Summary
DEPTOR, an inhibitor of mTORC1 and mTORC2, is degraded via ubiquitin-proteasome pathway by an unknown E3 ubiquitin ligase. Here we report that DEPTOR is a physiological substrate of SCFβTrCP E3 ligase for targeted degradation. Upon growth factor stimulation, RSK1 and S6K1 kinases are activated to phosphorylate DEPTOR, which is then recognized by the F-box protein, βTrCP via its degron sequence for subsequent ubiquitination and degradation by SCF E3. Endogenous DEPTOR levels are negatively regulated by βTrCP. DEPTOR half-life is shortened by βTrCP but extended by a dominant negative mutant of βTrCP, by RSK1/S6K1 inhibition, and by βTrCP degron site mutations. Biologically, DEPTOR accumulation upon βTrCP knockdown inactivates mTORC1 and activates AKT in cancer cells to confer resistance to rapamycin and paclitaxel. Furthermore, DEPTOR accumulates upon glucose deprivation and mTOR inhibition, to induce autophagy. Thus, βTrCP-DEPTOR-mTOR intertwine to regulate cell survival and autophagy.
Keywords: Autophagy, βTrCP, DEPTOR, mTOR, SCF E3 ligase, survival
Introduction
In response to various stimuli, such as growth factors, nutrient status, and various stresses, mammalian target of rapamycin (mTOR), an evolutionarily conserved serine/threonine protein kinase, is either activated or inactivated, leading to altered cellular processes, including cell growth and proliferation, cell survival, and autophagy (Corradetti and Guan, 2006; Jung et al., 2010; Sarbassov et al., 2005). In mammalian cells, mTOR forms two complexes with mTORC1 consisting of mTOR, raptor, PRAS40 and mLST8, and mTORC2 consisting of mTOR, rictor, mSin1, protor and mLST8 (Guertin and Sabatini, 2007; Peterson et al., 2009). While mTORC1 is mainly involved in regulation of protein translation, cell size, and cell proliferation by phosphorylating S6K1 and eIF-4E-BP1, two key regulators of protein synthesis, mTORC2 regulates cell survival via directly phosphorylating and activating AKT and SGK1 (Guertin and Sabatini, 2007). In addition, mTORC1 could negatively regulate AKT via down-regulating expression of IRS-1/2 via S6K to block PI3K activation (Proud, 2009; Sabatini, 2006). At the molecular level, mTOR itself is subjected to negative regulation by 1) TSC1/2 tumor suppressors through inactivation of Rheb, a Ras superfamily G-protein that directly activates mTOR (Corradetti and Guan, 2006; Inoki et al., 2003a), 2) FBXW7 tumor suppressor, an F-box protein of SCF (Skp1-Cullins-F box proteins) E3 ubiqutin ligase that promotes its ubiquitination and degradation (Mao et al., 2008), and 3) DEPTOR, a naturally occurring inhibitor of mTOR via directly binding to both mTORC1 and mTORC2 (Peterson et al., 2009). DEPTOR acts as a tumor suppressor by blocking mTORC1 and mTORC2 to inhibit protein synthesis, cell proliferation and survival effect of AKT. Under certain circumstance, however, DEPTOR acts as an oncogene by relieving the feedback inhibition from S6K1 to PI3K, thus activating AKT (Efeyan and Sabatini, 2010). Importantly, DEPTOR is subjected to proteasome-dependent degradation upon serum stimulation to ensure mTOR activation (Peterson et al., 2009). However, the E3 ubiquitin ligase responsible for DEPTOR degradation is unknown.
The SCF, a complex of SKP1, Cullins, F-box proteins, and a RING protein, RBX (RING Box protein), also known as ROC (Regulators of Cullins), is the largest family of E3 ubiquitin ligases and controls several biological processes through targeted ubiquitination and degradation of diverse regulatory proteins (Deshaies and Joazeiro, 2009; Nakayama and Nakayama, 2006). It is well-established that the substrate specificity of an SCF complex is determined by the F box proteins that bind to SKP1 and Cullins through its F-box domain and to substrates through its WD40 or LRR domains (Zheng et al., 2002). The human genome contains ~70 F-box proteins with a majority of them remaining uncharacterized (Jin et al., 2004). Among three well-studied F-box proteins, SKP2 and βTrCP are considered as oncogenes, whereas FBXW7 is a tumor suppressor (Frescas and Pagano, 2008; Welcker and Clurman, 2008). In order for an F-box protein to bind to its substrate, the substrate has to be phosphorylated by one or more kinases in a binding-specific degron motif (Deshaies and Joazeiro, 2009; Willems et al., 2004). Thus, the kinase(s), upon activation in response to various stimuli, phosphorylate the SCF substrates and facilitate their binding to the F-box proteins for subsequent ubiquitination and degradation, ensuring an adaptation to a new growth environment.
Here we report that DEPTOR is a physiological substrate of SCFβTrCP E3 ubiquitin ligase. Upon growth factor stimulation, DEPTOR is rapidly degraded by the ubiquitin-proteasome pathway to ensure a proper activation of mTOR for cell proliferation and survival. We identified that S6K1 and RSK1, which are activated by growth factors, are kinases that phosphorylate DEPTOR and facilitate its binding to βTrCP for subsequent degradation. DEPTOR, upon accumulation, regulates cell survival and autophagy. Our study revealed a previously unknown mechanism for oncogenic βTrCP to regulate survival positively and autophagy negatively by activating mTOR via targeting DEPTOR for degradation.
Results
DEPTOR binds to βTrCP and SCF complex and is negatively regulated by βTrCP
A recent study showed that DEPTOR, a naturally occurring mTOR inhibitor, is subjected to proteasome-dependent degradation (Peterson et al., 2009). However, the corresponding E3 ubiquitin ligase for DEPTOR degradation is unknown. To determine whether DEPTOR is a substrate of SCF E3 ubiquitin ligase, we examined the DEPTOR sequence for the consensus binding motifs of F-box proteins βTrCP and FBXW7 and found an evolutionarily conserved putative binding site (SSGYFS) for βTrCP (D-pS-G-X-X-pS) on codons 286-291 (Fig. S1A), and for FBXW7 (L-I/L/P-pT-P-X-X-X-X) on codons 319-326 (Fig. S1B). The exact motif sequence of SSGYFS, designated as DEPTOR degron, was also found on BimEL (BimEL degron), a newly characterized substrate of SCF-βTrCP E3 ligase (Dehan et al., 2009). We next determined potential binding of DEPTOR with βTrCP or FBXW7. FLAG-tagged DEPTOR was cotransfected with HA-tagged βTrCP2 or FBXW7 and was detected in immunoprecipitates pulled-down by HA antibody (Fig 1A), indicating that DEPTOR binds to both βTrCP2 and FBXW7 when overexpressed. Consistently, in a reciprocal experiment, either HA-βTrCP2 or HA-FBXW7 was detected in FLAG-DEPTOR immunoprecipitates (data not shown). On the other hand, however, transfection of HA-βTrCP2, but not of HA-FBXW7, pulled down endogenous DEPTOR in monomeric as well as higher molecular weight (HMW) forms, suggesting that DEPTOR is subjected to modification upon βTrCP transfection (Fig. 1B). Consistently, FLAG-βTrCP1, but not FLAG-FBXW7 (even expressed at a much higher level), pulled down both endogenous DEPTOR as well as its modified forms with HMW (Fig. S1C). Importantly, endogenous DEPTOR could pull-down endogenous βTrCP1 under the physiological growth conditions (Fig. 1C). Finally, we found that both RBX1 and Cullin-1, other two components of SCF, can pull down endogenous DEPTOR and its modified forms with HMW (Fig. S1D&E). These results indicate 1) an in vivo binding of DEPTOR with βTrCP and other components of SCF E3 ubiquitin ligase complex and 2) DEPTOR is likely subjected to ubiquitination modification by SCF E3 components. On the other hand, the lack of FBXW7 binding to endogenous DEPTOR could be attributable to its overlapping binding site with mTOR, which binds to DEPTOR on codons 324-409 (Peterson et al., 2009).
