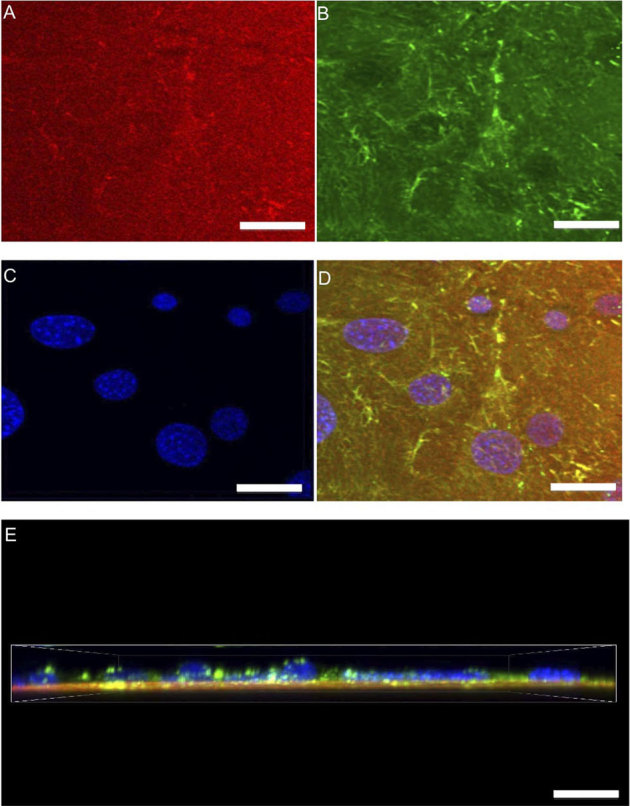
Z-stacks (0.5 μm steps) of unstimulated cells were captured to analyze the labeled substrate FN and its three-dimensional response modified during matrix formation. A top view displays substrate FN (A) cellular fibronectin (B) DAPI (C) and a merged image (D) of all structures. Also a merged side view (E) is presented. Unstimulated cells were observed to have randomly distributed FN fibrils with the substrate FN appearing to not change. (scale bars = 5 μm).
Figure 7. Probing cell-substrate interactions of cells stimulated by equibiaxial stretch through high-resolution confocal imaging.
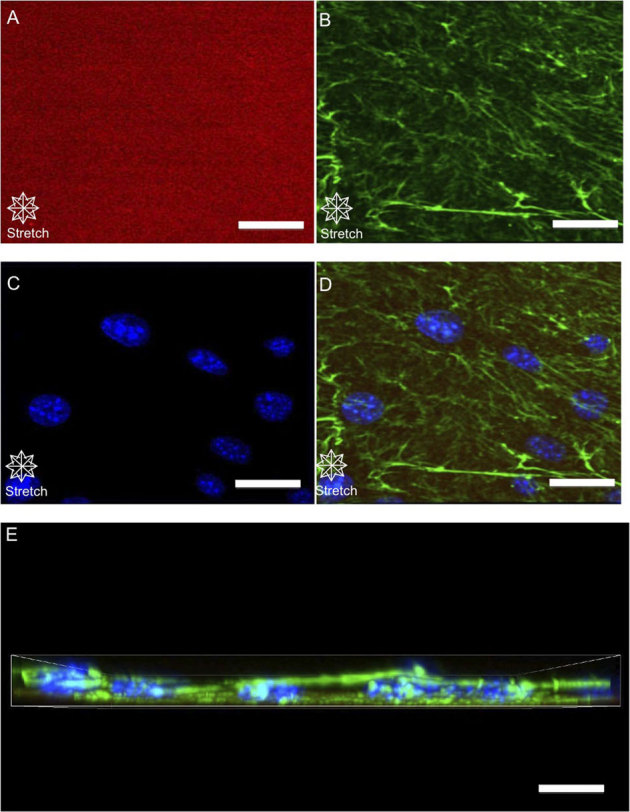
Z-stacks (0.5 μm steps) of equibiaxially stretched cells were captured to examine the FN under equibiaxial stretch. A top view displays substrate FN (A) cellular fibronectin (B) DAPI (C) and a merged image (D) of all structures, and a merged side view (E) Mechanically stimulated cells were observed to have a more structurally dense FN matrix compared to unstimulated while the substrate FN remained unstimulated. (scale bars = 5 μm).
