Abstract
Drug abuse and transmission of HIV during pregnancy are major public health problems that adversely affect pregnant women, their children and surrounding communities. Programs that address this vulnerable population have the ability to be cost effective due to resulting cost savings for mother, child and society. Economic evaluations of programs that address these issues are an important tool to better understand the costs of services and create sustainable healthcare systems. This study critically examined economic evaluations of drug abuse treatment and HIV prevention programs in pregnant women. A systematic review was conducted using the criteria recommended by the Panel on Cost-Effectiveness in Health and Medicine and the British Medical Journal (BMJ) checklist for economic evaluations. The search identified 6 economic studies assessing drug abuse treatment for pregnant women, and 12 economic studies assessing programs that focus on prevention of mother-to-child transmission (PMTCT) of HIV. Results show that many programs for drug abuse treatment and PMTCT among pregnant women are cost-effective or even cost-saving. This review identified several shortcomings in methodology and lack of standardization of current economic evaluations. Efforts to improve methodological challenges will help make future studies more comparable and have more influence on policy makers, clinicians and the public.
Keywords: systematic review, economic evaluation, drug abuse treatment, prevention of mother-to-child transmission (PMTCT), HIV prevention, pregnant women
1. Introduction
Illicit drug use, high risk HIV behaviors and transmission of the human immunodeficiency virus (HIV) during pregnancy are significant public health problems that adversely affect pregnant women, their children and surrounding communities. According to a national survey in 2002 in the United States (U.S.), over 3% of pregnant women reported unlawful drug use and roughly 9% of pregnant women reported drinking alcohol (1) resulting in over 200,000 infants exposed in utero to illicit substances and over 800,000 infants exposed in utero to alcohol (21). Trends show that drug and alcohol use has been increasing in pregnant women over the past several decades (17, 73). This could be due to an increase in the perceived availability of drugs or a shift in attitude about drug use and the opinion that such use is harmless or only slightly risky (36). Drug use has a negative impact on maternal and child health; studies have found associations between illegal drug use and negative maternal and child health outcomes, including prematurity, low birth weight, perinatal death, and cognitive, behavioral, and physical problems during infancy and childhood (4, 23, 35, 40, 42, 49, 65).
One of the most severe consequences of illicit substance use is transmission of HIV. Data show how pregnancy, drug use and HIV transmission are entwined. Two young people age 13 to 29 in the United States become infected with HIV every hour of every day (79). Young heterosexual women represent the upcoming wave in the HIV epidemic in the United States; those most at risk include young women from the ”communities of color” and those with socio-economic difficulties, women who use drugs and women with multiple sex partners (11). In a longitudinal HIV incidence study, 449 HIV negative women were followed for 30 months, during which 2.4% of women converted to HIV seropositivity and 3 of the 4 seroconverters were pregnant (12). Maternal HIV infection is associated with some adverse effects on infants, including premature membrane inflammation, preterm delivery, and delivery of infants that are low-birth weight or small for gestational age (9, 37), in addition to mother-to-child HIV transmission, one of the most problematic perinatal outcomes to offspring.
Programs that address drug use and HIV transmission in pregnant women have been shown to be effective in preventing drug use and HIV transmission. Such programs vary greatly in intervention approach and effectiveness. One randomized controlled trial comparing women in an AIDS prevention group, a health promotion group and a no-intervention group found that only the AIDS prevention group, which focused on skills and knowledge specific to AIDS, induced a “moderate, consistent increase in knowledge and safer sex behaviors” (30). Another study looking at sexual HIV-risk behavior in substance-dependent teens compared education to behavioral skills training interventions. Adolescents who received skills training showed greater decreases in high-risk sexual activity and had more positive attitudes toward prevention (71). A third study comparing brief counseling, enhanced counseling, and “didactic prevention messages” (usual care) found that women in both brief and enhanced counseling groups had significantly lower rates of new STDs and higher self-reported 100% condom use compared to the didactic message group (33). Cognitive-behavioral therapies (6), in particular, have been shown to be effective in reducing sex risk behavior and lowering susceptibility to HIV infection (18, 33, 34, 48, 64, 70, 71).
Because resources are limited, effectiveness and cost-effectiveness must be taken into consideration. The World Health Organization (WHO) considers interventions that cost three times per-person income or less per quality-adjusted life year gained – an intervention that costs approximately $100,000 or less per QALY – to be cost-effective (81). Programs that target pregnant women have the potential to be cost-effective, even cost saving, due to fewer infants being born with low-birth-weight or physical, cognitive and behavioral problems, fewer perinatal deaths, and the significant health benefits and subsequent lower healthcare costs for mothers. While cost-effectiveness is just one of many factors that affect policy decisions, economic evaluations are essential for rational decision-making and to identify sustainable services. Cost-effectiveness (CEA) and cost-utility (CUA) analyses, in particular, are useful tools to compare the costs per unit of health outcome (e.g. life years, quality-adjusted life years) between interventions and can help determine whether a program is worth implementing or including as a covered service by hospitals or insurance companies (27). Cost-benefit analysis (CBA) translates benefits into monetary units for cost benefit ratios (e.g. 3:1). Economic evaluations are needed to determine optimal resource allocation and to identify efficient programs that support a sustainable healthcare system.
In this systematic review we summarize the available economic evaluations on drug abuse treatment and HIV prevention programs for pregnant women in the U.S. and internationally. We assess cost and outcomes data for patients, hospitals, and society and discuss the clinical and public health implications of our findings.
2. Methods
2.1 Search strategy and selection criteria
We conducted a comprehensive literature search employing rigorous search strategies for identifying and selecting studies. As recommended and described elsewhere (10, 19, 20, 32, 41, 45, 61, 63, 72), a systematic search entails study search criteria and specific inclusion criteria pertinent to the interventions being studied. Inclusion criteria for this study were economic evaluations of drug abuse treatment programs and HIV prevention programs for pregnant women. We searched PubMed and the British National Health Service Economic Evaluation Database for English-language articles using the search terms “economic” or “cost” or “cost effectiveness” or “cost utility” or “cost benefit” combined with the search terms “drug treatment” or “substance use treatment” or “substance abuse treatment” or “HIV prevention” and “pregnancy” or “pregnant women.”
The initial search identified 27 articles on drug abuse programs for pregnant women and 49 articles on HIV prevention programs for pregnant women. Two reviewers inspected study abstracts and selected for inclusion according to these predetermined criteria: (1) study is directed at the question identified; (2) study undertakes original economic analysis (reviews excluded); (3) study used an appropriate outcome measurement (health outcomes such as life expectancy, quality-adjusted life years (QALYs), abstinence or quit rates). A separate reviewer fact checked these selections. Methodological quality was assessed using standard inclusion criteria for economic evaluation endorsed by the Guide to Community Prevention Services (10). Under these criteria, studies must use one of four analytical methods recommended by Drummond and colleagues (20) (see Table 1).
Table 1.