Figure 1. βTrCP binds to DEPTOR and regulates DEPTOR levels.
(A&B) βTrCP bound to exogenously expressed (A) or endogenous DEPTOR (B): The 293 cells were transfected with indicated plasmids, IP with anti-HA Ab and IB with indicated Abs. WCE: whole cell extract. (C) Binding of endogenous βTrCP and DEPTOR. HeLa cells were serum starved overnight, followed by serum addition for 4 hrs to stimulate DEPTOR phosphorylation. Cell lysates were then prepared for IP with anti-DEPTOR Ab and IB with anti-βTrCP1 Ab. (D) Inverse correlation between βTrCP and DEPTOR levels: Cell lysates were prepared from indicated breast cancer cell lines for IB. * indicates a non-specific band. (E) βTrCP silencing increases DEPTOR levels: Cancer cell lines were transfected with βTrCP siRNA and scrambled siRNA, followed by IB with indicated Abs. (F) βTrCP overexpression reduces DEPTOR levels. T47D and MCF7 cells were infected with retrovirus expressing βTrCP1, followed by puromycin selection. HeLa cells were transfected with plasmid expressing βTrCP2, followed by G418 selection. Stable clones were pooled for IB with indicated Abs.
We next observed an inversely correlated expression pattern between DEPTOR and βTrCP1 in multiple breast cancer cell lines. As shown in Figure 1D, three breast cancer lines (MDA-MB231, SK-BR3, and MDA-MB468) with relatively high levels of βTrCP1 had low or undetectable levels of DEPTOR, whereas other four lines (T47D, ZR75-1, MDA-MB453, and MCF7) with low levels of βTrCP1 had very high levels of DEPTOR. To determine a causal relationship, we used siRNA targeting both βTrCP1 and βTrCP2 in βTrCP1 high-expressing MDA-MB231 and SK-BR3 cells as well as HeLa cells, and found a significant accumulation of DEPTOR in all three lines, along with accumulation of β-catenin, a known substrate of SCFβTrCP E3, serving as a positive control (Fig. 1E). Accompanying DEPTOR accumulation, mTOR activity, as reflected by S6K1 phosphorylation, was reduced (Fig. 1E), consistent with the previous observation that DEPTOR is an inhibitor of mTOR (Peterson et al., 2009). In contrast, siRNA silencing of FBXW7 failed to cause DEPTOR accumulation, although accumulation of NOTCH1 and c-MYC, two well-known substrates of FBXW7 (Welcker and Clurman, 2008), was evident (Fig. S1F). Finally, we stably overexpressed βTrCP in βTrCP1 low-expressing T47D and MCF7 cells, along with HeLa cells, and found a consequent reduction of DEPTOR, but not other mTORC components, including mTOR, RAPTOR, RICTOR, and GβL, in all three lines tested (Fig. 1F). Thus, DEPTOR is selectively degraded by βTrCP.
βTrCP shortens DEPTOR protein half-life and promotes DEPTOR ubiquitination
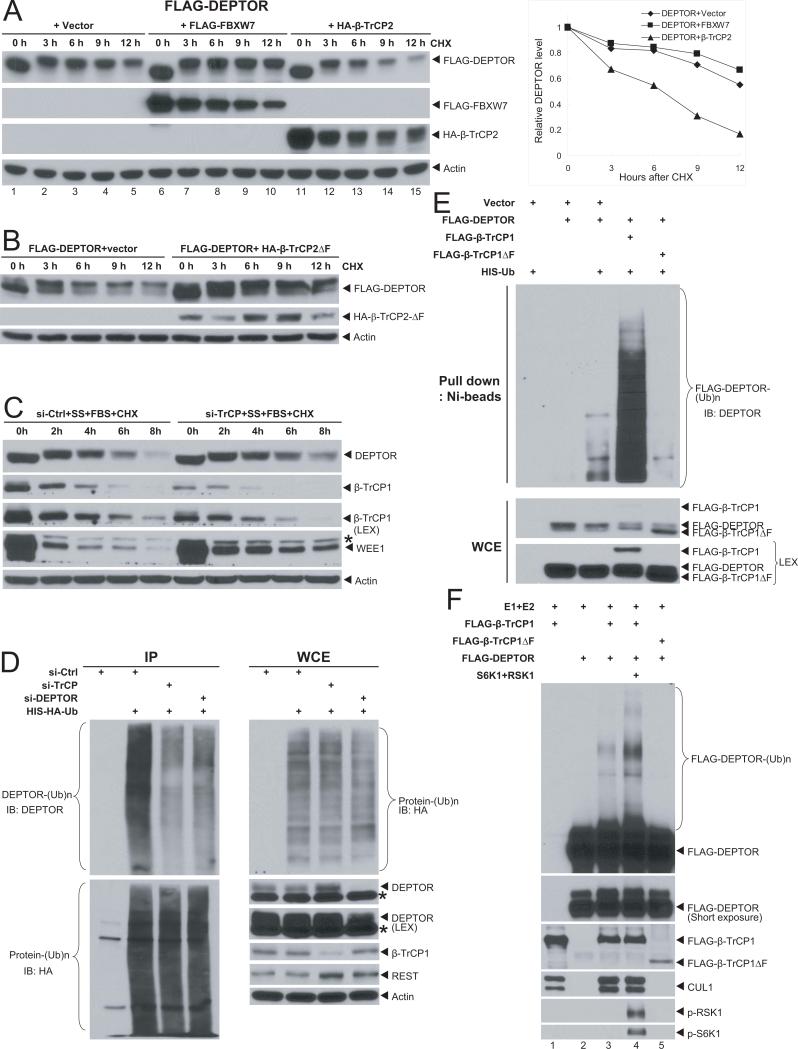
We next determined whether βTrCP shortened DEPTOR protein half-life. As shown in Figure 2A, the protein half-life of transfected DEPTOR is greater than 12 hrs (lanes 1-5), which was shortened to ~6 hrs upon βTrCP overexpression (lanes 11-15). Note that the band up-shifting upon addition of serum (by medium change) and CHX, as compared to the samples harvested at zero time point (without medium change, also see below), was due to serum-triggered DEPTOR phosphorylation (Peterson et al., 2009). On the other hand, overexpression of βTrCPΔF, a dominant negative mutant which bound to the substrates but failed to recruit other components of SCF E3 ligase (Tan et al., 2006; Yamoah et al., 2008), increased the DEPTOR basal level and extended its protein half-life (Fig. 2B), whereas siRNA knockdown of βTrCP extended protein half-life of endogenous DEPTOR whose degradation was triggered by addition of serum to serum-starved cells (Fig. 2C, Fig. S2A&B). In contrast, FBXW7 overexpression had no effect on exogenously expressed DEPTOR (Fig. 2A, lanes 6-10), whereas FBXW7 siRNA knockdown or gene deletion (Rajagopalan et al., 2004) had no effect on endogenous DEPTOR degradation, triggered by serum addition to serum starved HeLa or DLD1 colon cancer cells, respectively (Fig. S2C&D). These results further exclude the possible involvement of FBXW7 in promoting DEPTOR degradation. Finally, we determined whether manipulation of βTrCP levels would alter DEPTOR ubiquitination. Indeed, the in vivo ubiquitination assay showed that siRNA knockdown of βTrCP blocked ubiquitination of endogenous DEPTOR (Fig. 2D), whereas overexpression of βTrCP, but not its dominant negative form (ΔF), promoted ubiquitination of exogenously expressed DEPTOR (Fig. 2E). Consistently, in an in vitro ubiquitination assay, addition of immune-purified DEPTOR into a reaction mixture, containing E1, E2, and CUL1/βTrCP1 E3, co-purified by beads-conjugated immunoprecipitation (Yamoah et al., 2008), induced a βTrCP-dependent DEPTOR polyubiquitination (Fig. 2F, lanes 3 vs. 5), which was further enhanced by two kinases RSK1 and S6K1 (lanes 3 vs. 4, see below). Furthermore, DEPTOR polyubiquitination by βTrCP was dependent on the E2 as well as the degron-motif that mediates βTrCP-DEPTOR binding (see below) (Fig. S2E). Taken together, our results support the notion that DEPTOR is a substrate of SCFβTrCP E3 ligase, which ubiquitinates it and targets it for degradation.
