Abstract
Melanocortin 4 receptor (MC4R) plays an important role in the regulation of food intake and glucose homeostasis. Synthetic nonpeptide compound N- (3R)-1 4-tetrahydroisoquinolinium-3-ylcarbonyl -(1R)-1-(4-chlorobenzyl)-2- 4-cyclohexyl-4-(1H-1,2,4-triazol-1-ylmethyl)piperidin-1-yl -2-oxoethylamine (THIQ) is a potent agonist at MC4R but not at hMC2R. In this study, we utilized two approaches (chimeric receptor and site-directed mutagenesis) to narrow down the key amino acid residues of MC4R responsible for THIQ binding and signaling. Cassette substitutions of the second, third, fourth, fifth, and sixth transmembrane regions (TMs) of the human MC4R (hMC4R) with the homologous regions of hMC2R were constructed. Our results indicate that the cassette substitutions of these TMs of the hMC4R with homologous regions of the hMC2R did not significantly alter THIQ binding affinity and potency except the substitution of the hMC4R TM3, suggesting that the conserved amino acid residues in these TMs of the hMC4R are main potential candidates for THIQ binding and signaling while non conserved residues in TM3 of MC4R may also be involved. Nineteen MC4R mutants were then created, including 13 conserved amino acid residues and 6 non-conserved amino acid residues. Our results indicate that seven conserved residue [E100 (TM2), D122 (TM3), D126 (TM3), F254 (TM6), W258 (TM6), F261 (TM6), H264 (TM6)] are important for THIQ binding and three non-conserved residues [N123 (TM3), I129 (TM3) and S131 (TM3)] are involved in THIQ selectivity. In conclusion, our results suggest that THIQ utilize both conserved and non-conserved amino acid residues for binding and signaling at hMC4R and non conserved residues may be responsible for MC4R selectivity.
Introduction
Obesity is one of the major health threats for modern American society. Obese patients face an increased risk of morbidity and mortality due to obesity associated diseases such as Type II diabetes mellitus, hypertension, stroke and coronary artery diseases (1–3). However, obesity is a particularly challenging medical condition to treat because of its multi-factorial etiology and existing therapeutic limitations (4–6). In recent years, researchers have produced exciting new insights into obesity and the physiological systems of the brain governing metabolic, appetite, and energy expenditure regulation (7–13). Melanocortin system has been identified to play an important role in the regulation of food intake and glucose homeostasis (14–17). MC4R has been identified to play a key role in the regulation of food intake and body weight. MC4R is a seven transmembrane G-protein coupled receptor (GPCR) expressed mainly in the hypothalamus (16, 18–23). The endogenous agonist, α-melanocyte stimulating hormone (α-MSH), activates hMC4R and inhibits food intake while agouti-related protein (AGRP), the endogenous antagonist, inhibits hMC4R action and induces food intake (19, 24, 25). MC4R activation is therefore of interest as a possible therapeutic approach for the treatment of obesity. A better understanding of the molecular basis of the hMC4R function is therefore critical to understanding the development of human obesity and to guide the development of effective therapeutic strategies for its treatment.
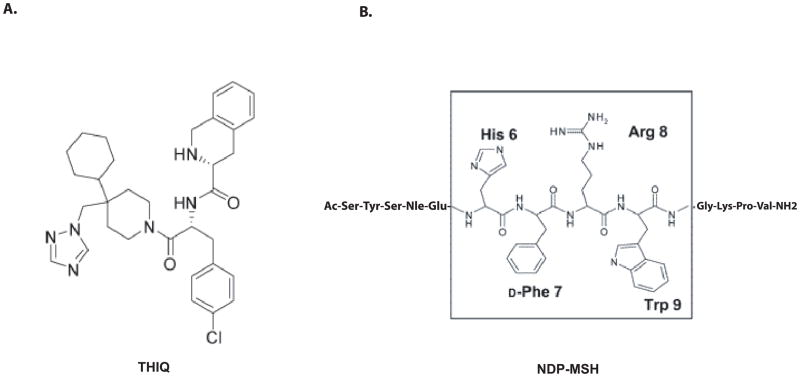
Melanocortins are peptides derived from the pro-opiomelanocortin (POMC) gene, including α-melanocyte-stimulating hormones (α-MSH) and adrenocorticotropic hormone (ACTH) and five melanocortin receptors have been cloned which belong to G-proteins coupled receptor family (GPCRs)(26–29). The molecular basis of MC4R responsible for peptide binding and signaling has been extensively studied and TMs of hMC4R have been identified to be involved in peptide binding and signaling (30–33). However, these peptides are non selective agonists at melanocortin receptors. In recent year, the pursuit of small molecule and selective agonists of the hMC4R has been intensified and numerous nonpeptide agonists and antagonists have been developed (19, 32, 34–43). THIQ is a selective agonist for MC4R (39) (Figure 1). Previously, Pogozheva et al reported that THIQ shares some binding sites with NDP-MSH at hMC4R using hypothesis-driven selection of receptor amino acid residue for site-directed mutagenesis study (32). They identified that the conserved amino acid residues in TM3 and TM6 of MC4R are involved in THIQ binding and signaling. Furthermore, Hogan et al also reported that some amino acid residues in TM6 and TM7 of MC4R are involved in THIQ binding using hypothesis driven site-directed mutagenesis study. However, whether these residues are solely residues for THIQ binding at hMC4R remain unclear. In this study, we systemically examined the molecular basis of MC4R responsible for THIQ binding and signaling by utilizing chimeric receptors and site-directed mutagenesis approaches. We have identified that key amino acid residues of the TM3 and TM6 of MC4R are responsible for THIQ binding using chimeric receptor and site-directed mutagenesis approaches. Our results indicate that THIQ utilizes not only conserved amino acid residues E100, D122, D126, F254, W258, F261 and H264 but also unique amino acid residues N123, I129 and S131 of the MC4R for specific binding and signaling.
Figure 1.
Sequence comparison between nonpeptide THIQ and peptide NDP-MSH.
Materials and Methods
Materials
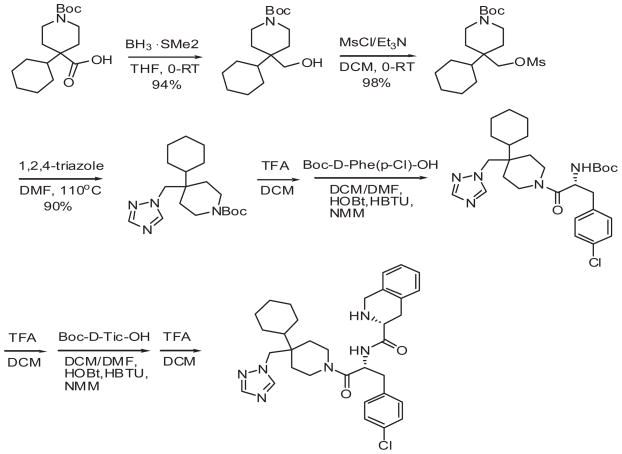
[Nle4-D-Phe7]-MSH (NDP-MSH) was purchased from Peninsula Laboratories (Belmont, CA). 3-Isobutyl-methylxanthine (IBMX) was from Sigma, and [125I] NDP-MSH was from Perkin-Elmer life Sciences (Boston, MA). DMEM, lipofectamine was from Life Technologies (Rockville MD). THIQ was synthesized using modified method by Sebhat (44). Briefly borane dimethylsulfide was used to reduce commercially available N-Boc-4 cyclohexylpiperidine-4-carboxylic acid. The hydroxy group was mesylated and replaced with triazole moiety. Triazole was coupled to Boc-D-Phe (pCl)-OH and then to Boc-D-Tic-OH followed by deprotection (Figure 2).
Figure 2.
