Abstract
The melanocortin-2 receptor (MC2R), also known as the adrenocorticotropic hormone (ACTH) receptor, plays an important role in regulating and maintaining adrenocortical function, specifically steroidogenesis. Mutations of the MC2R gene have also been identified in humans with familial glucocorticoid deficiency. However, the molecular basis of MC2R responsible for ligand binding and signaling remains unclear. In this study, both truncated ACTH peptides and site-directed mutagenesis studies were utilized to determine the molecular basis of MC2R responsible for ACTH binding and signaling. Our results indicate that ACTH1-16 is the minimal peptide required for MC2R binding and signaling and mutations of the conserved amino acid residues E80 in transmembrane domain 2 (TM2), D107 in TM3, F178 in TM4, F235, H238 in TM6 and F258 in TM7 significantly reduced ACTH binding affinity and signaling, which is similar to other MCRs. Furthermore, mutations of unique amino acids D104 and F108 in TM3, and F168 and F178 in TM4, significantly decreased ACTH binding and signaling. In conclusion, our results suggest that the residues in TM2, TM3 and TM6 of hMC2R share similar binding sites with other MCRs but the residues identified in TM4, TM7 of hMC2R are unique for ACTH binding. Our study suggests that hMC2R may have a broad binding pocket in which both conserved and unique amino acid residues are involved, which may be the reason why α-MSH was not able to bind hMC2R.
Keywords: stress, POMC, MC2R, agonist, GPCR
The melanocortin-2 receptor (MC2R), also known as the adrenocorticotropic hormone (ACTH) receptor, plays an important role in the regulation of adrenal cortisol secretion (9). Mutations of the hMC2R have been identified in a potentially fatal disease called familial glucocorticoid deficiency (FGD) in which affected individuals are deficient in cortisol and likely to succumb to hypoglycemia or overwhelming infection in infancy or childhood if they are not treated (8, 10, 16, 35, 39, 40, 44, 49, 50, 52, 58–60). Over expression of this receptor is also found in adrenal adenomas or hyperplasia associated with glucocorticoid overproduction (Cushing syndrome) (1, 22, 37, 38).
Extensive studies had been performed to determine the molecular basis of ACTH in receptor function in the last two decades but the results are not consistent. In early years, ACTH1-14, ACTH11-19 and ACTH11-24 were reported to increase corticosterone levels in the isolated adrenal cell system and ACTH11-24 plays an important role in adipocyte function (14, 24, 42, 45). However, ACTH1-14 was also reported not able to bind MC2R (48). The explanation for these variations is that different methods of adrenal cell preparation and separate receptors may be involved in these peptide activities (53). Further studies of some ACTH peptide truncations indicated that ACTH1-17 is the minimal peptide required for ligand binding and signaling (14, 24, 42, 45). However, detailed structure-function of ACTH responsible for MC2R binding and signaling is still unclear.
Cloning of melanocortin receptors (MCRs) has greatly improved understanding of the ligand receptor interactions between melanocortins and MCRs. Structure function studies and mutagenesis studies of the receptor have provided great insights into the ligand receptor interaction (26, 61, 63, 64). During the past several years, many studies have indicated that the transmembrane domains 2, 3 and 6 of melanocortin 1, 3 and 4 receptors (MCRs) are important for α-MSH binding and signaling (15, 26, 30, 46, 62, 64). However, the molecular determinants of the hMC2R responsible for agonist ACTH binding remain unknown.
MC2R is one of five known melanocortin receptors belonging to the seven transmembrane G-protein coupled receptor family (GPCR) (41). It shares a nearly 50% homology with other melanocortin receptor subtypes but it is unique among MCRs because of its ligand selectivity. ACTH is the only known endogenous agonist at MC2R, whereas ACTH as well as α–, β–, and γ–MSH bind to other MCRs (2, 4, 11–13, 48, 54, 55, 57). All melanocortins have a shared core amino acid sequence, His-Phe-Arg-Trp (HFRW), which is critical for melanocortin binding and signaling (27, 28, 47). In this study, both truncated ACTH peptides and site-directed mutagenesis studies were utilized to determine the molecular basis of MC2R responsible for ACTH binding and signaling. Our results indicate that ACTH1-16 is minimal peptide for MC2R binding and both conserved and unique residues in TMs of MC2R are involved in ACTH binding and signaling. Not only are TM2, TM3 and TM 6 of hMC2R are important for ACTH recognition but also TM4, TM5 and TM7 are required for ACTH binding and signaling.
Materials and Methods
Peptides
ACTH1-39 and the truncated ACTH peptides were purchased from Peninsula Laboratories, Inc. (Belmont, CA). ACTH1-15 was purchased from Genscript Corporation (Piscataway, NJ).
Site-directed mutagenesis
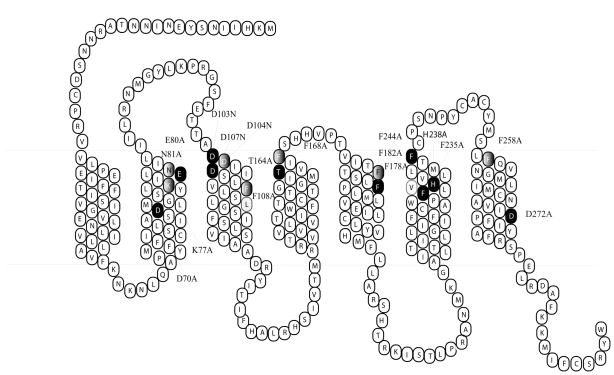
Single residue mutation was constructed using the Quick-Change Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA). The entire coding region of the mutated receptors was sequenced by the University of Alabama at Birmingham Sequence Core to confirm that the desired mutation sequences were present and that no sequence errors had been introduced. The mutated receptors are shown in Figure 1. The mutant receptors were subcloned into the eukaryotic expression vector pCDNA 3.1 (Invitrogen; Carlsbad, CA).
Figure 1.
Two-dimensional representation of the seven TM structure of the hMC2R. The conserved TM residues mutated in these experiments are denoted by gray highlighting. The unique TM residues mutated in these experiments are denoted by black highlighting.
Cell culture and transfection
The OS3 adrenal cell line was cultured in DMEM medium containing 10% bovine fetal serum 2% CBS and HEPES. Cells at 80% confluence were washed twice with DMEM, and the receptor constructs were transfected into cells using lipofectamine (Life Technologies, Rockville MD). The permanently transfected clonal cell lines were selected by resistance to the neomycin analogue G418 (62).
Binding Assays
After removal of media, OS3 cells expressing hMC2R wild type or mutants were incubated with various non-radioligand in 0.5 ml MEM (Fisher Scientific., Pittsburgh, PA) containing 0.2% BSA and radioligand. Binding experiments were performed using conditions previously described (62, 65). Briefly, 2 × 105 cpm of 125I-ACTH (Amersham, Arlington Heights, IL) was used in combination with non-radiolabeled ligand ACTH. Binding reactions were terminated by removing the media and washing the cells twice with MEM containing 0.2% BSA. The cells were lysed with 0.2 N NaOH, and the radioactivity in the lysate was quantified in an analytical gamma counter. Nonspecific binding was determined by measuring the amount of 125I-label bound in the presence of 10−6 M unlabeled ligand. Specific binding was calculated by subtracting nonspecifically bound radioactivity from total bound radioactivity.
cAMP Assay
cAMP generation was measured using a competitive binding assay (TRK 432, Amersham, Arlington Heights, IL). Briefly, OS3 cells stably expressing hMC2R were used in these assays (6, 65). Cell culture media was removed, and cells were incubated with 0.5 ml Earle’s Balanced Salt Solution (EBSS), containing ACTH (10−10–10−6 M), for one hour at 37°C in the presence of 10−3 M isobutylmethylxanthine. The reaction was stopped by adding ice-cold 100% ethanol (500μl/well). The cells in each well were scraped, transferred to a 1.5 ml tube, and centrifuged for 10 min at 1900 × g, and the supernatant was evaporated in a 55°C water bath with pre-purified nitrogen gas. cAMP content was measured according to instructions accompanying the assay kit. Each experiment was performed a minimum of three times with duplicate wells.
