Abstract
The fetal brain remains inaccessible to neurophysiological studies. Magnetoencephalography (MEG) is being assessed to fill this gap. We performed 40 fetal MEG (fMEG) recordings with gestational ages (GA) ranging from 30 to 37 weeks. The data from each recording were divided into 15 second epochs which in turn were classified as continuous (CO), discontinuous (DC), or artifact. The fetal behavioral state, quiet or active sleep, was determined using previously defined criteria based on fetal movements and heart rate variability. We studied the correlation between the fetal state, the GA and the percentage of CO and DC epochs. We also analyzed the Spectral Edge Frequency (SEF) and studied its relation with state and GA. We found that the odds of a DC epoch decreased by 6% per week as the GA increased (P=0.0036). This decrease was mainly generated by changes during quiet sleep, which showed 52% DC epochs before 35 weeks GA versus 38% after 35 weeks (P=0.0006). Active sleep did not show a significant change in DC epochs with GA. When both states were compared for MEG patterns within each GA group (before and after 35 weeks), the early group was found to have more DC epochs in quiet sleep (54%) compared to active sleep (42%) (P=0.036). No significant difference in DC epochs between the two states was noted in the late GA group. Analysis of SEF showed a significant difference (P=0.0014) before and after 35 weeks GA, with higher SEF noted at late GA. However, when both quiet and active sleep states were compared within each GA group, the SEF did not show a significant difference. We conclude that fMEG shows reproducible variations in gross features and frequency content, depending on GA and behavioral state. Fetal MEG is a promising tool to investigate fetal brain physiology and maturation.
Keywords: Magnetoencephalography, fetal behavioral states, continuous and discontinuous patterns, fetal brain
Introduction
The fetal brain remains practically inaccessible for electroencephalography (EEG) and other neurophysiological techniques. Magnetoencephalography (MEG) is being investigated to fill this gap (Haddad et al., 2006). In a recent paper, we demonstrated reproducible features in spontaneous fetal brain activity as recorded by MEG. The recordings showed more discontinuous patterns at early gestational ages (GA), consistent with the maturation seen in EEGs of premature infants (Eswaran et al., 2007). Sharp waves and delta brushes were also recorded and correctly mapped to the position of the fetal head within the gravid abdomen. These studies proved the viability of recording and characterizing fetal MEG (fMEG). Normative data need to be further established prior to investigating this technology in pathological conditions.
Our knowledge of neonatal EEG suggests that it correlates well with the behavioral state at late conceptional age, but does not offer a clear distinction between awake and sleep states early on. In healthy term newborns, there is a predictable and well-defined agreement between the behavioral and physiological observations corresponding to the various sleep and wake states, and the EEG patterns seen in such states. However, this concordance between the clinical and EEG expressions of state is not well developed in very premature infants (Stockard-Pope et al., 1992, Ebersole and Pedley 2003).
The fetal behavioral state can be characterized by monitoring a constellation of behavioral and physiological variables, including the variability in the heart rate in combination with eye and body movements. Nijhuis (Nijhuis et al., 1982; Nijhuis 1993) developed a classification of cycles with synchronic patterns of fetal heart rate (FHR), fetal eye and gross body movements representing behavioral states. The classification of states relies on an extensive ultrasound examination. Maeda et al (1999, 2004, 2005, 2006) developed a simplified investigation protocol using actocardiograms to classify the same behavioral states as defined by Nijhuis. An actogram is used to monitor fetal movements; a cardiogram is the registration of FHR. The simultaneous recording of fetal movements and FHR is an actocardiogram. The actocardiogram was first realized using single-sensor Doppler ultrasound measurements. Doppler detects body movements of the fetus in the same manner it detects movements of the cardiac wall and valves. To properly detect fetal body movements, the Doppler signals are filtered to eliminate artifacts from maternal movement. Since the introduction of actographic recordings, fetal movements have been used extensively to study fetal behavior (Maeda et al., 2005).
In fMEG studies, the fetal heart signals (i.e. fetal magnetocardiographic - fMCG signals) are obtained as a byproduct. It has been shown that the fetal MCG is capable of accurately and reliably detecting cardiac rhythm (Leuthold et al., 2002; Lowery et al., 2003; Stinstra et al., 2005). In general, the peak of the QRS is detected to acquire the RR intervals and calculate the heart rate (Lowery et al., 2003). Due to the high sensitivity of the fetal MCG to the orientation and position of the fetal heart, the magnitude of each QRS peak can be used to quantify gross body movements of the fetus (Zhao and Wakai, 2002). As the distance of the fetal heart to the sensor array increases, the magnitude of the QRS decreases and vice versa. Rotational changes will cause the magnitude of the fMCG to change also. By extracting the information related to gross body movement and heart rate pattern from fetal MCG obtained during fetal MEG recordings, we can classify the fetal behavioral state using criteria (Table 1) defined by Nijhuis (Nijhuis et al.,1982).