Types of Economic Evaluations
|
Adopted from Drummond (1997) (20)
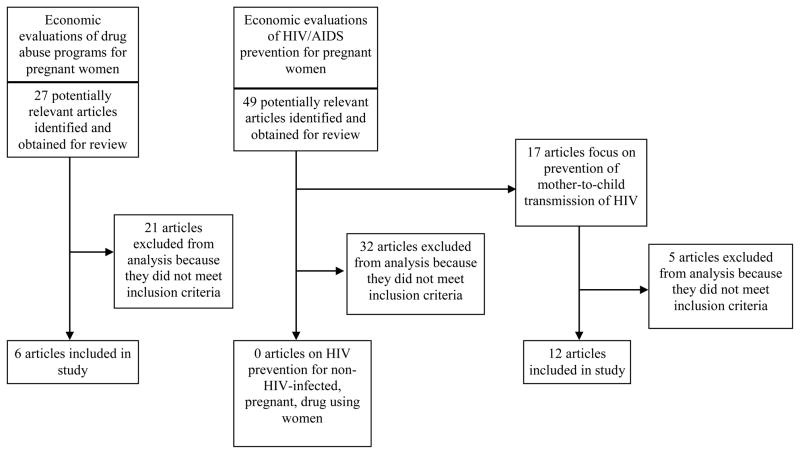
Of the 27 articles on drug abuse treatment programs for pregnant women identified, 21 were excluded because they did not meet inclusion criteria. This left six economic evaluations in our analysis. Of the 49 articles on HIV prevention for pregnant women identified, 17 articles focused on prevention of mother-to-child transmission (PMTCT) of HIV and 32 articles focused on another area of HIV prevention during pregnancy. Of the 17 PMTCT articles, 12 met inclusion criteria and were included in our analysis. Of the other HIV prevention articles, none of the 32 met inclusion criteria. Figure 1 summarizes our approach.
Figure 1.
Flow chart of study search and selection
2.2 Data extraction and analysis
Full copies of the final 18 economic evaluations were obtained and data was extracted by two people, one with training in decision analysis and cost, cost-benefit, cost-effectiveness, and cost-utility analyses. A separate reviewer fact checked data extractions for accuracy. Data extracted included type of economic evaluation, study design, main outcome measures, cost components, and study results (see Table 2).
Table 2.
Data extracted from included articles
|
Reprinted from Ruger and Emmons, 2008 (61)
The quality of included economic evaluations was assessed according to the Panel on Cost Effectiveness in Health and Medicine checklist for reporting reference-case cost-utility analyses, the British Medical Journal (BMJ) guidelines for economic submissions, and the data auditing form developed by researchers at the Harvard Center for Risk Analysis (29).
Differences in methodologies and interventions among studies prevented us from combining the original study results to obtain a summary cost per outcome metric of drug abuse treatments and HIV prevention programs. Due to the great heterogeneity in studies analyzed, we conducted a narrative synthesis (80) rather than a formal meta-analysis. Unlike a meta-analysis, narrative synthesis summarizes the type, statistical significance and distribution of program costs and effectiveness in lieu of a quantitative synthesis. Using a narrative synthesis allowed us to (1) examine studies as a whole while highlighting main features in study context; (2) compare primary study characteristics with each other; (3) identify major patterns in influencing factors, such as intervention type, comparison group, perspective, etc.; (4) consistently analyze studies vis-à-vis each other. In order to increase transparency, consistency and reproducibility often lacking in narrative syntheses, we relied on recommendations from expert review panels including the Guide to Community Prevention Services (10), as well as guidelines developed for the British Medical Journal (19) and the Panel on Cost Effectiveness in Health and Medicine (26).
3. Results
3.1 Economic evaluations of drug abuse treatment programs for pregnant women
Six studies meeting the inclusion criteria conducted economic evaluations of drug abuse treatment programs for pregnant women (see Table 3). Randomized-controlled trials are the gold standard of clinical evidence; economic evaluations should be planned in conjunction with randomized trials so that all cost components used during the interventions will be measured and valued in conjunction with the clinical trial. This improves the validity and reliability of intervention costs. No study used this design method, significantly limiting the study’s ability to reduce bias and confounding inherent in non-randomized study designs and to collect accurate cost data.
Table 3.
Summary of 6 economic evaluations of drug abuse treatment programs for pregnant women
| Characteristics | Svikis et al (1997) | Svikis et al (1998) | Jansson et al (1996) | Daley et al (2000) | Daley et al (2001) | Daley et al (2005) | |
|---|---|---|---|---|---|---|---|
| Method | Analysis type | CEA | CEA | CEA | CBA | CEA | CEA |
| Model type | Nonrandomized case-control | Nonrandomized case-control | Nonrandomized case-control | Multivariate regression | Multivariate regression | Multivariate regression | |
| Framing | Setting and population | Women with medical or psychosocial problems from drug use | Drug abusing women enrolled in urban, hospital-based obstetric clinic | Pregnant, drug-using women | Medicaid-eligible pregnant women receiving treatment in MA between 1992–1997 | Medicaid-eligible pregnant women receiving treatment in MA between 1992–1997 | Medicaid-eligible pregnant women receiving treatment in MA between 1992–1996 |
| Intervention (comparator) | 1 week residential care followed by intensive outpatient services through labor/delivery No treatment |
Weekly substance abuse support group No treatment |
Multidisciplinary CAPa program with individual and group counseling No treatment |
Methadone tx only Residential tx only Outpatient tx only Residential/outpatient tx Detoxification only |
Methadone tx only Residential tx only Outpatient tx only Residential/outpatient tx Detoxification only |
Methadone tx only Residential tx only Outpatient tx only Residential/outpatient tx Detoxification only |
|
| Perspective | Not reported | Not reported | Not reported | Taxpayers | Payer | Patient | |
| Time horizon | Birth and end of NICUb stay | 3 weeks after delivery | 1 year after birth | 1 year | Pregnancy and 6 months postpartum | Follow-up typically 6 months post-intake | |
| Effects | Main outcome and benefits measures | Need for and duration of NICU services | Maternal and neonatal outcomes (birth weights, 1min Apgar scores) | Need for and duration of NICU services | Time and costs of crime and of using criminal justice system | Infant birth weight | Quality of life index (QOLI) |
| Cost | Cost analysis (cost components) | Drug treatment costs NICU costs |
Short-term infant and maternal medical care costs | NICU costs | Drug treatment costs Cost of crime (victimization costs, criminal justice costs) |
Health care expenditure during pregnancy through 6 months postpartum | Drug treatment costs |
| Base year (costs) | 1991–1992 | 1989–1990 | Not available | 1998 | 1992–1997 | 1992–1996 | |
| Source (costs) | NICU rates, billing records | Hospital records | Hospital and program records | Bureau of Justice Statistics, National Crime Victimization Survey, National Police Budget | Addiction Severity Index, treatment records, Medicaid claims | Fiscal records from MA Department of Public Health Bureau of Substance Abuse Services | |
| Results | Summary results | Average net savings of $4,644 per mother/infant pair in treatment | Support group attendance decreased costs by $1,000 (maternal) and >$1,500 (infant/neonatal) and increased birth weight and 1min Apgar scores | Cost savings of nearly $5,000 per mother/infant pair | Net benefit ranged from $32,772 for residential only to $3,072 for detoxification | Compared to detoxification group, outpatient programs cost an additional $1,788 to increase birth weight by 139 grams and residential/outpatient tx cost an additional $17,211 to increase birth weight by 190g | Costs per QOLI ranged from $14,912 for detoxification to $44,291 for residential/outpatient |
Center for Addiction and Pregnancy (CAP)
Neonatal intensive care unit (NICU)
Three studies performed a cost-effectiveness analysis using a non-randomized study design. In the first study, researchers examined drug treatment and neonatal intensive care unit (NICU) costs for pregnant, drug-abusing women in either a multidisciplinary treatment program or a control group of women not receiving drug treatment (75). Outcome measures included infant birth weight, urine toxicology at delivery, APGAR scores and need for, and duration of, NICU services. NICU services and drug treatment costs were measured throughout the intervention. This study found that women in treatment had less drug use and that their infants had better clinical outcomes at delivery, including higher infant gestational age, birth weight and APGAR scores. Moreover, infants of women who had received drug treatment were less likely to need NICU services and had shorter duration of time in the NICU. In terms of total costs, this study found an average net savings of $4,644 per mother/infant pair for treatment patients. A second study examined differences in maternal and neonatal outcomes and medical cost data for pregnant, substance-abusing women who took part (N=54) in a weekly drug abuse support group and those who did not (N=67) (74). Investigators found that infants of support group participants had higher birth weights, better 1-minute APGAR scores, and that mean short-term medical care costs were nearly $1,000 (maternal) and over $1,500 (infant/neonatal) lower for support group participants compared to non-participants. Another study conducted a cost-effectiveness analysis on a matched cohort (31). Researchers looked at the Center for Addiction and Pregnancy (CAP) model of providing pediatric care, obstetric and gynecological services, family planning, and substance abuse treatment to mitigate obstacles to care for substance abusers during pregnancy. This study compared individuals who had participated in the CAP model with matched controls and found that participants enrolled in the CAP model reported almost $5,000 in cost savings.