Receptor Expression
For receptor protein expression studies, we utilized PCR to insert a FLAG tag onto the NH2 terminus of hMC2R in order to characterize receptor protein cell surface expression by flow cytometry using fluorescence-activated cell sorting (FACS). The FLAG protein is an eight amino acid peptide (Asp-Tyr-Lys-Asp-Asp-Asp-Asp-Lys), useful for immunoaffinity purification of fusion proteins (25, 57). hMC2R or mutant receptor transfected cells were harvested using 0.2% EDTA and washed twice with phosphate buffer saline (PBS). Aliquots of 3×106 cells were centrifuged and fixed with 3% paraformaldehyde in PBS (pH 7.4). The cells were incubated with 50 μl of 10 μg/ml murine anti-FLAG M1 monoclonal antibody(Sigma, catalog No. 316, St. Louis, MO) in incubation buffer for 45 minutes. Under these conditions, the primary antibody binds only to receptors located at the cell surface. The cells were collected by centrifugation and washed three times with incubation buffer. The cell pellets were suspended in 100 μl of incubation buffer containing CY™3-conjugated Affinity Pure Donkey Anti-Mouse Ig G (ImmunoResearch Lab, Inc., West Grove, PA) and incubated at room temperature for 30 minutes. Flow cytometry was performed on a fluorescence-activated cell sorter (Becton Dickinson FACStar plus six parameter cytometer/sorter with a dual Argon ion laser, San Jose, California). The results were analyzed using the software CellQuest (Beckton-Dickinson Immunocytometry Systems, San Jose, California).
Statistical analysis
Each experiment was performed at three separate times with duplicated wells. Data are expressed as mean ± SEM. The mean value of the dose-response data of binding and cAMP production was fit to a sigmoid curve with a variable slope factor using non-linear squares regression analysis (Graphpad Prism, Graphpad Software, San Diego, CA). Significant differences were assessed by one-way ANOVA, with p < 0.05 considered to be statistically significant.
Results
Structure-function study of ACTH peptides at hMC2R wild type
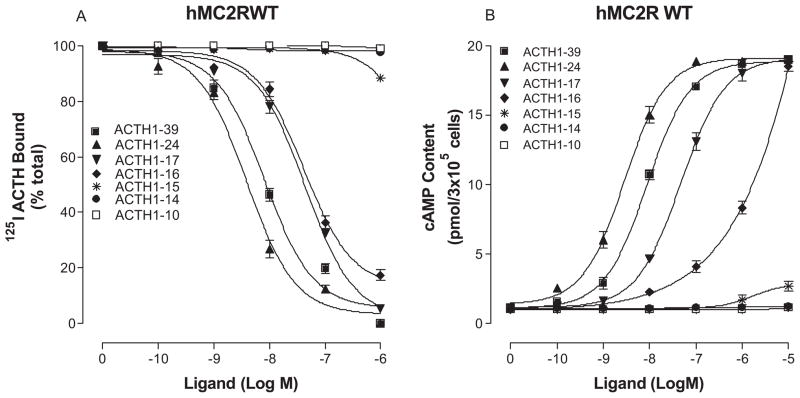
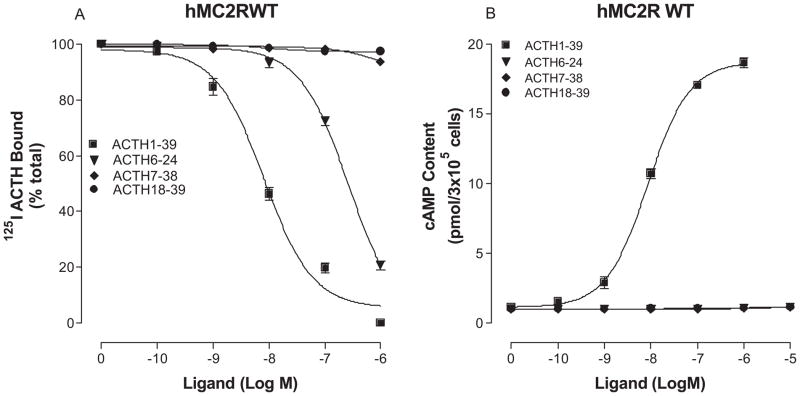
To determine which region of ACTH is essential for high affinity binding and potency at hMC2R, we performed structure-activity studies using the truncated ACTH peptides. The sequences of the truncated peptides are shown in Table 1. Our results indicate that ACTH1-39, ACTH1-24, ACTH1-17 and ACTH1-16 possess full agonist activities. ACTH1-24 is the most potent and ACTH1-16 is the least potent among the ACTH peptides tested (Figure 2 and table 1). The peptides with less than 16 amino acids lost their binding affinity and biological activities. Further studies indicate that the peptides without first six amino acids also lost their biological activities, suggesting that three key regions of ACTH are required for ligand binding and signaling at hMC2R which is different from that of other MCRs (Figure 3 and table 1).
Table 1.
Effect of ACTH truncations on 125I ACTH binding and cAMP generation
| Ki (nM) | EC50 (nM) | ||
|---|---|---|---|
| Ac-Ser-Tyr-Ser-Nle-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-AA14-39 | (ACTH1-39) | 9.8 ± 2.1 | 8.59 ± 0.65 |
| Ac-Ser-Tyr-Ser-Nle-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-AA14-24 | (ACTH1-24) | 5.4 ± 1.5* | 2.8 ± 0.5* |
| Ac-Ser-Tyr-Ser-Nle-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-Gly-Lys-Lys-Arg | (ACTH1-17) | 78 ± 3.7* | 46.8 ± 8.5* |
| Ac-Ser-Tyr-Ser-Nle-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-Gly-Lys-Lys | (ACTH1-16) | 92 ± 12.3* | 567 ± 34* |
| Ac-Ser-Tyr-Ser-Nle-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-Gly-Lys | (ACTH1-15) | No | NR |
| Ac-Ser-Tyr-Ser-Nle-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-Gly | (ACTH1-14) | No | NR |
| Ac-Ser-Tyr-Ser-Nle-Glu-His-Phe-Arg-Trp-Gly | (ACTH1-10) | No | NR |
| His-Phe-Arg-Trp-Gly-Lys-Pro-Val-Gly-Lys-Lys-Arg-Arg-Pro-Val-Lys-Val-Tyr-Pro | (ACTH6-24) | 839 ± 13.5* | NR |
| Phe-Arg-Trp-Gly-Lys-Pro-Val-Gly-Lys-Lys-Arg-Arg-Pro-Val-Lys-Val-Tyr-Pro-Asn-Gly-Ala-Glu-Asp-Glu-Ser-Ala-Glu-Ala-Phe-Pro-Leu-Glu | (ACTH7-38) | No | NR |
| Arg-Pro-Val-Lys-Val-Tyr-Pro-Asn-Gly-Ala-Glu-Asp-Glu-Ser-Ala-Glu-Ala-Phe-Pro-Leu-Glu-Phe | (ACTH18-30) | No | NR |
No: No binding; NR - No response n>3, mean ± S.E.M.