Table 1.
Proportion of DC patterns as a function of dichotomized GA and fetal state.
| 1A: Comparison of Early versus Late GA, by fetal state, in all fetuses | ||||
|---|---|---|---|---|
| State | Early GA† | Late GA† | difference | P-value |
| 1F | 0.52 (0.47-0.56) |
0.38 (0.33-0.44) |
−14% | 0.0006 |
| 2F | 0.43 (0.35–0.51) |
0.48 (0.44-0.52) |
5% | 0.24 |
| 1B: Comparison of Fetal States, by GA, in fetuses who change states | ||||
| GA | 1F State† | 2F State† | difference | P-value |
| Early | 0.54 (0.47-0.59) |
0.42 (0.35-0.51) |
−12% | 0.036 |
| Late | 0.41 (0.33-0.50) |
0.49 (0.45-0.53) |
8% | 0.15 |
Mean (95% Confidence Interval)
In the current study, we plan to further enhance the understanding of fMEG spontaneous patterns and explore their relationship with GA and behavioral state. We hypothesized that fMEG gross features and frequency content will vary between behavioral states (as is the situation with neonatal EEG), and that correlation may be dependent on the GA. The behavioral state information was extracted from the actocardiogram obtained from the MEG recordings. We scored the percentage of discontinuity in the recordings and studied its covariation with the behavioral state. We studied the influence of GA on such a correlation. We also analyzed the spectral edge frequency (SEF) and studied whether it varied between behavioral states and GAs. In our analysis we used SEF at 90% which is defined as the frequency below which 90% of the power in a power spectrum resides. SEF has been used for monitoring EEG in adults during anesthesia (Rampil and Matteo, 1987) and also in EEG of newborns to study the spectral differences in the sleep-wake patterns (Bell et al., 1991).
Methods
Instrument
The study was approved by the University of Arkansas for Medical Sciences Institutional Review Board. Recordings were performed at various GAs using the SARA fMEG system (Eswaran et al., 2007). This system consists of 151 primary superconducting sensors which detect magnetic fields generated in the body by various bioelectric sources such as the maternal and fetal heart, fetal brain, and uterine muscles. The sensor array is curved to fit the shape of the pregnant abdomen. The mother simply sits and leans forward against the smooth surface of the array allowing the sensors to receive signals from the entire maternal abdomen. To reduce the effects of environmental noise, SARA is installed in a magnetically shielded room (Vakuumschmelze, Germany).
Subjects and Recording Protocol
Forty MEG recordings were obtained between 30 and 37 weeks GA from fetuses of low-risk mothers. After giving consent, the mother is seated comfortably on the SARA system. Since the fMEG recordings are performed in the shielded room, there is negligible ambient noise and sounds outside the shielded room are kept to a minimum. The operator can communicate with and monitor the subject through intercom and video. At the start of the recording, the mother is encouraged to be relaxed and quiet with minimal movements unless she is uncomfortable and needs to communicate with the operator. All the recordings were performed in late morning or early afternoon.
After the subject sits down in front of the SARA array, we determine the fetal head position using a portable ultrasound scanner. A localization coil is then attached to the maternal abdomen at the head location, to provide positional information related to sensor coordinates. Another ultrasound exam to evaluate fetal head position is repeated at the end of the fMEG study.
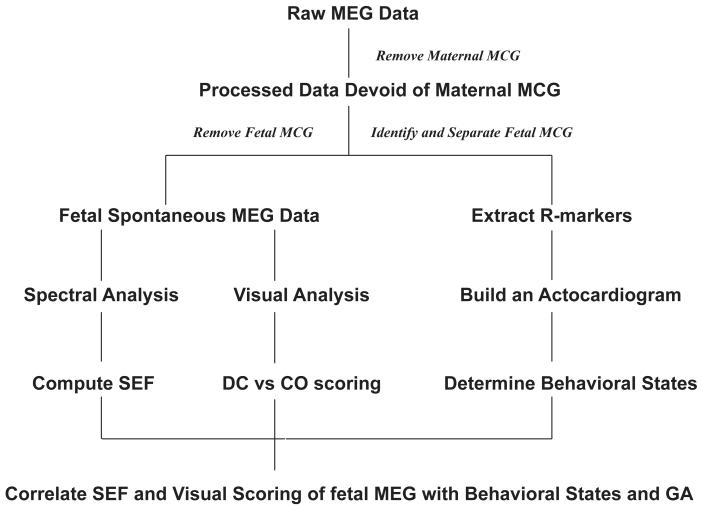
The MEG data were recorded in a continuous mode at a sampling rate of 312 Hz. The majority of recordings were 30 minutes long; in a few cases, the recordings were terminated early for maternal comfort, with the shortest recording time in the series being 16 minutes. The data was further processed using the procedure described below and the overall methodology is illustrated by a flowchart (Figure 1).