Three economic evaluations conducted multivariate regression analyses. One cost-benefit analysis sought to examine the effect of substance abuse treatment for pregnant women on crime (14). This work assessed differences in criminal involvement pre-and post-treatment for a sample of 439 pregnant women who partook of Massachusetts state-funded treatment programs between 1992 and 1997. Costs and benefits of five treatments were measured: detoxification only (the minimal treatment comparison), residential only, methadone only, outpatient only, and residential/outpatient combined. Controlling for baseline difference between treatment groups, women in the two residential programs had significantly greater reductions in crime. Total benefits, measured by the avoided costs of crime net of treatment costs, spanned from $32,772 for residential only treatment to $3,072 for detoxification. In another cost-effectiveness analysis, researchers compared infant birth weights and perinatal health care spending for 445 Medicaid eligible pregnant women in Massachusetts who received one of five treatments (detoxification only, methadone, residential, outpatient, residential/outpatient) between 1992 and 1997 (15). Measurement of costs and outcomes were based on treatment records, Medicaid claims, the Addiction Severity Index (ASI), and birth certificates. The study founda direct relationship between the amount of care received and birth weight. For an additional $1,788 over the detoxification-only program, the outpatient program raised birth weight by 139 grams and was the most cost-effective. A final study developed a quality of life index (QOLI) using ASI items and preference weights from a community sample to compare the cost-effectiveness of five addiction treatments for pregnant women (16). The cost of treatment ranged from $2,535 for detoxification only to $10,817 for combined residential and outpatient treatment. Cost per QOLI extended from $14,912 to $44,291.
3.2 Economic evaluations of HIV prevention programs for pregnant women
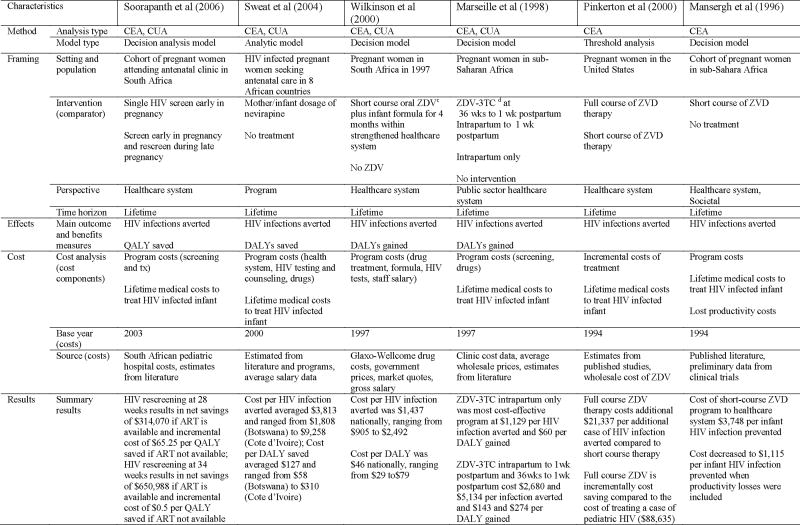
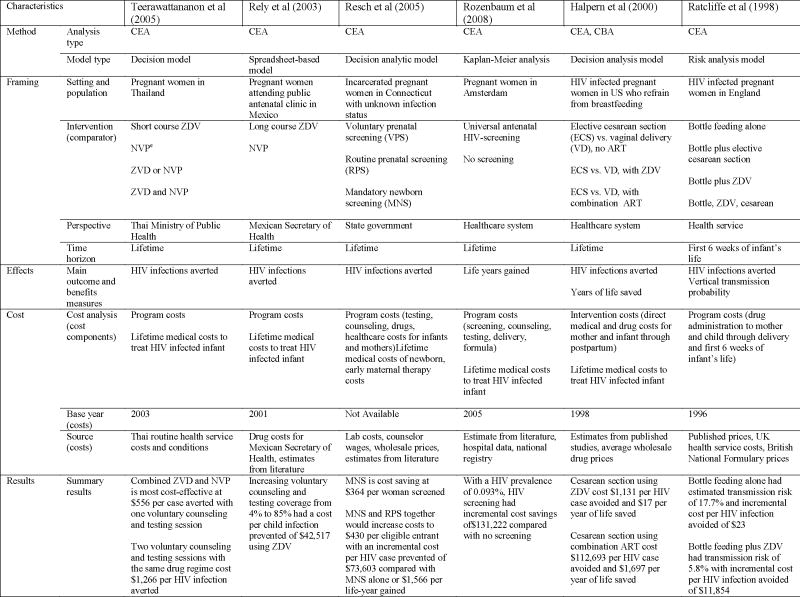
No studies conducting economic evaluations of HIV prevention programs for non-infected pregnant women were identified. Twelve studies conducting economic evaluations of programs to prevent mother-to-child transmission of HIV were analyzed (see Table 4). Of those, all were cost-effectiveness analyses: four were both CEA and CUA and one was both CEA and CBA. Although almost all twelve studies looked at infant infections averted as an outcome variable, study designs, perspectives and cost components differed substantially among studies.
Table 4.