P < 0.01 compared with ACTH1-39.
Figure 2.
Binding affinity and potency of truncated ACTH peptides in OS3 cells stably transfected with the wild-type hMC2R. Panel A shows that OS3 cells transfected with hMC2R were incubated with 125I-ACTH in the presence of the indicated amounts of unlabeled ligands, and total 125I-ACTH binding was determined. Panel B shows that the cells were incubated with the indicated amounts of peptides and total cAMP accumulation was determined. (n = 3; see Table 1 for Ki and EC50 values).
Figure 3.
Binding affinity and potency of truncated ACTH peptides in OS3 cells stably transfected with the wild-type hMC2R. Panel A shows that OS3 cells transfected with hMC2R were incubated with 125I-ACTH in the presence of the indicated amounts of unlabeled ligands, and total 125I-ACTH binding was determined. Panel B shows that the cells were incubated with the indicated amounts of peptides and total cAMP accumulation was determined. (n = 3; see Table 1 for Ki and EC50 values).
Substitutions of the conserved amino acid residues of hMC2R on ACTH binding and potency
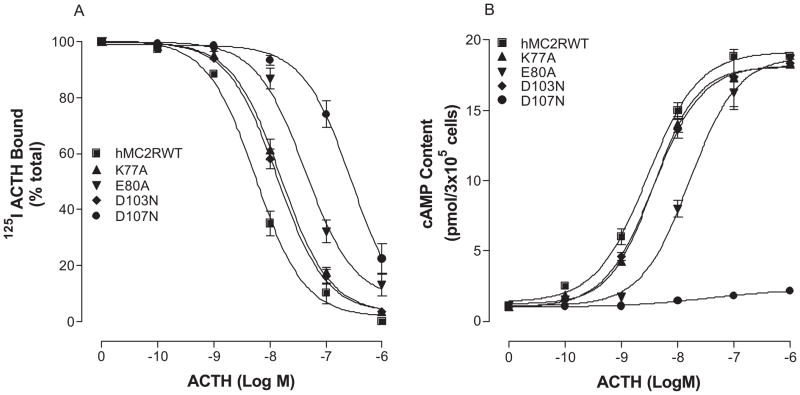
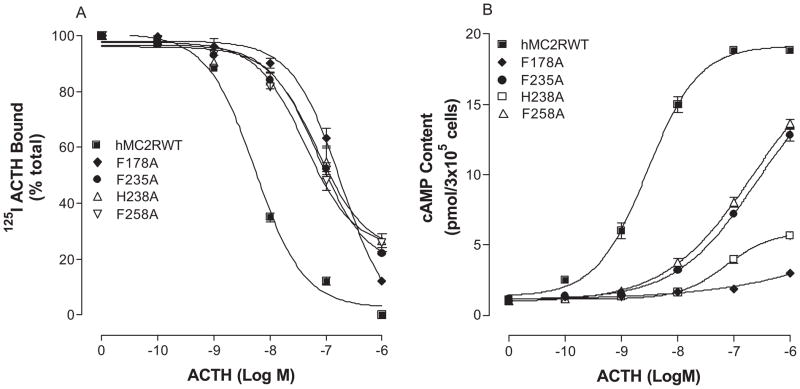
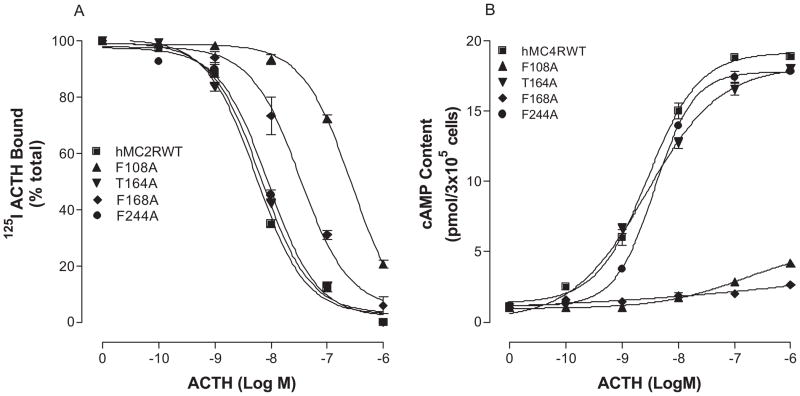
hMC2R shares 50% conserved amino acid residues with other MCRs and 67% conserved amino acid residues in the transmembrane domains (17–19, 41). However, the pharmacological profile of MC2R is substantially different from other MCRs. The rationale for selecting amino acids of hMC2R in the mutagenesis experiments is based on our previous studies of other melanocortin receptors. Our previous results indicate that the conserved amino acid residues in TMs of MCRs are important for ligand binding and signaling (6, 62, 64). Moreover, our recent studies of chimeric receptors with hMC4R and hMC2R indicate that the conserved amino acid residues of hMC2R are important for NDP-MSH binding and signaling (7). The substitutions of the TM2, 3, 4, 5 and 6 of the hMC4R with the corresponding regions of the hMC2R did not significantly alter or abolish NDP-MSH specific activity, suggesting that conserved amino acid residue of TMs may be crucial for NDP-MSH and ACTH binding and activity (7). Moreover, ACTH is a 39 amino acid peptide and does not have access to the receptor’s deep transmembrane region, the conserved ionic and aromatic amino acid residues in the upper region of the transmembrane regions of hMC2R are therefore potential candidates for ACTH binding and signaling. A sequence alignment of hMC2R with other MCRs was therefore compared and ten amino acid residues in TM2, TM3, TM4, TM5 and TM6 are identified conserved between hMC2R and other MCRs in the upper regions of the transmembrane regions of hMC2R. We hypothesized that these conserved amino acid residues of hMC2R are involved in ACTH binding and receptor activation which is similar to that of other MCRs. Besides the conserved amino acid residues, unique amino acid residues of hMC2R are also important for ACTH binding and signaling because MSH was not able to bind MC2R. Based on this theory, the conserved and unique ionic and aromatic amino acid residues in TMs of the hMC2R were selected in this study. To determine whether these amino acid residues are involved in ACTH binding and signaling, these residues were individually mutated with alanine and the ACTH binding affinity and potency were evaluated (Figure 1). Alanine is the generally accepted amino acid of choice for mutagenesis substitution in this type of analysis since its small neutral nature theoretically makes it unlikely to disturb receptor tertiary structure. Asparagine instead of alanine is utilized for some mutations when alanine resulted in complete loss of the receptor function (64). Our results indicate that all of the mutant receptors were expressed at cell surface and their expressions are shown in Table 2. The expressions of K77A, E80A, D103N, D107N, F178A, F235A, H238A and F258A are lower than that of the hMC2R-WT. Receptor function study indicates that ACTH dose dependently displaced 125I ACTH binding at the mutations, K77A, E80A, D103N, D107N, F178A, F235A, H238A and F258A, but their binding affinities for ACTH were significantly reduced compared to that of the hMC2R WT (Figure 4A, 5A and table 2). Consistent with the binding results, the mutations, K77A, E80A, D103N, D107N, F178A, F235A, H238A and F258A, significantly reduced ACTH mediated cAMP production (Figure 4B, 5B). Their Ki and EC50 are shown in Table 2.
Table 2.