Figure 2. βTrCP shortens DEPTOR half-life and promotes DEPTOR ubiquitination.
(A) DEPTOR half-life is shortened by βTrCP, but not by FBXW7: FLAG-DEPTOR was transfected into 293 cells, along with the vector control or plasmid expressing βTrCP2 or FBXW7. Cell were switched 48-hrs post transfection to fresh medium (10% FBS) containing cycloheximide (CHX) for indicated time periods and harvested for IB. Note that the samples harvested at zero time point (lanes 1, 6, 11) had no medium change, thus lack of serum-mediated DEPTOR phosphorylation (Peterson et al., 2009), and migrated faster. The band density was quantified using Image J software and plotted (right). (B&C) DEPTOR half-life is extended by a dominant negative βTrCP mutant (B) or by βTrCP siRNA silencing (C): The 293 cells were transfected with βTrCPΔF and the vector control (B) or HeLa cells were transfected with siRNA targeting βTrCP or control siRNA (C). Cells were harvested at indicated time points post CHX treatment for IB. SS: serum starvation, FBS: fetal bovine serum. (D-F) βTrCP promotes DEPTOR ubiquitination in vivo (D&E) and in vitro (F). The 293 cells were transfected with siRNA targeting βTrCP, DEPTOR and siRNA control. Twenty-four hrs later, cells were transfected with His-HA-Ub, followed by IP with bead-conjugated anti-HA Ab and IB using anti-DEPTOR Ab. Whole cell extracts were also subjected to IB (D). The 293 cells were transfected with indicated plasmids, lysed in under denatured condition at 6M guanidinium solution, followed by Ni-bead pull-down. Washed beads were boiled and subjected to IB for DEPTOR. LEX (longer exposure) (E). SCF E3 was prepared by FLAG bead IP using 293 cells transfected with βTrCP or βTrCPβF. DEPTOR was prepared by transfecting FLAG-DEPTOR into 293 cells, followed by FLAG-bead IP and 3xFLAG peptide elution. SCF E3, and DEPTOR as the substrate, were added into a reaction mixture containing ATP, ubiquitin, E1 and E2, in some reactions, S6K1 and RSK1, followed by constant mixing for 60 min. The reaction mixture was then loaded onto PAGE gel for IB using anti-DEPTOR Ab (F).
βTrCP-DEPTOR binding is dependent on degron motif and DEPTOR phosphorylation: mutations on degron motif abrogate DEPTOR ubiquitination and degradation
We then determined whether βTrCP-DEPTOR binding was dependent on the DEPTOR degron motif 286SSGYFS291 as well as its phosphorylation. While wild type DEPTOR bound well with βTrCP2, the binding was markedly reduced in any one of all three DEPTOR single-point mutants that had a serine-to-alanine substitution on codons, 286, 287 or 291, respectively (Fig. 3A). Furthermore, βTrCP1 or βTrCP2 bound to DEPTOR peptide containing phosphorylated, but not non-phosphorylated degron-motif, whereas there is no binding between FBXW7 and DEPTOR peptide regardless of phosphorylation (Figs. 3B, S3A). Consistently, ubiquitination of these three DEPTOR mutants, as well as a triple mutant with all three serine residues mutated to alanine (S286-7/291-3A) was remarkably reduced (Fig. 3C). Consequently, the protein half-life of three mutants (S287A, S291A, and S286-7/291-3A) was significantly extended (Figs. 3D and S3C), except S286A mutant which showed a moderate half-life extension (Fig. S3B). Thus, DEPTOR degron sequence determines the βTrCP binding and subsequent ubiquitination and degradation of DEPTOR.
Figure 3. βTrCP binding and ubiquitination/stability of DEPTOR are dependent on its degron sequence.
(A) Reduction of βTrCP-DEPTOR binding by degron site mutations: DEPTOR and its S→A mutants at the degron site were cotransfected with HA-βTrCP2, followed by IP with FLAG Ab and IB with indicated Abs. (B). βTrCP binds to phosphor-DEPTOR peptide: The 293 cells were transfected with indicated plasmids. Cells were lysed and incubated with bead-conjugated DEPTOR peptide with or without degron site phosphorylation, followed by IB using anti-FLAG Ab. (C) Reduction of DEPTOR ubiquitination by degron site mutation: The 293 cells were transfected with indicated plasmids, followed by IP using bead-conjugated anti-FLAG Ab and IB by anti-HA Ab. (D) DEPTOR mutants have a longer protein half-life: The 293 cells were transfected with two degron site mutants. Cells were switched 48-hrs post transfection to fresh medium containing CHX and harvested at indicated time points for IB using anti-FLAG Ab.
Serum-induced DEPTOR degradation is blocked by inhibition of S6K1 and RSK1 kinases via small molecule inhibitors or siRNAs
A recent report showed that RSK1 and ERK1, but not S6K1 kinase, phosphorylated the BimEL degron (SSGYFS) and facilitated its binding to βTrCP (Dehan et al., 2009). Since both DEPTOR and BimEL degrons showed the identical sequences, we first determined whether DEPTOR bound to each of these three kinases individually, and found that was the case upon co-transfection under overexpressed conditions, although DEPTOR did not bind to CHK1 kinase (served as a negative control) under the same conditions (Fig. S4A). Since DEPTOR level decreased upon serum addition to serum-starved cells (Peterson et al., 2009), we next determined a potential correlation between DEPTOR degradation and activation of all these three kinases upon serum stimulation. As shown in Figure 4A, serum addition to serum-starved HeLa cells caused a reduction of DEPTOR levels, starting at 2 hrs with continuous reduction thereafter and complete elimination at 8 hrs. Activation of all three kinases, as revealed by their phosphorylation, occurred at 5 min post serum addition. While S6K1 activation continued up to 8 hrs, RSK1 and ERK1/2 activation was bi-phasic with activation at 5-30 min, followed by deactivation at 1-2 hrs and reactivation at 4-8 hrs. Nevertheless, all three kinases were activated between 4-8 hrs post serum additions when DEPTOR expression was decreased or eliminated.