The procedure of the triazole-based non peptide THIQ synthesis
Construction of the melanocortin chimeric receptors
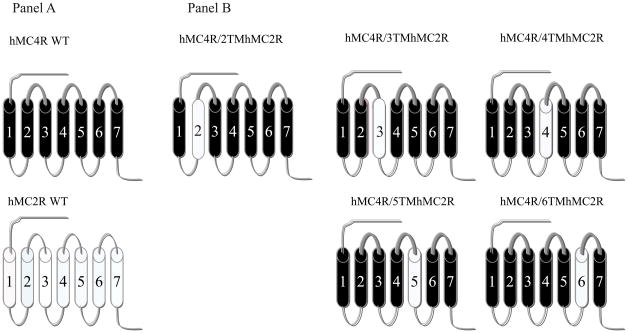
In order to determine which regions of the hMC4R are responsible for THIQ specific binding and activity, a domain-exchange strategy was used. Cassette substitutions of the TM2, TM3, TM4, TM5 and TM6 of the hMC4R with homologous regions of the hMC2R were performed. The human MC4R served as templates and the chimeras utilized in these studies are schematically diagramed in Figure 3. The first and seventh TMs were not chosen for investigation because our previous data suggested that they were not important in ligand binding (31, 45, 46). The amino acid sequences of the hMC2R and hMC4R were examined by hydrophobicity plot (Genetics Computer Group, Inc., Madison, WI) and manually by comparing their sequences to a previously published alignment of seven transmembrane G-protein coupled receptor α-helices (47). The chimeric receptors were constructed by polymerase chain reaction (PCR) using Pfu polymerase (Stratagene, La Jolla, CA) (48). Briefly, each chimeric receptor is realized by a three-step PCR-based method that allows the fusion of two PCR fragments corresponding to the respective segments that represent the chimeric receptor. The first PCR step corresponds to the amplification of the different fragments of the chimeric receptor. A second PCR step will generate full length of the chimeric receptor. During an initial round of PCR, partial-length receptor fragments were generated. The sequence of one of the PCR primer oligonucleotides consisted of the transmembrane domain of interest coupled to a portion of the extracellular domain required to form the chimeric receptor. The second oligonucleotide primer consisted of the 5′ or 3′ end of the MC4R. Receptor fragments were separated by agarose gel electrophoresis and used for a second round of PCR in which full-length chimeric receptor constructs were assembled by cycling the appropriate fragments together for 10 cycles prior to adding both 5′ and 3′ receptor primers. The chimeric receptors with FLAG were subcloned into the eukaryotic expression vector pcDNA 3.1 (Invitrogen, Carlsbad, CA) (49).
Figure 3.
Schematic representation of the chimeric human melanocortin receptors utilized in these studies. Panel A schematically depicts the seven transmembrane structures of the “wild-type” (WT) MC4R (drawn with heavy lines) and MC2R (drawn with thin lines). Panel B depicts the structure of the chimeric MC4R with the substituted TMs of the MC2R.
Site-directed mutagenesis of hMC4R
Single mutation was constructed using the Quick-Change Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA). The entire coding region of the mutated receptors was sequenced to confirm that the desired mutation sequences were present and that no sequence errors had been introduced by University of Alabama at Birmingham Sequence Core. The mutated receptors are shown in Figure 3. The mutant receptors were subcloned into the eukaryotic expression vector pCDNA 3.1 (Invitrogen; Carlsbad, CA).
Cell culture and transfection
OS3 and HEK293 cells, lacking endogenous melanocortin receptor, were cultured in DMEM medium containing 10% bovine fetal serum and HEPES. MC2R construct was transfected into OS3 cells and MC4R wild type, chimeric receptors and single mutants were transfected into HEK293 cells using lipofectamine (Life Technologies, Rockville MD). The permanently transfected clonal cell lines were selected by resistance to the neomycin analogue G418 (45).
Binding assays
Binding experiments were performed using the conditions previously described (30). Briefly, after removal of the media, cells with stably transfected with MC4R WT or the chimeric receptors were incubated with non-radioligand THIQ from 10−10 to 10−6M in 0.5 ml MEM containing 0.2% BSA and 2 × 105 cpm of 125I-NDP-MSH for one hour. The binding reactions were terminated by removing the media and washing the cells twice with MEM containing 0.2% BSA. The cells were then lysed with 0.2 N NaOH, and the radioactivity in the lysate was quantified in an analytical gamma counter (PerkinElmer, Shelton, CT). Nonspecific binding was determined by measuring the amount of 125I-label bound on the cells in the presence of excess 10−6 M unlabeled ligand. Specific binding was calculated by subtracting nonspecifically bound radioactivity from total bound radioactivity. Ki values for ligands will be calculated using the equation Ki = Kd = IC50 – [radioligand] (50).
cAMP assay
Cellular cAMP generation was measured using a competitive binding assay kit (TRK 432, Amersham, Arlington Heights, IL). Briefly, cell culture media was removed, and cells were incubated with 0.5 ml Earle’s Balanced Salt Solution (EBSS), containing the melanocortin agonist THIQ (10−10–10−6 M), for one hour at 37°C in the presence of 10−3 M isobutylmethylxanthine. The reaction was stopped by adding ice-cold 100% ethanol (500μl/well). The cells in each well were scraped, transferred to a 1.5 ml tube, and centrifuged for 10 min at 1900 × g, and the supernatant was evaporated in a 55°C water bath with pre-purified nitrogen gas. cAMP content was measured as previously described, according to instructions accompanying the assay kit (51).
Receptor expression by using FACs (52)
hMC4R transfected cells were harvested using 0.2% EDTA and washed twice with phosphate buffer saline (PBS). Aliquots of 3X106 cells were centrifuged and fixed with 3% paraformaldehyde in PBS (pH 7.4). The cells were incubated with 50 μl of 10 μg/ml murine anti-FLAG M1 monoclonal antibody(Sigma, catalog No. 316) in incubation buffer for 45 minutes. Under this condition the primary antibody binds only to receptors located at the cell surface. The cells were collected by centrifugation and washed three times with incubation buffer. The cell pellets were suspended in 100 μl of incubation buffer containing CY™3-conjugated Affinity Pure Donkey Anti-Mouse Ig G (ImmunoResearch Lab, Inc., West Grove, PA) and incubated at room temperature for 30 minutes. Flow cytometry was performed on a fluorescence-activated cell sorter (Becton Dickinson FACStar plus six parameter cytometer/sorter with a dual Argon ion laser, San Jose, California). The results were analyzed using the software CellQuest (Beckton-Dickinson Immunocytometry Systems, San Jose, California).
Statistical analysis
Each experiment was performed in duplicate wells with three separate times. The mean value of the dose-response data of binding and cAMP production was fit to a sigmoid curve with a variable slope factor using non-linear squares regression analysis (Graphpad Prism, Graphpad Software, San Diego, CA). Data are expressed as mean ± SEM. Significant difference was assessed by one-way ANOVA with P < 0.05 considered to be statistically significant.
Results
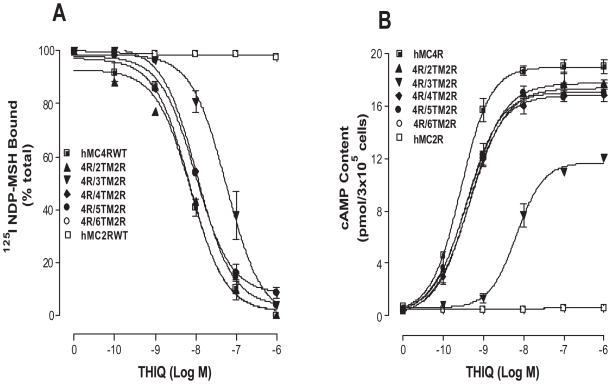
Substitutions of the TMs of the hMC4R with the corresponding regions of the hMC2R on THIQ binding
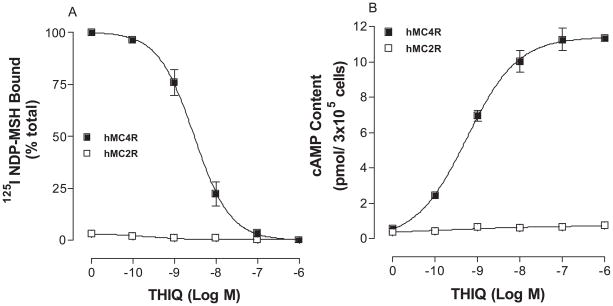
The melanocortin-2 receptor (MC2R) shares a nearly 50% homology with other melanocortin receptor subtypes but it is unique among MCRs because of its ligand selectivity. ACTH is the only known endogenous agonist at MC2R, whereas ACTH as well as α–, β–, and γ–MSH bind to other MCRs (53, 54). The binding affinity and potency of THIQ at wild type hMC4R and hMC2R were first examined. As shown in Figure 4A, THIQ dose-dependently displaced 125I NDP-MSH binding and possesses high binding affinity at wild type hMC4R but not at hMC2R. Consistent with the binding results, THIQ dose-dependently increased cAMP generation at hMC4R but not at hMC2R as expected (Figure 4B).