Effect of the substitutions of the conserved amino acid residues of hMC2R on 125I ACTH binding and cAMP production
| Receptor expression (% of WT) |
125I- ACTH binding Ki (nM) |
cAMP production EC50 (nM) |
|
|---|---|---|---|
| hMC2RWT | 100 | 5.6 ± 1.0 | 0.8 ± 0.2 |
| D70A | No | NB | NR |
| K77A | 73 ± 9.5 | 25.2 ± 4.1* | 6.2 ± 0.9* |
| E80A | 75 ± 12.3 | 65.2 ± 7.1* | 7.8 ± 1.1* |
| D103N | 68 ± 14 | 15.7 ± 0.7* | 5.8 ± 1.2* |
| D107N | 66 ± 11 | >103* | >103* |
| F178A | 67 ± 8.7 | >103* | >103* |
| F235A | 84 ± 6.8 | 98 ± 6.9* | 216 ± 18* |
| H238A | 69 ± 11.7 | >103* | >103* |
| F258A | 78 ± 12.7 | 78 ± 4.4* | 187 ± 10* |
| D272A | No | NB | NR |
P<0.05 compared with WT receptor.
Figure 4.
Effects of the mutations of the conserved charged amino acid hMC2R TM residues with alanine on ACTH binding affinity and receptor activity. Panel A shows the ACTH binding affinity of these mutants. Panel B shows the ability of ACTH stimulated cAMP production at these mutants. (n = 3; see Tables 2 for actual Ki and EC50 values).
Figure 5.
Effect of the mutations of various conserved aromatic amino acids in the hMC2R on ACTH binding affinity and receptor activity. Panel A shows the ACTH binding affinity to these mutant receptors. Panel B shows the ability of ACTH to stimulate cAMP production upon binding to the mutant receptors. (Panels A and B) (n = 3; see Tables 2 for actual Ki and EC50 values).
It has been reported that mutations of aspartic acid residues in TM2 and TM7 of hMC3R and hMC4R resulted in not only altered ligand binding, but also altered receptor signaling (6, 64). To determine whether the corresponding residues of hMC2R are also involved in agonist mediated receptor activation, we mutated residue D70 in TM2 and D272 in TM7 of hMC2R with alanine and tested their function. Our results indicate that mutations D70A and D272A resulted in a complete loss of receptor-ligand binding and activation. To examine the possibility that these mutations may not be properly expressed at the cell surface, we utilized FACS to determine whether the alanine substitution alters receptor expression. Our results show that strong signal was detected at FLAG tagged MC2R WT but no signal was detected in FLAG-tagged mutants D70A and D272A, suggesting that these two mutants were not properly expressed at the cell surface.
Substitutions of the unique amino acids in the transmembrane domains of hMC2R on ACTH binding and activation
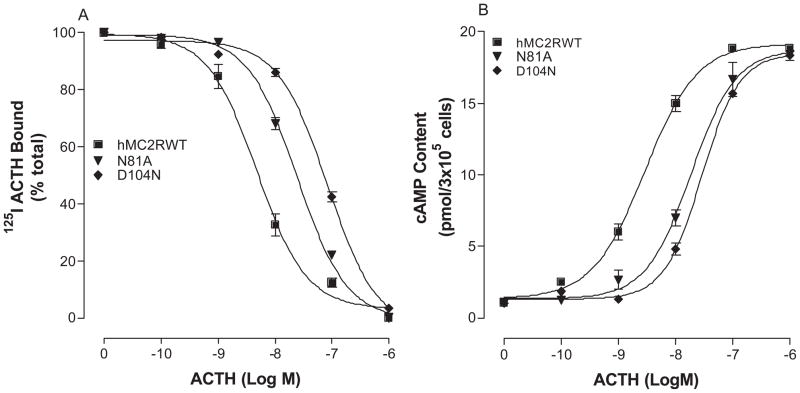
ACTH is the only endogenous ligand for hMC2R and three regions of ACTH are proposed for receptor ligand binding and signaling (3, 36, 48). Our results indicate that the residues 15–17 Lys15-Lys16-Arg17 are important for hMC2R binding and signaling because loss of this region (ACTH1-14) impairs receptor activation. This is a very positively charged domain and we therefore postulate that additional binding sites at hMC2R may be required for ACTH15-17 binding. Unique amino acid residues are therefore potential candidates responsible for this binding. The sequence of amino acids in TMs of hMC2R was compared with other MCRs and six amino acid residues are revealed to be unique. These residues are N81 in TM2, D104 and F108 in TM3, T164 and F168 in TM4 and F244 in TM6. To determine whether they are involved in ACTH specific binding and activity, these residues were individually mutated with alanine and evaluated. Our results indicate that these mutant receptors were expressed at cell surface but the expression levels of the mutations, N81A, D104N, F108A and F168A, are lower than that of the hMC2R-WT (Table 3). ACTH dose dependently displaced 125I ACTH binding at these mutations but the binding affinity of ACTH at mutations, N81, D104, F108 and F168, was significantly reduced (Figure 6A, 7A and Table 3). Consistent with the binding results, the mutations, N81A, D104A, F108A and F168A, significantly reduced ACTH mediated cAMP production (Figure 6B, 7B and Table 3).
Table 3.
Effect of the substitutions of unique amino acid residues of hMC2R on 125I ACTH binding and cAMP production
| Receptor expression (% of WT) |
125I- ACTH binding Ki (nM) |
cAMP production EC50 (nM) |
|
|---|---|---|---|
| hMC2RWT | 100 | 8.6 ± 1.0 | 4.8 ± 0.1 |
| N81A | 89 ± 12.4 | 15.9 ± 0.6* | 11.6 ± 1.2* |
| D104N | 78 ± 13.4 | 87.3 ± 9.8* | 87.7 ± 9.3* |
| F108A | 65 ± 10.2 | >103* | >103* |
| T164A | 93 ± 14.2 | 6.4 ± 0.7 | 3.9 ± 0.1 |
| F168A | 56 ± 9.5 | 217.8 ± 13* | 327.6 ± 30.3* |
| F244A | 95 ± 11.2 | 9.9 ± 0.9 | 4.9 ± 0.9 |
P<0.05 compared with WT receptor
Figure 6.
Effects of the mutations of unique charged amino acid hMC2R TM residues on ACTH binding affinity and receptor activity. Panel A shows the ACTH binding affinity of these mutants. Panel B shows the ability of ACTH stimulated cAMP production at these mutants. (n = 3; see Tables 3 for actual Ki and EC50 values).
Figure 7.
Effect of the mutations of various unique aromatic amino acids in the hMC2R on ACTH binding affinity and receptor activity. Panel A shows the ACTH binding affinity to these mutant receptors. Panel B shows the ability of ACTH to stimulate cAMP production upon binding to the mutant receptors. (Panels A and B) (n = 3; see Tables 3 for actual Ki and EC50 values).
