Abstract
Enteroaggregative Escherichia coli (EAEC) strains are etiologic agents of acute and persistent diarrhea. In this study, the results of phenotypic assays suggested that EAEC strains possess specialized iron acquisition systems. Genes required for the synthesis (iucA) or transport (fepC) of siderophores, and genes encoding siderophore (fyuA, ireA, and iroN) or heme transport (chu) receptors or hemoglobin proteases (pic and hbp), were sought in EAEC strains which have been characterized with respect to known virulence genes and phylogeny. The chuA, iucA, fyuA, fepC, and pic genes were detected in 33, 76.2, 85.7, 33, and 61.9% of these EAEC strains, respectively, and the other genes were absent. The majority of EAEC strains possessed genes encoding multiple iron transport systems, and there was no phylogenetic correlation in the distribution of the majority of these loci, as is typical for EAEC. The notable exceptions were chuA and fepC (which is associated with the prrA-modA-fepC pathogenicity island); these genes were restricted to the EAEC2 and DAEC2 phylogenetic groups, which could represent pathogenic subsets. When collections of EAEC strains isolated during case-control studies in Nigeria and Brazil were examined, no association of the presence of either chuA or iucA alone with diarrhea was seen, but both genes together were present in significantly more strains from cases than from controls in the Nigerian collection (P < 0.05). It is possible that the presence of both genes marks at least some virulent strains. The data also demonstrate geographical variation in the association of iron utilization genes with disease in EAEC.
The pattern of mannose-resistant adherence to intestinal epithelial cells has been used to broadly classify Escherichia coli diarrheal isolates (31). The majority of nonpathogenic E. coli strains are nonadherent, while E. coli strains that adhere in a localized manner are almost invariably found to be enteropathogenic E. coli (EPEC). Diffuse adherence is seen in diffusely adherent E. coli (DAEC) and to varying degrees in other categories. Intestinal isolates of E. coli that adhere to epithelial cells in a characteristic stacked-brick pattern are categorized as enteroaggregative E. coli (EAEC) (31, 55). EAEC and DAEC strains are often recovered from healthy individuals, and the factors that dictate their adherence patterns differ among strains in each category. It is likely that at least some EAEC and DAEC strains are pathogenic while others are not. EAEC and DAEC remain enigmatic in some sense because their epidemiology and molecular pathogenesis are less well understood than those of other diarrhea-causing categories—EPEC and enterohemorrhagic, enterotoxigenic, and enteroinvasive E. coli (EHEC, ETEC, and EIEC, respectively). EAEC strains were originally recognized as predominant etiologic agents of persistent diarrhea in developing countries (5). In recent epidemiological studies, EAEC has been increasingly associated with acute as well as protracted diarrhea in several parts of the world (reviewed in references 32 and 35).
The EAEC category is heterogeneous, and during epidemiological studies EAEC strains are usually recovered from healthy as well as diseased subjects (5, 10, 33, 35). This observation led to initial doubts about their pathogenicity (32, 35) until consistent epidemiological associations with disease and human volunteer challenge studies unequivocally demonstrated that at least some EAEC strains are true diarrheagenic pathogens (23, 28). Baudry et al. (2) developed an empiric diagnostic probe, CVD 432, for identifying EAEC. The probe consisted of a 1-kb fragment of the virulence plasmid of EAEC strain 17-2. Subsequent investigation demonstrated that the probe showed a sensitivity of 18 to 90% in various studies, again illustrating the heterogeneity of this pathogen (35). In this study, following a prescreen with well-characterized EAEC strains from around the world (10), we employed two epidemiological collections to evaluate the significance of selected genes in disease. Seventy-two percent of the EAEC strains from Brazil (45-47) and only 26% of the Nigerian isolates (33) were CVD 432 positive.
Multilocus enzyme electrophoretic (MLEE) patterns have been a useful tool for classifying E. coli isolates from diarrhea. For many diarrheagenic E. coli categories, notably EPEC and EHEC, MLEE-derived phylogeny has correlated with virulence gene distribution and provided clues about pathogen evolution (11). With EAEC, however, the distribution of previously identified virulence factors has not correlated with MLEE phylogeny (10). Although three major EAEC phylogenetic groups—EAEC1, EAEC2, and AA/DA (aggregative adherence/diffuse adherence)—have been identified based on this methodology, EAEC phylogeny overlaps with that of the (also heterogeneous) DAEC group, in which the DAEC1, DAEC2, and (to a lesser extent) AA/DA categories predominate. Furthermore, no previously described EAEC-related virulence factor is strictly distributed in accordance with this phylogenetic classification (10).
The virulence of EAEC, like that of other pathogens, is known to require a variety of virulence factors, but many of these have yet to be described (32, 35). During infection, EAEC strains have been shown to adhere closely to epithelial cells within a mucoid matrix as a mechanism to evade host defenses, digestive enzymes, and peristalsis and to compete for this niche with other bacterial species (29, 54). The ability of EAEC to obtain essential nutrients and multiply successfully in this environment is crucial. One essential nutrient for bacterial growth is iron, which is not readily available in human hosts, because intracellularly it is found mostly as heme or associated with storage proteins and extracellularly it is bound to transferrin and lactoferrin (38). Therefore, pathogenic microorganisms, such as EAEC, attempting to establish an infection must have the ability to scavenge iron and multiply within the host environment as a fundamental requirement for the production of disease.
Pathogenic E. coli strains have developed various strategies for acquiring iron, the most common of which involves the production of siderophores (41). Siderophores are low-molecular-weight, specific ferric ion-binding chelators produced by many members of the family Enterobacteriaceae. Enterobactin, the prototypical siderophore, is produced by all E. coli strains and is commonly found in clinical isolates of enterobacteriaceae; its biosynthesis involves the products of at least 14 chromosomally encoded genes, including the fep genes. One of these genes, fepC, encodes the ferric enterobactin transport ATP-binding protein. Guyer et al. (13) found that a second fepC gene (80% identical over 59% of the gene) was present within a pathogenicity island of uropathogenic E. coli strain CFT703. Ye and Xu (63) were subsequently able to demonstrate that part of this island, including the fepC homologue, was present in E. coli O157 strains. The reason behind the duplication is not known, and there is no evidence to suggest that the second fepC gene contributes to pathogenicity. Although adequate production of siderophores in vivo is one mechanism postulated to enhance the virulence of enteric bacteria, no evidence supporting the role of enterobactin production as a virulence determinant has been found (reviewed in reference 59).
Many E. coli strains also produce a second, unrelated siderophore known as aerobactin (41). The genes responsible for aerobactin synthesis are either located on plasmids or form part of a pathogenicity island (27, 57). A third siderophore found in E. coli strains is yersiniabactin (50). Yersiniabactin synthesis and uptake genes were originally described in Yersinia species and are encoded within the “high-pathogenicity island” (HPI), which is present in most EAEC strains. Aerobactin and yersiniabactin genes are commonly found in pathogenic E. coli strains, and both have been shown to be associated with virulence in extraintestinal E. coli (22, 49, 53, 61). More recently, the ireA gene, which may be involved in siderophore transport (42), and the iroN gene, encoding a siderophore receptor that contributes to urovirulence (43, 44), have been described in extraintestinal E. coli. iroN is also present in Salmonella enterica strains (4, 39).
