Abstract
Drug-naïve, non-deprived rats were trained to lever press for saccharin under fixed-ratio (FR) or variable-ratio (VR) schedules of reinforcement. Rats trained on the VR schedule in which saccharin reinforcement was not predicted by a fixed number of lever presses subsequently showed an enhanced locomotor response to a threshold amphetamine challenge injection (0.5 mg/kg IP) administered two weeks following the last saccharin session. This finding suggests that chronic exposure to gambling-like conditions of uncertain reinforcement can induce neuroadaptations in brain reward systems that are similar to those produced by repeated psychostimulant exposure and may lead to the development of addictive behaviors.
Keywords: addiction, sensitization, self-administration, sucrose, locomotion
Report
Repeated exposure to psychostimulants such as amphetamine leads to an enhancement of their behavioral and biochemical effects, known as sensitization. Neuroadaptations associated with sensitization occur in specific brain regions and may underlie the transition from casual drug use to craving and abuse [1, 2]. In animal models of drug abuse, sensitization has been manifested by enhanced locomotor activation and nucleus accumbens (NAcc) dopamine (DA) overflow in response to the drug, as well as increased drug self-administration [1, 3]. Sensitization has been observed following exposure to a number of drugs [4, 5] and cross-sensitization between drugs and non-drug reinforcers such as sucrose has been reported [6–8].
Pathological gambling and drug addiction share common neuronal substrates. Studies of cocaine- and alcohol-dependent individuals reveal a higher prevalence of pathological gambling than seen in the general population, often with gambling preceding drug addiction [9–12]. Furthermore, a priming dose of amphetamine given to pathological gamblers increases motivation to gamble and facilitates the reading of gambling-related words. These findings are consistent with the activation of common pathways in gambling and drug dependence [13, 14]. Human imaging studies conducted during gambling and the performance of unpredictable reward tasks have observed neuronal activation in the ventral tegmental area (VTA) and its subcortical dopaminergic terminal projections fields [15–20], brain regions intimately involved in the development and maintenance of addiction [1, 21, 22]. Collectively, these findings suggest that similar neuronal processes may underlie these disorders. The following experiment aimed to further characterize this relationship by studying how exposure to gambling-like intermittent reward affects the development of locomotor sensitization to amphetamine.
The effects of two different saccharin reinforcement schedules on amphetamine cross-sensitization were studied. Male Sprague-Dawley rats (Harlan Sprague-Dawley, Madison, WI) weighing 250–275g on arrival were individually housed with food and water available ad libitum in a reverse cycle room (12-h light/12-h dark). All tests were conducted during the dark phase of the light/dark cycle in individual sound and light attenuated operant chambers. Each chamber contained a lever, cue light, and liquid delivery spout connected to a syringe pump. Lever presses were recorded and a computer using proprietary software controlled delivery of liquid. Liquid not consumed was collected and measured at the end of each session.