Discussion
The melanocortin peptides α-MSH and ACTH belong to a group of neuropeptides derived from the pro-opiomelanocortin prohormone and contain the common amino acid sequence, His-Phe-Arg-Trp (23, 27, 51). These residues in MSH and ACTH were identified as important residues for ligand binding and biological activities at hMC1R, hMC3R and hMC4R (31–34, 56). However, this tetrapeptide was not able to bind and activate MC2R. As long with the cloning MC2R, the studies with cells expressing transfected hMC2R indicated that α-MSH was not able to bind to MC2R and ACTH1-17 is the minimal peptide for receptor binding and activation (3, 36, 48). In our study, we transfected hMC2R into OS3 cells which is an adrenal cell lines lacking endogenous MC2R and determined which region of ACTH is important for MC2R binding and activation. Our results indicate that ACTH1-24 is the most potent truncated peptide among the truncated peptides tested. The peptide loses its activity at MC2R if the length of ACTH peptide is less than 16 amino acids in N terminus, which is one amino acid less than the length of peptide reported before (36). Our results also indicate that N terminus of ACTH is also important for ligand binding and receptor activation because peptide ACTH6-24 completely loses its agonist activity which are consistent with the previous results (5, 24). Our results therefore supports the theory raised by other groups that three key regions of ACTH are important for ACTH binding and signaling at hMC2R (36). One region is located in ACTH1-5 which is important for receptor activation but not for ligand binding. The second region is located in ACTH 6-9 (HFRW) which is important for binding and activation. The third one is located between residue 14 and 16 of ACTH which is critical for hMC2R binding and activation, which is different from that of other MCRs. The results of truncated ACTH peptides also suggest that the binding pockets of MC2R may differ structurally from that of other MCRs.
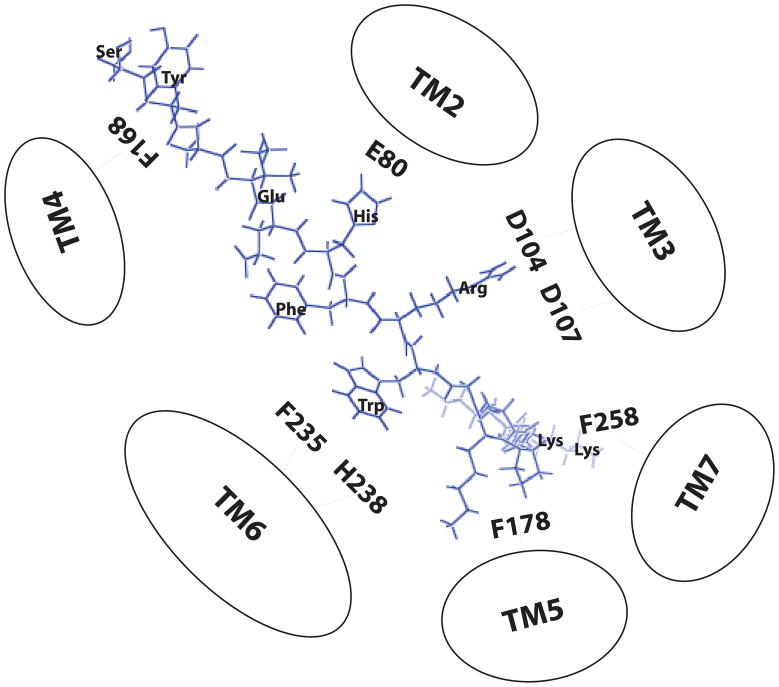
Extensive studies have been performed to examine the molecular basis of MC1R, MC3R and MC4R responsible for ligand binding and signaling (6, 62, 64). The results indicate that conserved residues in TM of melanocortin receptors are involved in MSH binding and signaling and electrostatic and hydrophobic forces have been proposed to be involved in MSH binding and receptor activation (29). Melanocortin receptor subtypes are believed to have the same basic molecular architecture and hMC2R has near 50% identity with other MCRs in the putative TM regions (18–20, 41). To determine the molecular basis of hMC2R responsible for ACTH binding and activity, we first examined the roles of the highly conserved residues across the MC2R, MC1R, MC3R and MC4R subtypes which have been identified to be crucial in other MCRs. We examined ten conserved amino acid residues of hMC2R that have previously been identified to be involved in ligand binding and activity in MC1R, MC3R and MC4R (6, 7, 15, 26, 30, 62, 64). Our results support this hypothesis and the conserved residues E80 in TM2, D107 in TM3, F178 in TM4, F235, H238 in TM6 are involved in ACTH binding which are similar to that of other MCRs (6, 15, 26, 62, 64). However, significant differences between MC2R and other MCRs were also identified in this study. hMC2R has three aspartic acids (D103, D104 and D107) in TM3 but other MCRs have only two (D117 and D121 in hMC1R; D154 and D158 in hMC3R, D122 and D126 in hMC4R). Residue D104 in TM3 of hMC2R is unique among the MCRs. Our results indicate that unlike MC1R, MC3R and MC4R, the residue D103 in hMC2R may be not important for ACTH binding because mutation of D103 did not dramatically alter ACTH binding affinity and potency. Instead of the residue D103, the residue D104 in TM3 is important in ACTH binding and activity because mutation of this residue significantly decreased ACTH binding affinity and potency. Our result of D107N is also consistent with the results identified in FGD in which mutation of D107N resulted in receptor malfunction (43). Our results suggest that hMC2R shares similar ionic binding sites with hMC1R, hMC3R and hMC4R. We incorporated our results into the model for hMC2R ligand receptor interaction which is shown in Figure 8. An ionic pocket formed by amino acid residues E80 in TM2 and D104, D107 in TM3 of hMC2R and a second hydrophobic binding pocket consisted of F235 and H238 in TM6 of hMC2R. In addition, two unique amino acid residues, F178 in TM5 and in TM7 of hMC2R may be involved in Lys15-Lys16 interaction.
Figure 8.
Two-dimensional representation of a proposed three dimensional model illustrating ACTH1-16 docked inside the hMC2R. Based on our results, three main receptor binding pockets are proposed. The first is a predominantly ionic pocket formed by E80, D104 and D107. The second binding pocket is formed by aromatic residues F235 and H238 in TM6. The third binding pocket is formed by F178 in TM5 and F258 in TM7.
MC2R is unique among MCRs due to the fact that ACTH is its only endogenous ligand. We speculate that the binding pockets of MC2R may be different from that of other MCRs and D104 may play an important role in differential recognition of ACTH or MSH at MC2R. We examined the effect of α-MSH at mutation D104N but this mutation did not increase MSH binding, hereby suggesting that this residue is not crucial for MSH binding (data not shown). Combined with truncated peptides and mutagenesis studies, our present data indicate that several similarities exist between the hMC2R and other hMCRs. First, all MCR subtypes are fully activated by the ACTH1-16. Secondly, the mutation of homologous residues in TM2, TM3, and TM6 of the hMC1R, hMC2R, hMC3R and hMC4R are found to affect agonist binding affinity (hMC1R residues E94, D117, D121, and H260, hMC3R residue E131, D154, D158 and H258, hMC4R residues E100, D122, D126 and H264 are homologous to hMC2R residue E80, D104, D107, H238) (6, 62, 64). However, the present studies did identify a potentially important subtype specific difference between the hMC2R and other hMCRs. TM4, TM5 and TM7 of hMC2R are important for ACTH binding and signaling because mutation of residue F168 (Y182 in MC1R; Y219 in MC3R, Y180 in MC5R; MC4R Y187) in TM4, F178 (L192 in MC1R; L229 in MC3R, L190 in MC5R) in TM5, and F258 in TM7 affects both ACTH binding affinity and potency. This observation suggests that hMC2R may have a broad binding pocket in which conserved amino acid residues are involved in ACTH binding which are similar to that of other MCRs but some unique amino acid residues are perhaps crucial for ACTH14-16 binding and activity. That may be the reason why α-MSH was not able to bind hMC2R although α-MSH shares the first 13 amino acids with ACTH1-39.