Bacterial strains can also acquire iron from the host through systems that bind heme and hemoproteins and then transport heme into the cell (reviewed in reference 9). One such mechanism requires outer membrane proteins that recognize the heme compounds and bind heme to the bacterial cell surface. Alternately, a second mechanism, the hemophore-dependent system, involves the binding of heme to a secreted bacterial protein that shuttles it back to a specific outer membrane receptor. In E. coli, there are two well-characterized heme transport systems; the Chu (Shu in Shigella dysenteriae) heme transport system and the Hbp hemophore-dependent system (36, 52, 62). Neither has been reported in EAEC previously, and chuA has recently been shown to be important for the virulence of a uropathogenic E. coli strain (53). Recently, Hernandez et al. (U. Hernandez, J. M. Villaseca, J. Molina, and C. Eslava, Abstr. 102nd Gen. Meet. Am. Soc. Microbiol., abstr. B-13, p. 34, 2002) demonstrated that Pic, a multifunctional autotransporter protease (15), was capable of proteolyzing hemoglobin, thus hypothesizing a role for this protein in iron acquisition. The pic gene lies within a pathogenicity island found in Shigella and EAEC (40).
In this study, we screened multiple EAEC strains, which have previously been characterized with respect to known virulence genes and phylogeny, for the presence of siderophore-dependent or heme transport systems. Our results indicate that most EAEC strains contain more than one iron transport system and that the distribution of two systems correlates with phylogeny. They also suggest that iron utilization may be important for the pathogenesis of EAEC in some epidemiological settings.
MATERIALS AND METHODS
Bacterial strains.
EAEC strains and other diarrheagenic E. coli strains from previously characterized strain collections were employed in this study. The first, or reference, collection comprised 21 EAEC and three coclustering DAEC strains. These strains have been phylogenetically classified by MLEE and probed for several virulence genes (10). At the start of the study, we confirmed the distribution of three loci—the AAF/I structural subunit gene (aagA), the HPI-encoded yersiniabactin gene (irp2), and the overlapping Shigella enterotoxin 1/Pic mucinase genes (she/pic)—in this collection, as well as confirming the adherence phenotype by the HEp-2 adherence assay using a previously described protocol (34). The ability of the reference strains to lyse erythrocytes on blood agar plates was also tested.
A second collection comprised 131 EAEC isolates from a Nigerian case-control study (33), and a third collection comprised 110 strains from Brazilian children with diarrhea and healthy controls (45-47). Ninety-eight strains belonging to other noninvasive diarrheagenic E. coli categories, including 40 EPEC, 25 ETEC, 13 nonphylogenetically classified DAEC, and 16 Shiga toxin-producing strains, were also evaluated in this study. These isolates were obtained from a recent epidemiological study (34) and archival stocks from the Center for Vaccine Development, University of Maryland. EHEC strain EDL933, uropathogenic E. coli strains 536 and CFT073 (20), Shigella flexneri 2a 2457T, and Shigella boydii 2954-72 were used as positive controls for phenotypic and PCR tests, and E. coli K-12 MG1655 (7) was used as a negative or baseline control.
Growth of EAEC strains in the absence of free iron and measurement of siderophore production.
The abilities of reference EAEC strains to utilize heme and hemoglobin as sole iron sources were evaluated by surface inoculation of T-medium plates (37) containing the restricted iron source and 2,2′-dipyridyl. Siderophore production in T-medium was measured by the liquid chrome-azurol S (CAS) assay (37). (This assay measures the abilities of catechol, hydoxamate, and potentially other siderophores secreted into the growth medium to remove iron from a chromogenic dye complex.) Uninoculated T-medium was employed as a blank, and a reference reading (Ar) was taken by measuring the iron affinity of the blank with test reagents. Test readings (As) for each strain were taken in triplicate, with dilution when necessary. Siderophore units were calculated as percentages by using the formula (Ar − As)/Ar × 100 (37). E. coli K-12 MG1655 (which produces only enterobactin) served as a baseline control, RW193 (an enterobactin mutant that produces no siderophores; kindly supplied by Charles Earhart) was used as a negative control, and pathogenic E. coli strains known to produce a range of siderophores were used as positive controls. T-medium was used as the zero reference.
Detection of genes encoding iron utilization systems.
The phylogenetically characterized reference collection was screened for a total of nine genes, representing separate iron utilization systems, by PCR and hybridization. Two of these, irp2 and pic (she), had been tested previously by Czeczulin et al. (10). Recombinant Taq polymerase and a PCR buffer from Gibco-BRL-Invitrogen were employed with 1 U of Taq polymerase, 2 mM MgCl2, and 1 μM oligonucleotide primer in each reaction. All amplifications began with a 2-min hot start at 94°C, followed by 30 cycles of denaturing at 94°C for 30 s, annealing for 30 s, and extension at 72°C. PCRs were templated with boiled bacterial colonies. Primers, annealing temperatures, extension times, and positive-control strains used for each amplification are given in Table 1.
TABLE 1.
Target genes, primers, and cycling conditions used to detect iron utilization systems
| Target gene | Description | F and Ra (5′→3′) | Amplicon size (kb) | Positive-control strain(s) used in this study | Annealing temp, extension time | Reference for PCR protocol |
|---|---|---|---|---|---|---|
| fyuA | Encodes the outer membrane Fe-yersiniabactin/pesticin receptor FyuA | F = GCGAC GGGAAGCGATTTA | 0.78 | EAEC 17-2 | 57°C, 1 min | 50 |
| R = CGCAGTAGGCACGATGTTGTA | ||||||
| iucA | Gene product is a synthetase involved in the modification of hydroxylysine during aerobactin synthesis | F = AGT CTG CAT CTT AAC CTT CA | 1.1 | S. flexneri 2457T and S. boydii 2954-72 | 52°C, 1.5 min | This study |
| R = CTC GTT ATG ATC GTT CAG AT | ||||||
| fepC (PAI encoded) | Homologue of the generic fepC gene, which encodes an ATP-binding component in the cytoplasmic membrane complex for ferric enterobactin transport | F = TACCTGGATAATGCTGTCGG | 0.35 | EHEC EDL 933 | 60°C, 30 s | 63 |
| R = ATGGTGTTGATGGGGCTG GC | ||||||
| chuA/shuA | Encodes the heme transport outer membrane receptor | F = ATC TGC TGC GTC ATG TTC CT | 1.7 | EDL933 (chuA) and S. flexneri 2457T (shuA) | 52°C, 1.5 min | This study |
| R = GTA GTG GTC ATA CCT TTG AGC | ||||||
| ireA | Encodes a putative siderophore receptor | F = TGGTCTTCAGCTATATGG | 0.4 | UPECb CFT073 | 57°C, 1 min | 42 |
| R = ATCTATGATTGTGTTGGT | ||||||
| iroN | Encodes a siderophore receptor protein in S. enterica and uropathogenic E. coli | F = AAGTCAAAGCAGGGGTTGCCCG | 0.67 | UPEC CFT073 | 60°C, 45 s | 19, 44 |
| R = GACGCCGACATTAAGACGCAG | ||||||
| hbp | Encodes an autotransporter hemoglobin protease | F = CTGACCTGACTCTTCAGAAT | 0.78 | UPEC 536 | 55°C, 45 s | 36 |
| R = GGTCTGCTGACGCATCTGTGA | ||||||
| pic | Encodes a multifunctional autotransporter hemoglobin protease | F = GGGTATTGTCCGTTCCGAT | 1.18 | S. flexneri 2457T | 55°C, 1 min | 10 |
| R = ACAACGATACCGTCTCCCG |
F, forward primer; R, reverse primer.