Figure 1.
Flowchart summarizing the overall methods and analysis.
Separation of Maternal and Fetal Heart
Fetal MEG data include interference from two major biological sources, namely, MCG signals from the fetal and maternal hearts. In the first step of the analysis, we attenuated the maternal MCG interference using the orthogonal projection algorithm (Vrba et al., 2004). The raw recordings were visually inspected and an initial template for the maternal QRS complex was determined by noting the time marks for the starting and ending sample of a typical QRS complex in the data. The template included up to ten channels (N) exhibiting the largest amplitude of the QRS complex of the MCG. The initial template was correlated with time slices of the raw data to obtain additional ones. The template was continuously updated by an averaged template based on the initial and marked templates. For the extraction of MCG signal, all time points in the averaged QRS complex showing a signal strength larger than 250 femtoTesla (fT) were used to construct a projection operator in the MCG signal space. This projection operator was applied to the whole dataset, leaving only the signals that are not related to maternal heart signals.
Actocardiogram
After the removal of maternal heart, the fetal RR-intervals were obtained using a peak-detection algorithm, and the peak amplitude of each QRS complex was recorded (Ulusar et al., 2009). At each R location, a center of gravity (COG) is defined as the product of the 3-dimensional sensor coordinates with their corresponding R amplitude, normalized by the sum of magnitude of R amplitudes from all the sensors. In a similar way, the COG was computed for all the R locations and the actogram is defined as the distance between the COG of each R location to the average COG of all the R locations (Govindan et al., 2010). The unit of actogram is in centimeters (cm). The fetal heart rate tracings were then plotted on graphs along with the actogram to obtain the actocardiogram.
Fetal State Determination
The actocardiogram plots were analyzed and the nomenclature of the fetal behavioral states was based on the Nijhuis criteria of using the presence of fetal gross body movements and FHR pattern persisting at least three minutes. Based on Nijhuis et al (1982) during 1F, the FHR pattern (pattern A) has a small bandwidth with only sporadic accelerations strictly associated with movement of the fetus. During state 2F, the bandwidth becomes larger, and accelerations in association with gross body movements are common (pattern B). During 3F, the bandwidth is larger than state 1F with a great amount of variability but seldom large accelerations and absence of gross body movements (pattern C). During 4F, the bandwidth is the largest, with numerous accelerations (pattern D) of 30-40 beats-per-minute (bpm) and vigorous, continual activity. 1F and 2F are very similar to neonatal quiet sleep (QS) and active sleep (AS) respectively, and occupy the majority of fetal time (Pillai and James 1990, van Woerden 1992, Nijhuis et al., 1993).
Since the magnetic signal corresponding to fetal eye movements is unknown, the presence or absence of eye movements was not used in assignment of behavioral states. As reported by Maeda et al., (2004), the inability to record fetal eye movements does not affect the proper assignment of fetal behavioral state. They concluded that fetal behavioral states 1F, 2F, and 4F can be identified using the actocardiogram alone, and state 3F can be identified using quantitative values of heart rate variability and accelerations.
The majority of the recordings in the current study exhibited the presence of states 1F and 2F; only two and one recordings respectively exhibited states 3F and 4F at least once. Because of the insufficient number of occurrences of the 3F and 4F states, further analysis was limited only to 1F and 2F.