Activation of GPCRs has been proposed to involve the rotation of TM domains with outward movement of their cytoplasmic ends (21). This theoretically would enable G proteins to interact with some of the intracellular loops as well as the C terminal tail of GPCRs. Some previous MCR studies support this theory since mutations of some TM residues of MCRs can result in inactivation of MCRs (6, 64). Our current results indicate that mutations of the homologous residues of hMC2R (D70A and D272A) abolish both ACTH binding and receptor signaling because these two mutated receptors are not fully expressed at the cell surface. Altered receptor function may reflect loss of cell surface receptor expression. Thus, this suggests that D70 and D272 in hMC2R play important roles in receptor expression on plasma membrane, and their role in hMC2R is likely similar to that of MC3R and MC4R.
In summary, our results indicate that ACTH1-16 is the minimal peptide required for MC2R binding and signaling. Both conserved and unique residues of MC2R are required to form a broad binding pocket for ACTH binding and signaling which includes TM2, TM3, TM4, TM6 and TM7 of hMC2R. These results may provide important information about the molecular determinants of hMC2R responsible for ACTH binding and receptor signaling.
Acknowledgments
This work has been funded by NIH Grants R03 HD047312-01A1 (Y-K, Yang)
Abbreviations
- MCR
Melanocortin receptor
- hMC2R
human melanocortin-2 receptor
- GPCR
G-protein coupled receptor
- ACTH
Adrenocorticotropic Hormone
- α–MSH
α-melanocyte stimulating hormone
- ASIP
Agouti-signaling protein
- TM
transmembrane domains
- IBMX
3-Isobutyl-methylxanthine
- PCR
Polymerase chain reaction
- FACs
Flow cytometry
References
- 1.Beauregard C, Dickstein G, Lacroix A. Classic and recent etiologies of Cushing’s syndrome: diagnosis and therapy. Treat Endocrinol. 2002;1:79–94. doi: 10.2165/00024677-200201020-00002. [DOI] [PubMed] [Google Scholar]
- 2.Bicknell AB. Identification of a receptor for N-POMC peptides. Endocr Res. 2002;28:309–314. doi: 10.1081/erc-120016801. [DOI] [PubMed] [Google Scholar]
- 3.Cammas FM, Kapas S, Barker S, Clark AJ. Cloning, characterization and expression of a functional mouse ACTH receptor. Biochem Biophys Res Commun. 1995;212:912–918. doi: 10.1006/bbrc.1995.2056. [DOI] [PubMed] [Google Scholar]
- 4.Catania A, Rajora N, Capsoni F, Minonzio F, Star RA, Lipton JM. The neuropeptide alpha-MSH has specific receptors on neutrophils and reduces chemotaxis in vitro. Peptides. 1996;17:675–679. doi: 10.1016/0196-9781(96)00037-x. [DOI] [PubMed] [Google Scholar]
- 5.Cavagnini F, Fossati R. ACTH 1-17 and pituitary-adrenal function in human. Ric Clin Lab. 1984;14:159–165. doi: 10.1007/BF02904968. [DOI] [PubMed] [Google Scholar]
- 6.Chen M, Aprahamian CJ, Celik A, Georgeson KE, Garvey WT, Harmon CM, Yang Y. Molecular characterization of human melanocortin-3 receptor ligand-receptor interaction. Biochemistry. 2006;45:1128–1137. doi: 10.1021/bi0521792. [DOI] [PubMed] [Google Scholar]
- 7.Chen M, Cai M, Aprahamian CJ, Georgeson KE, Hruby V, Harmon CM, Yang Y. Contribution of the conserved amino acids of the melanocortin-4 receptor in NDP-MSH binding and signaling. J Biol Chem. 2007 doi: 10.1074/jbc.M702285200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark AJ, McLoughlin L, Grossman A. Familial glucocorticoid deficiency associated with point mutation in the adrenocorticotropin receptor. Lancet. 1993;341:461–462. doi: 10.1016/0140-6736(93)90208-x. [DOI] [PubMed] [Google Scholar]
- 9.Clark AJ, Metherell LA. Mechanisms of Disease: the adrenocorticotropin receptor and disease. Nat Clin Pract Endocrinol Metab. 2006;2:282–290. doi: 10.1038/ncpendmet0165. [DOI] [PubMed] [Google Scholar]
- 10.Clark AJ, Weber A. Adrenocorticotropin insensitivity syndromes. Endocr Rev. 1998;19:828–843. doi: 10.1210/edrv.19.6.0351. [DOI] [PubMed] [Google Scholar]
- 11.Clark D, Thody AJ, Shuster S, Bowers H. Immunoreactive alpha-MSH in human plasma in pregnancy. Nature. 1978;273:163–164. doi: 10.1038/273163a0. [DOI] [PubMed] [Google Scholar]
- 12.Cooper ES, I, Greer A, Brooks AN. Placental proopiomelanocortin gene expression, adrenocorticotropin tissue concentrations, and immunostaining increase throughout gestation and are unaffected by prostaglandins, antiprogestins, or labor. J Clin Endocrinol Metab. 1996;81:4462–4469. doi: 10.1210/jcem.81.12.8954060. [DOI] [PubMed] [Google Scholar]
- 13.De Wied D, Jolles J. Neuropeptides derived from pro-opiocortin: behavioral, physiological, and neurochemical effects. Physiol Rev. 1982;62:976–1059. doi: 10.1152/physrev.1982.62.3.976. [DOI] [PubMed] [Google Scholar]
- 14.Elliott DA, Draper MW, Rizack MA. Evidence for separate peptide sequences related to the lipolytic and magnesium-accumulating activities of ACTH. Analogy with adrenergic receptors. J Med Chem. 1977;20:584–586. doi: 10.1021/jm00214a028. [DOI] [PubMed] [Google Scholar]
- 15.Fleck BA, Chen C, Yang W, Huntley R, Markison S, Nickolls SA, Foster AC, Hoare SR. Molecular interactions of nonpeptide agonists and antagonists with the melanocortin-4 receptor. Biochemistry. 2005;44:14494–14508. doi: 10.1021/bi051316s. [DOI] [PubMed] [Google Scholar]
- 16.Fluck CE, Martens JW, Conte FA, Miller WL. Clinical, genetic, and functional characterization of adrenocorticotropin receptor mutations using a novel receptor assay. J Clin Endocrinol Metab. 2002;87:4318–4323. doi: 10.1210/jc.2002-020501. [DOI] [PubMed] [Google Scholar]
- 17.Gantz I, Konda Y, Tashiro T, Shimoto Y, Miwa H, Munzert G, Watson SJ, DelValle J, Yamada T. Molecular cloning of a novel melanocortin receptor. J Biol Chem. 1993;268:8246–8250. [PubMed] [Google Scholar]
- 18.Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson SJ, DelValle J, Yamada T. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J Biol Chem. 1993;268:15174–15179. [PubMed] [Google Scholar]
- 19.Gantz I, Shimoto Y, Konda Y, Miwa H, Dickinson CJ, Yamada T. Molecular cloning, expression, and characterization of a fifth melanocortin receptor. Biochem Biophys Res Commun. 1994;200:1214–1220. doi: 10.1006/bbrc.1994.1580. [DOI] [PubMed] [Google Scholar]