Figure 8. Probing cell-substrate interactions of cells stimulated by shear fluid flow through high-resolution confocal imaging.
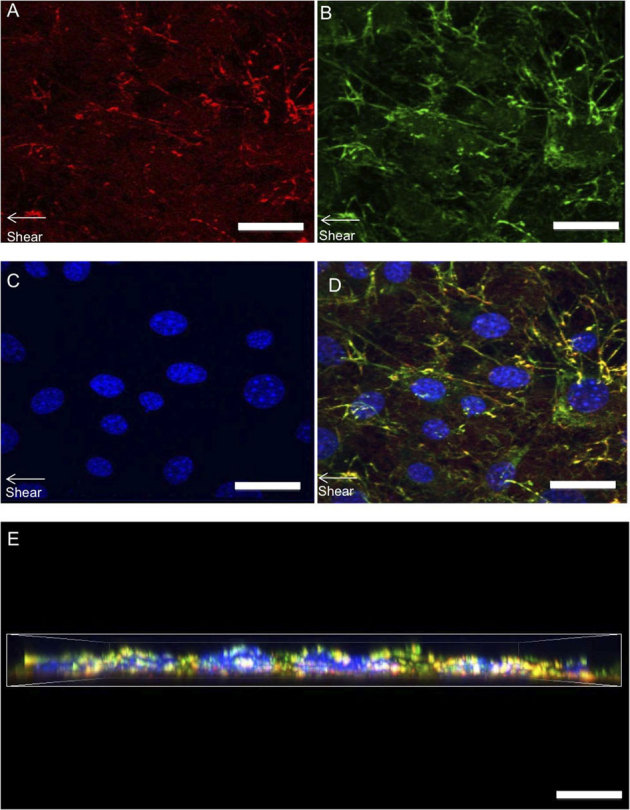
Z-stacks (0.5 μm steps) of sheared cells were taken to analyze FN response. A top view displays substrate FN (A) cellular fibronectin (B) DAPI (C) and a merged image (D) of all structures, and a merged side view (E). Mechanically stimulated cells were observed to have a more structurally dense FN matrix compared to unstimulated while the substrate FN was observed to be structurally modified in response to mechanical stimulation. (scale bars = 5 μm).
To quantify the behavior observed through confocal microscopy, Z-stacks of cellular FN were combined into a single, focused image (Figs. 9A–C) and analyzed using ImageJ. Results obtained from the analysis of the focused images of cells that were unstimulated (Figs. 9D and 9E) and exposed to equibiaxial stretch (Figs. 9F and 9G) or fluid shear stress (Figs. 9H and 9I) agreed closely with analyzed epifluorescent images (Figs. 5D–I). Unstimulated cells were observed to have randomly distributed fibronectin fibrils throughout the cell body while cells exposed to equibiaxial stretch and fluid shear stress were observed to contain fibronectin fibrils localized around the cell periphery.
Figure 9. Analysis of FN organization of cell-substrate interactions of cells exposured to mechanical stimulation.
Focused, Z-stack images were imported into ImageJ to analyze the distribution of unstimulated (A) and mechanically stimulated (equibiaxial stretch (B) shear fluid flow (C) FN across the cell. Representative graphs of intensity profiles plotted as a function of distance (measured in pixels) versus intensity (measured in gray-scale values) showed FN distribution for unstimulated cells (D) line 1–2 and (E) line 3–4. The multiple fluorescence intensity peaks in a diversity of areas revealed that FN did not seem to be localized in specific areas within the cell. Plots of intensity profiles of extracellular FN distribution after 24 hours of 10% equibiaxial stretch (F) line 1–2 and (G) line 3–4 revealed FN redistributed around the cell periphery. Analysis of FN distribution after 24 hours of 6±3 dynes/cm2 of fluid flow shear stress, (H) line 1–2 and (I) line 3–4 revealed that the FN was distributed around the cell periphery. (scale bar = 5 μm).