Figure 4. Inhibition of S6K1 and RSK1, but not MEK blocks serum-induced DEPTOR degradation.
HeLa cells were serum-starved for 36 hrs, followed by serum addition in the absence (A) or presence of rapamycin (100 nM) (B, top), BI-D1870 (20 μM) (B, middle) or U0126 (10 μM) (B, bottom). HeLa cells were serum-starved for 36 hrs, followed by serum addition in combination of CHX without or with rapamycin (100 nM) (C, top) or BI-D1870 (20 μM) (C, bottom). HeLa cells were transfected with siRNA targeting S6K1 (D, top) or RSK1 (D, bottom) and control siRNA, followed by serum starvation and serum-CHX addition. Cells were harvested at indicated time points for IB with indicated Abs.
To define which kinase(s) could mediate DEPTOR degradation at the individual level, we treated cells with selective inhibitor against each kinase and found that serum-induced DEPTOR degradation was completely abrogated by a) rapamycin, a mTOR inhibitor that eliminates S6K1 phosphorylation (Fig. 4B, top panel) or b) BI-D1870, an inhibitor of RSK1/2 (Sapkota et al., 2007), as reflected by reduced levels of pGSK3α/β, a known substrate of RSK1/2 (Sapkota et al., 2007) (Fig. 4B, middle panel), but not by U0126, a MEK inhibitor (Fig. 4B, bottom panel). We further found that either rapamycin or BI-D1870 increased the basal levels of endogenous DEPTOR (Fig. S4B-D), likely through the inhibition of mTOR in case of rapamycin (Peterson et al., 2009). Consistently, either drug extended the protein half-life of endogenous DEPTOR (Fig. 4C). More specifically, siRNA knockdown of either S6K1 or RSK1 also remarkably extended the protein half-life of DEPTOR with a more dominating effect seen upon RSK1 knockdown (Fig. 4D). In contrast, the protein half-life of DEPTOR was not affected by MEK inhibitor, U0126 (Fig. S4E), nor by siRNA knockdown of ERK1 (Fig. S4F). Taken together, both S6K1 and RSK1, but not ERK1/2, are involved in DEPTOR phosphorylation and subsequent degradation.
DEPTOR is phosphorylated by S6K1 and RSK1 on the degron serine residues upon serum stimulation
To further determine the nature of DEPTOR degradation in a phosphorylation-dependent manner, we raised a phospho-antibody against a DEPTOR peptide with all three serine residues (Ser286,287,291) phosphorylated on the DEPTOR degron (285GpSpSGYFpSSSPTLSKKC). We first confirmed that the antibody was phospho-specific in three assays: 1) detection of phosphor-DEPTOR by the antibody could be competed away by specific phospho-peptide, but not by nonphospho-peptide (Fig. S5A); 2) treatment of cell lysate with λPPase abrogated the detection of endogenous phospho-DEPTOR (Fig. S5B); and 3) the antibody only detected the phosphorylated wt DEPTOR, but not any of DEPTOR mutants (Fig. S5C). We then used this specific phospho-antibody to determine whether the endogenous DEPTOR is subjected to phosphorylation upon serum stimulation in serum-starved HeLa cells with inclusion of MG132 to block degradation of phospho-DEPTOR. We found that DEPTOR phosphorylation started to occur 2 hrs post serum addition and rearched its peak at 8 hrs (Fig. 5A). This pattern of DEPTOR phosphorylation was directly correlated with the pattern of DEPTOR degradation under the same treatment (Fig. 4A), suggesting that DEPTOR phosphorylation preceded its degradation. Furthermore, we found that DEPTOR phosphorylation was near completely abrogated by S6K1 inhibitor rapamycin or RSK1 inhibitor BI-D1870 in MCF 7 cells (Fig. 5B) as well as in 293 cells, but not by ERK inhibitor U0126 (Fig. 5C). In addition, we performed an in vitro kinase assay for DEPTOR phosphorylation at three serine residues (Ser286,287,291) on the degron site, and found that immuno-purified FLAG-tagged DEPTOR was barely phosphorylated by either S6K1 or RSK1 alone, but the combination of both kinases, added simultaneously, caused remarkably DEPTOR phosporylation on Ser286,287,291 (Fig. 5D). We further determined if Ser286,287,291 phosphorylation occurs in a sequential order by S6K1 then RSK1, or vice versa, and found that S6K1 pre-incubation, but not RSK1 pre-incubation, significantly enhanced DEPTOR phosphorylation on Ser286,287,291 (Fig. 5D). Finally, we observed that addition of S6K1 and RSK1 significantly enhanced poly-ubiquitination of DEPTOR by βTrCP in an in vitro ubiquitination assay (Fig. 2F, lanes 3 vs. 4). Taken together, our results suggest that S6K1 and RSK1 are DEPTOR kinases, and that S6K1 could acts as a prime kinase to facilitate DEPTOR phosphorylation on three serine residues at the degron motif by RSK1, leading to βTrCP binding and subsequent DEPTOR ubiquitination and degradation.
Figure 5. DEPTOR is phosphorylated upon serum stimulation: inhibition by rapamycin and BI-D1870.
HeLa (A) or MCF7 (B) cells were serum starved, followed by addition of serum and MG132 to block degradation in the absence (A) and presence of rapamycin (B, top) or BI-D1870 (B, bottom). Cells were harvested at indicated time points for IB with indicated Abs. (C) DEPTOR phosphorylation at the degron site was inhibited by rapamycin and BI-D1870, but not by U0126: The 293 cells were transfected with FLAG-DEPTOR. Forty-eight hrs post transfection, medium were changed (FBS), along with addition of indicated drugs (rapamycin, 100 nM; BI-D1870, 20 μM, or U0126, 10 μM). Cells were harvested 4 hrs later for IB. (D) In vitro phosphorylation of DEPTOR on the degron site. FLAG-tagged DEPTOR was transfected into 293 cells, purified by IP using bead-conjugated anti-FLAG Ab, and incubated with active S6K1 or RSK1 alone or in combination, added simultaneously or sequentially in an opposite order in a kinase reaction mixture. DEPTOR phosphorylation on the degron site was detected by IB using a DEPTOR phospho-specific Ab.