Extracting Fetal Spontaneous Brain Activity
The MEG data devoid of maternal MCG interference were further processed to remove the fetal heart artifacts in order to obtain spontaneous fetal brain data. The procedure is similar to the one described above for maternal MCG removal. The results were then band-pass filtered between 0·5-25 Hz. Thus, the maternal respiratory movement artifacts were attenuated with a high pass filter set at 0.5Hz. Channels corresponding to sensors located in close proximity to the fetal head, as identified by ultrasound, were saved for review. Each recording was divided into 15-second epochs, and these were classified as continuous (CO), discontinuous (DC) or artifact (AR) by a clinical neurophysiologist blinded to the behavioral state scoring. MEG signals that regularly varied between high amplitude bursts and low amplitude periods (interburst intervals) were called DC. The variability in amplitude between the bursts and interburst intervals had to exceed 50%, and the attenuated periods had to last for more than 3 seconds (to mimic the minimal criteria for trace alternant of neonatal EEG) (Stockard-Pope et al., 1992). Epochs that displayed relatively steady amplitude with smaller variability throughout were considered CO. Recordings with more than 60% of epochs marked as artifacts were excluded from the study. The number of CO and DC epochs was later counted in each recording in the different behavioral states.
With regards to MEG, a total of 2686 epochs were scored, out of which 1151 (43%) were continuous (CO) and 1026 (38%) were discontinuous (DC), while 509 (19%) were disregarded as artifacts, leaving a subtotal of 2177 (81%) scored as either CO or DC. Of this subtotal, the proportion displaying a DC pattern was analyzed via logistic regression.
Correlation of fMEG with GA and behavioral states
As a first step, all the recordings were investigated as to how their content of DC epochs varied before and after 35 weeks of GA for the same state. Clearly, the brain matures in a continuum but we chose a threshold for GA separation for the two groups to facilitate the analysis, as we did not have enough recordings to study each individual GA separately. The 35 week GA cut-off used to dichotomize the two groups was based on classical neonatal EEG knowledge that suggests features similar to term neonates being reached around that threshold age. (Stockard-Pope et al., 1992, Scher et al., 1995)
In the second step, to study the influence of the behavioral state (as a variable) within the same age group, only the recordings containing at least two behavioral states in the same study were included. Also, the fMEG signals corresponding to the different behavioral states were analyzed for the variation in the SEF across GA.
As the fMEG signals were initially scored in non-overlapping windows of 15-sec duration, each 3-min window of a behavioral state encompasses 12 contiguous windows of fMEG signal. The windows corresponding to the brain activity (CO and DC) were investigated for the SEF. SEFs were calculated as follows: In each recording, the 90th percentile of the spectral power distribution was measured in a 15-second window representing the brain activity (segments containing artifacts were excluded). The spectral analysis was performed on channels located in close proximity of the fetal head as identified by ultrasound and the power spectrum for each 15-sec window was calculated as the absolute of the fast Fourier transform of the signal, averaged over all the channels. For each dataset, SEF for each behavioral state of 3-min duration was calculated as the mean SEF of several 15-sec windows and the criteria required that the 3-minute block should have at least have two 15-sec window representing brain activity. The two-window minimum was imposed in order to assure that the average SEF was the average of more than a single number.
Statistical Analysis
The proportion of windows displaying a DC pattern was analyzed using logistic regression, with effects of interest being fetal state, GA (dichotomized as </≥35 weeks), and their interaction, and with generalized-estimating-equation methodology to model the non-independence between states from the same fetus during the same recording. SEF data were analyzed using the Mixed Procedure from SAS version 9.2 (The SAS Institute, Cary, NC). The analysis model was a repeated-measures ANOVA, with GA as the between-subject effect and fetal state as the within-subject effect, and with retention of the GA-by-state interaction only if statistically significant. Different variance-covariance models were compared via likelihood-ratio test (LRT) for goodness of fit to the error structure within recordings. The Satterthwaite approximation was used to calculate degrees of freedom for fixed-effect comparisons. All statistical tests were two-sided and employed an alpha=0.05 significance level.
Results
From a total of 40 recordings performed in the study, 28 recordings passed the artifact cut-off criterion. One more recording was excluded because the neonate had complications at birth, although the mother was initially recruited as a low-risk subject for the study. A total of 27 recordings were finally retained, out of which 17 were before 35 weeks and 10 were at 35 weeks or later. The median GA in the early and late GA groups was 33 and 36.5 weeks respectively.