Discussion
As one begins to probe the mechanisms by which abnormal cell behavior alters FN function, it is critically important to understand how FN is distributed in its native environment. Answering this question must, naturally, include an examination of physiologically relevant mechanical and chemical factors. This is important since abnormal FN function has many implications. For example diabetes-induced abnormal FN behavior has been linked to increased thickness and permeability of basement membranes of various tissues40,44. These diabetes-induced complications often lead to diseases such as micro- and macroangiopathy as well as atherosclerotic lesions44. In addition irregular FN function may also result in impaired wound healing31,45, a coordinated process that involves inflammation, matrix deposition, and remodeling by fibroblasts. Impairments to this process can cause a significant problem as well, in short wounds heal poorly46. In an effort to better understand the mechanisms that govern these phenomena, many studies have investigated abnormal and normal FN activity by chemical means, but limited studies probing mechanical contributions to this response exist, relative to the studies on chemical manipulations. Fibroblasts in general are known to be a vastly critical and mechanically diverse cell type that is responsible for ECM deposition and remodeling, secretion of various growth factors in important cell-cell communication processes, as well as the formation of many connective tissues in the body32, which implies that any alteration in behavior from their normal state could have extensive detrimental consequences. Many studies have attempted to better understand these phenomena40,47,48 by probing the abnormal expression of growth factors as well as abnormal matrix deposition and remodeling through various biochemical or biophysical techniques. However, few have investigated matrix deposition that may result from this with respect to the mechanical environment.
In this paper, our goal was to investigate the effects of mechanical stimulation on FN matrix formation and distribution, as FN is an ECM protein that has been found to be important in numerous cellular processes3,7. This protein also has many unique features including its ability to be structurally rearranged by the cell in response to mechanical stimulation. Here, fibroblasts exposed to equibiaxial stretch and fluid shear stress revealed an observable increase in FN fibrils and in denser FN matrix as measured through an increased immunofluorescent intensity that was quantified using ImageJ software. These findings support the proposed importance of mechanical forces in FN modulation. While strain-induced FN behavior has been demonstrated in previous studies28, few have connected this effect to other modes of mechanical stimulation, such as shear fluid flow. Upon further examination of the FN matrix, FN was observed to localize to the cell periphery after application of shear fluid flow and equibiaxial stretching while unstimulated cells contained randomly distributed FN throughout the cell. We further examined the response by coupling mechanical stimulation with chemical disruption of a pathway that is directly linked to FN and mechanical stimulation. In this approach, we chemically disrupted the Rho pathway with C3 transferase since proteins in the Rho family of GTPases have been demonstrated to be activated during abnormal FN synthesis in various cell types and tissues1. In addition, the inhibition of the Rho pathway (specifically with C3 transferase) has been demonstrated to affect FN matrix assembly13,14. Rho inhibition by C3 transferase caused significantly less visible FN fibrils. However, FN was observed to localize at the cell periphery after mechanical stimulation despite inhibition of Rho, suggesting that this phenomena may not be solely Rho dependent. Alternatively, there may be a mechanical threshold that must be reached before inhibition of Rho begins to take effect. Also when probing the interactions cells had with pre-coated substrate fibronectin, only cells exposed to fluid shear flow were observed to utilize the substrate fibronectin in response to mechanical stimulation. While the driving force behind this phenomenon is unknown we hypothesize that there may also be a mechanical threshold present that serves as part of a feedback loop, which governs this behavior. These results demonstrate the importance of mechanics on ECM production and distribution and provide an analysis of FN response in perturbed states that may be linked to many pathological abnormalities such as diabetes. We believe these studies will lay the groundwork for further examination of stimulation pathways and their role in ECM-related synthesis and matrix formation.
Methods
Cell culture
NIH 3T3 fibroblasts were seeded at a concentration of 1000 cells/cm2 on polydimethylsiloxane (PDMS) substrates that were coated with human fibronectin (10 μg in 1 mL PBS; BD Biosciences, San Jose, CA, USA; No.: 39410) or rhodamine fibronectin (10 μg in 1 mL PBS; Cytoskelton, Inc., Denver, CO, USA; No.:FNR01). A base-to-curing agent ratio of 10∶1 for the PDMS was used for this study resulting in a Young's Modulus of 2000 kPa for the polymer substrate (26). Cell cultures were maintained at 37°C under 5% carbon dioxide in Dulbecco's modified Eagle's medium supplemented with 10% calf serum, 0.3 mg/mL glutamine, 100 μg/mL streptomycin, 100 U/mL penicillin, and 20 mM N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid at pH of 7.4. Cells were incubated for 24 hours prior to testing to allow for assembly of the FN matrix; the media was also replenished prior to testing.