Figure 4.
Binding affinity and potency of THIQ at the wild-type hMC4R and hMC2R. Panel A depicts the binding affinity of THIQ as determined by inhibition of 125I NDP-MSH binding. Panel B demonstrates the ability of THIQ to stimulate the production of intracellular cAMP. Data points represent the mean ± SEM of at least 3 independent experiments.
To determine whether chimeric receptor proteins are expressed at the cell surface and to quantify receptor expression level we have utilized the antigenic epitope FLAG to examine the receptor expression. Our results indicate that FLAG signal fluorescence emitted by cells expressing the hMC4R WT and chimeric receptors can be detected by FACs. The protein expression levels of the chimeric receptors showed no significant variation compared with that of wild-type receptor (Table 1).
Table 1. Receptor domain exchanges of hMC4R on THIQ binding and siganaling.
All determinations were on cells transfected with wild-type or chimeric receptors. Ki values for THIQ were determined from displacement of 125I-NDP-MSH binding, as described under “Experimental Procedures”. The data shown are mean ± SE of at least three independent experiments.
| Receptor expression (% WT) | THIQ Ki (nM) | THIQ (EC50 (nM)) | |
|---|---|---|---|
| hMC4RWT | 100 | 5.9 ± 1.1 | 3.3 ± 0.7 |
| hMC4R/TM2 hMC2R | 97 ± 10.5 | 6.2 ± 0.2 | 3.1 ± 0.3 |
| hMC4R/TM3 hMC2R | 95 ± 5.4 | 76.2 ± 17.3* | 91 ± 6.2* |
| hMC4R/TM4 hMC2R | 93 ± 4.7 | 7.2 ± 2.3 | 3.5 ± 0.3 |
| hMC4R/TM5 hMC2R | 92 ± 6.5 | 6.7 ± 3.4 | 3.9 ± 0.4 |
| hMC4R/TM6 hMC2R | 96 ± 5.5 | 5.3 ± 0.2 | 2.8 ± 0.6 |
| hMC2RWT | 100 | No | NR |
NO: no binding; NR: no response.
To evaluate THIQ binding affinity at these chimeric receptors, experiment of unlabelled THIQ to displace labeled 125I NDP-MSH was performed (Figure 5A). Our results indicate that replacement of a hMC4R transmembrane domain with that of hMC2R did not significantly alter THIQ binding affinity except the chimeric receptor, hMC4R/TM3hMC2R, and their Ki values are summarized in Table 1. To examine the ability of THIQ to activate hMC4/hMC2 chimeric receptors, cAMP production was determined. Our results indicate that THIQ increased cAMP generation in a dose-dependent manner at these chimeric receptors except the chimeric receptor hMC4R/TM3hMC2R. Substitution of the hMC4R TM3 with the corresponding region of the hMC2R (hMC4R/TM3hMC2R) significantly decreased THIQ binding affinity and potency (Figure 5B). Their EC50 are shown in Table 1.
Figure 5.
Binding affinity and potency of THIQ at the chimeric receptors. Panel A depicts the binding affinity of THIQ as determined by inhibition of 125I NDP-MSH binding. Nonspecific binding was determined by measuring the amount of 125I-label bound on the cells in the presence of excess 10−6 M unlabeled ligand. Specific binding was calculated by subtracting nonspecifically bound radioactivity from total bound radioactivity. Panel B demonstrates the ability of THIQ to stimulate the production of intracellular cAMP. Data points represent the mean ±SEM of at least three independent experiments.
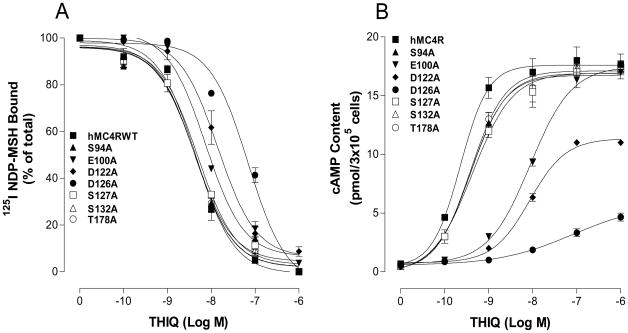
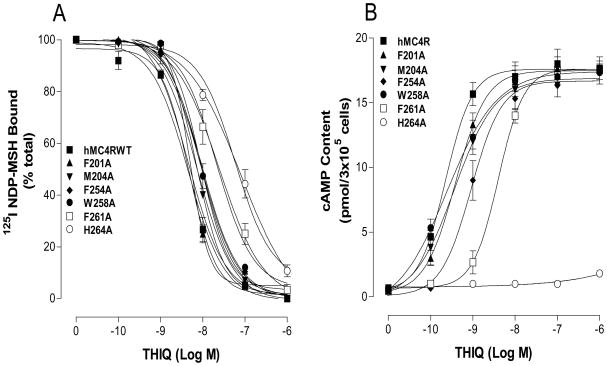
Mutations of the conserved amino acid residues in TM2, TM3, TM4, TM5 and TM6 of the hMC4R for THIQ binding and signaling
Our chimeric receptor study suggests that conserved amino acid residue in TMs of the MC4R may be crucial for THIQ specific binding and activity. Thirteen amino acid residues in TM2, TM3, TM4, TM5 and TM6 are conserved between hMC4R and hMC2R in the upper regions of the transmembrane regions of hMC4R (Figure 6). To determine whether these amino acid residues are involved in THIQ binding and signaling, these residues were individually mutated with alanine and the THIQ binding affinity and potency were evaluated. Our results indicate that all of the mutant receptors were expressed at cell surface and their expressions are shown in Table 2. The protein expression levels of mutation S94A (TM2), S127A (TM3), S132A (TM3), T178A (TM4), F201A (TM5), M204A (TM5), F254A (TM6) and W258A (TM6) are not significantly different from that of the hMC4R-WT. However, the expression levels of mutation E100A, D122A, D126A, F261A and H264A are lower than that of the hMC4R-WT. Our results indicate that THIQ dose dependently displaced 125I NDP-MSH binding at the mutations of the amino acid residues, E100A, D122A, D126A, F254A, W258A, F261A and H264A but their binding affinities for THIQ were significantly reduced compared to that of the hMC4R WT (Figure 7A and 8A). Mutation D126A significantly reduced THIQ binding and signaling. However, mutations of S94A, S127A, S132A, T178A, F201A and M204A remain similar binding affinity compared to that of the hMC4R-WT. Consistent with the binding results, the mutations of the amino acid residues, E100A, D122A, D126A, F254A, W258A, F261A and H264A significantly reduced THIQ mediated cAMP production but S94A, S127A, S132A, T178A, F201A and M204A maintain high THIQ stimulated cAMP production (Figure 7B and 8B). Their Ki and EC50 are shown in Table 2.
Figure 6.
Receptor sequence comparison between MC4R and MC2R. The mutated conserved TM residues in these experiments are denoted by bold. The mutations of the residues affected THIQ binding were highlighted by bold italic.
Table 2. Effect of the substitutions of the conserved amino acid residues of hMC4R on THIQ binding and cAMP production.