Summary of 12 economic evaluations of preventing mother-to-child transmission of HIV/AIDS
|
|
Zidovudine (ZDV)
Lamivudine (3TC)
Nevirapine (NVP)
Four studies reported outcomes in terms of cost per disability-adjusted life years (DALYs) or quality-adjusted life years (QALYs). One study assessed the cost of late-pregnancy HIV rescreening in South Africa and other resource-limited settings in a cohort of 100,000 women (69). Costs included expected program costs and lifetime costs associated with treating infant HIV infections. This study found that HIV screening late in pregnancy (34 weeks) in women being treated with Zidovudine plus a single-dose of nevirapine resulted in a net savings of $650,988 if antiretroviral therapy (ART) was available and an incremental cost of $0.50 per QALY saved if ART was not available. A second study measuring the cost-effectiveness of nevirapine prophylaxis in eight African countries found the cost per HIV infection averted ranged from $1,808 (Botswana) to $9,258 (Cote d’Ivoire). The cost per DALY saved ranged from $58 (Botswana) to $310 (Cote d’Ivoire) (76). A third study in South Africa compared short course zidovudine plus infant formula within a strengthened health system to no zidovudine intervention and found that costs per HIV infection averted averaged $1,437 nationally with a cost per DALY of $46 nationally (82). A fourth study compared the cost of antiviral drug therapy administered at different times to each other and to no drug treatment in a hypothetical population in sub-Sahara Africa (39). Drug therapy during intrapartum only was found to be the most cost-effective, with a cost of $1,129 per HIV infection averted and a cost of $60 per DALY gained.
Four studies measured the cost-effectiveness of different drug treatment programs. One study compared the cost-effectiveness of full and short course zidovudine therapy in a model population in the U.S. (50). The study showed that full course zidovudine therapy costs an additional $21,337 per additional infant HIV infection prevented compared to short course therapy. This is substantially less than the estimated $88,635 it would cost to treat a case of pediatric HIV. Another study compared short-course zidovudine to no treatment in a hypothetical cohort in sub-Sahara Africa (38). Short-course zidovudine cost the healthcare system $3,748 per infant HIV infection prevented; when productivity losses were factored in, costs decreased to $1,115 per infant HIV infection prevented. A third study compared one or two voluntary counseling sessions with four antiretroviral therapy regimes and found that the cost of one voluntary counseling and testing session with zidovudine and nevirapine was the most cost-effective at $556 per HIV case averted (78). Two voluntary counseling and testing session with the same drug regimen cost $1,266 per infant HIV infection averted. In a cost-effectiveness analysis of HIV-infected pregnant women in Mexico, a low-prevalence setting, Zidovudine combined with increasing voluntary counseling and testing coverage from 4% to 85% resulted in a cost per child infection prevented of $42,517 (54).
Two studies looked at the cost-effectiveness of HIV screening programs. One economic analysis compared routine prenatal screening, voluntary prenatal screening, and mandatory newborn screening of incarcerated pregnant women who were unaware of their infection status (57). The results found that mandatory newborn screening is cost-saving, at a cost of $364 per woman screened. Mandatory newborn screening and routine prenatal screening together are most effective at preventing new infant HIV infections but increase costs per women screened to $430 with an incremental cost per HIV case averted of $73,603 and incremental cost per life-year gained of $1,566. A study of pregnant women in Amsterdam compared the costs of universal antenatal HIV screening to no screening in preventing HIV infant infections (58). This study found that, with an HIV prevalence rate of 0.093%, HIV screening had an incremental cost savings of $131,222 compared to no screening.
The last two studies measured the cost-effectiveness of elected cesarean sections versus vaginal delivery for preventing infant HIV infections. One study modeling a population of HIV infected women in the United States found that elective cesarean sections among women using Zidovudine cost $1,131 per infant infection avoided and $17 per year of life saved (28). The second study, using a model of alternative intervention strategies to prevent mother-to-child HIV transmission, found that bottle feeding alone was the most cost effective, with an estimated transmission risk of 18% and an incremental cost per HIV infection avoided of $23. Bottle feeding plus zidovudine decreased HIV transmission risk to 5.8% at an incremental cost per HIV infection prevented of $11,854 (53).
4. Discussion
Our systematic review analyzes a wide variety of economic evaluations for drug abuse treatment and HIV prevention programs for pregnant women. Results show that economic evaluations differ greatly in approach and evaluation methods used (e.g., decision analysis model, multivariate regression, nonrandomized comparisons; different measures of effectiveness; different cost components) when reporting economic findings of drug abuse treatment and HIV prevention programs. Differences in data definition and estimation, model assumptions, discount rates, and perspectives limit our methodologically ability to draw direct comparisons about the effectiveness and cost-effectiveness of the various programs. This diversity also makes it difficult to determine which of the different program characteristics (e.g., targeted populations and intervention types) lead one program to appear more cost-effective than others.
While many of the economic studies in this review provide useful program value assessments, they have some substantive and methodological limitations. First, no economic evaluation is conducted from a randomized controlled study design, the gold standard of clinical evidence. Randomized designs help improve the validity and reliability of intervention costs and effectiveness and are thus preferred; alternative study designs significantly limit the ability to reduce bias and confounding inherent in non-randomized study designs. Second, not all studies used standardized cost measures, which limit the reliability and validity of cost estimates and our ability to compare intervention costs between studies. Third, many studies did not conduct incremental cost-effectiveness or cost-utility analyses, limiting the ability to draw conclusions about the incremental or marginal differences in costs and outcomes of different interventions vis-à-vis each other. Also relevant for analyzing prevention program investment is the outcome of resource savings.
Lack of standardization in economic evaluation makes it difficult to compare across the studies that currently exist. In 1996, the U.S. Panel on Cost-Effectiveness in Health and Medicine published recommendations and guidelines to promote comparability of cost-effectiveness analyses. Guidelines recommend that (1) all costs are reported from the societal perspective, (2) cost estimates are converted to a common year, (3) a rate of 3% is used to discount future outcomes and costs to present value, (4) quality-adjusted life years are used as the outcome metric, (5) sensitivity analysis should be performed to buffer for possible error (26). Since publication of the Panel’s recommendations, there has been some improvement in the methods used over time (3, 7, 8, 43, 44, 47), however this review, and others (7, 25, 61), indicate economic evaluations are still not entirely adopting the Panel’s recommendations nor being diffused widely (68). The persistence of methodological problems in economic evaluations suggests there remains room for substantial improvement.
There are several methodological challenges pertaining to the economic evaluation of substance abuse treatment and HIV prevention programs (24), especially for pregnant women. The first challenge is to assess the benefits of different prevention and treatment programs with the same outcome measures (66, 67). Many times health benefits are measured in prevention-specific terms (i.e. number of HIV cases prevented) rather than outcomes common across diverse interventions. Similar outcome units would allow policy makers and clinicians to assess whether the benefits of a program outweigh its costs and to determine which particular drug prevention and treatment efforts are more cost-effective than others. A second challenge is to create standardized outcome measures that also allow comparisons of economic efficiency across sectors of care – for example, comparing substance dependence prevention with law enforcement and treatment. This will allow drug abuse and HIV prevention programs to be compared to other interventions and may highlight areas where funds currently used in other programs may be used more efficiently and ethically if put into drug treatment and HIV prevention programs. Cost-benefit analysis is often promoted for this purpose, yet many oppose translating health and other social outcomes into monetary units. A third and final challenge is for cost-effectiveness researchers to measure outcomes in terms of Quality-Adjusted Life Years (QALYs). This will make study findings more comparable for treatment policy development (5).