Mechanical stimulation-equibiaxial stretching
Mechanical stimulation by equibiaxial stretching was performed in an incubator under cell culture conditions previously described using the method previously described41,42 for 24 hours. Our system uses compressed air to induce a hydrostatic pressure across the bottom surface of a PDMS membrane. The circular boundary conditions of our system, allow us to apply an equibiaxial strain of 10% to the basal surface of cells cultured on the center of the flexible silicone membranes21.
Mechanical stimulation-shear fluid flow
A custom-fabricated device that we previously developed42 was used to induce shear fluid flow on cells in an incubator while maintaining cell culture conditions. In brief, the device was a rectangular chamber (length = 5 cm, width = 1 cm and height = 2 cm) that used a variable-flow peristaltic pump (Fisher Scientific, Pittsburgh, PA; No.13-867-2) to produce a unidirectional, laminar fluid flow across the apical surface of single cells cultured on PDMS membranes. This design was advantageous since it allowed for probing the effects of shear stress on cell behavior while the cells were on a flexible substrate. Through this PDMS substrate, the stiffness can be adjusted to become more physiologically relevant when compared to other methods, which generally use substrates that are significantly stiffer and much less physiologically relevant from a mechanical environment perspective49,50. Our device used a peristaltic flow pump to impose shear stresses in the range of 6±3 dynes/cm2 for 24 hours.
Chemical modulator - Rho inhibitor
Cell-permeable C3 transferase (Cytoskeleton, Denver, CO, USA; No.:CT04) was used to disrupt Rho activity through the inactivation of Rho A, Rho B, and Rho C. This was accomplished by incubating cells with 5 μg/mL of the C3 transferase in serum free media 24 hours prior to testing. Successful Rho disruption was observed through optical microscopy and verified (according to manufacturer's instructions) as having decreased cell spreading; which has been shown by others as well10,13. After C3 transferase treatment, the medium was replaced with serum-containing medium and mechanical stimulation of cells began.
Immunostaining
Unstimulated, mechanically stimulated, and chemically stimulated cells attached to the PDMS substrate were immediately fixed with 4% paraformaldahyde and prepared for visualization. FN was labelled by permeabilizing cells with 0.2% Triton-X 100, followed by staining with a monoclonal mouse anti-FN primary antibody (Sigma-Aldrich, Saint Louis, MO, USA; No.:F0791) or using polyclonal rabbit anti-FN primary antibody (Sigma-Aldrich, Saint Louis, MO, USA; No.:F3648) and Alexa Flour® 594 goat anti-mouse IgG (Molecular Probes, Carlsbad, CA, USA; No.:A-11032) or Alexa Flour® 488 goat anti-rabbit IgG (Molecular Probes, Carlsbad, CA, USA; No.:A-11034) secondary antibody. Following staining, the cells were mounted with fluoromount-G or fluoromout-G with DAPI, and sealed under a coverslip.
Epifluorescence and confocal microscopy
Following immunostaining, cells were examined using an optical microscope (Zeiss Axiovert 200; Zander IVF, Vero Beach, FL, USA) with a 63X high numerical aperture (NA = 1.4) oil immersion objective. This approach allowed us to image FN using a tetramethylrhodamine isothiocyanate (TRITC; correspondingly pseudo-colored red) filter set with epifluorescence microscopy. All immunofluorescent images of FN were captured with an InsightTM QE Monochrome CCD camera at 17.4 frames per second with a 0.4 ms exposure time and brightness intensity of 80% to ensure that all imaging was performed under consistent lighting conditions. Cell morphology was visualized using phase contrast microscopy. Also, high resolution Z–stacks were acquired using a Nikon A1 laser confocal system (0.5 μm steps) combined with an optical microscope (Nikon Eclipse TI series) and 60X (NA = 1.4) oil immersion objective which allowed for imaging of rhodamine FN, Alexa Flour 488, and DAPI.
Image analysis
The images used to study the effects of exogenous mechanical forces applied using our system on FN were processed using ImageJ software (downloaded from the National Institutes of Health; http://rsb.info.nih.gov/ij/download.html). Epifluorescent images of FN were imported into the ImageJ software and deconvolved to visualize FN. The FN images were separately overlayed on their corresponding phase contrast images to visualize the ECM's location in individual cells. This was followed by placing horizontal and vertical lines of various orientations and lengths across the length of the cell and measuring the intensity in gray-scale values at each point along the line. Thus, we could measure changes in intensity related to the FN staining across the width of cell bodies. Similar methods have been used by previous groups51,52. Also on average over 20 lines were drawn on each cell. The lines displayed on deconvolved, immunofluorescent images (Figs. 3, 5, and 9) used for fluorescence intensity and therefore FN localization analysis are representative of the analysis done, although many more were drawn during analysis. More details on this analysis are shown in Supplementary Figure 2. In addition, Nikon NIS-Elements was used to create a volume rendering of Z–stacks. The extended depth of focus (EDF) module within NIS–Elements was used to combine multiple Z–stack images of cellular FN into a single, focused image, that was imported into ImageJ and analyzed using the same method previously described for epifluorescent images. ImageJ was also used to enhance image quality by adjusting brightness and contrast.