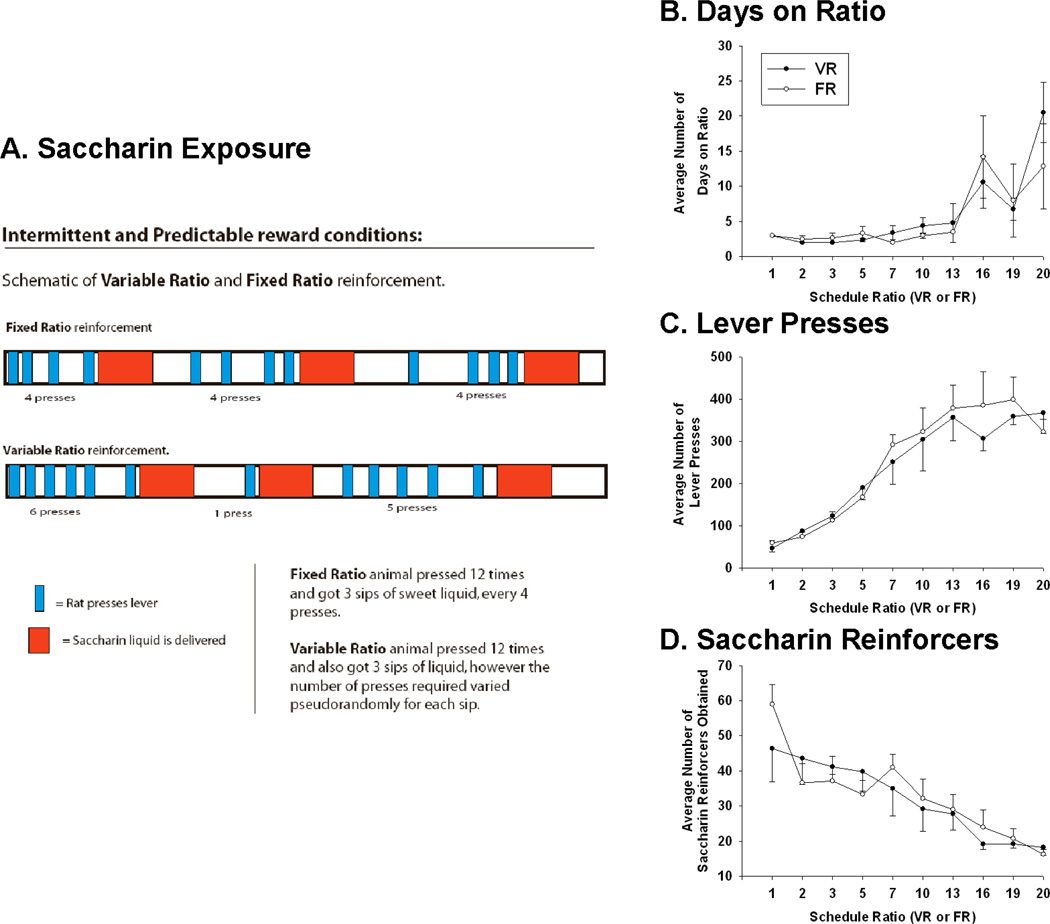
In order to manipulate chronic exposure to unpredictable reinforcement, the pattern of lever pressing necessary to obtain saccharin reward during each session differed between two groups of rats. Rats were exposed to either predictable fixed ratio (FR; n=6) or unpredictable variable ratio (VR; n=5) schedules of non-caloric saccharin reinforcement (0.3% saccharin in water) during 1-hour sessions given 6 days/week (Figure 1A). The fixed or average number of lever presses necessary to receive reward was escalated when rats were able to obtain saccharin at least 20 times in two consecutive sessions. After 55 days of training, rats from both groups worked reliably to achieve an average press-to-payout ratio of 20 per session (FR20 or VR20). For each VR ratio, the minimum number of lever presses needed to obtain reward was always 1, whereas the maximum was double the average (ex. 40 lever presses for the VR20 schedule). It is important to note that although VR exposure may mimic the unpredictable nature of gambling, other aspects of gambling, such as pursuit of reward despite negative consequences, were not tested and may involve similar or different neuronal circuitry.
Figure 1. Examples of variable and fixed ratio reinforcement conditions.
(A) Rats were trained to lever press for saccharin on either a fixed ratio (FR; n=6) or variable ratio (VR; n=5) schedule of reinforcement. Lever pressing for saccharin lasted for 55 days and rats from both groups worked reliably to achieve FR20 or VR20 reinforcement schedules. Over the course of the sessions, the average number of days worked on each ratio (B), lever presses made (C), and amount of saccharin reward obtained (D) was statistically the same for FR and VR animals.
During the first 5 days of training (schedules FR1 and FR2 or VR1 and VR2) there was no significant difference in the saccharin left unconsumed between the FR (20.33 ± 2.89 ml) and VR ( 15.0 ± 3.29 ml) rats (t9=1.22, ns). Rats reliably consumed all saccharin rewarded after the fifth training session. Taking into account these results, over the course of 55 days of training there was no significant difference in the total amount of saccharin consumed for the FR (265.71 ± 18.43 ml) and VR (283.01 ± 21.39 ml) rats (t9=0.55, ns). Over the course of all training sessions, repeated between-within ANOVAs with group (FR or VR) as the between factor and schedule ratio as the within factor showed no significant group differences between FR and VR rats in the days worked on each schedule (Figure 1B; F1,5=2.14; ns), total lever presses made (Figure 1C; F1,5=1.24; ns), and saccharin reinforcements received (Figure 1D; F1,5=0.496; ns). A significant effect of schedule was found for each of these measures (Days, F9,45=15.9, p<0.001; Presses, F9,45=22.8, p<0.001; Reinforcers F9,45=6.78, p<0.001), but no significant interaction between group and schedule was found for any measure (Days, F9,45=0.391, ns; Presses, F9,45=1.36, ns; Reinforcers F9,45=0.657, ns).