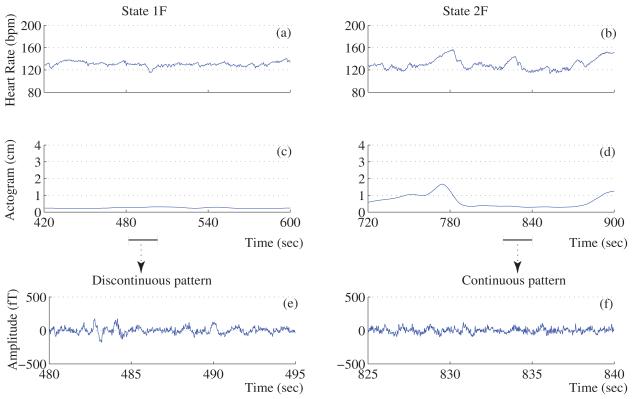
The actocardiograms and the corresponding brain activity for representative fetuses in quiet and active sleep states are shown in Figure 2. Upon separating fetuses by early and late GA (</=>35 weeks), we found that the early-GA fetuses had occurrence rates of 17 (100%) for state 1F and 10 (59%) for state 2F, while the late-GA fetuses had occurrence rates of 9 (90%) for state 1F and 8 (80%) for state 2F. Finally, 17 of the 27 datasets exhibited transitions between states 1F and 2F in the same recording.
Figure 2.
Actocardiogram and Fetal brain activity. Panel (a) and (b) show the heart rate of a fetus in 1F and 2F states respectively. Panel (c) and (d) represent their corresponding movement. (e, i) and (f, j) representative examples of continuous and discontinuous brain pattern observed during quiet sleep state. (h, l) and (g, k) represent the same for the active sleep state.
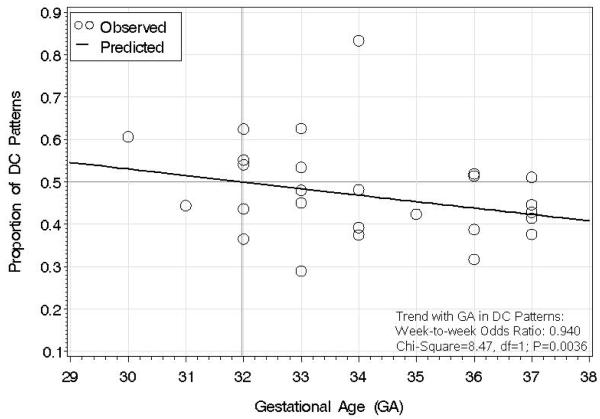
The results of the DC pattern analyzed via logistic regression are shown in Figure 3. The trend line of predicted proportions shows that DC becomes less likely with increasing GA; the magnitude of the trend is expressed as a week-to-week odds ratio of 0.940 (P=0.0036), which denotes (a) a 6% decrease in odds of DC at any week compared to the previous week, and (b) a 35% decrease in odds of DC at 37 weeks compared to 30 weeks. The trend in proportion CO (data not shown) was equal and opposite, by virtue of the fact that proportion CO equals one minus proportion DC.
Figure 3.
Proportion of discontinuous (DC) patterns across gestational age (GA) in weeks. Logistic regression was used to generate the trend line of predicted proportions. The trend line shows that, at each week, there is a 6% decrease in Odds of a DC pattern compared to the previous week (week-to-week Odds Ratio: 0.940; P=0.0036).
In further analysis of DC pattern frequency, GA was dichotomized at </≥35 weeks into early and late, so that the influence of fetal state on the effect of GA could be studied more readily; Table 1, shows the logistic-regression results. In late GA compared to early GA (Table 1A), the proportion DC decreased by a large and significant amount when fetuses were in state 1F (Late=0.38, Early=0.52; P=0.0006), but did not show a statistically significant change when fetuses were in state 2F (Late=0.48, Early=0.43, P=0.24). The state-by-GA interaction was statistically significant (P=0.019), indicating that the two fetal states behave differently with respect to GA.
Next, we analyzed how DC pattern frequency changes between fetal states in each GA group. To assure that each fetal recording would serve as its own control, we included only those fetal observations that underwent a state transition during the recording; Table 1B shows logistic-regression results on the 17 fetal recordings that qualified. In the 1F state compared to the 2F state, the proportion of DC patterns increased by a large and significant amount in the early GA group (1F:0.54, 2F:0.42; P=0.036), but there was no statistically significant difference between the two states in the late GA group (1F:0.41, 2F:0.49; P=0.15). The GA-by-state interaction was statistically significant (P=0.044), indicating that the DC response to fetal state was different between the early versus late GA groups.