Author Contributions
R.L.S.Jr. performed experiments, analyzed data, and wrote manuscript. J.D.Y. performed experiments. C.-M.C., R.M.B. and P.R.L. designed experiments and revised the manuscript.
Supplementary Material
Supporting Information
Supplementary movie 1
Supplementary movie 2
Supplementary movie 3
Acknowledgments
We would like to acknowledge Gregory Gibson, Donna Stolz at the Center for Biologic Imaging, at the University of Pittsburgh for the training and use of the Nikon A1 confocal microscope. This work was supported in part by the National Science Foundation (CMMI-0856187, CMMI-1013748) and the Office of Naval Research (N000140910215) (P.R.L.). start-up funds, and a grant for Interactive Nano/MicroElectroMechanical Components and Systems from National Tsing Hua University, Taiwan (C.-M.C.). R. L. S. Jr. was supported by the National Science Foundation Graduate Research Fellowship.
References
- Lam S. et al.. Glucose-induced fibronectin and collagen type III expression in renal fibroblasts can occur independent of TGF-beta1. Kidney Int 63, 878–888 (2003). [DOI] [PubMed] [Google Scholar]
- Midwood K. S., Valenick L. V., Hsia H. C. & Schwarzbauer J. E. Coregulation of fibronectin signaling and matrix contraction by tenascin-C and syndecan-4. Mol Biol Cell 15, 5670–5677 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. Cell surface interactions with extracellular materials. Ann. Rev. Biochem. 52, 761–799 (1983). [DOI] [PubMed] [Google Scholar]
- Wierzbicka-Patynowski I. & Schwarzbauer J. E. The ins and outs of fibronectin matrix assembly. J Cell Sci 116, 3269–3276 (2003). [DOI] [PubMed] [Google Scholar]
- Woods A. , Longley R. L., Tumova S., Couchman J. R. Syndecan-4 binding to the high affinity heparin-binding domain of fibronectin drives focal adhesion formation in fibroblasts. Arch. Biochem. and Biophys 374, 66–72 (2000). [DOI] [PubMed] [Google Scholar]
- Wierzbicka-Patynowski I. & Schwarzbauer J. E. Regulatory role for SRC and phosphatidylinositol 3-kinase in initiation of fibronectin matrix assembly. J Biol Chem 277, 19703–19708 (2002). [DOI] [PubMed] [Google Scholar]
- Hynes R. Molecular biology of fibronectin. Annu Rev Cell Biol 1, 67–90 (1985). [DOI] [PubMed] [Google Scholar]
- Ohashi T., Kiehart D. P. & Erickson H. P. Dual labeling of the fibronectin matrix and actin cytoskeleton with green fluorescent protein variants. J Cell Sci 115, 1221–1229 (2002). [DOI] [PubMed] [Google Scholar]
- Birchmeier C., Kreis T. E., Eppenberger H. M., Winterhalter K. H. & Birchmeier W. Corrugated attachment membrane in WI-38 fibroblasts: alternating fibronectin fibers and actin-containing focal contacts. Proc Natl Acad Sci U S A 77, 4108–4112 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdoulous S., Orend G., MacKenna D. A., Pasqualini R. & Ruoslahti E. Fibronectin matrix regulates activation of RHO and CDC42 GTPases and cell cycle progression. J Cell Biol 143, 267–276 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science 279, 509–514 (1998). [DOI] [PubMed] [Google Scholar]
- Hall A. & Nobes C. D. Rho GTPases: molecular switches that control the organization and dynamics of the actin cytoskeleton. Philos Trans R Soc Lond B Biol Sci 355 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Peyruchaud O., French K. J., Magnusson M. K. & Mosher D. F. Sphingosine 1-phosphate stimulates fibronectin matrix assembly through a Rho-dependent signal pathway. Blood 93, 2984–2990 (1999). [PubMed] [Google Scholar]