Two weeks after the last training session, rats received an intraperitoneal (IP) saline injection followed by a threshold amphetamine challenge (0.5 mg/kg, IP; S(+)-amphetamine sulfate, Sigma Inc., St. Louis, MO; dose refers to the weight of the salt). Horizontal locomotor activity was monitored for 3 hours after each injection in activity boxes (22 X 43 X 33 cm) constructed of opaque plastic (rear and two side walls), a Plexiglas front-hinged door, and a tubular stainless steel ceiling and floor. Photocells, positioned 2.5 cm above the floor and spaced evenly along the longitudinal axis of each box, were used to quantify locomotion. Separate interruptions of photocell beams were detected and recorded via an electrical interface by a computer situated in an adjacent room using locally developed software.
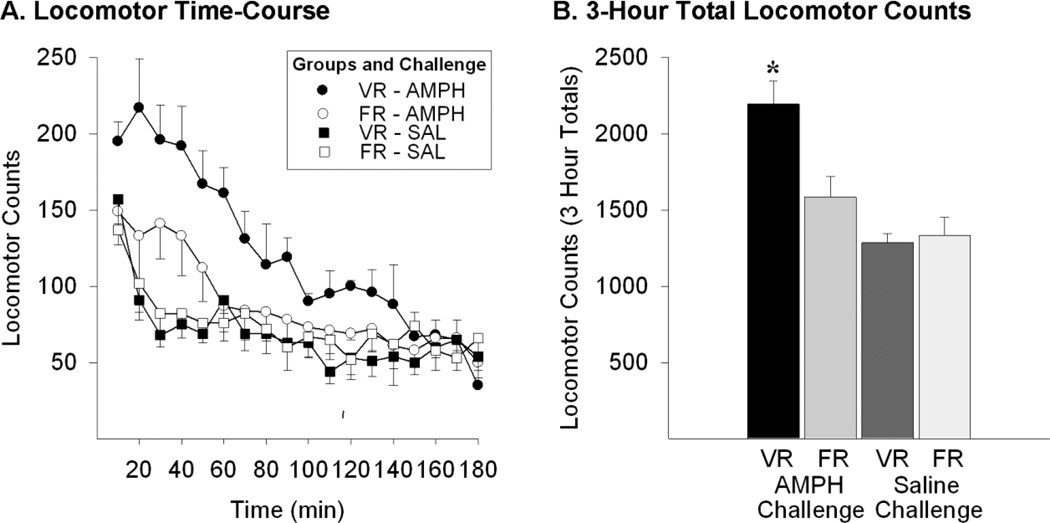
A two-way between-within ANOVA on three hour total locomotor counts was performed with saccharin exposure (FR or VR) as the group factor and challenge (saline or amphetamine) as the within factor (Figure 2). The ANOVA revealed a significant challenge effect (F1,9=46.8; p<0.001), as well as a significant group×challenge interaction (F1,9=15.1; p<0.01), but no significant group effect (F1,9=3.44; ns). Post-hoc Sheffé comparisons confirmed that amphetamine significantly enhanced locomotor responding in VR rats compared to saline (*p<0.01) and that this effect was enhanced in VR relative to FR rats (*p<0.01). FR-trained rats showed a trend towards enhanced locomotor responding to amphetamine compared to saline challenged rats, but the effect was not significant, a finding that may be explained by the low threshold dose of amphetamine administered. No significant difference in locomotor responding to the saline injection was found between the two groups. These results indicate that long-term exposure to unpredictable variable-, as opposed to predictable fixed-, ratio schedules of saccharin reinforcement produce cross-sensitization to amphetamine.
Figure 2. Rats previously exposed to conditions of uncertain reinforcement (VR) show significantly enhanced locomotor responding to a threshold dose of amphetamine (0.5 mg/kg, IP) compared to rats exposed to predictable reinforcement (FR).