This review highlights some overall trends in drug abuse treatment and HIV prevention programs with important implications for clinicians, patients, and policy makers. The broader literature on HIV prevention shows that programs employing small-group, safer sex skills building, cognitive-behavioral sessions, or peer leader community-level norm change foci as intervention techniques and programs aimed at high-risk groups (51) typically emerge as particularly cost-effective intervention modalities. For example, a program for gay and bisexual male youth and young adults (55) that featured optional HIV testing and counseling, peer education, risk reduction counseling, and referral to medical and psycho-social services was found to be moderately cost-effective (77). While this program was tailored to the specific needs of young men who have sex with men and it is of limited relevance to programs targeted specifically for at-risk women, several studies in this review (31, 74, 75) suggest programs targeted to drug abusing pregnant women are similarly cost-effective. Policy makers and clinicians will want to continue to support similar programs that use cognitive-behavioral techniques and target high-risk groups, as they are shown to be particularly effective.
While prevention is generally associated with cost-savings (13), studies show that prevention programs are not always cost-effective; prevention programs may increase or decrease overall healthcare costs, depending on the program’s effectiveness, target population and other variables (62). For example, because cervical cancer is generally a slow-moving disease, the costs of annual cervical cancer screening programs for the general public are large, thus annual screening programs are considered less cost-effective (22). A promising trend that emerges from this study is that drug-abuse treatment and HIV prevention programs generally appear to be cost-effective (costs less than approximately $100,000 per QALY (81)) by international standards. Of the one study that assessed incremental quality-adjusted life years (69), three studies that assessed costs per disability-adjusted life years (39, 76, 82), and one study that assessed costs per quality of life index (16), all were considered cost-effective by this standard. These studies provide examples of cost-effective programs and suggest that similar drug abuse treatment and HIV prevention programs will be cost-effective by this yardstick. Furthermore, using the WHO’s threshold to measure whether a program is cost-effective supports the Panel’s recommendations that future economic evaluations should measure outcome metrics in terms of quality-adjusted life years. Program outcomes reported in QALYs will be easier to compare to other programs. This review aims to highlight the importance of cost-effectiveness evaluations and call for a more systematic, standardized methodology that can produce useful inputs for health policy decision making, such as the deliberations of the National Institute for Health and Clinical Excellence (NICE) in the U.K. or the Centers for Medicare and Medicaid Services (CMS) in the U.S.
Despite the need for efficient resource allocation and sustainable healthcare programs, research shows that policy makers rarely factor in cost-effectiveness analyses when making decisions (7). For example, economic evaluations on childhood vaccinations appear to play only a small role in shaping immunization policy in the United States. While many recommended childhood vaccinations have proven to be cost-effective and even cost-saving, not all are; the meningococcal polysaccharide vaccine (MCV-4) is estimated to cost $121,000 per life year saved and $138,000 per QALY saved (27). Several explanations for physician and public wariness of adopting CEAs include skepticism of motives and concern that costs, rather than effectiveness, will be the primary influencing factor (2, 52), fear of rationing of healthcare services and desire for the newest medical technology (46), and lack of trust in economic evaluation methodology (46, 56). CEA should be incorporated into health policy in a step-wise manner, beginning with cost and cost-minimization analyses. (59, 60)
This review attempts to examine the available economic evaluations on drug abuse treatment and HIV prevention programs for pregnant women in a systematic and transparent way to help researchers, clinicians and the general public better understand economic analyses and its useful role in identifying high-quality programs that help contain costs. It also highlights important cost and effectiveness data on drug abuse treatment and HIV prevention programs that can aid decisions about resource allocation for sustainable healthcare systems. In addition, this study highlights the paucity of economic evaluations of drug abuse treatment and HIV prevention programs for pregnant women in the literature. Of the original 76 studies identified by our search terms, only 18 (24%) conducted appropriate economic evaluations to be included in our analysis. This scarcity of economic evaluations in the literature may be due to a combination of a lack of appreciation for the usefulness of economic evaluations, limited funding for such studies, or insufficient interest or expertise in conducting economic evaluations. An increased emphasis on the importance and usefulness of economic evaluations is needed to spur greater interest and funding for these studies. Greater transparency in economic evaluation methodology and a continued effort to improve the quality and comparability of economic evaluations are needed if policy makers, clinicians and the public are to accept and incorporate findings from economic evaluations.
Our review has several limitations. First, our literature search was conducted using key words to identify appropriate studies and may have missed some relevant articles that were not picked up from database searches. Second, our analysis was limited to economic studies assessing drug treatment and HIV prevention among pregnant women, and does not include economic evaluations of programs targeted to different groups. Third, considerable heterogeneity among study methods, interventions, outcome variables, and cost components limits our ability to directly compare studies and determine specific policy recommendations. Fourth, we included only published studies in our analysis. If studies with favorable economic findings are published more often than studies with unfavorable findings, publication bias can make economic analysis of drug abuse treatment and PMTCT studies appear more cost-effective than the broader literature actually suggests. Narrative syntheses have several limitations, the most important being that it does not allow study results to be aggregated into a single empirical estimate. This makes it less useful in quantitatively calculating and comparing cost effectiveness of programs vis-à-vis each other. Second, unlike other types of syntheses, narrative syntheses are not well developed by the scientific community. Despite these general drawbacks this study and others like it attempt to provide useful quasi-empirical information and transparent and reproducible study methods for policy makers and clinical and public health practitioners.
Conclusion
Continued work to address methodological challenges related to cost components and outcome measures of economic evaluation will eventually lead to more standardization. Attention to enumerating cost categories and determining and measuring health benefits will improve the comparability and generalizability of economic evaluations. Such efforts, together with established standards, will make future studies more transparent, more rigorous, and more comparable and useful to policy makers, clinicians and patients, thereby increasing the influence of economic evaluations on policy and resource allocation decisions.
Highlights.
Research Highlights:
Drug abuse treatment and PMTCT programs for pregnant women are cost-effective
Cognitive-behavioral programs targeting high-risk groups appear more cost-effective
Policymakers should consider both effectiveness and cost-effectiveness
Economic evaluations should become part of evidence base
Greater standardization and transparency in economic analysis will improve value
Acknowledgments
Dr. Ruger is supported in part by a Career Development Award from the U.S. National Institutes of Health (grant K01DA016358) and an Investigator Award from the Patrick and Catherine Weldon Donaghue Medical Research Foundation (grant DF06-112). This research was supported by a grant from the National Institutes of Health (grant R01DA025555). We thank Nora Ng for research and editing assistance.
Role of Funding Sources
JR is supported in part by a Career Development Award from the U.S. National Institutes of Health (grant K01DA016358) and an Investigator Award from the Patrick and Catherine Weldon Donaghue Medical Research Foundation (grant DF06-112). This research was supported by a grant from the National Institutes of Health (grant R01DA025555).
Footnotes
Contributors
JR and CL conducted literature searches, provided summaries of research studies and contributed to the final manuscript.