UPEC, uropathogenic E. coli.
Colony hybridization with digoxigenin-labeled probes was performed to validate the PCR results. Colony lifts of test and control strains cultured in brain heart infusion medium (Oxoid, Basingstoke, England) were prepared in a 96-well format on nylon membranes (Hybond-N; Amersham Biosciences). Membranes were denatured in 0.5 M NaOH-1.5 M NaCl, neutralized in 1.5 M NaCl-0.5 M Tris HCl-1 mM EDTA, dried, and fixed by UV exposure. DNA probes were prepared by PCR using the primers listed in Table 1 with positive-control strains as templates. They were labeled by using the PCR DIG labeling mix (Roche), according to the manufacturer's instructions. Following 2 h of prehybridization at 42°C, the membranes were hybridized with a denatured probe at 42°C, with continuous, gentle agitation in a hybridization solution containing 50% formamide, 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 5% blocking reagent, 0.1% N-lauryl sarcosine, and 0.02% sodium dodecyl sulfate (SDS). Membranes were washed three times in 2× SSC-0.1% SDS and then three times in 0.1× SSC-0.1% SDS. Signals were detected by using the DIG nucleic acid detection kit (Roche) in accordance with the manufacturer's instructions.
Statistical analysis.
The significance of differences observed in the prevalence of target DNA among strains from patients with diarrhea and healthy controls was assessed by the chi-square test and Fisher's exact test.
RESULTS
Growth under iron-limited conditions and siderophore expression.
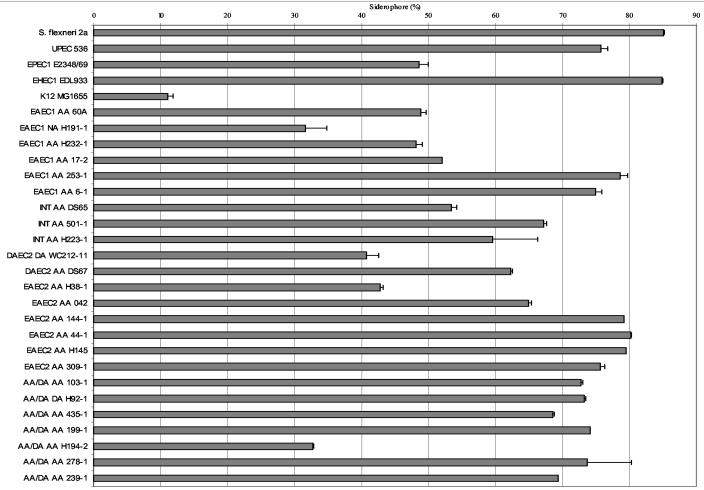
As shown in Table 2, 19 (90.5%) of the EAEC reference strains were capable of growth utilizing heme and hemoglobin as sole iron sources. In comparison, Table 3 shows that only 42 (47.2%) of E. coli strains belonging to other diarrheagenic, noninvasive categories were able to grow under these conditions, and 20 of these were attaching-and-effacing E. coli (EPEC and EHEC) strains, which adhere intimately during infection. These data suggest that the ability to sequester iron may be essential for pathogens that adhere in close proximity to the intestinal mucosa and that EAEC strains possess specialized iron recruitment systems. The results of the CAS assay (Fig. 1) demonstrate that total siderophore production by all the EAEC strains was greater than that for the baseline control, MG1655. Seventy-eight percent of the strains expressed siderophores within ranges approaching or comparable to those of control strains EHEC O157 EDL933, uropathogenic E. coli 536, and S. flexneri and greater than that of EPEC strain E2348/69 (Fig. 1). Only two EAEC strains, AA H38-1 (EAEC1) and AA H194-2 (AA/DA), were relatively deficient in siderophore production (though both still produced significantly greater levels of siderophores than the baseline control, MG1655), and these strains demonstrated a corresponding inability to utilize host-bound assay sources. Only three of the phylogenetically characterized strains produced siderophores below the level seen with EPEC, and two of the three were non-EAEC strains (NA H191 and DA WC192-11). Within the EAEC category, there were variations associated with phylogenetic group, although these were not statistically significant. The mean siderophore percentage for the six strains in the EAEC2 category, which demonstrated the highest levels of siderophore production, was 77.37%. For the six EAEC1 and seven AA/DA strains, the respective values were 55.67 and 66.33%. (For comparison, the 23 ETEC strains gave a mean siderophore value of 52.41%.) Although hemolytic activity has been proposed as a virulence property of EAEC (12), it was seen in only five (23.8%) of the EAEC reference strains.
TABLE 2.