- Zhang Q., Magnusson M. K. & Mosher D. F. Lysophosphatidic acid and microtubule-destabilizing agents stimulate fibronectin matrix assembly through Rho-dependent actin stress fiber formation and cell contraction. Mol Biol Cell 8, 1415–1425 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy K. & Ross J. M. Shear stress prevents fibronectin binding protein-mediated Staphylococcus aureus adhesion to resting endothelial cells. Infect Immun 69 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen M., Wei C. & Yamada K. M. Cell and fibronectin dynamics during branching morphogenesis. J Cell Sci 119, 3376–3384 (2006). [DOI] [PubMed] [Google Scholar]
- Trinh L. A. & Stainier D. Y. Fibronectin regulates epithelial organization during myocardial migration in zebrafish. Dev Cell 6, 371–382 (2004). [DOI] [PubMed] [Google Scholar]
- Sottile J., Hocking D. C. & Swiatek P. J. Fibronectin matrix assembly enhances adhesion-dependent cell growth. J Cell Sci 111 ( Pt 19), 2933–2943 (1998). [DOI] [PubMed] [Google Scholar]
- Niklason L. E. et al.. Functional arteries grown in vitro. Science 284, 489–493 (1999). [DOI] [PubMed] [Google Scholar]
- Fornaro M. et al.. Fibronectin protects prostate cancer cells from tumor necrosis factor-alpha-induced apoptosis via the AKT/survivin pathway. J Biol Chem 278, 50402–50411 (2003). [DOI] [PubMed] [Google Scholar]
- Bellin R. M. et al.. Defining the role of syndecan-4 in mechanotransduction using surface-modification approaches. Proc Natl Acad Sci U S A 106, 22102–22107 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol 292, H1209–1224 (2007). [DOI] [PubMed] [Google Scholar]
- Owan I. et al.. Mechanotransduction in bone: osteoblasts are more responsive to fluid forces than mechanical strain. Am J Physiol 273, C810–815 (1997). [DOI] [PubMed] [Google Scholar]
- Lin Y. W., Cheng C. M., Leduc P. R. & Chen C. C. Understanding sensory nerve mechanotransduction through localized elastomeric matrix control. PLoS One 4, e4293 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C. M., Steward R. L. Jr & LeDuc P. R. Probing cell structure by controlling the mechanical environment with cell-substrate interactions. J Biomech 42, 187–192 (2009). [DOI] [PubMed] [Google Scholar]
- Chou S. Y., Cheng C. M. & LeDuc P. R. Composite polymer systems with control of local substrate elasticity and their effect on cytoskeletal and morphological characteristics of adherent cells. Biomaterials 30, 3136–3142 (2009). [DOI] [PubMed] [Google Scholar]
- Wang J. H., Thampatty B. P., Lin J. S. & Im H. J. Mechanoregulation of gene expression in fibroblasts. Gene 391, 1–15 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourgeon E., Xu J., Tanswell A. K., Liu M. & Post M. Mechanical strain-induced posttranscriptional regulation of fibronectin production in fetal lung cells. Am J Physiol 277, L142–149 (1999). [DOI] [PubMed] [Google Scholar]
- Defilippi P. et al.. Actin cytoskeleton organization in response to integrin-mediated adhesion. Microsc Res Tech 47, 67–78 (1999). [DOI] [PubMed] [Google Scholar]
- Mitra S. K., Hanson D. A. & Schlaepfer D. D. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol 6, 56–68 (2005). [DOI] [PubMed] [Google Scholar]
- Lerman O. Z., Galiano R. D., Armour M., Levine J. P. & Gurtner G. C. Cellular dysfunction in the diabetic fibroblast: impairment in migration, vascular endothelial growth factor production, and response to hypoxia. Am J Pathol 162, 303–312 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood M., McGrouther D. A. & Brown R. A. Fibroblast responses to mechanical forces. Proc Inst Mech Eng H 212, 85–92 (1998). [DOI] [PubMed] [Google Scholar]
- Danciu T. E., Gagari E., Adam R. M., Damoulis P. D. & Freeman M. R. Mechanical strain delivers anti-apoptotic and proliferative signals to gingival fibroblasts. J Dent Res 83, 596–601 (2004). [DOI] [PubMed] [Google Scholar]
- Grierson J. P. & Meldolesi J. Shear stress-induced [Ca2+]i transients and oscillations in mouse fibroblasts are mediated by endogenously released ATP. J Biol Chem 270, 4451–4456 (1995). [DOI] [PubMed] [Google Scholar]