All determinations were on cells transfected with wild-type or mutant receptors. The receptor concentration was determined by FLAG-tagged receptor expression and expressed as % of Wild type receptor protein. Ki values for THIQ were determined from displacement of 125I-NDP-MSH binding, as described under “Experimental Procedures”.
| Receptor expression (% WT) | THIQ Ki (nM) | THIQ (EC50 (nM)) | Max (100% WT) | |
|---|---|---|---|---|
| hMC4R | 100 | 5.6 ± 1.0 | 0.8 ± 0.2 | 100 |
| S94A | 93 ±5.8 | 4.3 ± 0.9 | 1.2 ± 0.2 | 82 ± 9.2 |
| E100A | 65 ± 7.5 | 19 ± 5.9* | 5.7 ± 0.5* | 96 ± 4.5 |
| D122A | 78 ±11 | 38 ± 3.6* | 16 ± 3.2* | 52 ± 8.3 |
| D126A | 35 ± 10 | ND | 453 ± 43* | 26 ± 8.2 |
| S127A | 97 ± 5.8 | 5.7 ± 0.6 | 1.4 ± 0.5 | 94 ±4.5 |
| S132A | 96 ± 3.8 | 5.9 ± 0.7 | 1.3 ± 0.4 | 95 ± 6.1 |
| T178A | 94 ± 2.7 | 6.4 ± 0.4 | 1.7 ± 0.9 | 91 ± 9.9 |
| F201A | 95 ± 6.8 | 5.3 ± 0.3 | 1.0 ± 0.2 | 95 ± 3.9 |
| M204A | 98 ± 5.5 | 6.0 ± 0.9 | 1.1 ± 0.3 | 93 ± 8.8 |
| F254A | 91 ± 6.3 | 16 ±2.9* | 4.9 ± 0.9* | 85 ± 11.2 |
| W258A | 89 ± 3.9 | 21 ± 3.9* | 11 ± 1.3* | 90 ± 12 |
| F261A | 81 ± 8.3 | 98 ±6.9* | 216 ± 18* | 93 ± 3.2 |
| H264A | 78 ±4.5 | >103* | >103* | 3 ± 0.5 |
P<0.05 compared with WT receptor.
Figure 7.
Binding affinity and potency of THIQ at the mutations of the conserved amino acid residues of the hMC4Rs. Panel A shows the binding affinity of unlabeled THIQ to displace 125I NDP-MSH. Nonspecific binding was determined by measuring the amount of 125I-label bound on the cells in the presence of excess 10−6 M unlabeled ligand. Specific binding was calculated by subtracting nonspecifically bound radioactivity from total bound radioactivity. Panel B shows that THIQ to stimulate cAMP production. Data points represent the mean ±SEM of at least three independent experiments with duplicated wells.
Figure 8.
Binding affinity and potency of THIQ at the mutations of the conserved amino acid residues of the hMC4Rs. Panel A shows the binding affinity of unlabeled THIQ to displace 125I NDP-MSH. Nonspecific binding was determined by measuring the amount of 125I-label bound on the cells in the presence of excess 10−6 M unlabeled ligand. Specific binding was calculated by subtracting nonspecifically bound radioactivity from total bound radioactivity. Panel B shows that THIQ to stimulate cAMP production. Data points represent the mean ±SEM of at least three independent experiments with duplicated wells.
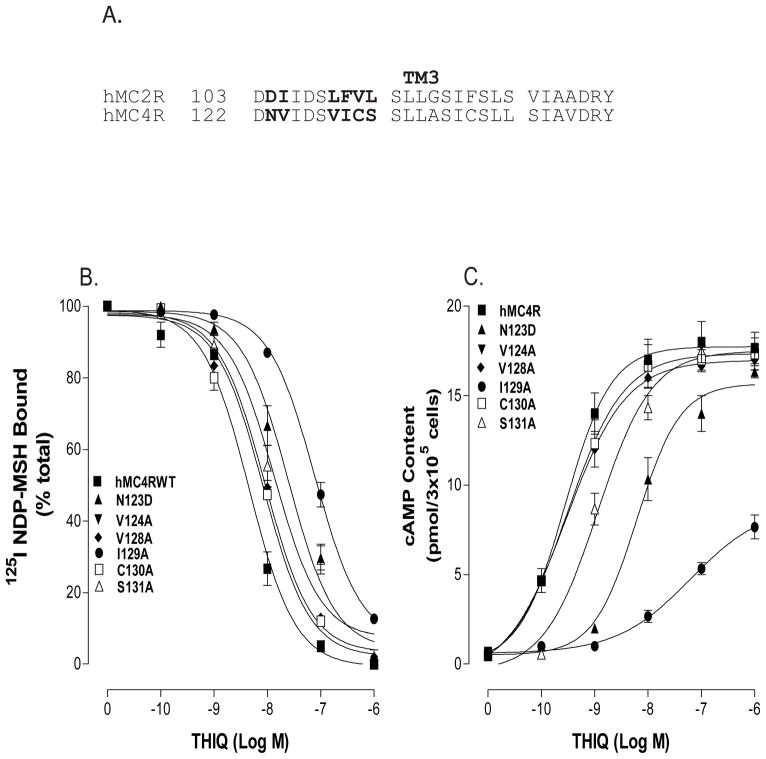
Mutations of non conserved amino acid residues in TM3 of the hMC4R for THIQ binding and signaling
Because substitution of TM3 of the hMC4R with the corresponding TM3 region of the hMC2R significantly alters THIQ binding affinity and potency, we speculate that non conserved amino acid residues of the TM3 of the hMC4R may also be involved in THIQ specific binding. The sequence of amino acids in TM3 between hMC4R and hMC2R was compared, and depicted in Figure 9A. A sequence alignment of the hMC2R and the hMC4R revealed that six amino acid residues are non-conserved in upper region of the TM3. To determine whether these amino acid residues are also involved in THIQ specific binding and activity, these residues were individually mutated with alanine and their functions were evaluated. Our results indicate that these non-conserved mutant receptors were expressed at cell surface and the expression levels were not significantly different from that of wild type receptor (Table 3). THIQ dose dependently displaced 125I NDP-MSH binding at V124A, V128A, C130A, and their binding affinities for THIQ were not significantly different from that of hMC4R-WT. However, THIQ binding affinity at N123D, I129A and S131A was significantly decreased (Figure 9B). Their IC50 are shown in Table 3. Consistent with binding results, the ability of THIQ to stimulate cAMP production at N123D, I129A and S131A was significantly decreased (figure 9C). Their EC50 are shown in Table 3.
Figure 9.
Binding affinity and potency of THIQ at the mutations of non-conserved amino acid residues of the hMC4Rs. Panel A shows the amino acid residue differences between hMC4R and hMC2R. Panel B shows the binding affinity of unlabeled THIQ to displace 125I NDP-MSH. Nonspecific binding was determined by measuring the amount of 125I-label bound on the cells in the presence of excess 10−6 M unlabeled ligand. Specific binding was calculated by subtracting nonspecifically bound radioactivity from total bound radioactivity. Panel C shows that THIQ to stimulate cAMP production. Data points represent the mean ±SEM of at least three independent experiments with duplicated wells.
Table 3. Effect of the substitutions of the non-conserved amino acid residues of hMC4R on THIQ binding and cAMP production.
All determinations were on cells transfected with wild-type or mutant receptors. The receptor concentration was determined by FLAG-tagged receptor expression and expressed as % of Wild type receptor protein. Ki values for THIQ were determined from displacement of 125I-NDP-MSH binding, as described under “Experimental Procedures”.
| Receptor expression (% WT) | Ki (nM) | EC50 (nM) | Max (100% WT) | |
|---|---|---|---|---|
| 4R-WT | 100 | 5.9 ± 1.0 | 3.3 ± 1.0 | 100 |
| N123D | 78 ± 11 | 29.3 ± 0.2* | 76 ± 5.9* | 82 ± 9.2 |
| V124A | 95 ± 6.5 | 5.0 ± 0.4 | 3.5 ± 0.5 | 94 ± 4.5 |
| V128A | 98 ± 4.5 | 5.6 ± 0.3 | 3.7 ± 0.3 | 95 ± 6.1 |
| I129A | 88 ± 5.5 | 57 ± 0.1* | >103* | 45 ± 4.5 |
| C130A | 95 ± 4.7 | 3.9 ± 0.3 | 3.8 ± 0.7 | 93 ±3.2 |
| S131A | 91 ± 9.1 | 15.1 ± 0.4* | 24 ±3.2* | 90 ±5.5 |
P <0.05 compared with WT receptor.