Conflict of Interest
All authors declare that they have no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Administration SAaMHS. Results from the 2002 national survey on drug use and health: national findings. Rockville, MD: Office of Applied Studies; 2003. Available http://www.oas.samhsa.gov/nhsda/2k2nsduh/results/2k2Results.htm. [Google Scholar]
- 2.Asch DA, Jepson C, Hershey JC, Baron J, Ubel PA. When money is saved by reducing healthcare costs, where do U.S. primary care physicians think the money goes? Am J Manag Care. 2003;9:438–442. [PubMed] [Google Scholar]
- 3.Banta HD, de Wit GA. Public Health Services and Cost-Effectiveness Analysis. Annu Rev Public Health. 2008;29:383–97. doi: 10.1146/annurev.publhealth.29.020907.090808. [DOI] [PubMed] [Google Scholar]
- 4.Bard KA, Coles CD, Platzman KA, Lynch ME. The effects of prenatal drug exposure, term status, and caregiving on arousal and arousal modulation of 8-week-old infants. Dev Psychobiol. 2000;36:194–212. [PubMed] [Google Scholar]
- 5.Barnett PG, Hui SS. The cost-effectiveness of methadone maintenance. Mt Sinai J Med. 2000;67(5–6):365–74. [PubMed] [Google Scholar]
- 6.Boyer CB, Barrett DC, Peterman TA, Bolan G. Sexually transmitted disease (STD) and HIV risk in heterosexual adults attending a public STD clinic: evaluation of a randomized controlled behavioral risk-reduction intervention trial. AIDS. 1997;11(3):359–67. doi: 10.1097/00002030-199703110-00014. [DOI] [PubMed] [Google Scholar]
- 7.Brauer CA, Neumann PJ, Rosen AB. Trends in Cost Effectiveness Analyses in Orthopaedic Surgery. Clin Ortho Relat Res. 2007;457:42–8. doi: 10.1097/BLO.0b013e31803372c9. [DOI] [PubMed] [Google Scholar]
- 8.Brauer CA, Rosen AB, Greenberg D, Neumann PJ. Trends in the measurement of health utilities in published cost-utility analyses. Value Health. 2006;9:213–8. doi: 10.1111/j.1524-4733.2006.00116.x. [DOI] [PubMed] [Google Scholar]
- 9.Brocklehurst P, French R. The association between maternal HIV infection and perinatal outcome: a systematic review of the literature and meta-analysis. Br J Obstet Gynaecol. 1998;105(8):836–48. doi: 10.1111/j.1471-0528.1998.tb10227.x. [DOI] [PubMed] [Google Scholar]
- 10.Carande-Kulis VG, Maciosek MV, Briss PA, Teutsch SM, Zaza S, Truman BI, et al. Methods for systematic reviews of economic evaluations for the Guide to Community Preventive Services. Am J Prev Med. 2000;18(1 Suppl):75–91. doi: 10.1016/s0749-3797(99)00120-8. [DOI] [PubMed] [Google Scholar]
- 11.CDC. HIV/AIDS among Women. 2008 August; Available http://img.thebody.com/cdc/pdfs/women_hiv.pdf.
- 12.Chirgwin KD, Feldman J, Dehovitz JA, Minkoff H, Landesman SH. Incidence and risk factors for heterosexually acquired HIV in an inner-city cohort of women: temporal association with pregnancy. J Acquir Immune Defic Sydnr Hum Retrovirol. 1999;20(3):295–9. doi: 10.1097/00042560-199903010-00013. [DOI] [PubMed] [Google Scholar]
- 13.Cohen JT, Neumann PJ, Weinstein MC. Does preventive care save money? Health economics and the presidential candidates. N Engl JMed. 2008;358:661–3. doi: 10.1056/NEJMp0708558. [DOI] [PubMed] [Google Scholar]
- 14.Daley M, Argeriou M, McCarty D, Callahan JJ, Jr, Shepard DS, Williams CN. The costs of crime and the benefits of substance abuse treatment for pregnant women. J Subst Abuse Treat. 2000;19(4):445–58. doi: 10.1016/s0740-5472(00)00138-0. [DOI] [PubMed] [Google Scholar]
- 15.Daley M, Argeriou M, McCarty D, Callahan JJ, Jr, Shepard DS, Williams CN. The impact of substance abuse treatment modality on birth weight and health care expenditures. J Psychoactive Drugs. 2001;33(1):57–66. doi: 10.1080/02791072.2001.10400469. [DOI] [PubMed] [Google Scholar]
- 16.Daley M, Shepard DS, Bury-Maynard D. Changes in quality of life for pregnant women in substance user treatment: developing a quality of life index for the addictions. Subst Use Misuse. 2005;40(3):375–94. doi: 10.1081/ja-200030798. [DOI] [PubMed] [Google Scholar]
- 17.Dicker M, Leighton EA. Trends in the U.S. prevalence of drug-using parturient women and drug-affected newborns, 1979 through 1990. Am J Public Health. 1994;84:1433–8. doi: 10.2105/ajph.84.9.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DiClemente RJ, Wingood GM. A randomized controlled trial of an HIV sexual risk-reduction intervention for young African-American women. JAMA. 1995;274(16):1271–6. [PubMed] [Google Scholar]
- 19.Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. BMJ. 1996;313:275–83. doi: 10.1136/bmj.313.7052.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drummond MF, O’Brien BJ, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. New York: Oxford University Press; 1997. [Google Scholar]
- 21.Ebrahim SH, Gfroerer J. Pregnancy-related substance use in the United States during 1996–1998. Obstet Gynecol. 2003;101(2):374–9. doi: 10.1016/s0029-7844(02)02588-7. [DOI] [PubMed] [Google Scholar]
- 22.Eddy DM. Screening for cervical cancer. Ann Intern Med. 1990;113:214–26. doi: 10.7326/0003-4819-113-3-214. [DOI] [PubMed] [Google Scholar]
- 23.Frank DA, Augustyn M, Knight WG, Pell T, Zuckerman B. Growth, development, and behavior in early childhood following prenatal cocaine exposure. JAMA. 2001;285:1613–25. doi: 10.1001/jama.285.12.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.French MT, Drummond M. A research agenda for economic evaluation of substance abuse services. J Subst Abuse Treat. 2005;29:125–37. doi: 10.1016/j.jsat.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Giacomini M, Miller F, O’Brien BJ. Economic considerations for health insurance coverage of emerging genetic tests. Commun Genet. 2003;6:61–73. doi: 10.1159/000072998. [DOI] [PubMed] [Google Scholar]
- 26.Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 27.Grosse SD, Teutsch SM, Haddix AC. Lessons from cost-effectiveness research for United States public health policy. Annu Rev Public Health. 2007;28:365–91. doi: 10.1146/annurev.publhealth.28.021406.144046. [DOI] [PubMed] [Google Scholar]
- 28.Halpern MT, Read JS, Ganoczy DA, Harris DR. Cost-effectiveness of cesarean section delivery to prevent mother-to-child transmission of HIV-1. AIDS. 2000;14(6):691–700. doi: 10.1097/00002030-200004140-00008. [DOI] [PubMed] [Google Scholar]
- 29.Harvard Center for Risk Analysis. Available http://www.hcra.harvard.edu/