DEPTOR accumulation by βTrCP knockdown increased cell survival by activating AKT
It was recently reported that DEPTOR could regulate cell survival via interacting with mTOR and AKT (Peterson et al., 2009). To determine the biological significance of DEPTOR degradation by βTrCP, we knocked down βTrCP and detected an expected accumulation of DEPTOR (Fig. 6A and Fig. S6A&B). Using both the ATP-lite cell viability assay and clonogenic cell survival assay, we found that under the βTrCP-knockdown/DEPTOR accumulated condition, cancer cells become more resistant to rapamycin, a mTOR inhibitor, whose anti-proliferation activity depends on mTOR activity (Mao et al., 2008) (Fig. 6B), as well as to paclitaxel, an agent with poor activity against DEPTOR overexpressing ovarian cancer (Foster et al., 2010) in multiple cancer lines (Figs. S6C-F). The resistance to both drugs can be partially or completely rescued by knockdown of DEPTOR, indicating a causal effect (Fig. 6B and Figs. S6C-F). Mechanistically, we found that DEPTOR accumulation, upon βTrCP knockdown, inhibited mTORC1, as reflected by reduced S6K1 phosphorylation (Fig. 6A, Figs. S6A&B), and increased AKT phosphorylation, which can be rescued by DEPTOR knockdown (Fig. 6C and Figs. S6D-F). We further elucidated the mechanism of paclitaxel resistance upon DEPTOR accumulation (as a result of βTrCP-knockdown) by 1) siRNA knockdown of AKT or RICTOR, and 2) inhibition of mTORC1/2 by Torin (Thoreen et al., 2009) or PI3K by LY294003 (Fig. 6C), and found that blockage of mTORC1/2 and PI3K/AKT pathways by these approaches largely abrogated paclitaxel resistance (Fig. 6D), indicating their causal involvement.
Figure 6. βTrCP and DEPTOR regulates cell proliferation and survival.
MCF7 cells were transfected with siRNA targeting βTrCP alone or in combination with siRNA targeting DEPTOR, along with scrambled siRNA, followed by IB (A), or ATPlite cell proliferation assay and clonogenic assay (with 400 cell seeded) (B). MCF7 cells were transfected with the siRNA oligos or treated with different drugs (Torin, 7.5 nM; LY294003, 1.25 μM; or paclitaxel, 50 nM) for 24 hrs or as indicated, followed by IB with indicated Abs (C&F), ATP-lite cell viability assay (D), and trypan blue exclusion assay (E). Shown are mean ± SEM from three independent experiments, each run in duplicate.
Finally, we determined the effect of DEPTOR on paclitaxel-induced growth suppression, resulting from inhibition of proliferation and/or induction of apoptosis. As shown in Figure 6E, DEPTOR accumulation (via βTrCP knockdown) had no effect on cell proliferation when cells were grown in paclitaxol-free or low paclitaxol (2 nM) conditions (left and middle panels). However, DEPTOR accumulation inhibited cells death, when cells were grown at 5 nM paclitaxel, which was near completely abrogated by simultaneous DEPTOR knockdown (right panel). Consistently, DEPTOR accumulation activated AKT and inhibited paclitaxel-induced PARP cleavage, a hallmark of apoptosis (Fig. 6F, lanes 4-6 vs. 1-3), which was again abrogated by simultaneous knockdown of DEPTOR (Fig. 6F, lanes 7-9), RICTOR or AKT itself, as well as by the treatment with Torin or LY294003 (Fig. S6G). Thus, DEPTOR mainly plays a survival role against paclitaxel-induced apoptosis.
DEPTOR knockdown blocks autophagy induced by glucose deprivation & mTOR inactivation
mTOR is a well-established negative regulator of autophagy; whereas energy depletion, such as glucose deprivation, is a potent inducer of autophagy via inhibition of mTOR (Corradetti and Guan, 2006; Jung et al., 2010; Shintani and Klionsky, 2004). Since mTOR inhibits DEPTOR expression (Peterson et al., 2009), we determined if mTOR inactivation by glucose deprivation would cause DEPTOR accumulation and autophagy induction. Indeed, glucose deprivation caused a time-dependent DEPTOR accumulation and mTOR inactivation (as reflected by reduced S6K1 phosphorylation), as well as autophagy induction, as evidenced by accumulation of LC3-II (lipid-conjugated form of LC3, localized to the membranes of autophagosomes), a hallmark of autophagy (Figs. 7A&B, and S7A&B). We next determined if accumulation of DEPTOR is causally related to autophagy induction and found that siRNA knockdown of DEPTOR partially abrogated glucose deprivation-induced autophagy as demonstrated by reduced levels of LC3-II (Figs. 7A&B, and S7A&B). Furthermore, we found that the levels of p62, an autophagy adaptor/receptor and a LC3 binding protein, which is selectively degraded under most autophagic conditions (Johansen and Lamark, 2011; Komatsu and Ichimura, 2010), did not change significantly upon glucose depletion within 24 hrs in most lines tested (Figs. 7A&B, and S7A&B), but decreased after 48 hr glucose deprivation in HeLa cells (Fig 7B, bottom panels). Interestingly, in every 4 cell lines tested, DEPTOR knockdown significantly reduced the basal levels of p62, possibly through mTOR activation or via other unknown mechanisms. On the other hand, DEPTOR knockdown during glucose deprivation facilitated the return of reduced p62 level gradually, reaching the basal level at 24 hrs, suggesting that DEPTOR knockdown may inhibit autophagy induced by glucose deprivation, leading to p62 accumulation (Figs. 7A&B, and S7A&B). Nevertheless, DEPTOR regulation of autophagy via mTOR might be more complicated and stage-dependent, given a recent report showing that mTOR signaling is inhibited during initiation of autophagy upon starvation, but reactivated later to facilitate the reformation of lysosomes from autolysosomes (Yu et al., 2010). Finally, we used acridine orange (AO) staining for detection of acidic vesicular organelles as an independent measure of autophagy (Paglin et al., 2001; Vucicevic et al., 2010) and found that glucose deprivation remarkably increased the number of AO positive cells, which was significantly inhibited upon DEPTOR knockdown (Fig. S7C). Thus, energy depletion inactivates mTOR to cause DEPTOR accumulation, which is responsible, at least in part, for subsequent induction of autophagy (Fig. 7C).
Figure 7. DEPTOR regulates autophagy induced by glucose deprivation.
Cells were transfected with siRNA targeting DEPTOR and control siRNA, and subjected to glucose deprivation by growth in glucose-free medium for various times, followed by IB with indicated Abs. (LEX: longer exposure) (A&B). (C) A model for SCFβTrCP-DEPTOR-mTOR interplay in regulation cell survival and autophagy (see text for details).
DISCUSSION
In this study we identified and characterized DEPTOR, a naturally occurring inhibitor of mTOR, as a physiological substrate of SCFβTrCP E3 ubiquitin ligase. Our conclusion is supported by the following lines of evidence: 1) DEPTOR binds to βTrCP and is in the complex of RBX1-CUL1-SCFβTrCP E3 ubiquitin ligase; 2) DEPTOR-βTrCP binding is dependent on an evolutionarily conserved βTrCP consensus binding site (degron) on DEPTOR; 3) DEPTOR-βTrCP binding leads to DEPTOR ubiquitination; 4) DEPTOR protein half-life is shortened by βTrCP, but extended by a dominant negative mutant of βTrCP or by βTrCP siRNA knockdown; 5) DEPTOR is phosphorylated by RSK1 and S6K1, and DEPTOR protein half-life is extended either by RSK1/S6K1 specific inhibitor or by RSK1/S6K1 knockdown; 6) Cellular levels of DEPTOR are inversely correlated with the levels of βTrCP and can be increased or decreased by βTrCP silencing or overexpression, respectively; and finally 7) S6K1/RSK1 and βTrCP are required for ubiquitination and degradation of endogenous DEPTOR upon mitogen stimulation. Taken together, our results indicated that DEPTOR is a physiological substrate of SCFβTrCP E3 ubiquitin ligase, and that DEPTOR phosphorylation by S6K1 and RSK1 and degradation by SCFβTrCP E3 in response to serum stimulation is a physiologically relevant event, leading to mitogen-induced mTOR activation (Fig 7C).