Iron uptake and utilization genes in EAEC strains that have been phylogenetically classified
| Strain | Adherence pattern on HEp-2 cells | MLEE-determined phylogenetic groupa | Country of isolation | Reaction to CVD 432 probeb,c | Hemolysis on blood agar | Heme utilization | Hemoglobin utilization | FeCl 3 utilization | fepC (PAI)d | chuA | iucA | irp2c | fyuA | ireA | iroN | hbp | picc |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA 60A | Aggregative | EAEC1 | Mexico | + | − | + | + | + | − | − | + | + | + | − | − | − | + |
| NA H191-1 | Weak diffusee | EAEC1 | Peru | − | − | + | + | + | − | − | + | − | − | − | − | − | + |
| AA H232-1 | Aggregative-detaching | EAEC1 | Peru | + | − | + | + | + | − | − | + | + | + | − | − | − | + |
| AA 17-2 | Aggregative-detaching | EAEC1 | Chile | + | + | + | + | + | − | − | + | + | + | − | − | − | − |
| AA 253-1 | Aggregative | EAEC1 | Thailand | + | − | + | + | + | − | − | + | + | + | − | − | − | − |
| AA 6-1 | Aggregative | EAEC1 | Thailand | + | + | + | + | + | − | − | + | + | + | − | − | − | − |
| AA DS65-R2 | Weak localized-aggregative | INT1 | Philippines | − | − | + | + | + | − | − | − | − | − | − | − | − | − |
| AA 501-1 | Aggregative | INT2 | Thailand | − | + | + | + | + | − | − | − | − | − | − | − | − | − |
| AA H223-1 | Aggregative | INT2 | Peru | + | − | + | + | + | − | − | + | − | + | − | − | − | − |
| DA WC212-11 | Strong diffusee | DAEC2 | Thailand | − | + | + | + | + | + | + | + | + | − | + | − | − | − |
| AA DS67-R2 | Aggregative | DAEC2 | Philippines | + | − | + | + | + | + | + | + | − | − | − | − | − | − |
| AA H38-1 | Aggregative | EAEC2 | Peru | + | − | − | − | + | + | + | + | + | + | − | − | − | − |
| AA 042 | Aggregative | EAEC2 | Peru | + | − | + | + | + | + | + | − | + | + | − | − | − | + |
| AA 144-1 | Aggregative-detaching | EAEC2 | Thailand | + | + | + | + | + | + | + | + | + | + | − | − | − | − |
| AA 44-1 | Aggregative | EAEC2 | Thailand | + | + | + | + | + | + | + | + | + | + | − | − | − | + |
| AA H145-1 | Aggregative | EAEC2 | Peru | + | − | + | + | + | + | + | + | + | + | − | − | − | + |
| AA 309-1 | Aggregative | EAEC2 | Thailand | + | − | + | + | + | + | + | − | + | + | − | − | − | + |
| AA 103-1 | Aggregative | AA/DA | Thailand | + | − | + | + | + | − | − | + | + | + | − | − | − | − |
| DA H92-1 | Strong diffusee | AA/DA | Peru | − | − | + | + | + | − | − | − | − | + | − | − | − | − |
| AA 435-1 | Aggregative | AA/DA | Thailand | + | − | + | + | + | − | − | + | + | + | − | − | − | + |
| AA 199-1 | Aggregative | AA/DA | Thailand | + | − | + | + | + | − | − | − | + | − | − | − | − | + |
| AA H194-2 | Aggregative | AA/DA | Peru | + | − | − | − | + | − | − | + | + | + | − | − | − | + |
| AA 278-1 | Aggregative | AA/DA | Thailand | + | − | + | + | + | − | − | + | + | + | − | − | − | + |
| AA 239-1 | Aggregative | AA/DA | Thailand | + | − | + | + | + | − | − | + | + | + | − | − | − | + |
EAEC1 and EAEC2, MLEE-defined clusters composed largely of EAEC; DAEC2; one of two clusters composed largely of DAEC; AA/DA, cluster containing both EAEC and DAEC; INT, intercluster group.
CVD 432, empiric probe for the EAEC virulence plasmid (2). Not all EAEC strains are detected by this probe (29).
Data from Czeczulin et al. (10).
PAI, pathogenicity island.
Three strains that did not show classic aggregative adherence were included because they cluster with EAEC strains and share EAEC-associated genes.
TABLE 3.
Numbers and percentages of noninvasive diarrheagenic E. coli strains utilizing heme and hemoglobin and possessing iron uptake genes
| Category and subgroup | No. tested | No. (%) of strains showing:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Heme utilization | Hemoglobin utilization | FeCl 3 utilization | chuA | iucA | fepC (PAI)a | ireA | iroN | hbp | ||
| EAEC (reference strains) | 21 | 19 (90.5) | 19 (90.5) | 21 (100) | 7 (33.3) | 16 (76.2) | 7 (33.3) | 0 (0) | 0 (0) | 0 (0) |
| EHEC and other Shiga toxin- producing E. coli strains | ||||||||||
| O157 | 3 | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 0 (0) | 3 (100) | 0 (0) | 0 (0) | 0 (0) |
| Non-O157 | 13 | 3 (23.1) | 10 (7.69) | 13 (100) | 0 (0) | 6 (46.2) | 0 (0) | 1 (7.7) | 0 (0) | 0 (0) |
| EPEC | ||||||||||
| EPEC1 | 18 | 11 (61.11) | 17 (94.4) | 18 (100) | 17 (94.4) | 1 (5.6) | 15 (83.3) | 0 (0) | 0 (0) | 0 (0) |
| EPEC2 | 12 | 3 (25.0) | 11 (91.7) | 12 (100) | 1 (8.3) | 0 (0) | 1 (8.3) | 0 (0) | 0 (0) | 0 (0) |
| Non-EPEC, non-EHEC attaching and effacing E. colib | 4 | 0 (0) | 4 (100) | 4 (100) | 0 (0) | 1 (25.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| ETEC | 23 | 20 (87.0) | 21 (91.3) | 23 (100) | 0 (0) | 0 (0) | 9 (39.1) | 0 (0) | 4 (17.4) | 0 (0) |
| DAEC | 16 | 4 (25.0) | 10 (62.5) | 16 (100) | 1 (6.3) | 2 (12.5) | 1 (6.3) | 4 (25.0) | 4 (25.0) | 5 (31.3) |
PAI, pathogenicity island.
One strain each of porcine, rabbit, canine, and human origin.
FIG. 1.
Siderophore production by reference strains belonging to the EAEC1, EAEC2, AA/DA, and other (DAEC2 and intergroup) branches of the tree generated by Czeczulin et al. (10). UPEC, uropathogenic E. coli.
Iron utilization genes in EAEC reference strains.
All but two of the reference strains were positive for at least one of the iron utilization genes sought, and the majority (85.8%) of EAEC reference strains harbored genes for multiple systems. As shown in Table 2, the yersiniabactin (fyuA) genes were the most highly prevalent iron utilization systems in EAEC: they were present in 85.7% of the reference strains. The CFT703/O157 pathogenicity island-encoded fepC gene was present in 33% of the strains, as determined by PCR. One hundred percent of the strains hybridized to the fepC probe, presumably due to cross-reaction with the native fepC gene, which is present in all E. coli strains. The chuA, iucA, and pic genes were present in 33, 76.2, and 61.9% of the strains, respectively. iroN, ireA, and hbp, all of which are associated with extraintestinal E. coli, were not found in EAEC, but ireA was detected in coclustering DAEC strains. The iron utilization systems sought in this study were much less common in ETEC, and the range of systems in other classes of diarrheagenic E. coli was less diverse than that for EAEC (Table 3). Of note was the restriction of chuA and the CFT703/O157 pathogenicity island-encoded fepC to the EAEC2 and DAEC2 phylogenetic groups. These two genes are the first loci that have been shown to be restricted to any EAEC phylogenetic group. Among EPEC strains, chuA and the pathogenicity island-encoded gene fepC were restricted to the MLEE-defined phylogenetic group EPEC1; among EHEC strains, they were restricted to O157 strains, which belong to phylogenetic group EHEC1. We did not find an association between the presence of any of the iron utilization loci sought and previously described EAEC virulence genes such as those encoding the AAF/I fimbrial subunit (aafA), the AAF/II fimbrial subunit (aagA), plasmid-encoded enterotoxin (pet), dispersin (aap), aggregative regulator (aggR), or the empiric probe locus (CVD 432) (35, 51).
Screening EAEC collections from case-control studies by a duplex PCR for chuA and iucA.