- 20.Gantz I, Yamada T, Tashiro T, Konda Y, Shimoto Y, Miwa H, Trent JM. Mapping of the gene encoding the melanocortin-1 (alpha-melanocyte stimulating hormone) receptor (MC1R) to human chromosome 16q24.3 by Fluorescence in situ hybridization. Genomics. 1994;19:394–395. doi: 10.1006/geno.1994.1080. [DOI] [PubMed] [Google Scholar]
- 21.Gether U. Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr Rev. 2000;21:90–113. doi: 10.1210/edrv.21.1.0390. [DOI] [PubMed] [Google Scholar]
- 22.Girard J, Eberle AN, Baumann JB. Plasma ACTH in diagnosis and control of adrenal disorders. Acta Endocrinol Suppl (Copenh) 1986;279:254–258. doi: 10.1530/acta.0.112s254. [DOI] [PubMed] [Google Scholar]
- 23.Goldman JM, Hadley ME. Evidence for separate receptors for melanophore stimulating hormone and catecholamine regulation of cyclic AMP in the control of melanophore responses. Br J Pharmacol. 1970;39:160–166. doi: 10.1111/j.1476-5381.1970.tb09565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goverde HJ, Smals AG. The anomalous effect of some ACTH-fragments missing the amino acid sequence 1–10 on the corticosteroidogenesis in purified isolated rat adrenal cells. FEBS Lett. 1984;173:23–26. doi: 10.1016/0014-5793(84)81009-1. [DOI] [PubMed] [Google Scholar]
- 25.Guan XM, Kobilka TS, Kobilka BK. Enhancement of membrane insertion and function in a type IIIb membrane protein following introduction of a cleavable signal peptide. J Biol Chem. 1992;267:21995–21998. [PubMed] [Google Scholar]
- 26.Haskell-Luevano C, Cone RD, Monck EK, Wan YP. Structure activity studies of the melanocortin-4 receptor by in vitro mutagenesis: identification of agouti-related protein (AGRP), melanocortin agonist and synthetic peptide antagonist interaction determinants. Biochemistry. 2001;40:6164–6179. doi: 10.1021/bi010025q. [DOI] [PubMed] [Google Scholar]
- 27.Haskell-Luevano C, Hendrata S, North C, Sawyer TK, Hadley ME, Hruby VJ, Dickinson C, Gantz I. Discovery of prototype peptidomimetic agonists at the human melanocortin receptors MC1R and MC4R. J Med Chem. 1997;40:2133–2139. doi: 10.1021/jm960840h. [DOI] [PubMed] [Google Scholar]
- 28.Haskell-Luevano C, Sawyer TK, Hendrata S, North C, Panahinia L, Stum M, Staples DJ, Castrucci AM, Hadley MF, Hruby VJ. Truncation studies of alpha-melanotropin peptides identify tripeptide analogues exhibiting prolonged agonist bioactivity. Peptides. 1996;17:995–1002. doi: 10.1016/0196-9781(96)00141-6. [DOI] [PubMed] [Google Scholar]
- 29.Haskell-Luevano C, Sawyer TK, Trumpp-Kallmeyer S, Bikker JA, Humblet C, Gantz I, Hruby VJ. Three-dimensional molecular models of the hMC1R melanocortin receptor: complexes with melanotropin peptide agonists. Drug Des Discov. 1996;14:197–211. [PubMed] [Google Scholar]
- 30.Hogan K, Peluso S, Gould S, Parsons I, Ryan D, Wu L, Visiers I. Mapping the binding site of melanocortin 4 receptor agonists: a hydrophobic pocket formed by I3.28(125), I3.32(129), and I7.42(291) is critical for receptor activation. J Med Chem. 2006;49:911–922. doi: 10.1021/jm050780s. [DOI] [PubMed] [Google Scholar]
- 31.Holder JR, Bauzo RM, Xiang Z, Haskell-Luevano C. Structure-activity relationships of the melanocortin tetrapeptide Ac-His-DPhe-Arg-Trp-NH(2) at the mouse melanocortin receptors. 1. Modifications at the His position. J Med Chem. 2002;45:2801–2810. doi: 10.1021/jm0104872. [DOI] [PubMed] [Google Scholar]
- 32.Holder JR, Bauzo RM, Xiang Z, Haskell-Luevano C. Structure-activity relationships of the melanocortin tetrapeptide Ac-His-DPhe-Arg-Trp-NH(2) at the mouse melanocortin receptors: part 2 modifications at the Phe position. J Med Chem. 2002;45:3073–3081. doi: 10.1021/jm010524p. [DOI] [PubMed] [Google Scholar]
- 33.Holder JR, Marques FF, Xiang Z, Bauzo RM, Haskell-Luevano C. Characterization of aliphatic, cyclic, and aromatic N-terminally “capped” His-D-Phe-Arg-Trp-NH2 tetrapeptides at the melanocortin receptors. Eur J Pharmacol. 2003;462:41–52. doi: 10.1016/s0014-2999(03)01322-0. [DOI] [PubMed] [Google Scholar]
- 34.Holder JR, Xiang Z, Bauzo RM, Haskell-Luevano C. Structure-activity relationships of the melanocortin tetrapeptide Ac-His-DPhe-Arg-Trp-NH2 at the mouse melanocortin receptors. Part 3: modifications at the Arg position. Peptides. 2003;24:73–82. doi: 10.1016/s0196-9781(02)00278-4. [DOI] [PubMed] [Google Scholar]
- 35.Imamine H, Mizuno H, Sugiyama Y, Ohro Y, Sugiura T, Togari H. Possible relationship between elevated plasma ACTH and tall stature in familial glucocorticoid deficiency. Tohoku J Exp Med. 2005;205:123–131. doi: 10.1620/tjem.205.123. [DOI] [PubMed] [Google Scholar]
- 36.Kapas S, Cammas FM, Hinson JP, Clark AJ. Agonist and receptor binding properties of adrenocorticotropin peptides using the cloned mouse adrenocorticotropin receptor expressed in a stably transfected HeLa cell line. Endocrinology. 1996;137:3291–3294. doi: 10.1210/endo.137.8.8754753. [DOI] [PubMed] [Google Scholar]
- 37.Labeur M, Arzt E, Stalla GK, Paez-Pereda M. New perspectives in the treatment of Cushing’s syndrome. Curr Drug Targets Immune Endocr Metabol Disord. 2004;4:335–342. doi: 10.2174/1568008043339703. [DOI] [PubMed] [Google Scholar]
- 38.Lampron A, Bourdeau I, Hamet P, Tremblay J, Lacroix A. Whole genome expression profiling of GIP- and ACTH-dependent adrenal hyperplasias reveals novel targets for the study of GIP-dependent Cushing’s syndrome. J Clin Endocrinol Metab. 2006;91:3611–3618. doi: 10.1210/jc.2006-0221. [DOI] [PubMed] [Google Scholar]
- 39.Matsuura H, Shiohara M, Yamano M, Kurata K, Arai F, Koike K. Novel compound heterozygous mutation of the MC2R gene in a patient with familial glucocorticoid deficiency. J Pediatr Endocrinol Metab. 2006;19:1167–1170. doi: 10.1515/jpem.2006.19.9.1167. [DOI] [PubMed] [Google Scholar]
- 40.Migeon CJ, Kenny EM, Kowarski A, Snipes CA, Spaulding JS, Finkelstein JW, Blizzard RM. The syndrome of congenital adrenocortical unresponsiveness to ACTH. Report of six cases. Pediatr Res. 1968;2:501–513. doi: 10.1203/00006450-196811000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257:1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura M. Studies on the role of basic amino acid residues of ACTH peptide in steroidogenesis by isolated adrenal cells. J Biochem (Tokyo) 1972;71:1029–1041. doi: 10.1093/oxfordjournals.jbchem.a129851. [DOI] [PubMed] [Google Scholar]