- 30.Hobfoll SE, Jackson AP, Lavin J, Britton PJ, Shepherd JB. Reducing inner-city women’s AIDS risk activities: A study of single, pregnant women. Health Psychol. 1994;13(5):397–403. doi: 10.1037//0278-6133.13.5.397. [DOI] [PubMed] [Google Scholar]
- 31.Jansson LM, Svikis D, Lee J, Paluzzi P, Rutigliano P, Hackerman F. Pregnancy and addiction. A comprehensive care model. J Subst Abuse Treat. 1996;13(4):321–9. doi: 10.1016/s0740-5472(96)00070-0. [DOI] [PubMed] [Google Scholar]
- 32.Jefferson T, Demicheli V, Vale L. Quality of systematic reviews of economic evaluations in health care. JAMA. 2002;287:2809–12. doi: 10.1001/jama.287.21.2809. [DOI] [PubMed] [Google Scholar]
- 33.Kamb ML, Fishbein M, Douglas JM, Jr, Rhodes F, Rogers J, Bolan G, et al. Efficacy of risk-reduction counseling to prevent human immunodeficiency virus and sexually transmitted diseases: A randomized controlled trial. Project RESPECT Study Group. JAMA. 1998;280(13):1161–7. doi: 10.1001/jama.280.13.1161. [DOI] [PubMed] [Google Scholar]
- 34.Kelly JA, Murphy DA, Washington CD, Wilson TS, Koob JJ, Davis DR, et al. The effects of HIV/AIDS intervention groups for high-risk women in urban clinics. Am J Public Health. 1994;84(12):1918–22. doi: 10.2105/ajph.84.12.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelly JJ, Davis PG, Henschke PN. The drug epidemic: effects on newborn infants and health resource consumption at a tertiary perinatal centre. J Paediatr Child Health. 2000;36:262–4. doi: 10.1046/j.1440-1754.2000.00492.x. [DOI] [PubMed] [Google Scholar]
- 36.Kokkevi A, Loukadakis M, Plagianakou S, Politikou K, Stefanis C. Sharp increase in illicit drug use in Greece: trends from a general population survey on licit and illicit drug use. Eur Addict Res. 2000;6:42–9. doi: 10.1159/000019008. [DOI] [PubMed] [Google Scholar]
- 37.Kumar RM, Uduman SA, Khurranna AK. Impact of maternal HIV-1 infection on perinatal outcome. Int J Gynecol Obstet. 1995;49(2):137–43. doi: 10.1016/0020-7292(95)02356-h. [DOI] [PubMed] [Google Scholar]
- 38.Mansergh G, Haddix AC, Steketee RW, Nieburg PI, Hu DJ, Simonds RJ, Rogers M. Cost-effectiveness of short-course zidovudine to prevent perinatal HIV type 1 infection in a sub-Saharan African developing country setting. JAMA. 1996;276(2):139–45. [PubMed] [Google Scholar]
- 39.Marseille E, Kahn JG, Saba J. Cost-effectiveness of antriviral drug therapy to reduce mother-to-child HIV transmission in sub-Saharan Africa. AIDS. 1998;12(8):939–48. doi: 10.1097/00002030-199808000-00017. [DOI] [PubMed] [Google Scholar]
- 40.Merrick JC. Maternal substance abuse during pregnancy. Policy implications in the United States. J Leg Med. 1993;14:57–71. doi: 10.1080/01947649309510903. [DOI] [PubMed] [Google Scholar]
- 41.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Lancet. 1999;354:1896–900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 42.Ness RB, Grisso JA, Hirschinger N, Markovic N, Shaw LM, Day NL, et al. Cocaine and tobacco use and the risk of spontaneous abortion. N Engl J Med. 1999;340:333–9. doi: 10.1056/NEJM199902043400501. [DOI] [PubMed] [Google Scholar]
- 43.Neumann PJ, Fang CH, Cohen JT. 30 years of pharmaceutical cost-utility analyses: growth, diversity, and methodological improvement. Pharmacoeconomics. 2009;27(10):861–72. doi: 10.2165/11312720-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 44.Neumann PJ, Greenberg D, Olchanski NV, Stone PW, Rosen AB. Growth and quality of the cost-utility literature, 1976–2001. Value Health. 2005;8:3–9. doi: 10.1111/j.1524-4733.2005.04010.x. [DOI] [PubMed] [Google Scholar]
- 45.Neumann PJ, Stone PW, Chapman RH, Sandberg EA, Bell CM. The quality of reporting in published cost-utility analyses, 1976–1997. Ann Intern Med. 2000;132:964–72. doi: 10.7326/0003-4819-132-12-200006200-00007. [DOI] [PubMed] [Google Scholar]
- 46.Neumann PJ. Why don’t Americans use cost-effectiveness analysis? Am J Manag Care. 2004;10:308–12. [PubMed] [Google Scholar]
- 47.Neumann PJ. Using cost-effectiveness analysis to improve health care. New York: Oxford University Press; 2005. [Google Scholar]
- 48.NIMH. The NIMH multisite HIV prevention trial: reducing HIV sexual risk behavior. Science. 1998;280:1889–94. doi: 10.1126/science.280.5371.1889. [DOI] [PubMed] [Google Scholar]
- 49.Ostrea EM, Ostrea AR, Simpson PM. Mortality within the first two years in infants exposed to cocaine, opiate, or cannabinoid during gestation. Pediatrics. 1997;100:79–83. doi: 10.1542/peds.100.1.79. [DOI] [PubMed] [Google Scholar]
- 50.Pinkerton SD, Holtgrave DR, Layde PM. Incremental cost-effectiveness of two zidovudine regimens to prevent perinatal HIV transmission in the United States. Prev Med. 2000;30(1):64–9. doi: 10.1006/pmed.1999.0601. [DOI] [PubMed] [Google Scholar]
- 51.Pinkerton SD, Johnson-Masotti AP, Otto-Salaj LL, Stevenson LY, Hoffmann RG. Cost-effectiveness of an HIV prevention intervention for mentally ill adults. Ment Health Serv Res. 2001;3(1):45–55. doi: 10.1023/a:1010112619165. [DOI] [PubMed] [Google Scholar]
- 52.Prosser LA, Koplan JP, Neumann PJ, Weinstein MC. Barriers to using cost-effectiveness analysis in managed care decision making. Am J Manag Care. 1999;6:173–9. [PubMed] [Google Scholar]
- 53.Ratcliffe J, Ades AE, Gibb D, Sculpher MJ, Briggs AH. Prevention of mother-to-child transmission of HIV-1 infection: alternative strategies and their cost-effectiveness. AIDS. 1998;12(11):1381–8. doi: 10.1097/00002030-199811000-00021. [DOI] [PubMed] [Google Scholar]
- 54.Rely K, Bertozzi SM, Avila-Figueroa C, Guijarro MT. Cost-effectiveness of strategies to reduce mother-to-child HIV transmission in Mexico, a low-prevalence setting. Health Policy Plan. 2003;18(3):290–8. doi: 10.1093/heapol/czg035. [DOI] [PubMed] [Google Scholar]
- 55.Remafedi G. Cognitive and behavioral adaptations to HIV/AIDS among gay and bisexual adolescents. J Adolesc Health. 1994;15(2):142–8. doi: 10.1016/1054-139x(94)90541-x. [DOI] [PubMed] [Google Scholar]
- 56.Rennie D, Luft HS. Pharmacoeconomic analyses: making them transparent, making them credible. JAMA. 2000;283:2158–60. doi: 10.1001/jama.283.16.2158. [DOI] [PubMed] [Google Scholar]