Discussion
In the present study, we examined MC4R residues conferring nonpeptide THIQ binding and signaling by utilizing both chimeric receptors and site-directed mutagenesis studies. Our results demonstrate that both the conserved residues and non-conserved residue in TMs of hMC4R are crucial for nonpeptide agonist THIQ specific binding and signaling.
In the last decade, great effort has been made in the design and development of selective nonpeptide agonists for MC4R with properties not present in the endogenous peptides. Towards this end, substantial progress has been obtained in the development of nonpeptide molecules for the MC4R and many nonpeptide MC4R selective agonists have been developed (35–43). THIQ is a nonpeptide ligand of the MC4R which inhibits food intake in rodent models and THIQ has a completely different structure from MSH (44, 55, 56). Endogenous agonist α-MSH is a 13 amino acid peptide but THIQ has a tripeptide scaffold, with 4-CI derivative of D-Phe in the central position, an N-terminal part with charged amino group, and a C-terminal portion formed by 4, 4-disubstituted piperidine (44). The amino acid residues in transmembrane (TM) domains 2, 3, 4, 5 and 6 of the hMC4R are reported in α-MSH binding and signaling (30, 45, 46, 57). Previously, Pogozheva et al reported that THIQ shares some binding sites with NDP-MSH at hMC4R using hypothesis-driven selection of receptor amino acid residue for site-directed mutagenesis study (32). They identified that D122, D126, F261 and H264 of the hMC4R are involved in THIQ binding and signaling. Furthermore, Hogan et al also reported that Y268, Y287 I125, I129, and I291 are likely to have direct contacts with THIQ using same method (58). However, whether these residues are solely residues for THIQ binding are unclear. In this study, we utilized two different approaches to narrow down the key amino acid residues of MC4R for THIQ binding and signaling. We utilize the different pharmacological characteristics between MC4R and MC2R to distinguish which residue in MC4R is crucial for THIQ binding. THIQ is a potent agonist for MC4R but not for MC2R. The hMC4R and hMC2R share a number of structural, functional and pharmacologic similarities. In the putative TM regions, the hMC4R and hMC2R show overall identities of 67% identity and conserved amino acid residues in TM3 and TM6 of the hMC2R and hMC4R are identified to be involved in ACTH binding. The utilization of MC4R/MC2R chimeric receptor approach can first determine whether or not conserved amino acid residues between MC4R and MC2R are involved in THIQ binding and signaling. Further site-directed mutagenesis studies will further identify which amino acid residues are crucial for THIQ binding (48, 59, 60). We anticipate that if substitution of TM of the hMC4R with the corresponding region of the hMC2R results in the decrease THIQ binding affinity and potency, it will imply that non-conserved residues are involved in THIQ binding and potency. In contrary, if substitution does not significantly alter THIQ binding affinity and potency, it will imply that conserved residues are involved in THIQ binding. Our chimeric receptor results suggest that conserved amino acid residues between the hMC4R and hMC2R are crucial for THIQ specific binding and activity. We then performed site-directed mutagenesis study to examine which conserved residues of MC4R are responsible for THIQ binding and signaling. Our previous results indicate that most ionic residues and aromatic residues in upper regions of the TMs of the hMC4R are involved in ligand binding and signaling (30, 49, 60). We therefore mainly focused on the residues in the upper regions of the hMC4R. We have identified that seven conserved amino acid residues, E100 in TM2, D122, and D126 in TM3, F254, W258, F261 and H264 in TM6 of the hMC4R, are crucial for THIQ binding and signaling. Our chimeric study also indicated that the non-conserved amino acid residues of MC4R TM3 may participate THIQ binding. Site-directed mutagenesis study of TM3 of the hMC4R indicates that non conserved amino acid residues N123, I129 and S131 of hMC4R are involved in THIQ specific binding. Through both chimeric receptor and site-directed mutagenesis studies, we have limited seven conserved amino acid residues and three non-conserved amino acid residues of hMC4R responsible for THIQ specific binding and activity. Hogan et al reported that Y268 (TM6) is important for THIQ binding (58). However, our chimeric receptor study indicates that substitution of TM6 of the MC4R with the corresponding region of hMC2R did not alter THIQ binding and activity, suggesting that this non conserved residue is not involved in THIQ binding. In addition to the role of the TM region of MC4R for THIQ binding and signaling, our previous results indicate that the extracellular loops of the MC4R are not crucial for NDP-MSH binding and signaling (48, 60). Our studies also show that the extracellular loops of hMC4R are not important for THIQ binding and signaling (data not shown).
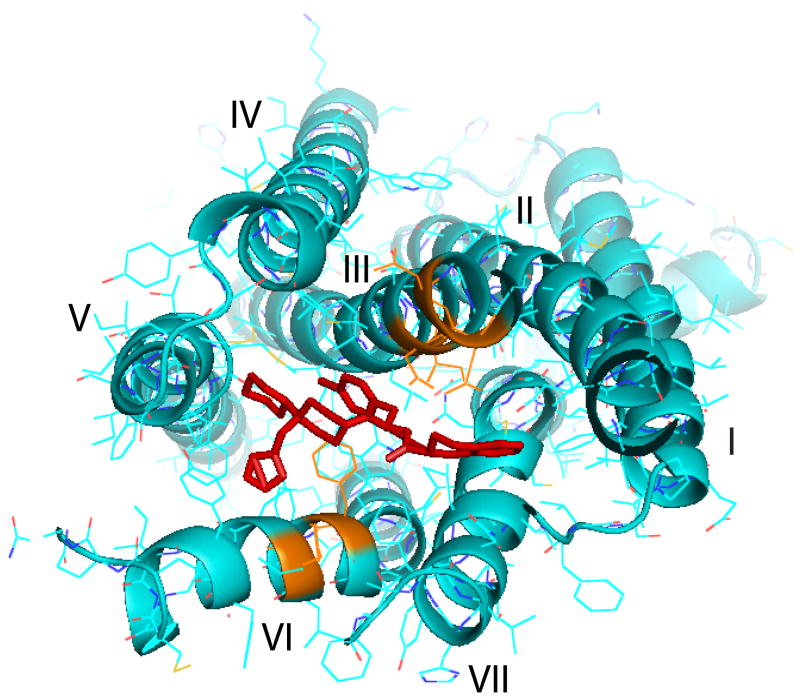
MCR family is a member of G-protein-coupled receptor families that share an endogenous ligand. While ligand selectivity for melanocortin receptor family is usually achievable, it is often difficult to obtain selectivity among members of a family. Theoretically, the orthosteric site is under selective pressure and it would be evolutionarily unfavorable for a broad divergence to develop in the amino acid sequence that composes the binding domain. Thus selective orthosteric ligands, which presumably interact with the same amino acid sequences, would tend to be non-selective within a family. Assuming that the allosteric sites on MCRs are not bound by an endogenous ligand the amino acid sequence composing these sites would be less restricted by selective pressure and therefore, free to vary. Selectivity at the allosteric site allowed for the pharmacological separation of receptors that previously could not be distinguished using orthosteric ligands. Peptides as endogenous orthosteric ligands may be more amenable to small molecule discovery efforts directed at producing allosteric ligands. This is based on the concept that any compound acting at the orthosteric site would likely need to bridge distant binding partners engaged by the larger peptide. Therefore, an allosteric interaction with the receptor may be able to produce a significant conformational change while acting in a more spatially restricted fashion. THIQ is a selective MC4R agonist and it may have specific binding sites at MC4R. The crystal structure of the THIQ shows that THIQ has an extended polypeptide backbone, piperidine and cyclohexane group which forms the biologically active conformation (44, 61). In the conformations of THIQ, the 4-chlorophenyl group and piperidine ring are close while the aromatic ring of D-Tic is oriented in the opposite direction and the 1, 2, 4-triazol ring is exposed to the environment. Although the structure of THIQ is totally different from that of MSH, NMR study indicates that three dimensional structure of the THIQ was similar to DPhe-Arg-Trp of MSH (32). If THIQ was docked into the binding pocket of the hMC4R, it may have a similar spatial arrangement of common pharmacophore group with that of NDP-MSH. Based on the THIQ structure and our current results, the possible ligand receptor interactions occurred between THIQ and hMC4R may be as following: The side chain of the central D-Phe (4-Cl) residue of the THIQ interacts with I129 and N123 which may be important for binding and activation of THIQ. The charged amino acidic group may form ionic interactions with several acidic residues from TM2 and TM3, including the most functionally important E100, D122 and D126, which forms an H-bond with the backbone amid group of D-Phe, which restrains the position of the ligand inside the binding pocket which is similar to that of NDP-MSH in which Arg8 interacts with these residues (30, 31). The triazole part of THIQ may interact with F261 and H264 (TM6). Figure 10 was generated by pyMol system via Pogozheva’s earlier work (32). In the picture, the binding site on receptor is showing orange color.