Testing was conducted two weeks after the last VR or FR reinforcement exposure session. Rats were first injected with saline and three hours later with amphetamine. Data are shown in A as group mean (± SEM) locomotor counts observed throughout the 3-hr saline and amphetamine sessions. Group mean (+ SEM) total locomotor counts observed in the three hours after saline or after amphetamine are shown in B. Post-hoc Sheffé comparisons confirmed that amphetamine significantly enhanced locomotor responding in VR rats compared to saline (*p<0.01) and that this effect was enhanced in VR relative to FR rats (*p<0.01). n=5–6/group.
The present results suggest that the actions of drug and non-drug reinforcers converge on similar brain substrates. Several lines of evidence support this possibility. At the behavioral level, exposure to sugar has been shown to produce cross-sensitization to the locomotor effects of amphetamine and cocaine [6, 8]. In addition, compared to rats bred to have a low preference for saccharin (LoS), high saccharin intake (HiS) rats escalate cocaine self-administration more rapidly and respond more on the drug paired lever during maintenance, extinction, and cocaine-induced reinstatement [23]. Conversely, drug exposure or genetic predisposition to administer drugs enhances the psychomotor effects of sugar. Repeated exposure to amphetamine cross-sensitizes sugar-induced locomotion and enhances sugar consumption [24]. In addition, alcohol-preferring breeds of rats display increased intake of sweet substances compared to breeds with low or no alcohol preference [25, 26].
Both drugs of abuse and natural reinforcers, such as sugar, produce similar biochemical effects. Like psychostimulants [1, 3], self-administration or sham-feeding of sugar leads to an increase in NAcc DA levels during intake [27–30]. In addition, in food-deprived rats placed on chronic schedules of intermittent bingeing for sucrose, the expression levels of dopamine D2 receptors are decreased and D1-type receptors are increased in several brain areas, including the NAcc [31–33]. This feeding schedule has also been shown to increase protein expression and mRNA levels of the dopamine transporter [34]. Studies from drug or alcohol dependent patients and animals are consistent with these findings [35–38]. Electrophysiological recordings of NAcc neurons obtained during operant responding for water/food or cocaine have demonstrated non-overlapping firing patterns, however, suggesting that separate neural circuits are nonetheless recruited during reinforced responding for drugs and natural rewards [39–41]. In addition, similar to how different abused drugs have various pharmacological actions, different natural rewards, such as food and sex, also may have different neurobiological substrates [42, 43]. Furthermore, the magnitude of effects and incentive salience of drugs and natural rewards may be different, with drugs often hijacking natural reward processing systems, leading to exaggerated responses. For example, cocaine reward elicits a greater NAcc neuronal response than a juice reward [42]. Despite these differences, the present results suggest that exposure to VR saccharin may influence similar neuronal circuitry by which amphetamine produces its locomotor sensitization effects.
One possible explanation for the locomotor sensitization observed in VR relative to FR rats may be that variable, but not fixed, ratio saccharin reinforcement elicits phasic dopaminergic responses from midbrain neurons that resemble neuronal activation patterns induced by repeated psychostimulant exposure. When animals are exposed to maximal conditions of uncertainty (50% chance of reward), VTA neurons fire at a maximal rate from the time of cue exposure to that of expected reward [44]. In contrast, phasic dopaminergic activity is decreased during this time period if there is predictability of either reward or lack thereof. To the extent that the variable ratio exposed rats performed under conditions of uncertainty regarding the procurement of reward in the present experiment, it is likely that dopamine neuron activation was also elevated during the saccharin sessions in these rats. This potential repeated phasic enhancement of dopamine levels in the midbrain, sites of induction of stimulant sensitization [1], would be predicted to lead to cross-sensitization with amphetamine.
Long-term changes in dopamine receptor subtype expression or sensitivity resulting from increased dopamine release may underlie the enhanced behavioral response to amphetamine observed in the VR-exposed rats. Support for these long-lasting receptor changes comes from both the gambling and addiction literature. Individuals with a genetic D2-receptor deficiency have an increased risk of developing various forms of addiction [45, 46], and administering D2-receptor antagonists to pathological gamblers enhances the rewarding effects of gambling [47]. Interestingly, lower D2 expression levels have also been shown to correlate with increased liking of psychostimulant effects in humans [48, 49]. Furthermore, reduced D2/D3 receptor function has also been show to govern impulsivity and cocaine self-administration in rodents [50]. Thus, it is possible that exposure to unpredictability and intermittently administered psychostimulants each produces reductions in D2-mediated signaling. Such a reduction may consequentially lead to increased amphetamine-induced dopamine release [51].