The F-box protein, βTrCP is the substrate recognition subunit of SCF E3 ligase with two family members, βTrCP1 and βTrCP2. βTrCP is one of few well-studied F box proteins with ~40 known substrates characterized (Skaar et al., 2009). In some tissues, βTrCP is characterized as an oncoprotein for targeted degradation of tumor suppressors (Frescas and Pagano, 2008), such as p53 (Xia et al., 2009), and survival regulatory genes, including proapoptotic proteins BimEL (Dehan et al., 2009) and procaspase-3 (Tan et al., 2006) as well as IκB (Winston et al., 1999), whose degradation leads to activation of survival transcription factor NFκB. Genetic studies showed that transgenic expression of βTrCP1 in mammary glands promotes tumor formation via activating NFκB (Kudo et al., 2004). Consistently, βTrCP was overexpressed during mouse skin carcinogenesis induced by DMBA/TPA, leading to IκB degradation and NFκB activation and enhanced tumor growth (Gu, 2007). Here we revealed an additional mechanism by which oncogenic βTrCP positively regulates protein synthesis, cell proliferation and survival via activating mTOR signals through targeting DEPTOR for degradation. Our finding, in combination with a recent study showing βTrCP-mediated degradation of PDCD4, an EIF4A inhibitor and a negative regulator of protein translation (Dorrello et al., 2006), clearly demonstrates that SCFβTrCP E3 ligase plays an essential role in governing protein synthesis through the mTOR pathway upon mitogen stimulation.
Our study defined that either S6K1 or RSK1 is required for DEPTOR phosphorylation for subsequent degradation. Identification of S6K1 as one of DEPTOR kinases is consistent with a recent observation that DEPTOR phosphorylation is mTOR dependent (Peterson et al., 2009). In fact, S286 residue was identified as a DEPTOR phosphorylation site, although S287 or S291 residues were not detected by a mass spectrometry based analysis (Peterson et al., 2009). Consistently, RSK1 was reported to phosphorylate the BimEL degron (SSGYFS) (Dehan et al., 2009) with identical sequence to the DEPTOR degron sequence. Future study could be directed to define which serine residues on the DEPTOR degron is subjected to phosphorylation by S6K1 or RSK1 after raising phosphor-specific antibody against each individual serine residue.
Development of drug resistance in cancer cells is one of the major reasons for therapeutic failure. We found that cancer cells become drug resistant upon DEPTOR accumulation in response to βTrCP knockdown. It is conceivable that resistance to rapamycin is attributable to DEPTOR-mediated mTOR inhibition, since cancer cells with higher mTOR activity are in general more sensitive to the drug (Mao et al., 2008). On the other hand, developed resistance to paclitaxel is likely due to activation of AKT as a result of relieving feedback inhibition from mTORC1 to PI3K (Peterson et al., 2009; Proud, 2009), since blockage of AKT/mTOR pathway by small molecule inhibitors or siRNA knockdown abrogates drug resistance (Fig. 6 and S6). A causal effect of DEPTOR accumulation in drug resistance was demonstrated by DEPTOR knockdown which abrogated/rescued S6K1 inactivation and AKT activation, along with the abrogation of drug resistance partially or completely in a cell line dependent manner. The lack of complete rescue in some lines is likely attributable to the accumulation of other substrates of SCFβTrCP, such as oncogenic β-catenin (Fig. 1E) upon βTrCP knockdown. Our study may have clinical implication for cancer treatment: rapamycin should not be used in combination with MLN4924, a newly discovered SCF E3 ligase inhibitor, currently in clinical development (Soucy et al., 2009a; Soucy et al., 2009b), since MLN4924 would confer the resistance of cancer cells to rapamycin by inducing DEPTOR accumulation (data not shown).
Several recent studies showed that energy depletion (e.g. glucose deprivation) inactivates mTOR via AMPK/TSC2 (Corradetti et al., 2004; Inoki et al., 2003b), or GAPDH-RHEB (Lee et al., 2009) pathway. It is well established that mTOR is a negative regulator of autophagy. While mTORC1 inhibits autophagosome formation, mTORC2 represses the expression of some autophagy-related genes (ATG) and other autophagy regulators (Cardenas et al., 1999; Levine and Klionsky, 2004; Narita and Young, 2009). Likewise, rapamycin, a potent inhibitor of mTOR, is commonly used as an autophagy inducer (Jung et al.; Kondo et al., 2005). We sought to determine the potential effect of DEPTOR on autophagy, given the fact that mTOR inhibits DEPTOR expression at the transcriptional and posttranslational levels (Peterson et al., 2009) and mTOR inhibition would lead to DEPTOR accumulation. Indeed, we found that upon glucose deprivation, DEPTOR accumulates with corresponding induction of autophagy. The causal effect of DEPTOR in the process was confirmed by a partial abrogation of autophagy by DEPTOR knockdown. Autophagy is a process by which cytoplasmic materials including organelles reach lysosomes for degradation (Levine and Kroemer, 2008) and is one effective mean for cells to “re-feed” themselves upon starvation (Mizushima et al., 2010). Our study suggests a DEPTOR-mediated cellular protective mechanism in response to energy deprivation via induction of autophagy.
In summary, our study reveals an important interplay among βTrCP-DEPTOR-mTOR in regulation of cell survival, and autophagy in response to environmental changes. Upon mitogen stimulation (e.g. serum addition), RSK1 and S6K1 are activated to phosphorylate DEPTOR at the βTrCP binding degron, which is then recognized by βTrCP for binding and subsequent degradation by SCF E3 ligase. Degradation of DEPTOR removes its inhibitory binding to mTORC1/2, leading to activation of S6K and AKT to promote cell proliferation and survival. Termination of growth signals inactivates RSK1 and S6K1, which no longer phosphorylate newly synthesized DEPTOR, leading to its accumulation to bind and inhibit mTORC1/2, and shut down mTOR mediated protein synthesis for energy-saving. Thus, the balance between DEPTOR-mTOR plays an essential role in the maintenance of cellular homeostasis under physiological conditions. Upon βTrCP1 silencing, DEPTOR accumulates to block mTORC1, leading to drug resistance and enhanced cell survival, likely due to releasing feedback inhibition from mTORC1/S6K1 to IRS1/PI3K signaling, leading to AKT activation. Upon glucose deprivation which inhibits mTORC1, DEPTOR accumulates to induce autophagy as a cellular protective mechanism via recycling sub-cellular organelles as the energy sources (Fig. 7C).