Figure 10.
Two-dimensional representation of a proposed three dimensional model illustrating the synthetic melanocortin THIQ docked inside the hMC4R. Three receptor binding pockets are hypothesized. The first is a predominantly ionic pocket formed by Asp122 and Asp 126. The second a hydrophobic pocket formed by aromatic residues in TM6., F261 and H264 are included. The third one is THIQ specific, including N123 and I129.
Interestingly, our results also indicate that activation of the hMC4R by nonpeptide is very sensitive to minor receptor alterations (i.e., mutations). Single amino acid substitutions within the putative ligand pocket of hMC4R markedly decrease potency of THIQ compared to that of peptide NDP-MSH. THIQ becomes partial agonist at D122A, D126A and I129A. THIQ can not tolerate single receptor residue mutation which is consistent with other G-protein coupled receptor angiotensin AT1 receptor in which single amino acid substitutions within the putative ligand pocket markedly decrease efficacy of nonpeptide molecules without significantly altering the function of the peptide, angiotensin II (62). This may be due to more extensive and redundant interactions between the receptor and peptide or due to peptide flexibility, which allows adjustment inside the modified receptor. It may be reasonable that under evolutionary pressure, both the endogenous ligands and the receptor may have adapted structures, which ensure optimal receptor activation, independent of single amino acid differences. This would also explain why different species homologous of the hMC4R, which share 90% amino acid identity (mouse, dog, human), show an equal response to MSH but demonstrate marked differences in the response to synthetic drugs.
In summary, THIQ specific binding at MC4R is different from that of NDP-MSH. THIQ utilizes both conserved residues (E100, D122, D126, F254, W258, F261, and H264) and unique residues (N123, I129 and S131) for specific binding and signaling at hMC4R.
References
- 1.Hotu S, Carter B, Watson P, Cutfield W, Cundy T. Increasing prevalence of type 2 diabetes in adolescents. J Paediatr Child Health. 2004;40:201–204. doi: 10.1111/j.1440-1754.2004.00337.x. [DOI] [PubMed] [Google Scholar]
- 2.Bhargava SK, Sachdev HS, Fall CH, Osmond C, Lakshmy R, Barker DJ, Biswas SK, Ramji S, Prabhakaran D, Reddy KS. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med. 2004;350:865–875. doi: 10.1056/NEJMoa035698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nammi S, Koka S, Chinnala KM, Boini KM. Obesity: An overview on its current perspectives and treatment options. Nutr J. 2004;3:3. doi: 10.1186/1475-2891-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bray G. Drug treatment of obesity: don’t throw the baby out with the bath water. Am J Clin Nutr. 1998;67:1–2. doi: 10.1093/ajcn/67.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Bray GA. Obesity: a time bomb to be defused. Lancet. 1998;352:160–161. doi: 10.1016/S0140-6736(98)22029-0. [DOI] [PubMed] [Google Scholar]
- 6.Bray GA, Tartaglia LA. Medicinal strategies in the treatment of obesity. Nature. 2000;404:672–677. doi: 10.1038/35007544. [DOI] [PubMed] [Google Scholar]
- 7.Boston BA. Pro-opiomelanocortin and weight regulation: from mice to men. J Pediatr Endocrinol Metab. 2001;14(Suppl 6):1409–1416. [PubMed] [Google Scholar]
- 8.Boutin P, Froguel P. Genetics of human obesity. Best Pract Res Clin Endocrinol Metab. 2001;15:391–404. doi: 10.1053/beem.2001.0153. [DOI] [PubMed] [Google Scholar]
- 9.Hu Y, Bloomquist BT, Cornfield LJ, DeCarr LB, Flores-Riveros JR, Friedman L, Jiang P, Lewis-Higgins L, Sadlowski Y, Schaefer J, Velazquez N, McCaleb ML. Identification of a novel hypothalamic neuropeptide Y receptor associated with feeding behavior. J Biol Chem. 1996;271:26315–26319. [PubMed] [Google Scholar]
- 10.Bultman SJ, Michaud EJ, Woychik RP. Molecular characterization of the mouse agouti locus. Cell. 1992;71:1195–1204. doi: 10.1016/s0092-8674(05)80067-4. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 12.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:1. doi: 10.1016/s0092-8674(02)09256-5. page following 696. [DOI] [PubMed] [Google Scholar]
- 13.Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670–674. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- 14.Baltatzi M, Hatzitolios A, Tziomalos K, Iliadis F, Zamboulis C. Neuropeptide Y and alpha-melanocyte-stimulating hormone: interaction in obesity and possible role in the development of hypertension. Int J Clin Pract. 2008;62:1432–1440. doi: 10.1111/j.1742-1241.2008.01823.x. [DOI] [PubMed] [Google Scholar]
- 15.Kaelin CB, Candille SI, Yu B, Jackson P, Thompson DA, Nix MA, Binkley J, Millhauser GL, Barsh GS. New ligands for melanocortin receptors. Int J Obes (Lond) 2008;32(Suppl 7):S19–27. doi: 10.1038/ijo.2008.234. [DOI] [PubMed] [Google Scholar]
- 16.Millington GW. The role of proopiomelanocortin (POMC) neurones in feeding behaviour. Nutr Metab (Lond) 2007;4:18. doi: 10.1186/1743-7075-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nogueiras R, Wiedmer P, Perez-Tilve D, Veyrat-Durebex C, Keogh JM, Sutton GM, Pfluger PT, Castaneda TR, Neschen S, Hofmann SM, Howles PN, Morgan DA, Benoit SC, Szanto I, Schrott B, Schurmann A, Joost HG, Hammond C, Hui DY, Woods SC, Rahmouni K, Butler AA, Farooqi IS, O’Rahilly S, Rohner-Jeanrenaud F, Tschop MH. The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest. 2007 doi: 10.1172/JCI31743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 19.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 20.Giraudo SQ, Billington CJ, Levine AS. Feeding effects of hypothalamic injection of melanocortin 4 receptor ligands. Brain Res. 1998;809:302–306. doi: 10.1016/s0006-8993(98)00837-3. [DOI] [PubMed] [Google Scholar]
- 21.Cone RD. The Central Melanocortin System and Energy Homeostasis. Trends Endocrinol Metab. 1999;10:211–216. doi: 10.1016/s1043-2760(99)00153-8. [DOI] [PubMed] [Google Scholar]
- 22.Fisher SL, Yagaloff KA, Burn P. Melanocortin-4 receptor: a novel signalling pathway involved in body weight regulation. Int J Obes Relat Metab Disord. 1999;23(Suppl 1):54–58. doi: 10.1038/sj.ijo.0800796. [DOI] [PubMed] [Google Scholar]
- 23.Emmerson PJ, Fisher MJ, Yan LZ, Mayer JP. Melanocortin-4 receptor agonists for the treatment of obesity. Curr Top Med Chem. 2007;7:1121–1130. doi: 10.2174/156802607780906636. [DOI] [PubMed] [Google Scholar]