Of the nine loci for which the reference collection was screened, only fepC (pathogenicity island encoded), iucA, chuA, and the HPI-encoded irp2 and fyuA loci were present in more than one reference strain. The generic enterobactin system is documented to be present in all E. coli strains (commensal and pathogenic), and although uropathogenic E. coli and E. coli O157 have been shown to possess a second fepC gene (13, 63), FepC has not been found associated with virulence. The HPI genes (irp2 and fyuA) have been studied extensively in EAEC (21, 22, 48, 50, 58). Both chuA and iucA were present in a significant proportion of EAEC reference strains and have been demonstrated to play a potential role in virulence in pathogenic E. coli strains belonging to other categories (24, 25, 52, 53, 60). We therefore sought to determine the role, if any, played by these genes in EAEC virulence. In the absence of a suitable disease model for EAEC, we developed a duplex PCR protocol for simultaneously screening strains for chuA and iucA in order to evaluate the importance of these genes in EAEC pathogenicity by molecular epidemiologic methods. This protocol was used to screen EAEC strains from two sets derived from case-control studies. In each case, we found that neither gene was significantly more common in isolates from children with diarrhea than in isolates from controls. However, EAEC strains that carried both chuA and iucA were more common in Nigerian children with diarrhea than in matched controls (P = 0.03) (Table 4). In the strains from Brazilian children, no significant differences between isolates from patients and isolates from controls were observed for any of the genes or for the combination.
TABLE 4.
Distribution of chuA and iucA genes in EAEC isolates from children with diarrhea and apparently healthy controls
| Probe or gene(s) | No. (%) of positive isolates
|
||
|---|---|---|---|
| From children with diarrhea | From controls | Total | |
| Nigerian EAEC isolatesa | |||
| CVD 432 probe | 20 (27.4) | 14 (24.1) | 34 (26.0) |
| chuA | 26 (35.6) | 19 (32.8) | 45 (34.4) |
| iucA | 32 (43.8) | 28 (48.3) | 60 (45.8) |
| chuA and iucAb | 25 (34.2) | 10 (17.2) | 35 (26.7) |
| Brazilian EAEC isolatesc | |||
| CVD 432 probe | 49 (75.4) | 31 (68.9) | 80 (72.7) |
| chuA | 22 (33.9) | 15 (33.3) | 37 (33.6) |
| iucA | 30 (46.2) | 27 (60.0) | 57 (51.8) |
| chuA and iucA | 12 (18.5) | 13 (28.9) | 25 (27.7) |
Comprising 73 isolates from children with diarrhea and 58 from controls, for a total of 131.
Differences between isolates from children with diarrhea and those from controls were significant (P < 0.03).
Comprising 65 isolates from children with diarrhea and 45 from controls, for a total of 110.
DISCUSSION
The iron utilization systems of invasive pathogens such as Shigella, EIEC, and E. coli that causes extraintestinal infections have been extensively investigated. Relatively few studies have examined other diarrhea-causing E. coli strains, and very little has been reported about iron utilization systems in EAEC. In this study we determined that many EAEC strains are able to utilize heme or hemoglobin as their sole iron source and are capable of siderophore production comparable to that of Shigella spp., EHEC, and uropathogenic E. coli. Furthermore, EAEC strains are more likely to produce high levels of siderophores than other noninvasive diarrhea-causing E. coli strains, and most EAEC strains possess genes associated with multiple iron utilization systems.
As has been reported previously (10, 50), the fyuA and irp2 genes, found within the Yersinia HPI, were present in the majority (85.7%) of the EAEC reference strains. The iucA gene, which is involved in the synthesis of the siderophore aerobactin and was originally detected in Shigella, was found in 76.2% of EAEC strains. It therefore appears that the genes for aerobactin and yersinabactin siderophore production are widely distributed among EAEC strains. This provides a plausible explanation for the high levels of siderophore activity detected in the majority of the strains. However, we cannot rule out the possibility that EAEC strains are only secreting enterobactin. Additional experiments to identify the nature of these siderophores are in progress in our laboratory. Headley et al. (14) observed that Shigella spp. synthesize aerobactin genes in the extracellular milieu but not intracellularly. The high prevalence of these genes in EAEC strains, generally considered to be extracellular pathogens, fits the current model for their pathogenicity.
Wyckoff et al. (62) examined EHEC, EPEC, EIEC, ETEC, and extraintestinal E. coli for the presence of the shuA gene. They found that shuA (chuA in EHEC O157:H7) was not restricted to Shigella but was also present in the related EIEC strains as well as in hypervirulent phylogenetic groups within the EPEC and EHEC categories (62), a feature we also observed in this study. In the EPEC category, shuA/chuA is restricted to EPEC1 strains, which show a greater association with outbreaks than the chuA-negative EPEC2 strains. Similarly, in the EHEC category, EHEC1, which includes serogroup O157, is more likely to cause life-threatening hemorrhagic uremic syndrome than chuA-negative EHEC2 strains (30, 52, 62). It therefore appears that the presence of the chuA gene may be critical for hypervirulence. However, it must be mentioned that in both these cases, the hypervirulent categories appear to possess a more sophisticated array of potential virulence factors than their chuA-negative counterparts, and therefore we cannot rule out the possibility that chuA merely serves as a useful marker for pathogenic phylogenetic groups. MLEE-classified phylogenetic groups of other diarrheagenic E. coli strains have correlated with virulence gene distribution and provided a framework for evolutionary hypotheses. A previous screen for eight loci in EAEC did not identify any gene whose presence was universal or restricted to specific phylogenetic groups (10). In this study, using strains from that collection, we identified two loci, chuA and the pathogenicity island-encoded gene fepC, which were present in all strains belonging to the EAEC2 and DAEC2 categories and absent in other EAEC strains. These appear to be the first genes whose presence in EAEC strains correlates with MLEE-derived phylogeny. The detection of these genes solely in the DAEC2 and EAEC2 categories suggests that these categories may comprise hypervirulent strains. Interestingly, strains in these categories produced the highest levels of siderophores. In support of this hypothesis was the finding that EAEC2 strain 042 produced diarrhea in adult volunteers, whereas EAEC1 strains JM221 and 17-2 and AA/DA strain 34b were nonpathogenic in the same study (28).
Examination of the reference and case-control EAEC isolates revealed that the iucA gene is widespread among EAEC strains, although its presence does not appear to correlate with isolation from diarrhea patients. Following the identification of the Shiga toxins, Shigella enterotoxins 1 and 2 as well as the pic pathogenicity island (16, 26, 56), the identification of this gene among EAEC strains suggests that virulence loci commonly associated with Shigella are common features of EAEC strains. EAEC strains also share virulence genes with other enteric pathogens. The previously detected HPI (originally described in Yersina spp.) and the chuA/shuA gene of Shigella and EHEC were found in this study and further attest to the propensity for EAEC strains to harbor horizontally acquired genes.