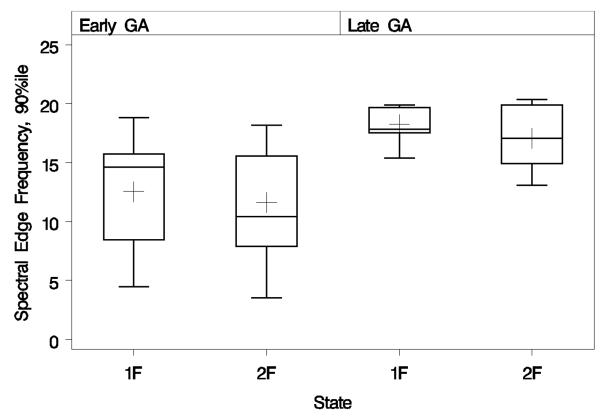
We also examined the SEF in the 17 fetal recordings that underwent state transition. The box- and-whiskers plot (Figure 4) shows the distribution of spectral edge frequencies by fetal state and GA. Figure 4 shows that the mean SEFs (plus signs inside boxes) changed with GA by similar amounts in both the 1F and 2F states, consistent with the finding of a non-significant GA-by-state interaction (F=2.43; DFs=1 and 7.3; P=0.16). Upon removing the interaction from the analysis model, we found that SEF increased considerably from early to late GA (difference=6.52 Hz, ∣t∣=4.38, DF=9.9; P=0.0014), but decreased slightly from the 1F to 2F state without reaching statistical significance (difference=1.00 Hz, ∣t∣=1.86, DF=12.1; P=0.087). The mean SEF in early GA for 1F and 2F states were 12.4 and 11.4 Hz respectively. Similarly the mean SEF for late GA for 1F and 2F states was 18.9 and 17.9 Hz respectively. The unequal box heights indicate that early GA has considerably larger variance than late GA (LRT chi-square=9.38, DF=3; P=0.025), and also that 2F tends to have larger variance than 1F (LRT chi-square=7.46, DF=2; P=0.024). However, the repeated-measures correlation between 1F and 2F was similar in both GA groups: 93% for early GA and 96% for late GA.
Figure 4.
Box-and-whiskers plots of spectral edge frequencies calculated on fetal recordings, grouped by gestational age (GA) and fetal state. Boxes extend vertically from the distributions’ 25th to 75th percentiles, while lower and upper whiskers extend to their minima and maxima, respectively. Inside each box, the horizontal line denotes the median while the plus sign denotes the mean. On average, spectral edge frequencies increase significantly from early to late GA.
Discussion
Based on the actocardiogram recordings, the fetal behavioral state classifications showed that the majority of the fetuses exhibited either quiet or active sleep state. This result conforms to the general observation by ultrasound studies that the fetus spends a significant portion of time in sleep state (Pillai and James 1990, van Woerden 1992, Nijhuis et al., 1993). The FHR pattern and the gross body movements provide a reasonable classification of the fetal behavioral state even before 35 weeks of GA. Although the synchronization of FHR patterns and body movements increase with gestation, the criteria to classify the FHR patterns, which is a major component of characterization of behavioral states, have been applied starting at 32 weeks of GA (Nijhuis et al.,1982, Schneider et al., 2008). Moreover classification and evaluation of fetal behavior studies with actocardiogram by Maeda et al. (2006) have included fetuses as early as 28 weeks of GA.
In this study we developed a more elaborate description of spontaneous fetal brain signals in normal fetuses by fMEG. In previous publications (Eswaran et al., 2007, Rose and Eswaran 2004), we have shown that fetal spontaneous brain activity can be recorded and its features identified using the MEG technique. We were able to detect discontinuous patterns, delta brushes and sharp transients that correctly mapped to the fetal head position in the maternal abdomen. In the current study, the observation of more discontinuity at early GA, with a gradual decrease with later GA, is consistent with the overall pattern of maturation seen in EEGs of premature neonates, and confirms similar preliminary findings in previous fMEG reports (Eswaran et al., 2007, Rose and Eswaran 2004).
This study partially reproduces features of EEG neonatal brain maturation as it relates to the behavioral state. In neonatal EEG studies, discontinuity prevails at early GA, occupying all periods of quiet sleep, while continuous activity is only seen in parts of the active sleep stage. This finding is reproduced with the current fMEG study, confirming the predominance of discontinuous epochs in quiet sleep compared to active sleep before 35 weeks of GA. Another concordant feature is the decrease noted in the percentage of discontinuous MEG epochs in quiet sleep as the fetus matures with greater GA; discontinuous EEG patterns are known to be partially replaced by more continuous activity closer to term even during quiet sleep, with only epochs of “trace alternant” as remnants of earlier discontinuous EEG (Ebersole and Pedley 2003).