- Garanich J. S., Mathura R. A., Shi Z. D. & Tarbell J. M. Effects of fluid shear stress on adventitial fibroblast migration: implications for flow-mediated mechanisms of arterialization and intimal hyperplasia. Am J Physiol Heart Circ Physiol 292, H3128–3135 (2007). [DOI] [PubMed] [Google Scholar]
- Galante L. L. & Schwarzbauer J. E. Requirements for sulfate transport and the diastrophic dysplasia sulfate transporter in fibronectin matrix assembly. J Cell Biol 179, 999–1009 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan-Thanh L., Robert L., Derouette J. C. & Labat-Robert J. Increased biosynthesis and processing of fibronectin in fibroblasts from diabetic mice. Proc Natl Acad Sci U S A 84, 1911–1915 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Sala R., Cagliero E. & Lorenzi M. Overexpression of fibronectin induced by diabetes or high glucose: phenomenon with a memory. Proc Natl Acad Sci U S A 87, 404–408 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby I. A., Bisucci T., Hewitson T. D. & MacLellan D. G. Apoptosis is increased in a model of diabetes-impaired wound healing in genetically diabetic mice. Int J Biochem Cell Biol 29, 191–200 (1997). [DOI] [PubMed] [Google Scholar]
- Qian Y., Feldman E. & Pennathur S., Kretzler M., Brosius F. C. 3rd From fibrosis to sclerosis: mechanisms of glomerulosclerosis in diabetic nephropathy. Diabetes 57, 1439–1445 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicek J. D., Brelsford S., Ahluwalia P. & Leduc P. R. Integrated lithographic membranes and surface adhesion chemistry for three-dimensional cellular stimulation. Langmuir 20, 11552–11556 (2004). [DOI] [PubMed] [Google Scholar]
- Steward R. L. Jr, Cheng C. M., Wang D. L. & Leduc P. R. Probing Cell Structure Responses Through a Shear and Stretching Mechanical Stimulation Technique. Cell BIochem Biophys 56, 115–124 (2009). [DOI] [PubMed] [Google Scholar]
- Richard M. N., Deniset J. F., Kneesh A. L., Blackwood D. & Pierce G. N. Mechanical stretching stimulates smooth muscle cell growth, nuclear protein import, and nuclear pore expression through mitogen-activated protein kinase activation. J Biol Chem 282, 23081–23088 (2007). [DOI] [PubMed] [Google Scholar]
- Tsilibary E. C. Microvascular basement membranes in diabetes mellitus. J Pathol 200, 537–546 (2003). [DOI] [PubMed] [Google Scholar]
- Brem H. & Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest 117, 1219–1222 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitar M. S. Glucocorticoid dynamics and impaired wound healing in diabetes mellitus. Am J Pathol 152, 547–554 (1998). [PMC free article] [PubMed] [Google Scholar]
- Kanters S. D. et al.. Plasma levels of cellular fibronectin in diabetes. Diabetes Care 24, 323–327 (2001). [DOI] [PubMed] [Google Scholar]
- Loot M. A. et al.. Fibroblasts derived from chronic diabetic ulcers differ in their response to stimulation with EGF, IGF-I, bFGF and PDGF-AB compared to controls. Eur J Cell Biol 81, 153–160 (2002). [DOI] [PubMed] [Google Scholar]
- Albuquerque M. L., Waters C. M., Savla U., Schnaper H. W. & Flozak A. S. Shear stress enhances human endothelial cell wound closure in vitro. Am J Physiol Heart Circ Physiol 279, H293–302 (2000). [DOI] [PubMed] [Google Scholar]
- Brown T. D. Techniques for mechanical stimulation of cells in vitro: a review. J Biomech 33, 3–14 (2000). [DOI] [PubMed] [Google Scholar]
- Yi Q. & Coppolino M. G. Automated classification and quantification of F-actin-containing ruffles in confocal micrographs. Biotechniques 40, 745–746 (2006). [DOI] [PubMed] [Google Scholar]
- Swedlow J. R. Quantitative fluorescence microscopy and image deconvolution. Methods Cell Biol 72, 349–367 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supplementary movie 1
Supplementary movie 2
Supplementary movie 3