This study indicated that most EAEC strains carry genes for multiple iron utilization systems and that this is relatively uncommon in EPEC, ETEC, and DAEC strains. Multiple iron utilization systems are a common feature of EHEC and Shigella. EAEC strains were shown to share many iron utilization genes with these organisms but were less likely to harbor ireA, iroN, and hbp, systems found in extraintestinal E. coli (3, 19, 36, 42, 43). Surprisingly, these genes were relatively common among DAEC strains, which are at least as heterogeneous as EAEC strains, and this finding opens up the possibility that at least some of them are potential extraintestinal pathogens. A recent report identified the shuA/chuA and iucB genes in C1845, a DAEC2 strain (6). These genes were detected in WC212-11, the DAEC2 diffusely adherent strain used in this study (Table 2), but not in any other DAEC strain (Table 4). It is likely that a molecular epidemiological study of iron utilization genes in phylogenetically characterized DAEC strains could shed more light on the heterogeneity and pathogenesis of this category.
Human volunteer studies have confirmed that not all EAEC strains cause diarrhea and that there is some host variation in susceptibility to pathogenic EAEC. Molecular epidemiology may therefore be a useful tool for evaluating the pathogenicity of EAEC strains and has been used to identify the aafA gene, which encodes the structural subunit of AAF/II fimbriae, as a putative marker for potentially pathogenic strains (8, 33). In this study, we employed strain collections obtained during case-control studies. Because geographical variation in the distribution of virulence genes has been reported previously for EAEC (1), we examined collections of strains from two widely separated geographical areas where EAEC is highly prevalent. Although strains from both collections were identified as EAEC by using the HEp-2 adherence assay, the two collections were dissimilar in the proportion of strains positive for CVD 432, an empirically derived probe for the aggregative prototype plasmid (2), to which only a subset of EAEC strains hybridize (35). The Brazilian collection comprised chiefly strains that hybridized to CVD 432 (72.7% were probe positive) (45-47), and the majority of the Nigerian strains (74%) were negative for this probe (33). In neither collection were probe-positive strains associated with diarrheal disease. CVD 432 probe positivity also was not associated with the presence of chuA or iucA in the reference or case-control study collections. We specifically sought to study the distribution of chuA and iucA, two genes that were common in the reference collection, that were reputed to play a role in the virulence of other pathogenic E. coli strains, and whose presence in EAEC had not previously been reported. We found, in both the Brazilian and Nigerian collections, that neither chuA nor iucA alone was significantly associated with disease. The proportions of strains carrying both iucA and chuA were similar in the two collections. However, the presence of both genes was observed more frequently in strains from children with diarrhea than in strains from controls for Nigerian (P < 0.03) but not Brazilian isolates, an observation that provides further evidence of the geographical heterogeneity of EAEC pathogenicity. Although differences in strains from different areas offer one explanation, recent findings suggest that variations in host susceptibility may play an important role (17).
The close association of chuA with the EAEC2 and DAEC2 phylogenetic groups in the reference collection is suggestive of an involvement of similar strains in EAEC-induced diarrhea in Nigeria, but not in Brazil, where the distribution of chuA and iucA among controls was inverse to the results from the Nigerian collection. Another, but not contradictory, explanation of the data from the Nigerian strains is that strains with both ChuA and aerobactin systems are likely to be more virulent, at least in that population; this hypothesis should be further investigated. Such a hypothesis is supported by the observation of Torres and Payne (52), who in working with the uropathogenic strain CFT703 found that neither the iucA- nor the chuA-associated system alone correlated with pathogenicity in mice. However, multiple iron utilization systems collectively confer a competitive advantage over E. coli strains that are negative for these factors. Multiple iron utilization systems are frequently seen in uropathogenic and other invasive E. coli strains (18). It is possible that such an advantage would exist for EAEC strains closely associated with the host mucosa, where the supply of iron would be limited.
Acknowledgments
We are grateful to James P. Nataro and Charles Earhart for strains, to James B. Kaper for mentorship, and to Gavin Atkinson and Joanna Carder for technical support.
A.G.T. was supported by research supplements for underrepresented minorities from the NIDDK, NIH, and by startup funds from UTMB. I.N.O. received career development support from the University of Bradford.
REFERENCES
- 1.Adachi, J. A., Z. D. Jiang, J. J. Mathewson, M. P. Verenkar, S. Thompson, F. Martinez-Sandoval, R. Steffen, C. D. Ericsson, and H. L. DuPont. 2001. Enteroaggregative Escherichia coli as a major etiologic agent in traveler's diarrhea in 3 regions of the world. Clin. Infect. Dis. 32:1706-1709. [DOI] [PubMed] [Google Scholar]
- 2.Baudry, B., S. J. Savarino, P. Vial, J. B. Kaper, and M. M. Levine. 1990. A sensitive and specific DNA probe to identify enteroaggregative Escherichia coli, a recently discovered diarrheal pathogen. J. Infect. Dis. 161:1249-1251. [DOI] [PubMed] [Google Scholar]
- 3.Bauer, R. J., L. Zhang, B. Foxman, A. Siitonen, M. E. Jantunen, H. Saxen, and C. F. Marrs. 2002. Molecular epidemiology of 3 putative virulence genes for Escherichia coli urinary tract infection—usp, iha, and iroNE. coli. J. Infect. Dis. 185:1521-1524. [DOI] [PubMed] [Google Scholar]
- 4.Baumler, A. J., T. L. Norris, T. Lasco, W. Voight, R. Reissbrodt, W. Rabsch, and F. Heffron. 1998. IroN, a novel outer membrane siderophore receptor characteristic of Salmonella enterica. J. Bacteriol. 180:1446-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhan, M. K., P. Raj, M. M. Levine, J. B. Kaper, N. Bhandari, R. Srivastava, R. Kumar, and S. Sazawal. 1989. Enteroaggregative Escherichia coli associated with persistent diarrhea in a cohort of rural children in India. J. Infect. Dis. 159:1061-1064. [DOI] [PubMed] [Google Scholar]
- 6.Blanc-Potard, A. B., C. Tinsley, I. Scaletsky, C. Le Bouguenec, J. Guignot, A. L. Servin, X. Nassif, and M. F. Bernet-Camard. 2002. Representational difference analysis between Afa/Dr diffusely adhering Escherichia coli and nonpathogenic E. coli K-12. Infect. Immun. 70:5503-5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blattner, F., G. R. Plunkett, C. Bloch, N. Perna, V. Burland, M. Riley, J. Collado-Vides, J. Glasner, C. Rode, G. Mayhew, J. Gregor, N. Davis, H. Kirkpatrick, M. Goeden, D. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 8.Bouzari, S., A. Jafari, A. Azizi, M. Oloomi, and J. P. Nataro. 2001. Characterization of enteroaggregative Escherichia coli isolates from Iranian children. Am. J. Trop. Med. Hyg. 65:13-14. [DOI] [PubMed] [Google Scholar]