These findings notwithstanding, it has also been shown that certain populations of dopamine-deficient Parkinsonian patients may be vulnerable to pathological gambling following treatment with dopamine D2/D3 receptor agonists [52–56]. Additionally, when given the choice, rats treated with the D2/D3 receptor agonist pramipexole choose a more difficult and gamblinglike variable- rather than fixed ratio-schedule of reinforcement to earn food reward [57]. Interestingly, both treatments for Parkinson’s disease and psychostimulant exposure lead to an upregulation of D3 receptors [58–60]. It is believed that the receptor does not mediate the direct reinforcing effects of abused drugs, but instead may control motivation to take drug under schedules of reinforcement with high response requirements [61]. Altogether, it is conceivable that the difficult and gambling-like variable ratio schedule of saccharin reinforcement used in the present study may have elevated D3 receptor levels, thereby supporting cross-sensitization to amphetamine.
The present experiments demonstrate that animals previously exposed to conditions of uncertain reinforcement with saccharin show enhanced locomotor responding to amphetamine compared to animals previously exposed to predictable reinforcement. This finding suggests that the variable reinforcement schedule elicited neuroplastic changes similar to those produced by repeated psychostimulant exposure known to sensitize responding. Indeed, considerable evidence suggests that gambling and drug addiction usurp similar brain substrates and that the disorders are often comorbid. The present findings support this relationship, by showing that repeated exposure to gambling-like reinforcement promotes cross-sensitization to psychostimulant effects.
Highlights.
Drug-naïve rats were trained to self-administer saccharin
Saccharin reinforcement was either predictable or unpredictable
Unpredictable saccharin reinforcement produced cross-sensitization to amphetamine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 3.Vezina P, Lorrain DS, Arnold GM, Austin JD, Suto N. Sensitization of midbrain dopamine neuron reactivity promotes the pursuit of amphetamine. J Neurosci. 2002;22:4654–4662. doi: 10.1523/JNEUROSCI.22-11-04654.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vezina P, McGehee DS, Green WN. Exposure to nicotine and sensitization of nicotine-induced behaviors. Prog in Neuro-Psychopharmacology and Biol Psychiat. 2007;31:1625–1638. doi: 10.1016/j.pnpbp.2007.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- 6.Avena NM, Hoebel BG. A diet promoting sugar dependency causes behavioral cross-sensitization to a low dose of amphetamine. Neurosci. 2003;122:17–20. doi: 10.1016/s0306-4522(03)00502-5. [DOI] [PubMed] [Google Scholar]
- 7.Avena NM, Hoebel BG. Amphetamine-sensitized rats show sugar-induced hyperactivity (cross-sensitization) and sugar hyperphagia. Pharmacol Biochem and Be. 2003;74:635–639. doi: 10.1016/s0091-3057(02)01050-x. [DOI] [PubMed] [Google Scholar]
- 8.Gosnell BA. Sucrose intake enhances behavioral sensitization produced by cocaine. Brain Res. 2005;1031:194–201. doi: 10.1016/j.brainres.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 9.Baldo V, Cristofoletti M, Majori S, Cibin M, Peron C, Dal Zotto A, Zampieri N, Saia M, Trivello R. Relationship between pathological gambling, alcoholism and drug addiction. Ann Ig. 2006;18:147–153. [PubMed] [Google Scholar]
- 10.Sellman JD, Adamson S, Robertson P, Sullivan S, Coverdale J. Gambling in mild-moderate alcohol-dependent outpatients. Subst Use Misuse. 2002;37:199–213. doi: 10.1081/ja-120001977. [DOI] [PubMed] [Google Scholar]
- 11.Steinberg MA, Kosten TA, Rounsaville BJ. Cocaine Abuse and Pathological Gambling. Am J Addiction. 1992;1:121–132. [Google Scholar]