The spectral edge frequency reported here is a quantitative measure and provides the frequency below which 90% of the spectral power is concentrated. This analysis replicates some of the results reported by Bell et al, (1991). In their study, they observed a mean SEF value of 10.4 Hz in preterm babies and 24 Hz in term babies. Our results show a similar trend with significant increase in the mean SEF between the early GA (12 Hz) and late GA (18 Hz) fetuses. This implies that there is an overall increase in the SEF with increasing GA. This finding is also consistent with trends noted in spectral power studies of neonatal EEG at variable GAs (Scher et al., 1994, 1995, 1997). These studies show a higher concentration of delta power in preterm infants as opposed to term infants where there is a trend towards an increase in higher frequencies.
It is worth noting that few of our results differed from existing reports on neonatal EEG; the lack of predominance of continuous fMEG epochs in active sleep especially close to term was unexpected. This state is known to correspond to an almost always continuous EEG closer to term (Ebersole and Pedley 2003). Also, in contrast to the observation by Bell et al (1991), we do not see any significant change in the SEF between quiet and active sleep states. They observed an increase in SEF in active sleep in comparison to quiet sleep. The lack of concordance between fetal MEG and known neonatal EEG with respect to these two aspects is unclear and needs further investigation.
Conclusions
In summary, fMEG is a useful non invasive tool to investigate the fetal brain. We were able to show reproducible features, many in concordance with what we know about neonatal EEG, but some differed. Before fMEG can be investigated in pathological situations, some normative data need to be obtained and standardized. This paper is an effort towards such a goal. We are developing automated algorithms to help in the analysis of fMEG. Future studies will attempt to quantify better the CO versus DC ratio at each GA, with the aim of establishing normative ranges for each GA. We will also study in more detail the burst/interburst interval duration and frequency content for each GA. Once sufficient normative data is obtained, potential deviations in pathological scenarios can be investigated.
Acknowledgements
This study was supported by a grant from the National Institutes of Health (NIH) R01 EB007826-01A1, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bell AH, McClure BG, McCullagh PJ, McClelland RJ. Spectral edge frequency of the EEG in healthy neonates and variation with behavioural state. Biol Neonate. 1991;60(2):69–74. doi: 10.1159/000243390. [DOI] [PubMed] [Google Scholar]
- Carrozzi M, Accardo A, Bouquet F. Analysis of sleep-stage characteristics in full-term newborns by means of spectral and fractal parameters. Sleep. 2004;27(7):1384–93. doi: 10.1093/sleep/27.7.1384. [DOI] [PubMed] [Google Scholar]
- Ebersole JS, Pedley TA. Current Practice of Clinical Electroencephalography. Lippincott Williams and Wilkins; Philadelphia: 2003. [Google Scholar]
- Eswaran H, Haddad N, Shihabuddin BS, Preissl H, Siegel ER, Murphy P, Lowery CL. Non invasive Detection and Identification of Brain Activity Patterns in the Developing Fetus. Clinical Neurophysiol. 2007;118(9):1940–46. doi: 10.1016/j.clinph.2007.05.072. [DOI] [PubMed] [Google Scholar]
- Govindan RB, Vairavan S, Ulusar UD, Preissl H, Murphy P, Lowery CL. Detection of Fetal Movement using Spatio-Temporal Magnetocardiogram. Frontiers in Neuroscience. Conference Proceedings Biomag 2010 - 17th International Conference on Biomagnetism; doi:10.3389/conf.fnins.2010.06.00167. [Google Scholar]
- Haddad N, Shihabuddin B, Lowery CL, Holst M, Eswaran H. Magnetoencephalography in Healthy Neonates. Clinical Neurophysiol. 2006;117:289–94. doi: 10.1016/j.clinph.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Leuthold A, Wakai RT, Martin CB. Noninvasive in utero assessment of PR and QRS intervals from the fetal magnetocardiogram. Early Hum Dev. 1999;54(3):235–43. doi: 10.1016/s0378-3782(98)00100-5. [DOI] [PubMed] [Google Scholar]