- 24.Fong TM, Mao C, MacNeil T, Kalyani R, Smith T, Weinberg D, Tota MR, Van der Ploeg LH. ART (protein product of agouti-related transcript) as an antagonist of MC-3 and MC-4 receptors. Biochem Biophys Res Commun. 1997;237:629–631. doi: 10.1006/bbrc.1997.7200. [DOI] [PubMed] [Google Scholar]
- 25.Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 26.Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257:1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- 27.Gantz I, Konda Y, Tashiro T, Shimoto Y, Miwa H, Munzert G, Watson SJ, DelValle J, Yamada T. Molecular cloning of a novel melanocortin receptor. J Biol Chem. 1993;268:8246–8250. [PubMed] [Google Scholar]
- 28.Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson SJ, DelValle J, Yamada T. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J Biol Chem. 1993;268:15174–15179. [PubMed] [Google Scholar]
- 29.Gantz I, Shimoto Y, Konda Y, Miwa H, Dickinson CJ, Yamada T. Molecular cloning, expression, and characterization of a fifth melanocortin receptor. Biochem Biophys Res Commun. 1994;200:1214–1220. doi: 10.1006/bbrc.1994.1580. [DOI] [PubMed] [Google Scholar]
- 30.Yang YK, Fong TM, Dickinson CJ, Mao C, Li JY, Tota MR, Mosley R, Van Der Ploeg LH, Gantz I. Molecular determinants of ligand binding to the human melanocortin-4 receptor. Biochemistry. 2000;39:14900–14911. doi: 10.1021/bi001684q. [DOI] [PubMed] [Google Scholar]
- 31.Haskell-Luevano C, Cone RD, Monck EK, Wan YP. Structure activity studies of the melanocortin-4 receptor by in vitro mutagenesis: identification of agouti-related protein (AGRP), melanocortin agonist and synthetic peptide antagonist interaction determinants. Biochemistry. 2001;40:6164–6179. doi: 10.1021/bi010025q. [DOI] [PubMed] [Google Scholar]
- 32.Pogozheva ID, Chai BX, Lomize AL, Fong TM, Weinberg DH, Nargund RP, Mulholland MW, Gantz I, Mosberg HI. Interactions of human melanocortin 4 receptor with nonpeptide and peptide agonists. Biochemistry. 2005;44:11329–11341. doi: 10.1021/bi0501840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nickolls SA, Fleck B, Hoare SR, Maki RA. Functional selectivity of melanocortin 4 receptor peptide and nonpeptide agonists: evidence for ligand-specific conformational states. J Pharmacol Exp Ther. 2005;313:1281–1288. doi: 10.1124/jpet.105.083337. [DOI] [PubMed] [Google Scholar]
- 34.Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O’Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348:1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 35.Arasasingham PN, Fotsch C, Ouyang X, Norman MH, Kelly MG, Stark KL, Karbon B, Hale C, Baumgartner JW, Zambrano M, Cheetham J, Tamayo NA. Structure-activity relationship of (1-aryl-2-piperazinylethyl)piperazines: antagonists for the AGRP/melanocortin receptor binding. J Med Chem. 2003;46:9–11. doi: 10.1021/jm0255522. [DOI] [PubMed] [Google Scholar]
- 36.Chen C, Pontillo J, Fleck BA, Gao Y, Wen J, Tran JA, Tucci FC, Marinkovic D, Foster AC, Saunders J. 4-{(2R)-[3-Aminopropionylamido]-3-(2,4-dichlorophenyl)propionyl}-1-{2-[(2- thienyl)ethylaminomethyl]phenyl}piperazine as a potent and selective melanocortin-4 receptor antagonist--design, synthesis, and characterization. J Med Chem. 2004;47:6821–6830. doi: 10.1021/jm049278i. [DOI] [PubMed] [Google Scholar]
- 37.Ye Z, Guo L, Barakat KJ, Pollard PG, Palucki BL, Sebhat IK, Bakshi RK, Tang R, Kalyani RN, Vongs A, Chen AS, Chen HY, Rosenblum CI, MacNeil T, Weinberg DH, Peng Q, Tamvakopoulos C, Miller RR, Stearns RA, Cashen DE, Martin WJ, Metzger JM, Strack AM, MacIntyre DE, Van der Ploeg LH, Patchett AA, Wyvratt MJ, Nargund RP. Discovery and activity of (1R,4S,6R)-N-[(1R)-2-[4-cyclohexyl-4-[[(1,1-dimethylethyl)amino]carbonyl]-1-piperidinyl]-1-[(4-fluorophenyl)methyl]-2-oxoethyl]-2-methyl-2-azabicycl o[2.2.2]octane-6-carboxamide (3, RY764), a potent and selective melanocortin subtype-4 receptor agonist. Bioorg Med Chem Lett. 2005;15:3501–3505. doi: 10.1016/j.bmcl.2005.05.109. [DOI] [PubMed] [Google Scholar]
- 38.Palucki BL, Park MK, Nargund RP, Tang R, MacNeil T, Weinberg DH, Vongs A, Rosenblum CI, Doss GA, Miller RR, Stearns RA, Peng Q, Tamvakopoulos C, Van der Ploeg LH, Patchett AA. 2-Piperazinecarboxamides as potent and selective melanocortin subtype-4 receptor agonists. Bioorg Med Chem Lett. 2005;15:1993–1996. doi: 10.1016/j.bmcl.2005.02.068. [DOI] [PubMed] [Google Scholar]
- 39.Palucki BL, Park MK, Nargund RP, Ye Z, Sebhat IK, Pollard PG, Kalyani RN, Tang R, Macneil T, Weinberg DH, Vongs A, Rosenblum CI, Doss GA, Miller RR, Stearns RA, Peng Q, Tamvakopoulos C, McGowan E, Martin WJ, Metzger JM, Shepherd CA, Strack AM, Macintyre DE, Van der Ploeg LH, Patchett AA. Discovery of (2S)-N-[(1R)-2-[4-cyclohexyl-4-[[(1,1-dimethylethyl)amino]carbonyl]-1-pipe ridinyl]-1-[(4-fluorophenyl)methyl]-2-oxoethyl]-4-methyl-2-piperazinecarbo xamide (MB243), a potent and selective melanocortin subtype-4 receptor agonist. Bioorg Med Chem Lett. 2005;15:171–175. doi: 10.1016/j.bmcl.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 40.Fotsch C, Han N, Arasasingham P, Bo Y, Carmouche M, Chen N, Davis J, Goldberg MH, Hale C, Hsieh FY, Kelly MG, Liu Q, Norman MH, Smith DM, Stec M, Tamayo N, Xi N, Xu S, Bannon AW, Baumgartner JW. Melanocortin subtype-4 receptor agonists containing a piperazine core with substituted aryl sulfonamides. Bioorg Med Chem Lett. 2005;15:1623–1627. doi: 10.1016/j.bmcl.2005.01.060. [DOI] [PubMed] [Google Scholar]
- 41.Fleck BA, Chen C, Yang W, Huntley R, Markison S, Nickolls SA, Foster AC, Hoare SR. Molecular interactions of nonpeptide agonists and antagonists with the melanocortin-4 receptor. Biochemistry. 2005;44:14494–14508. doi: 10.1021/bi051316s. [DOI] [PubMed] [Google Scholar]
- 42.Xi N, Hale C, Kelly MG, Norman MH, Stec M, Xu S, Baumgartner JW, Fotsch C. Synthesis of novel melanocortin 4 receptor agonists and antagonists containing a succinamide core. Bioorg Med Chem Lett. 2004;14:377–381. doi: 10.1016/j.bmcl.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 43.Vos TJ, Caracoti A, Che JL, Dai M, Farrer CA, Forsyth NE, Drabic SV, Horlick RA, Lamppu D, Yowe DL, Balani S, Li P, Zeng H, Joseph IB, Rodriguez LE, Maguire MP, Patane MA, Claiborne CF. Identification of 2-[2-[2-(5-bromo-2- methoxyphenyl)-ethyl]-3-fluorophenyl]-4,5-dihydro-1H-imidazole (ML00253764), a small molecule melanocortin 4 receptor antagonist that effectively reduces tumor-induced weight loss in a mouse model. J Med Chem. 2004;47:1602–1604. doi: 10.1021/jm034244g. [DOI] [PubMed] [Google Scholar]