- 9.Braun, V. 2001. Iron uptake mechanisms and their regulation in pathogenic bacteria. Int. J. Med. Microbiol. 291:67-79. [DOI] [PubMed] [Google Scholar]
- 10.Czeczulin, J., T. Whittam, I. Henderson, F. Navarro-Garcia, and J. Nataro. 1999. Phylogenetic analysis of virulence genes in enteroaggregative and diffusely adherent Escherichia coli. Infect. Immun. 67:2692-2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donnenberg, M. S., and T. S. Whittam. 2001. Pathogenesis and evolution of virulence in enteropathogenic and enterohemorrhagic Escherichia coli. J. Clin. Investig. 107:539-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez-Prada, C., B. D. Tall, S. E. Elliott, D. L. Hoover, J. P. Nataro, and M. M. Venkatesan. 1998. Hemolysin-positive enteroaggregative and cell- detaching Escherichia coli strains cause oncosis of human monocyte-derived macrophages and apoptosis of murine J774 cells. Infect. Immun. 66:3918-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guyer, D. M., J. S. Kao, and H. L. Mobley. 1998. Genomic analysis of a pathogenicity island in uropathogenic Escherichia coli CFT073: distribution of homologous sequences among isolates from patients with pyelonephritis, cystitis, and catheter-associated bacteriuria and from fecal samples. Infect. Immun. 66:4411-4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Headley, V., M. Hong, M. Galko, and S. M. Payne. 1997. Expression of aerobactin genes by Shigella flexneri during extracellular and intracellular growth. Infect. Immun. 65:818-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henderson, I., J. Czeczulin, C. Eslava, F. Noriega, and J. Nataro. 1999. Characterization of Pic, a secreted protease of Shigella flexneri and enteroaggregative Escherichia coli. Infect. Immun. 67:5587-5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iyoda, S., K. Tamura, K. Itoh, H. Izumiya, N. Ueno, K. Nagata, M. Togo, J. Terajima, and H. Watanabe. 2000. Inducible stx2 phages are lysogenized in the enteroaggregative and other phenotypic Escherichia coli O86:HNM isolated from patients. FEMS Microbiol. Lett. 191:7-10. [DOI] [PubMed] [Google Scholar]
- 17.Jiang, Z. D., P. C. Okhuysen, D. C. Guo, R. He, T. M. King, H. L. DuPont, and D. M. Milewicz. 2003. Genetic susceptibility to enteroaggregative Escherichia coli diarrhea: polymorphism in the interleukin-8 promotor region. J. Infect. Dis. 188:506-511. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, J. R., M. A. Kuskowski, T. T. O'Bryan, and J. N. Maslow. 2002. Epidemiological correlates of virulence genotype and phylogenetic background among Escherichia coli blood isolates from adults with diverse-source bacteremia. J. Infect. Dis. 185:1439-1447. [DOI] [PubMed] [Google Scholar]
- 19.Johnson, J. R., T. A. Russo, P. I. Tarr, U. Carlino, S. S. Bilge, J. C. Vary, Jr., and A. L. Stell. 2000. Molecular epidemiological and phylogenetic associations of two novel putative virulence genes, iha and iroNE. coli, among Escherichia coli isolates from patients with urosepsis. Infect. Immun. 68:3040-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kao, J.-S., D. M. Stucker, J. W. Warren, and H. L. T. Mobeley. 1997. Pathogenicity island sequences of pyelonephritogenic Escherichia coli CFT073 are associated with virulent uropathogenic strains. Infect. Immun. 65:2812-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karch, H., S. Schubert, D. Zhang, W. Zhang, H. Schmidt, T. Olschlager, and J. Hacker. 1999. A genomic island, termed high-pathogenicity island, is present in certain non-O157 Shiga toxin-producing Escherichia coli clonal lineages. Infect. Immun. 67:5994-6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koczura, R., and A. Kaznowski. 2003. The Yersinia high-pathogenicity island and iron-uptake systems in clinical isolates of Escherichia coli. J. Med. Microbiol. 52:637-642. [DOI] [PubMed] [Google Scholar]
- 23.Mathewson, J. J., P. C. Johnson, and H. L. DuPont. 1986. Pathogenicity of enteroadherent Escherichia coli in adult volunteers. J. Infect. Dis. 154:524-527. [DOI] [PubMed] [Google Scholar]
- 24.Montgomerie, J. Z., A. Bindereif, J. B. Neilands, G. M. Kalmanson, and L. B. Guze. 1984. Association of hydroxamate siderophore (aerobactin) with Escherichia coli isolated from patients with bacteremia. Infect. Immun. 46:835-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montgomerie, J. Z., G. M. Kalmanson, and L. B. Guze. 1979. Enterobactin and virulence of Escherichia coli in pyelonephritis. J. Infect. Dis. 140:1013. [DOI] [PubMed] [Google Scholar]
- 26.Morabito, S., H. Karch, P. Mariani-Kurkdjian, H. Schmidt, F. Minelli, E. Bingen, and A. Caprioli. 1998. Enteroaggregative, Shiga toxin-producing Escherichia coli O111:H2 associated with an outbreak of hemolytic-uremic syndrome. J. Clin. Microbiol. 36:840-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moss, J. E., T. J. Cardozo, A. Zychlinsky, and E. A. Groisman. 1999. The selC-associated SHI-2 pathogenicity island of Shigella flexneri. Mol. Microbiol. 33:74-83. [DOI] [PubMed] [Google Scholar]
- 28.Nataro, J. P., Y. Deng, S. Cookson, A. Cravioto, S. J. Savarino, L. D. Guers, M. M. Levine, and C. O. Tacket. 1995. Heterogeneity of enteroaggregative Escherichia coli virulence demonstrated in volunteers. J. Infect. Dis. 171:465-468. [DOI] [PubMed] [Google Scholar]
- 29.Nataro, J. P., S. Hicks, A. D. Phillips, P. A. Vial, and C. L. Sears. 1996. T84 cells in culture as a model for enteroaggregative Escherichia coli pathogenesis. Infect. Immun. 64:4761-4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nataro, J. P., J. B. Kaper, R. Robins-Browne, V. Prado, P. Vial, and M. M. Levine. 1987. Patterns of adherence of diarrheagenic Escherichia coli to HEp-2 cells. Pediatr. Infect. Dis. J. 6:829-831. [DOI] [PubMed] [Google Scholar]
- 32.Nataro, J. P., T. Steiner, and R. L. Guerrant. 1998. Enteroaggregative Escherichia coli. Emerg. Infect. Dis. 4:251-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okeke, I. N., A. Lamikanra, J. Czeczulin, F. Dubovsky, J. B. Kaper, and J. P. Nataro. 2000. Heterogeneous virulence of enteroaggregative Escherichia coli strains isolated from children in Southwest Nigeria. J. Infect. Dis. 181:252-260. [DOI] [PubMed] [Google Scholar]
- 34.Okeke, I. N., A. Lamikanra, H. Steinrück, and J. B. Kaper. 2000. Characterization of Escherichia coli strains from cases of childhood diarrhea in provincial South-Western Nigeria. J. Clin. Microbiol. 38:7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okeke, I. N., and J. P. Nataro. 2001. Enteroaggregative Escherichia coli. Lancet Infect. Dis. 1:304-313. [DOI] [PubMed] [Google Scholar]