- Lowery CL, Campbell JQ, Wilson JD, Murphy P, Preissl H, Malak SF, Ewaran H. Noninvasive antepartum recording of fetal S-T segment with a newly developed 151-channel magnetic sensor system. Am J Obstet Gynecol. 2003;188:1491–97. doi: 10.1067/mob.2003.367. [DOI] [PubMed] [Google Scholar]
- Maeda K, Tatsumura M, Utsu M. Analysis of fetal movements by Doppler actocardiogram and fetal B-mode imaging. Clin Perinatol. 1999;26(4):829–51. [PubMed] [Google Scholar]
- Maeda K. Fetal monitoring and actocardiogram in the evaluation of fetal behavior. Ultrasound Rev Obstet Gynecol. 2004;4(1):12–25. [Google Scholar]
- Maeda K. Quantitative Studies on Fetal Actocardiogram. Croatian Medical Journal. 2005;45:792–96. [PubMed] [Google Scholar]
- Maeda K, Tatsumura M, Serizawa M. Classification and evaluation of fetal behavior with actocardiogram. Ultrasound Rev Obstet Gynecol. 2006;6(3-4):163–71. [Google Scholar]
- Nijhuis JG, Prechtl HFR, Martin CB, Jr., Bots RSGM. Are there behavioral states in the human fetus? Early Hum Dev. 1982;6:177–195. doi: 10.1016/0378-3782(82)90106-2. [DOI] [PubMed] [Google Scholar]
- Nijhuis JG. Fetal behavioral states. In: Chervnak FA, Isaacson GC, Campbell S, editors. Ultrasound in obstetrics and gynecology. Vol.1. Little, Brown and Co.; Boston (MA): 1993. pp. 447–55. [Google Scholar]
- Pillai M, James D. Development of human fetal behavior: a review. Fetal Diagn Ther. 1990;5(1):15–32. doi: 10.1159/000263530. [DOI] [PubMed] [Google Scholar]
- Rampil IJ, Matteo RS. Changes in EEG spectral edge frequency correlate with the hemodynamic response to laryngoscopy and intubation. Anesthesiology. 1987;67(1):139–42. doi: 10.1097/00000542-198707000-00033. [DOI] [PubMed] [Google Scholar]
- Rose DF, Eswaran H. Spontaneous neuronal activity in fetuses and newborns. Exp Neurol. 2004;190(Suppl 1):S37–43. doi: 10.1016/j.expneurol.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Scher MS, Sun M, Steppe DA, Guthrie RD, Sclabassi RJ. Comparisons of EEG spectral and correlation measures between healthy term and preterm infants. Pediatr Neurol. 1994;10(2):104–8. doi: 10.1016/0887-8994(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Scher MS, Steppe DA, Banks DL, Guthrie RD, Sclabassi RJ. Maturational trends of EEG-sleep measures in the healthy preterm neonate. Pediatr Neurol. 1995;12(4):314–22. doi: 10.1016/0887-8994(95)00052-h. [DOI] [PubMed] [Google Scholar]
- Scher MS, Steppe DA, Sclabassi RJ, Banks DL. Regional differences in spectral EEG measures between healthy term and preterm infants. Pediatr Neurol. 1997;17(3):218–23. doi: 10.1016/s0887-8994(97)00101-x. [DOI] [PubMed] [Google Scholar]
- Schneider U, Frank B, Fiedler A, Kaehler C, Hoyer D, Liehr M, Haueisen J, Schleussner E. Human fetal heart rate variability-characteristics of autonomic regulation in the third trimester of gestation. J. Perinat. Med. 2008;36:433–41. doi: 10.1515/JPM.2008.059. [DOI] [PubMed] [Google Scholar]
- Stinstra J, Golbach E, Van Leeuwen P, Lange S, Menendez T, Moshage W, et al. Multicentre study on the fetal cardiac time intervals using magnetocardiography. British J of Ob and Gyn. 2002;109:1235–43. doi: 10.1046/j.1471-0528.2002.01057.x. [DOI] [PubMed] [Google Scholar]
- Stockard-Pope JE, Werner SS, Bickford RG. Atlas of Neonatal Electroencephalography. Raven Press; New York: 1992. pp. 105–75. [Google Scholar]
- Ulusar UD, Govindan RB, Wilson JD, Lowery CL, Preissl H, Eswaran H. Adaptive rule based fetal QRS complex detection using Hilbert transform. Conf Proc IEEE Eng Med Biol Soc.; 2009. pp. 4666–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Woerden EE, van Geijn HP. Heart-rate patterns and fetal movements. In: Nijhuis JG, editor. Fetal Behaviour: Developmental and Perinatal Aspects. Oxford University Press; London: 1992. pp. 41–72. [Google Scholar]
- Vrba J, Robinson SE, McCubbin J, Lowery CL, Eswaran H, Wilson JD, Murphy P, Preissl H. Fetal MEG redistribution by projection operators. IEEE Trans. Biomed. Eng. Jul. 2004;51(7):1207–18. doi: 10.1109/TBME.2004.827265. [DOI] [PubMed] [Google Scholar]
- Zhao H, Wakai RT. Simultaneity of fetal heart rate acceleration and fetal trunk movement determined by fetal magnetocardiogram and actocardiography. Phys Med Biol. 2002;47:839–46. doi: 10.1088/0031-9155/47/5/310. [DOI] [PubMed] [Google Scholar]