- 57.Resch S, Altice FL, Paltiel AD. Cost-effectiveness of HIV screening for incarcerated pregnant women. J Acquir Immune Defic Syndr. 2005;38(2):163–73. doi: 10.1097/01.qai.0000146598.40301.e6. [DOI] [PubMed] [Google Scholar]
- 58.Rozenbaum MH, Verweel G, Folkerts DK, Dronkers F, van den Hoek JA, Hartwig NG, et al. Cost-effectiveness estimates for antenatal HIV testing in the Netherlands. Int J STD AIDS. 2008;19(10):668–75. doi: 10.1258/ijsa.2008.008077. [DOI] [PubMed] [Google Scholar]
- 59.Ruger JP. Health and social justice. Oxford: Oxford University Press; 2009. [Google Scholar]
- 60.Ruger JP. The cost side of health morality. under review. [Google Scholar]
- 61.Ruger JP, Emmons KM. Economic evaluations of smoking cessation and relapse prevention programs for pregnant women: a systematic review. Value Health. 2008;11(2):180–90. doi: 10.1111/j.1524-4733.2007.00239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Russell LB. Prevention’s potential for slowing the growth of medical spending. Washington, DC: National Coalition on Health Care; 2007. Oct, Available http://www.ihhcpar.rutgers.edu/downloads/RussellNCHC2007.pdf. [Google Scholar]
- 63.Saha S, Hoerger TJ, Pignone MP, Teutsch SM, Helfand M, Mandelblatt JS Cost Work Group, Third US Preventive Services Task Force. The art and science of incorporating cost effectiveness into evidence-based recommendations for clinical preventive services. Am J Prev Med. 2001;20(3 Suppl):36–43. doi: 10.1016/s0749-3797(01)00260-4. [DOI] [PubMed] [Google Scholar]
- 64.Shain RN, Piper JM, Newton ER, Perdue ST, Ramos R, Champion JD, Guerra FA. A randomized, controlled trial of a behavioral intervention to prevent sexually transmitted disease among minority women. N Engl J Med. 1999;340(2):93–100. doi: 10.1056/NEJM199901143400203. [DOI] [PubMed] [Google Scholar]
- 65.Sherwood RA, Keating J, Kavvadia V, Greenough A, Peters TJ. Substance misuse in early pregnancy and relationship to fetal outcome. Eur J Pediatr. 1999;158:488–92. doi: 10.1007/s004310051126. [DOI] [PubMed] [Google Scholar]
- 66.Sindelar J, Manning W. Cost-benefit and cost-effectiveness analyses: issues in the evaluation of the treatment of illicit drug abuse. In: Egertson JA, Fox DM, Leshner AI, editors. Treating Drug Abusers Effectively. Cambridge, MA: Blackwell; 1997. pp. 187–222. [Google Scholar]
- 67.Sindelar J. Social costs of alcohol. J Drug Issues. 1998;28(3):763–80. [Google Scholar]
- 68.Sonnad S, Greenberg D, Rosen A, Neumann P. Diffusion of published cost-utility analyses in the field of health policy and practice. Int J Technol Assess Health Care. 2005;21:399–402. doi: 10.1017/s026646230505052x. [DOI] [PubMed] [Google Scholar]
- 69.Soorapanth S, Sansom S, Bulterys M, Besser M, Theron G, Fowler MG. Cost-effectiveness of HIV rescreening during late pregnancy to prevent mother-to-child HIV transmission in South Africa and other resource-limited settings. J Acquir Immune Defic Syndr. 2006;42(2):213–21. doi: 10.1097/01.qai.0000214812.72916.bc. [DOI] [PubMed] [Google Scholar]
- 70.St Lawrence JS, Brasfield TL, Jefferson KW, Alleyne E, O’Bannon RE, 3rd, Shirley A. Cognitive-behavioral intervention to reduce African American adolescents’ risk for HIV infection. J Consult Clin Psychol. 1995;63(2):221–37. doi: 10.1037//0022-006x.63.2.221. [DOI] [PubMed] [Google Scholar]
- 71.St Lawrence JS, Jefferson KW, Alleyne E, Brasfield TL. Comparison of education versus behavioral skills training interventions in lowering sexual HIV-risk behavior of substance-dependent adolescents. J Consult Clin Psychol. 1995;63(1):154–7. doi: 10.1037//0022-006x.63.1.154. [DOI] [PubMed] [Google Scholar]
- 72.Stone PW, Teutsch SM, Chapman RH, Bell C, Goldie SJ, Neumann PJ. Cost-utility analyses of clinical preventive services: published ratios, 1976–1997. Am J Prev Med. 2000;19:15–23. doi: 10.1016/s0749-3797(00)00151-3. [DOI] [PubMed] [Google Scholar]
- 73.Streissguth AP, Grant TM, Barr HM, Brown ZA, Martin JC, Mayock DE, et al. Cocaine and the use of alcohol and other drugs during pregnancy. Am J Obstet Gynecol. 1991;164:1239–43. doi: 10.1016/0002-9378(91)90691-j. [DOI] [PubMed] [Google Scholar]
- 74.Svikis D, McCaul M, Feng T, Stuart M, Fox M, Stokes E. Drug dependence during pregnancy. Effect of an on-site support group. J Reprod Med. 1998;43(9):799–805. [PubMed] [Google Scholar]
- 75.Svikis DS, Golden AS, Huggins GR, Pickens RW, McCaul ME, Velez ML, et al. Cost-effectiveness of treatment for drug-abusing pregnant women. Drug Alcohol Depend. 1997;45(1–2):105–13. doi: 10.1016/s0376-8716(97)01352-5. [DOI] [PubMed] [Google Scholar]
- 76.Sweat MD, O’Reilly KR, Schmid GP, Denison J, de Zoysa I. Cost-effectiveness of nevirapine to prevent mother-to-child HIV transmission in eight African countries. AIDS. 2004;18(12):1661–71. doi: 10.1097/01.aids.0000131353.06784.8f. [DOI] [PubMed] [Google Scholar]
- 77.Tao G, Remafedi G. Economic evaluation of an HIV prevention intervention for gay and bisexual male adolescents. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:83–90. doi: 10.1097/00042560-199801010-00013. [DOI] [PubMed] [Google Scholar]
- 78.Teerawattananon Y, Vos T, Tangcharoensathien V, Mugford M. Cost-effectiveness of models for prevention of vertical HIV transmission – voluntary counseling and testing and choices of drug regimen. Cost Eff Resour Alloc. 2005;3:7. doi: 10.1186/1478-7547-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.The Foundation for AIDS Research (amfAR) Young People and HIV/AIDS. 2010 November; Available http://www.amfar.org/abouthiv/article.aspx?id=3596.
- 80.United Kingdom National Health Service Centre for Reviews and Dissemination. CRD Report. 2. Vol. 4. York: University of York; 2001. Mar, Undertaking systematic reviews of research on effectiveness: CRD’s guidance for those carrying out or commissioning reviews. Available http://www.medepi.net/meta/guidelines/Overview_CRD_Guidelines.pdf. [Google Scholar]
- 81.Weinstein M. Can cost-effective health care = better health care? Harvard Public Health Review. 2010 Winter; Available http://www.hsph.harvard.edu/news/hphr/winter-2010/winter10assessment.html.
- 82.Wilkinson D, Floyd K, Gilks CF. National and provincial estimated costs and cost effectiveness of a programme to reduce mother-to-child HIV transmission in South Africa. S Afr Med J. 2000;90(8):794–8. [PubMed] [Google Scholar]