- 12.Hall GW, Carriero NJ, Takushi RY, Montoya ID, Preston KL, Gorelick DA. Pathological gambling among cocaine-dependent outpatients. Am J Psychiat. 2000;157:1127–1133. doi: 10.1176/appi.ajp.157.7.1127. [DOI] [PubMed] [Google Scholar]
- 13.Zack M, Poulos CX. Parallel roles for dopamine in pathological gambling and psychostimulant addiction. Curr Drug Abuse Rev. 2009;2:11–25. doi: 10.2174/1874473710902010011. [DOI] [PubMed] [Google Scholar]
- 14.Zack M, Poulos CX. Amphetamine primes motivation to gamble and gambling-related semantic networks in problem gamblers. Neuropsychopharmacol. 2004;29:195–207. doi: 10.1038/sj.npp.1300333. [DOI] [PubMed] [Google Scholar]
- 15.Berns GS, McClure SM, Pagnoni G, Montague PR. Predictability modulates human brain response to reward. J Neurosci. 2001;21:2793–2798. doi: 10.1523/JNEUROSCI.21-08-02793.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–639. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- 17.D'Ardenne K, McClure SM, Nystrom LE, Cohen JD. BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science. 2008;319:1264–1267. doi: 10.1126/science.1150605. [DOI] [PubMed] [Google Scholar]
- 18.Linnet J, Moller A, Peterson E, Gjedde A, Doudet D. Dopamine release in ventral striatum during Iowa Gambling Task performance is associated with increased excitement levels in pathological gambling. Addiction. 2011;106:383–390. doi: 10.1111/j.1360-0443.2010.03126.x. [DOI] [PubMed] [Google Scholar]
- 19.Elliott R, Newman JL, Longe OA, Deakin JF. Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: a parametric functional magnetic resonance imaging study. J Neurosci. 2003;23:303–307. doi: 10.1523/JNEUROSCI.23-01-00303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galvan A, Hare TA, Davidson M, Spicer J, Glover G, Casey BJ. The role of ventral frontostriatal circuitry in reward-based learning in humans. J Neurosci. 2005;25:8650–8656. doi: 10.1523/JNEUROSCI.2431-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volkow ND, Wang G, Fowler JS, Logan J, Gatley SJ, Wong C, Hitzemann R, Pappas NR. Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D2 receptors. J Pharmacol Exp Ther. 1999;291:409–415. [PubMed] [Google Scholar]
- 22.Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M, Benkelfat C. Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Arch Gen Psychiatry. 2006;63:1386–1395. doi: 10.1001/archpsyc.63.12.1386. [DOI] [PubMed] [Google Scholar]
- 23.Perry JL, Morgan AD, Anker JJ, Dess NK, Carroll ME. Escalation of i.v. cocaine selfadministration and reinstatement of cocaine-seeking behavior in rats bred for high and low saccharin intake. Psychopharmacology. 2006;186:235–245. doi: 10.1007/s00213-006-0371-x. [DOI] [PubMed] [Google Scholar]
- 24.Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: Behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav R. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stewart RB, Russell RN, Lumeng L, Li T, Murphy JM. Consumption of sweet, salty, sour, and bitter solutions by selectively bred alcohol-preferring and alcohol-nonpreferring lines of rats. Alcohol Clin Exp Res. 1994;18:375–381. doi: 10.1111/j.1530-0277.1994.tb00028.x. [DOI] [PubMed] [Google Scholar]
- 26.Eiler WJA, II, Woods JE, II, Masters J, McKay PF, Hardy L, III, Goergen JJ, Mensah-Zoe B, Cook JB, Johnson NJ, June HL. Brain stimulation reward performance and sucrose maintained behaviors in alcohol-preferring and -nonpreferring rats. Alcohol Clin Exp Res. 2005;29:571–583. doi: 10.1097/01.alc.0000158934.50534.b7. [DOI] [PubMed] [Google Scholar]
- 27.Doyon WM, Ramachandra V, Samson HH, Czachowski CL, Gonzales RA. Accumbal dopamine concentration during operant self-administration of a sucrose or a novel sucrose with ethanol solution. Alcohol. 2004;34:261–271. doi: 10.1016/j.alcohol.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol - Reg I. 2004;286:R31–R37. doi: 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- 29.Hajnal A, Norgren R. Repeated access to sucrose augments dopamine turnover in the nucleus accumbens. NeuroReport. 2002;13:2213–2216. doi: 10.1097/00001756-200212030-00010. [DOI] [PubMed] [Google Scholar]