EXPERIMENTAL PROCEDURES
The In vitro ubiquitination assay
Cullin-βTrCP E3 complex was precipitated from 293 cells either overexpressing both proteins with epitope tags (Yamoah et al., 2008), or being transiently transfected with βTrCP or βTrCPΔF. FLAG-tagged DEPTOR was pulled down by FLAG-bead after transfection into 293 cells and eluted with 3x FLAG peptide (Sigma), followed by incubation with Cullin-βTrCP E3 complex in the presence of E1 and E2 in a ubiquitin reaction buffer (Swaroop et al., 2000). Polyubiquitinated DEPTOR was resolved by SDS-PAGE and detected by IB with anti-DEPTOR Ab. In some reactions, S6K1 and RSK1 were added to show an enhancement of DEPTOR polyubiquitination, whereas in others, E2 was omitted to ensure that the E3-mediated polyubiquitination is E2 dependent.
The In vivo ubiquitination assay
To detect endogenous DEPTOR ubiquitination, cells were transfected with siRNA oligonucleotide targeting both βTrCP1&2, along with scrambled control siRNA. Twenty-four hrs post transfection, cells were transfected with His-HA tagged ubiquitin, followed by IP with beads-conjugated anti-HA Ab. Ubiquitinated DEPTOR was detected by IB with anti-DEPTOR Ab. To determine DEPTOR ubiquitination by βTrCP, cells were cotransfected with DEPTOR, βTrCP and His-Ub, along with empty vector or βTrCPΔF controls. Cells were lysed in 6M guanidinium denaturing solution, as described (Gu et al., 2007). DEPTOR-poly-Ub was purified by Ni-bead pull-down, and detected by IB using anti-DEPTOR Ab. To detect ubiquitination of overexpressed DEPTOR and its mutants, cells were cotransfected with FLAG-DEPTOR and His-HA-ubiquitin, followed by IP with bead-conjugated anti-FLAG Ab and IB with anti-HA Ab.
Generation of phospho-DEPTOR antibody
A peptide polyclonal Ab against phospho-DEPETOR (GpSpSGYFpSSSPTLSKK) within the βTrCP binding motif (DSGxxS) on DEPTOR (Fig S1A) was generated, followed by two sequential rounds of affinity purification by YenZym Antibodies, LLC (San Francisco, CA).
The In vitro binding of DEPTOR peptide and βTrCP
The 293 cells were transfected with FLAG-βTrCP1 or FLAG-FBXW7. Cells were lysed and incubated for 2hr under rotation with bead-conjugated DEPTOR peptides either with or without degron site phosphorylation. Beads were then washed with lysis buffer, boiled and subjected to IB using anti-FLAG Ab (Yada et al., 2004).
The in vitro kinase assay
FLAG-tagged DEPTOR was pulled down by bead-conjugated anti-FLAG Ab after transfection into 293 cells. A kinase reaction (Dehan et al., 2009) was initiated by incubating bead-conjugated FLAG-DEPTOR with active S6K1 or RSK1 kinases (In Vitrogen, CA) in a kinase reaction buffer (50 mM Tris pH 7.5, 10 mM MgCl2, 0.6 mM DTT, 0.01% Triton X-100, 2 mM ATP) at 30°C for 30 mins in constant vortexing. S6K1 or RSK1 was added individually or in combination either simultaneously or in opposite sequential orders with bead-washing step in between to remove the first kinase. The phosphorylated DEPTOR on the degron site was detected by IB with anti-DEPTOR phospho-specific Ab.
Supplementary Material
Acknowledgements
We thank Drs. B. Clurman and K. Nakayama for providing us FBXW7 expressing plasmids; Dr. K. Guan for providing S6K1, ERK1, RSK1 expressing plasmids; Dr. Z. Pan for 293 cells overexpressing cullin-1 and βTrCP; and Dr. D. Sabatini for Torin-1. This work was supported by the NCI grants (CA111554, CA118762, and CA156744) to YS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cardenas ME, Cutler NS, Lorenz MC, Di Como CJ, Heitman J. The TOR signaling cascade regulates gene expression in response to nutrients. Genes Dev. 1999;13:3271–3279. doi: 10.1101/gad.13.24.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradetti MN, Guan KL. Upstream of the mammalian target of rapamycin: do all roads pass through mTOR? Oncogene. 2006;25:6347–6360. doi: 10.1038/sj.onc.1209885. [DOI] [PubMed] [Google Scholar]
- Corradetti MN, Inoki K, Bardeesy N, DePinho RA, Guan KL. Regulation of the TSC pathway by LKB1: evidence of a molecular link between tuberous sclerosis complex and Peutz-Jeghers syndrome. Genes Dev. 2004;18:1533–1538. doi: 10.1101/gad.1199104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehan E, Bassermann F, Guardavaccaro D, Vasiliver-Shamis G, Cohen M, Lowes KN, Dustin M, Huang DC, Taunton J, Pagano M. betaTrCP- and Rsk1/2-mediated degradation of BimEL inhibits apoptosis. Mol Cell. 2009;33:109–116. doi: 10.1016/j.molcel.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies RJ, Joazeiro CA. RING domain E3 ubiquitin ligases. Annu Rev Biochem. 2009;78:399–434. doi: 10.1146/annurev.biochem.78.101807.093809. [DOI] [PubMed] [Google Scholar]
- Dorrello NV, Peschiaroli A, Guardavaccaro D, Colburn NH, Sherman NE, Pagano M. S6K1- and betaTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science. 2006;314:467–471. doi: 10.1126/science.1130276. [DOI] [PubMed] [Google Scholar]
- Efeyan A, Sabatini DM. mTOR and cancer: many loops in one pathway. Curr Opin Cell Biol. 2010;22:169–176. doi: 10.1016/j.ceb.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster H, Coley HM, Goumenou A, Pados G, Harvey A, Karteris E. Differential expression of mTOR signalling components in drug resistance in ovarian cancer. Anticancer Res. 2010;30:3529–3534. [PubMed] [Google Scholar]
- Frescas D, Pagano M. Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat Rev Cancer. 2008;8:438–449. doi: 10.1038/nrc2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Bowden TG, Normolle D, Sun Y. SAG/ROC2 E3 ligase regulates skin carcinogenesis by stage dependent targeting of c-Jun/AP1 and IkB/NF-kB. J Cell Biol. 2007;178:1009–1023. doi: 10.1083/jcb.200612067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Tan M, Sun Y. SAG/ROC2/Rbx2 is a novel activator protein-1 target that promotes c-Jun degradation and inhibits 12-O-tetradecanoylphorbol-13-acetate-induced neoplastic transformation. Cancer Res. 2007;67:3616–3625. doi: 10.1158/0008-5472.CAN-06-4020. [DOI] [PubMed] [Google Scholar]
- Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003a;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003b;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Jia L, Soengas MS, Sun Y. ROC1/RBX1 E3 ubiquitin ligase silencing suppresses tumor cell growth via sequential induction of G2-M arrest, apoptosis, and senescence. Cancer Res. 2009;69:4974–4982. doi: 10.1158/0008-5472.CAN-08-4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L, Yang J, Hao X, Zheng M, He H, Xiong X, Xu L, Sun Y. Validation of SAG/RBX2/ROC2 E3 Ubiquitin Ligase as an Anticancer and Radiosensitizing Target. Clin Cancer Res. 2010;16:814–824. doi: 10.1158/1078-0432.CCR-09-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Cardozo T, Lovering RC, Elledge SJ, Pagano M, Harper JW. Systematic analysis and nomenclature of mammalian F-box proteins. Genes Dev. 2004;18:2573–2580. doi: 10.1101/gad.1255304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen T, Lamark T. Selective autophagy mediated by autophagic adapter proteins. Autophagy. 2011;7 doi: 10.4161/auto.7.3.14487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Lett. 2010;584:1287–1295. doi: 10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Ichimura Y. Physiological significance of selective degradation of p62 by autophagy. FEBS Lett. 2010;584:1374–1378. doi: 10.1016/j.febslet.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5:726–734. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- Kudo Y, Guardavaccaro D, Santamaria PG, Koyama-Nasu R, Latres E, Bronson R, Yamasaki L, Pagano M. Role of F-box protein betaTrcp1 in mammary gland development and tumorigenesis. Mol Cell Biol. 2004;24:8184–8194. doi: 10.1128/MCB.24.18.8184-8194.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MN, Ha SH, Kim J, Koh A, Lee CS, Kim JH, Jeon H, Kim DH, Suh PG, Ryu SH. Glycolytic flux signals to mTOR through glyceraldehyde-3-phosphate dehydrogenase-mediated regulation of Rheb. Mol Cell Biol. 2009;29:3991–4001. doi: 10.1128/MCB.00165-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao JH, Kim IJ, Wu D, Climent J, Kang HC, DelRosario R, Balmain A. FBXW7 targets mTOR for degradation and cooperates with PTEN in tumor suppression. Science. 2008;321:1499–1502. doi: 10.1126/science.1162981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama KI, Nakayama K. Ubiquitin ligases: cell-cycle control and cancer. Nat Rev Cancer. 2006;6:369–381. doi: 10.1038/nrc1881. [DOI] [PubMed] [Google Scholar]
- Narita M, Young AR. Autophagy facilitates oncogene-induced senescence. Autophagy. 2009;5:1046–1047. doi: 10.4161/auto.5.7.9444. [DOI] [PubMed] [Google Scholar]
- Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E, Domingo D, Yahalom J. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001;61:439–444. [PubMed] [Google Scholar]
- Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, Gray NS, Sabatini DM. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137:873–886. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proud CG. Dynamic balancing: DEPTOR tips the scales. J Mol Cell Biol. 2009;1:61–63. doi: 10.1093/jmcb/mjp012. [DOI] [PubMed] [Google Scholar]
- Rajagopalan H, Jallepalli PV, Rago C, Velculescu VE, Kinzler KW, Vogelstein B, Lengauer C. Inactivation of hCDC4 can cause chromosomal instability. Nature. 2004;428:77–81. doi: 10.1038/nature02313. [DOI] [PubMed] [Google Scholar]
- Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- Sapkota GP, Cummings L, Newell FS, Armstrong C, Bain J, Frodin M, Grauert M, Hoffmann M, Schnapp G, Steegmaier M, et al. BI-D1870 is a specific inhibitor of the p90 RSK (ribosomal S6 kinase) isoforms in vitro and in vivo. Biochem J. 2007;401:29–38. doi: 10.1042/BJ20061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306:990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaar JR, D'Angiolella V, Pagan JK, Pagano M. SnapShot: F Box Proteins II. Cell. 2009;137:1358, 1358, e1351. doi: 10.1016/j.cell.2009.05.040. [DOI] [PubMed] [Google Scholar]
- Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S, Brownell JE, Burke KE, Cardin DP, Critchley S, et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009a;458:732–736. doi: 10.1038/nature07884. [DOI] [PubMed] [Google Scholar]
- Soucy TA, Smith PG, Rolfe M. Targeting NEDD8-activated cullin-RING ligases for the treatment of cancer. Clin Cancer Res. 2009b;15:3912–3916. doi: 10.1158/1078-0432.CCR-09-0343. [DOI] [PubMed] [Google Scholar]
- Swaroop M, Wang Y, Miller P, Duan H, Jatkoe T, Madore S, Sun Y. Yeast homolog of human SAG/ROC2/Rbx2/Hrt2 is essential for cell growth, but not for germination: Chip profiling implicates its role in cell cycle regulation. Oncogene. 2000;19:2855–2866. doi: 10.1038/sj.onc.1203635. [DOI] [PubMed] [Google Scholar]
- Tan M, Gallegos JR, Gu Q, Huang Y, Li J, Jin Y, Lu H, Sun Y. SAG/ROC-SCFbeta-TrCP E3 ubiquitin ligase promotes pro-caspase-3 degradation as a mechanism of apoptosis protection. Neoplasia. 2006;8:1042–1054. doi: 10.1593/neo.06568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009;284:8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucicevic L, Misirkic M, Kristina J, Vilimanovich U, Sudar E, Isenovic E, Prica M, Harhaji-Trajkovic L, Kravic-Stevovic T, Vladimir B, et al. Compound C induces protective autophagy in cancer cells through AMPK inhibition-independent blockade of Akt/mTOR pathway. Autophagy. 2010;7:40–50. doi: 10.4161/auto.7.1.13883. [DOI] [PubMed] [Google Scholar]
- Welcker M, Clurman BE. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer. 2008;8:83–93. doi: 10.1038/nrc2290. [DOI] [PubMed] [Google Scholar]
- Willems AR, Schwab M, Tyers M. A hitchhiker's guide to the cullin ubiquitin ligases: SCF and its kin. Biochim Biophys Acta. 2004;1695:133–170. doi: 10.1016/j.bbamcr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Winston JT, Strack P, Beer-Romero P, Chu CY, Elledge SJ, Harper JW. The SCFbeta-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IkappaBalpha and beta-catenin and stimulates IkappaBalpha ubiquitination in vitro. Genes Dev. 1999;13:270–283. doi: 10.1101/gad.13.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Padre RC, De Mendoza TH, Bottero V, Tergaonkar VB, Verma IM. Phosphorylation of p53 by IkappaB kinase 2 promotes its degradation by beta-TrCP. Proc Natl Acad Sci U S A. 2009;106:2629–2634. doi: 10.1073/pnas.0812256106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yada M, Hatakeyama S, Kamura T, Nishiyama M, Tsunematsu R, Imaki H, Ishida N, Okumura F, Nakayama K, Nakayama KI. Phosphorylation-dependent degradation of c-Myc is mediated by the F-box protein Fbw7. EMBO J. 2004;23:2116–2125. doi: 10.1038/sj.emboj.7600217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamoah K, Oashi T, Sarikas A, Gazdoiu S, Osman R, Pan ZQ. Autoinhibitory regulation of SCF-mediated ubiquitination by human cullin 1's C-terminal tail. Proc Natl Acad Sci U S A. 2008;105:12230–12235. doi: 10.1073/pnas.0806155105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, McPhee CK, Zheng L, Mardones GA, Rong Y, Peng J, Mi N, Zhao Y, Liu Z, Wan F, et al. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature. 2010;465:942–946. doi: 10.1038/nature09076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng N, Schulman BA, Song L, Miller JJ, Jeffrey PD, Wang P, Chu C, Koepp DM, Elledge SJ, Pagano M, et al. Structure of the Cul1-Rbx1-Skp1-F boxSkp2 SCF ubiquitin ligase complex. Nature. 2002;416:703–709. doi: 10.1038/416703a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.