- 43.Naville D, Barjhoux L, Jaillard C, Faury D, Despert F, Esteva B, Durand P, Saez J, Begeot M. Mutations of ACTH receptor gene and familial syndrome of glucocorticoid deficiency. Ann Endocrinol (Paris) 1996;57:101–106. [PubMed] [Google Scholar]
- 44.Naville D, Barjhoux L, Jaillard C, Faury D, Despert F, Esteva B, Durand P, Saez JM, Begeot M. Demonstration by transfection studies that mutations in the adrenocorticotropin receptor gene are one cause of the hereditary syndrome of glucocorticoid deficiency. J Clin Endocrinol Metab. 1996;81:1442–1448. doi: 10.1210/jcem.81.4.8636348. [DOI] [PubMed] [Google Scholar]
- 45.Opmeer FA, van Ree JM, de Wied D. ACTH-induced lipolysis in rat adipocytes: structure-activity relationships. Naunyn Schmiedebergs Arch Pharmacol. 1978;302:31–36. doi: 10.1007/BF00586593. [DOI] [PubMed] [Google Scholar]
- 46.Pogozheva ID, Chai BX, Lomize AL, Fong TM, Weinberg DH, Nargund RP, Mulholland MW, Gantz I, Mosberg HI. Interactions of human melanocortin 4 receptor with nonpeptide and peptide agonists. Biochemistry. 2005;44:11329–11341. doi: 10.1021/bi0501840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sawyer TK, Castrucci AM, Staples DJ, Affholter JA, De Vaux A, Hruby VJ, Hadley ME. Structure-activity relationships of [Nle4, D-Phe7]alpha-MSH. Discovery of a tripeptidyl agonist exhibiting sustained bioactivity. Ann N Y Acad Sci. 1993;680:597–599. doi: 10.1111/j.1749-6632.1993.tb19749.x. [DOI] [PubMed] [Google Scholar]
- 48.Schioth HB, Chhajlani V, Muceniece R, Klusa V, Wikberg JE. Major pharmacological distinction of the ACTH receptor from other melanocortin receptors. Life Sci. 1996;59:797–801. doi: 10.1016/0024-3205(96)00370-0. [DOI] [PubMed] [Google Scholar]
- 49.Selva KA, LaFranchi SH, Boston B. A novel presentation of familial glucocorticoid deficiency (FGD) and current literature review. J Pediatr Endocrinol Metab. 2004;17:85–92. doi: 10.1515/jpem.2004.17.1.85. [DOI] [PubMed] [Google Scholar]
- 50.Shepard TH, Landing BH, Mason DG. Familial Addison’s disease; case reports of two sisters with corticoid deficiency unassociated with hypoaldosteronism. AMA J Dis Child. 1959;97:154–162. [PubMed] [Google Scholar]
- 51.Shizume K, Lerner AB, Fitzpatrick TB. In vitro bioassay for the melanocyte stimulating hormone. Endocrinology. 1954;54:553–560. doi: 10.1210/endo-54-5-553. [DOI] [PubMed] [Google Scholar]
- 52.Slavotinek AM, Hurst JA, Dunger D, Wilkie AO. ACTH receptor mutation in a girl with familial glucocorticoid deficiency. Clin Genet. 1998;53:57–62. doi: 10.1034/j.1399-0004.1998.531530112.x. [DOI] [PubMed] [Google Scholar]
- 53.Szalay KS, De Wied D, Stark E. Effects of ACTH-(11–24) on the corticosteroid production of isolated adrenocortical cells. J Steroid Biochem. 1989;32:259–262. doi: 10.1016/0022-4731(89)90261-6. [DOI] [PubMed] [Google Scholar]
- 54.Tatro JB. Receptor biology of the melanocortins, a family of neuroimmunomodulatory peptides. Neuroimmunomodulation. 1996;3:259–284. doi: 10.1159/000097281. [DOI] [PubMed] [Google Scholar]
- 55.Tatro JB, Reichlin S. Specific receptors for alpha-melanocyte-stimulating hormone are widely distributed in tissues of rodents. Endocrinology. 1987;121:1900–1907. doi: 10.1210/endo-121-5-1900. [DOI] [PubMed] [Google Scholar]
- 56.Todorovic A, Holder JR, Bauzo RM, Scott JW, Kavanagh R, Abdel-Malek Z, Haskell-Luevano C. N-terminal fatty acylated His-dPhe-Arg-Trp-NH(2) tetrapeptides: influence of fatty acid chain length on potency and selectivity at the mouse melanocortin receptors and human melanocytes. J Med Chem. 2005;48:3328–3336. doi: 10.1021/jm0490843. [DOI] [PubMed] [Google Scholar]
- 57.Tritos NA, Maratos-Flier E. Two important systems in energy homeostasis: melanocortins and melanin-concentrating hormone. Neuropeptides. 1999;33:339–349. doi: 10.1054/npep.1999.0055. [DOI] [PubMed] [Google Scholar]
- 58.Tsigos C, Arai K, Hung W, Chrousos GP. Hereditary isolated glucocorticoid deficiency is associated with abnormalities of the adrenocorticotropin receptor gene. J Clin Invest. 1993;92:2458–2461. doi: 10.1172/JCI116853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weber A, Clark AJ. Mutations of the ACTH receptor gene are only one cause of familial glucocorticoid deficiency. Hum Mol Genet. 1994;3:585–588. doi: 10.1093/hmg/3.4.585. [DOI] [PubMed] [Google Scholar]
- 60.Weber A, Toppari J, Harvey RD, Klann RC, Shaw NJ, Ricker AT, Nanto-Salonen K, Bevan JS, Clark AJ. Adrenocorticotropin receptor gene mutations in familial glucocorticoid deficiency: relationships with clinical features in four families. J Clin Endocrinol Metab. 1995;80:65–71. doi: 10.1210/jcem.80.1.7829641. [DOI] [PubMed] [Google Scholar]
- 61.Yang Y, Chen M, Lai Y, Gantz I, Yagmurlu A, Georgeson KE, Harmon CM. Molecular determination of agouti-related protein binding to human melanocortin-4 receptor. Mol Pharmacol. 2003;64:94–103. doi: 10.1124/mol.64.1.94. [DOI] [PubMed] [Google Scholar]
- 62.Yang Y, Dickinson C, Haskell-Luevano C, Gantz I. Molecular basis for the interaction of [Nle4,D-Phe7]melanocyte stimulating hormone with the human melanocortin-1 receptor. J Biol Chem. 1997;272:23000–23010. doi: 10.1074/jbc.272.37.23000. [DOI] [PubMed] [Google Scholar]
- 63.Yang YK, Dickinson CJ, Zeng Q, Li JY, Thompson DA, Gantz I. Contribution of melanocortin receptor exoloops to Agouti-related protein binding. J Biol Chem. 1999;274:14100–14106. doi: 10.1074/jbc.274.20.14100. [DOI] [PubMed] [Google Scholar]
- 64.Yang YK, Fong TM, Dickinson CJ, Mao C, Li JY, Tota MR, Mosley R, Van Der Ploeg LH, Gantz I. Molecular determinants of ligand binding to the human melanocortin-4 receptor. Biochemistry. 2000;39:14900–14911. doi: 10.1021/bi001684q. [DOI] [PubMed] [Google Scholar]
- 65.Yang YK, Thompson DA, Dickinson CJ, Wilken J, Barsh GS, Kent SB, Gantz I. Characterization of Agouti-related protein binding to melanocortin receptors. Mol Endocrinol. 1999;13:148–155. doi: 10.1210/mend.13.1.0223. [DOI] [PubMed] [Google Scholar]