- 30.Avena NM, Rada P, Moise N, Hoebel BG. Sucrose sham feeding on a binge schedule releases accumbens dopamine repeatedly and eliminates the acetylcholine satiety response. Neurosci. 2006;139:813–820. doi: 10.1016/j.neuroscience.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 31.Bello NT, Lucas LR, Hajnal A. Repeated sucrose access influences dopamine D2 receptor density in the striatum. NeuroReport. 2002;13:1575–1578. doi: 10.1097/00001756-200208270-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spangler R, Wittkowski KM, Goddard NL, Avena NM, Hoebel BG, Leibowitz SF. Opiate-like effects of sugar on gene expression in reward areas of the rat brain. Mol Brain Res. 2004;124:134–142. doi: 10.1016/j.molbrainres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 33.Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet J, Schwartz GJ, Moran TH, Hoebel BG. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. NeuroReport. 2001;12:3549–3552. doi: 10.1097/00001756-200111160-00035. [DOI] [PubMed] [Google Scholar]
- 34.Bello NT, Sweigart KL, Lakoski JM, Norgren R, Hajnal A. Restricted feeding with scheduled sucrose access results in an upregulation of the rat dopamine transporter. Am J Physiol - Reg I. 2003;284:R1260–R1268. doi: 10.1152/ajpregu.00716.2002. [DOI] [PubMed] [Google Scholar]
- 35.Spangler R, Goddard NL, Avena NM, Hoebel BG, Leibowitz SF. Elevated D3 dopamine receptor mRNA in dopaminergic and dopaminoceptive regions of the rat brain in response to morphine. Mol Brain Res. 2003;111:74–83. doi: 10.1016/s0169-328x(02)00671-x. [DOI] [PubMed] [Google Scholar]
- 36.Unterwald EM, Kreek MJ, Cuntapay M. The frequency of cocaine administration impacts cocaine-induced receptor alterations. Brain Res. 2001;900:103–109. doi: 10.1016/s0006-8993(01)02269-7. [DOI] [PubMed] [Google Scholar]
- 37.Shilling PD, Kelsoe JR, Segal DS. Dopamine transporter mRNA is up-regulated in the substantia nigra and the ventral tegmental area of amphetamine-sensitized rats. Neurosci Lett. 1997;236:131–134. doi: 10.1016/s0304-3940(97)00768-4. [DOI] [PubMed] [Google Scholar]
- 38.Hietala J, West C, Syvalahti E, Nagren K, Lehikoinen P, Sonninen P, Ruotsalainen U. Striatal D2 dopamine receptor binding characteristics in vivo in patients with alcohol dependence. Psychopharmacology. 1994;116:285–290. doi: 10.1007/BF02245330. [DOI] [PubMed] [Google Scholar]
- 39.Carelli RM, Deadwyler SA. A comparison of nucleus accumbens neuronal firing patterns during cocaine self-administration and water reinforcement in rats. J Neurosci. 1994;14:7736–7746. doi: 10.1523/JNEUROSCI.14-12-07735.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carelli RM, Ijames SG, Crumling AJ. Evidence that separate neural circuits the nucleus accumbens encode cocaine versus 'natural' (water and food) reward. J Neurosci. 2000;20:4255–4266. doi: 10.1523/JNEUROSCI.20-11-04255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carelli RM. Nucleus accumbens cell firing during goal-directed behaviors for cocaine vs. 'natural' reinforcement. Physiol Behav. 2002;76:379–387. doi: 10.1016/s0031-9384(02)00760-6. [DOI] [PubMed] [Google Scholar]
- 42.Deadwyler SA. Electrophysiological correlates of abused drugs: Relation to natural rewards. Ann NY Acad Sci. 2010;1187:140–147. doi: 10.1111/j.1749-6632.2009.05155.x. [DOI] [PubMed] [Google Scholar]
- 43.Lajtha A, Sershen H. Heterogeneity of reward mechanisms. Neurochem Res. 2010;35:851–867. doi: 10.1007/s11064-009-0096-4. [DOI] [PubMed] [Google Scholar]
- 44.Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- 45.Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, Comings DE. Dopamine D2 receptor gene variants: association and linkage studies in impulsive-addictive-compulsive behaviour. Pharmacogenetics. 1995;5:121–141. doi: 10.1097/00008571-199506000-00001. [DOI] [PubMed] [Google Scholar]