- 44.Sebhat IK, Martin WJ, Ye Z, Barakat K, Mosley RT, Johnston DB, Bakshi R, Palucki B, Weinberg DH, MacNeil T, Kalyani RN, Tang R, Stearns RA, Miller RR, Tamvakopoulos C, Strack AM, McGowan E, Cashen DE, Drisko JE, Hom GJ, Howard AD, MacIntyre DE, van der Ploeg LH, Patchett AA, Nargund RP. Design and pharmacology of N-[(3R)-1,2,3,4-tetrahydroisoquinolinium- 3-ylcarbonyl]-(1R)-1-(4-chlorobenzyl)- 2-[4-cyclohexyl-4-(1H-1,2,4-triazol- 1-ylmethyl)piperidin-1-yl]-2-oxoethylamine (1), a potent, selective, melanocortin subtype-4 receptor agonist. J Med Chem. 2002;45:4589–4593. doi: 10.1021/jm025539h. [DOI] [PubMed] [Google Scholar]
- 45.Yang Y, Dickinson C, Haskell-Luevano C, Gantz I. Molecular basis for the interaction of [Nle4,D-Phe7]melanocyte stimulating hormone with the human melanocortin-1 receptor. J Biol Chem. 1997;272:23000–23010. doi: 10.1074/jbc.272.37.23000. [DOI] [PubMed] [Google Scholar]
- 46.Haskell-Luevano C, Sawyer TK, Trumpp-Kallmeyer S, Bikker JA, Humblet C, Gantz I, Hruby VJ. Three-dimensional molecular models of the hMC1R melanocortin receptor: complexes with melanotropin peptide agonists. Drug Des Discov. 1996;14:197–211. [PubMed] [Google Scholar]
- 47.Baldwin JM. The probable arrangement of the helices in G protein-coupled receptors. Embo J. 1993;12:1693–1703. doi: 10.1002/j.1460-2075.1993.tb05814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang YK, Dickinson CJ, Zeng Q, Li JY, Thompson DA, Gantz I. Contribution of melanocortin receptor exoloops to Agouti-related protein binding. J Biol Chem. 1999;274:14100–14106. doi: 10.1074/jbc.274.20.14100. [DOI] [PubMed] [Google Scholar]
- 49.Chen M, Cai M, Aprahamian CJ, Georgeson KE, Hruby V, Harmon CM, Yang Y. Contribution of the conserved amino acids of the melanocortin-4 receptor in [corrected] [Nle4,D-Phe7]-alpha-melanocyte-stimulating [corrected] hormone binding and signaling. J Biol Chem. 2007;282:21712–21719. doi: 10.1074/jbc.M702285200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeBlasi A, O’Reilly K, Motulsky HJ. Calculating receptor number from binding experiments using same compound as radioligand and competitor. Trends Pharmacol Sci. 1989;10:227–229. doi: 10.1016/0165-6147(89)90266-6. [DOI] [PubMed] [Google Scholar]
- 51.Yang YK, Ollmann MM, Wilson BD, Dickinson C, Yamada T, Barsh GS, Gantz I. Effects of recombinant agouti-signaling protein on melanocortin action. Mol Endocrinol. 1997;11:274–280. doi: 10.1210/mend.11.3.9898. [DOI] [PubMed] [Google Scholar]
- 52.Conrad DM, Hanniman EA, Watson CL, Mader JS, Hoskin DW. Ryanodine receptor signaling is required for anti-CD3-induced T cell proliferation, interleukin-2 synthesis, and interleukin-2 receptor signaling. J Cell Biochem. 2004;92:387–399. doi: 10.1002/jcb.20064. [DOI] [PubMed] [Google Scholar]
- 53.Schioth HB, Chhajlani V, Muceniece R, Klusa V, Wikberg JE. Major pharmacological distinction of the ACTH receptor from other melanocortin receptors. Life Sci. 1996;59:797–801. doi: 10.1016/0024-3205(96)00370-0. [DOI] [PubMed] [Google Scholar]
- 54.Chen M, Aprahamian CJ, Kesterson RA, Harmon CM, Yang Y. Molecular identification of the human melanocortin-2 receptor responsible for ligand binding and signaling. Biochemistry. 2007;46:11389–11397. doi: 10.1021/bi700125e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van der Ploeg LH, Martin WJ, Howard AD, Nargund RP, Austin CP, Guan X, Drisko J, Cashen D, Sebhat I, Patchett AA, Figueroa DJ, DiLella AG, Connolly BM, Weinberg DH, Tan CP, Palyha OC, Pong SS, MacNeil T, Rosenblum C, Vongs A, Tang R, Yu H, Sailer AW, Fong TM, Huang C, Tota MR, Chang RS, Stearns R, Tamvakopoulos C, Christ G, Drazen DL, Spar BD, Nelson RJ, MacIntyre DE. A role for the melanocortin 4 receptor in sexual function. Proc Natl Acad Sci U S A. 2002;99:11381–11386. doi: 10.1073/pnas.172378699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martin WJ, McGowan E, Cashen DE, Gantert LT, Drisko JE, Hom GJ, Nargund R, Sebhat I, Howard AD, Van der Ploeg LH, MacIntyre DE. Activation of melanocortin MC(4) receptors increases erectile activity in rats ex copula. Eur J Pharmacol. 2002;454:71–79. doi: 10.1016/s0014-2999(02)02479-2. [DOI] [PubMed] [Google Scholar]
- 57.Chen M, Aprahamian CJ, Celik A, Georgeson KE, Garvey WT, Harmon CM, Yang Y. Molecular characterization of human melanocortin-3 receptor ligand-receptor interaction. Biochemistry. 2006;45:1128–1137. doi: 10.1021/bi0521792. [DOI] [PubMed] [Google Scholar]
- 58.Hogan K, Peluso S, Gould S, Parsons I, Ryan D, Wu L, Visiers I. Mapping the binding site of melanocortin 4 receptor agonists: a hydrophobic pocket formed by I3.28(125), I3.32(129), and I7.42(291) is critical for receptor activation. J Med Chem. 2006;49:911–922. doi: 10.1021/jm050780s. [DOI] [PubMed] [Google Scholar]
- 59.Kristiansen K. Molecular mechanisms of ligand binding, signaling, and regulation within the superfamily of G-protein-coupled receptors: molecular modeling and mutagenesis approaches to receptor structure and function. Pharmacol Ther. 2004;103:21–80. doi: 10.1016/j.pharmthera.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 60.Yang Y, Chen M, Lai Y, Gantz I, Georgeson KE, Harmon CM. Molecular determinants of human melanocortin-4 receptor responsible for antagonist SHU9119 selective activity. J Biol Chem. 2002;277:20328–20335. doi: 10.1074/jbc.M201343200. [DOI] [PubMed] [Google Scholar]
- 61.Mutulis F, Yahorava S, Mutule I, Yahorau A, Liepinsh E, Kopantshuk S, Veiksina S, Tars K, Belyakov S, Mishnev A, Rinken A, Wikberg JE. New substituted piperazines as ligands for melanocortin receptors. Correlation to the X-ray structure of “THIQ”. J Med Chem. 2004;47:4613–4626. doi: 10.1021/jm0311285. [DOI] [PubMed] [Google Scholar]
- 62.Perlman S, Costa-Neto CM, Miyakawa AA, Schambye HT, Hjorth SA, Paiva AC, Rivero RA, Greenlee WJ, Schwartz TW. Dual agonistic and antagonistic property of nonpeptide angiotensin AT1 ligands: susceptibility to receptor mutations. Mol Pharmacol. 1997;51:301–311. doi: 10.1124/mol.51.2.301. [DOI] [PubMed] [Google Scholar]