- 36.Otto, B. R., S. J. van Dooren, J. H. Nuijens, J. Luirink, and B. Oudega. 1998. Characterization of a hemoglobin protease secreted by the pathogenic Escherichia coli strain EB1. J. Exp. Med. 188:1091-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Payne, S. M. 1994. Detection, isolation, and characterization of siderophores. Methods Enzymol. 235:329-344. [DOI] [PubMed] [Google Scholar]
- 38.Payne, S. M. 1993. Iron acquisition in microbial pathogenesis. Trends Microbiol. 1:66-69. [DOI] [PubMed] [Google Scholar]
- 39.Rabsch, W., W. Voigt, R. Reissbrodt, R. M. Tsolis, and A. J. Baumler. 1999. Salmonella typhimurium IroN and FepA proteins mediate uptake of enterobactin but differ in their specificity for other siderophores. J. Bacteriol. 181:3610-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rajakumar, K., C. Sasakawa, and B. Adler. 1997. Use of a novel approach, termed island probing, identifies the Shigella flexneri she pathogenicity island which encodes a homolog of the immunoglobulin A protease-like family of proteins. Infect. Immun. 65:4606-4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ratledge, C., and L. G. Dover. 2000. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 54:881-941. [DOI] [PubMed] [Google Scholar]
- 42.Russo, T. A., U. B. Carlino, and J. R. Johnson. 2001. Identification of a new iron-regulated virulence gene, ireA, in an extraintestinal pathogenic isolate of Escherichia coli. Infect. Immun. 69:6209-6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russo, T. A., U. B. Carlino, A. Mong, and S. T. Jodush. 1999. Identification of genes in an extraintestinal isolate of Escherichia coli with increased expression after exposure to human urine. Infect. Immun. 67:5306-5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Russo, T. A., C. D. McFadden, U. B. Carlino-MacDonald, J. M. Beanan, T. J. Barnard, and J. R. Johnson. 2002. IroN functions as a siderophore receptor and is a urovirulence factor in an extraintestinal pathogenic isolate of Escherichia coli. Infect. Immun. 70:7156-7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scaletsky, I. C., S. H. Fabbricotti, K. R. Aranda, M. B. Morais, and U. Fagundes-Neto. 2002. Comparison of DNA hybridization and PCR assays for detection of putative pathogenic enteroadherent Escherichia coli. J. Clin. Microbiol. 40:1254-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scaletsky, I. C., S. H. Fabbricotti, R. L. Carvalho, C. R. Nunes, H. S. Maranhao, M. B. Morais, and U. Fagundes-Neto. 2002. Diffusely adherent Escherichia coli as a cause of acute diarrhea in young children in Northeast Brazil: a case-control study. J. Clin. Microbiol. 40:645-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scaletsky, I. C., M. Z. Pedroso, C. A. Oliva, R. L. Carvalho, M. B. Morais, and U. Fagundes-Neto. 1999. A localized adherence-like pattern as a second pattern of adherence of classic enteropathogenic Escherichia coli to HEp-2 cells that is associated with infantile diarrhea. Infect. Immun. 67:3410-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schubert, S., S. Cuenca, D. Fischer, and J. Heesemann. 2000. High-pathogenicity island of Yersinia pestis in enterobacteriaceae isolated from blood cultures and urine samples: prevalence and functional expression. J. Infect. Dis. 182:1268-1271. [DOI] [PubMed] [Google Scholar]
- 49.Schubert, S., B. Picard, S. Gouriou, J. Heesemann, and E. Denamur. 2002. Yersinia high-pathogenicity island contributes to virulence in Escherichia coli causing extraintestinal infections. Infect. Immun. 70:5335-5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schubert, S., A. Rakin, H. Karch, E. Carniel, and J. Heesemann. 1998. Prevalence of the “high-pathogenicity island” of Yersinia species among Escherichia coli strains that are pathogenic to humans. Infect. Immun. 66:480-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sheikh, J., J. R. Czeczulin, S. Harrington, S. Hicks, I. R. Henderson, C. Le Bouguenec, P. Gounon, A. Phillips, and J. P. Nataro. 2002. A novel dispersin protein in enteroaggregative Escherichia coli. J. Clin. Investig. 110:1329-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Torres, A., and S. M. Payne. 1997. Haem iron-transport system in enterohaemorrhagic Escherichia coli O157:H7. Mol. Microbiol. 23:825-833. [DOI] [PubMed] [Google Scholar]
- 53.Torres, A. G., P. Redford, R. A. Welch, and S. M. Payne. 2001. TonB-dependent systems of uropathogenic Escherichia coli: aerobactin and heme transport and TonB are required for virulence in the mouse. Infect. Immun. 69:6179-6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tzipori, S., J. Montanaro, R. M. Robins-Browne, P. Vial, R. Gibson, and M. M. Levine. 1992. Studies with enteroaggregative Escherichia coli in the gnotobiotic piglet gastroenteritis model. Infect. Immun. 60:5302-5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vial, P. A., R. Robins-Browne, H. Lior, V. Prado, J. B. Kaper, J. P. Nataro, D. Maneval, A. Elsayed, and M. M. Levine. 1988. Characterization of enteroadherent-aggregative Escherichia coli, a putative agent of diarrheal disease. J. Infect. Dis. 158:70-79. [DOI] [PubMed] [Google Scholar]
- 56.Vila, J., M. Vargas, I. R. Henderson, J. Gascon, and J. P. Nataro. 2000. Enteroaggregative Escherichia coli virulence factors in traveler's diarrhea strains. J. Infect. Dis. 182:1780-1783. [DOI] [PubMed] [Google Scholar]
- 57.Vokes, S. A., S. A. Reeves, A. G. Torres, and S. M. Payne. 1999. The aerobactin iron transport system genes in Shigella flexneri are present within a pathogenicity island. Mol. Microbiol. 33:63-73. [DOI] [PubMed] [Google Scholar]
- 58.Wang, Y., H. Wang, Q. Xiang, S. X. Sun, and S. Y. Yu. 2002. Detection of the high-pathogenicity island of Yersinia enterocolitica in enterotoxigenic and enteropathogenic E. coli strains. Di Yi Jun Yi Da Xue Xue Bao 22:580-583. (Abstract.) [PubMed] [Google Scholar]
- 59.Weinberg, E. 1995. Acquisition of iron and other nutrients in vivo, p. 79-93. In J. Roth et al. (ed.), Virulence mechanisms of bacterial pathogens, 2nd ed. ASM Press, Washington, D.C.
- 60.Williams, P. H. 1979. Novel iron uptake system specified by ColV plasmids: an important component in the virulence of invasive strains of Escherichia coli. Infect. Immun. 26:925-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams, P. H., and P. J. Warner. 1980. ColV plasmid-mediated, colicin V-independent iron uptake system of invasive strains of Escherichia coli. Infect. Immun. 29:411-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wyckoff, E. E., D. Duncan, A. G. Torres, M. Mills, K. Maase, and S. M. Payne. 1998. Structure of the Shigella dysenteriae haem transport locus and its phylogenetic distribution in enteric bacteria. Mol. Microbiol. 28:1139-1152. [DOI] [PubMed] [Google Scholar]
- 63.Ye, C., and J. Xu. 2001. Prevalence of iron transport gene on pathogenicity-associated island of uropathogenic Escherichia coli in E. coli O157:H7 containing Shiga toxin gene. J. Clin. Microbiol. 39:2300-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]