- 46.Noble EP. Addiction and its reward process through polymorphisms of the D2 dopamine receptor gene: a review. Eur J Psychiatry. 2000;15:79–89. doi: 10.1016/s0924-9338(00)00208-x. [DOI] [PubMed] [Google Scholar]
- 47.Zack M, Poulos CX. A D2 antagonist enhances the rewarding and priming effects of a gambling episode in pathological gamblers. Neuropsychopharmacol. 2007;32:1678–1686. doi: 10.1038/sj.npp.1301295. [DOI] [PubMed] [Google Scholar]
- 48.Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Gifford A, Hitzemann R, Ding YS, Pappas N. Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am J Psychiat. 1999;156:1440–1443. doi: 10.1176/ajp.156.9.1440. [DOI] [PubMed] [Google Scholar]
- 49.Volkow ND, Wang GJ, Fowler JS, Thanos PP, Logan J, Gatley SJ, Gifford A, Ding YS, Wong C, Pappas N. Brain DA D2 receptors predict reinforcing effects of stimulants in humans: replication study. Synapse. 2002;46:79–82. doi: 10.1002/syn.10137. [DOI] [PubMed] [Google Scholar]
- 50.Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pehek EA. Comparison of effects of haloperidol administration on amphetamine-stimulated dopamine release in the rat medial prefrontal cortex and dorsal striatum. J Pharmacol Exp Ther. 1999;289:14–23. [PubMed] [Google Scholar]
- 52.Dodd ML, Klos KJ, Bower JH, Geda YE, Josephs KA, Ahlskog JE. Pathological gambling caused by drugs used to treat Parkinson disease. Arch Neurol. 2005;62:1377–1381. doi: 10.1001/archneur.62.9.noc50009. [DOI] [PubMed] [Google Scholar]
- 53.Driver-Dunckley E, Samanta J, Stacy M. Pathological gambling associated with dopamine agonist therapy in Parkinson's disease. Neurology. 2003;61:422–423. doi: 10.1212/01.wnl.0000076478.45005.ec. [DOI] [PubMed] [Google Scholar]
- 54.Grosset KA, Macphee G, Pal G, Stewart D, Watt A, Davie J, Grosset DG. Problematic gambling on dopamine agonists: Not such a rarity. Mov Disord. 2006;21:2206–2208. doi: 10.1002/mds.21110. [DOI] [PubMed] [Google Scholar]
- 55.Molina JA, Sainz-Artiga MJ, Fraile A, Jimenez-Jimenez FJ, Villanueva C, Orti-Pareja M, Bermejo F. Pathologic gambling in Parkinson's disease: a behavioral manifestation of pharmacologic treatment? Mov Disord. 2000;15:869–872. doi: 10.1002/1531-8257(200009)15:5<869::aid-mds1016>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 56.Dagher A, Robbins TW. Personality, addiction, dopamine: insights from Parkinson's disease. Neuron. 2009;61:502–510. doi: 10.1016/j.neuron.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 57.Johnson PS, Madden GJ, Brewer AT, Pinkston JW, Fowler SC. Effects of acute pramipexole on preference for gambling-like schedules of reinforcement in rats. Psychopharmacology (Berl) 2011;213:11–18. doi: 10.1007/s00213-010-2006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bordet R, Ridray S, Carboni S, Diaz J, Sokoloff P, Schwartz JC. Induction of dopamine D3 receptor expression as a mechanism of behavioral sensitization to levodopa. Proc Natl Acad Sci U S A. 1997;94:3363–3367. doi: 10.1073/pnas.94.7.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Segal DM, Moraes CT, Mash DC. Up-regulation of D3 dopamine receptor mRNA in the nucleus accumbens of human cocaine fatalities. Brain Res Mol Brain Res. 1997;45:335–339. doi: 10.1016/s0169-328x(97)00025-9. [DOI] [PubMed] [Google Scholar]
- 60.Staley JK, Mash DC. Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. J Neurosci. 1996;16:6100–6106. doi: 10.1523/JNEUROSCI.16-19-06100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Le Foll B, Goldberg SR, Sokoloff P. The dopamine D3 receptor and drug dependence: effects on reward or beyond? Neuropharmacology. 2005;49:525–541. doi: 10.1016/j.neuropharm.2005.04.022. [DOI] [PubMed] [Google Scholar]