DGKα and DGKζ negatively regulate the DAG/RasGRP1/Ras pathway in T cells. Study of the specific contribution of each isoform to DAG metabolism during immune synapse formation by use of a combination of RNAi and videomicroscopy techniques identifies DGKζ as mainly responsible for DAG consumption at the immunological synapse.
Abstract
Diacylglycerol (DAG) generation at the T cell immunological synapse (IS) determines the correct activation of antigen-specific immune responses. DAG kinases (DGKs) α and ζ act as negative regulators of DAG-mediated signals by catalyzing DAG conversion to phosphatidic acid (PA). Nonetheless, the specific input of each enzyme and their spatial regulation during IS formation remain uncharacterized. Here we report recruitment of endogenous DGKα and DGKζ to the T cell receptor (TCR) complex following TCR/CD28 engagement. Specific DGK gene silencing shows that PA production at the activated complex depends mainly on DGKζ, indicating functional differences between these proteins. DGKζ kinase activity at the TCR is enhanced by phorbol-12-myristate-13-acetate cotreatment, suggesting DAG-mediated regulation of DGKζ responsiveness. We used GFP-DGKζ and -DGKα chimeras to assess translocation dynamics during IS formation. Only GFP-DGKζ translocated rapidly to the plasma membrane at early stages of IS formation, independent of enzyme activity. Finally, use of a fluorescent DAG sensor confirmed rapid, sustained DAG accumulation at the IS and allowed us to directly correlate membrane translocation of active DGKζ with DAG consumption at the IS. This study highlights a DGKζ-specific function for local DAG metabolism at the IS and offers new clues to its mode of regulation.
INTRODUCTION
T lymphocytes respond to antigen-presenting cell (APC) interactions through T cell receptor (TCR)–induced signals that promote the formation of a surface subdomain at the T cell–APC contact zone, termed the immunological synapse (IS) (Smith-Garvin et al., 2009; Fooksman et al., 2010). At the IS, signaling components and adaptor proteins are recruited and reorganized, orchestrating T cell responses (Lin and Weiss, 2001). TCR triggering leads to phosphatidylinositol-4,5 bisphosphate hydrolysis by phospholipase Cγ1 (PLCγ1) to generate inositol-1,4,5 trisphosphate and diacylglycerol (DAG), which control intracellular Ca2+ intake and activation of DAG effectors, respectively. TCR-triggered DAG accumulation at the IS results in DAG-mediated recruitment and allosteric activation of proteins with conserved protein kinase C type I (C1) domains. These include the Ras-guanyl nucleotide–releasing protein 1 (RasGRP1), classic and novel protein kinase C (PKC) family members, protein kinase D, and β-chimaerin (Ebinu et al., 2000; Spitaler et al., 2006; Siliceo and Merida, 2009; Quann et al., 2011). The need for a polarized DAG pool to ensure appropriate T cell polarization and activation suggests that generation and consumption of this lipid must be precisely regulated (Carrasco and Merida, 2007; Quann et al., 2009).
Diacylglycerol kinases (DGKs) participate in DAG metabolism, catalyzing its conversion to phosphatidic acid (PA). There are 10 mammalian DGKs, classified in five groups (types I–V) based on specific regulatory domains (Merida et al., 2008). Type I DGKα and type IV DGKζ are the two main isozymes expressed in lymphoid tissues, and their exact contribution to TCR-triggered, DAG-mediated signals is of great interest. Gain-of-function experiments in the Jurkat T cell line demonstrated a role for both isoforms as negative regulators of Ras activation by impairing the DAG-mediated membrane localization and activation of RasGRP1 (Sanjuan et al., 2003; Zhong et al., 2003). These early data suggest overlapping functions for the two isoforms in the regulation of the Ras/ERK pathway, although structural–functional analysis shows striking differences probably based on their different mechanisms of regulation. DGKα contains Ca2+-responsive regions at its N-terminus, whose deletion improves its negative regulation of RasGRP1 membrane localization (Sanjuan et al., 2003). Removal of the DGKζ C‑terminal region, containing ankyrin repeats and a PDZ-binding motif, enhances its negative regulation of TCR-induced Ras-ERK1/2 activation (Zhong et al., 2002).
The phenotypes of knockout (KO) mouse models for these two isoforms confirmed their major role as brakes on TCR-mediated RasGRP1-Ras-ERK1/2 activation. DGKα- and DGKζ-deficient mice show enhanced Ras activation and increased ERK1/2 phosphorylation compared with wild-type littermates. As a result, DGKα- and DGKζ-deficient mice are resistant to anergy, a nonresponsive T cell state caused by TCR triggering in the absence of costimulation and originated by selective generation of Ca2+ signals without simultaneous activation of the RasGRP1-Ras-ERK1/2-AP1 pathway (Zhong et al., 2003; Olenchock et al., 2006). Anergy induction helps to maintain peripheral T cell tolerance, and its impairment is predicted to result in self-reactive T cell activation and autoimmunity. Nonetheless, DGKα- and DGKζ-deficient T cells do not develop autoimmune diseases, suggesting that one isoform may compensate for the loss of the other to maintain T cell tolerance. In agreement with this argument, mice deficient in both isoforms develop chronic autoimmune hepatitis (Guo et al., 2008), confirming DGKα and DGKζ synergy in the control of T cell tolerance and development.
The analysis of genetically modified mice has greatly helped to confirm the physiological relevance of DGKα and DGKζ. There are nonetheless important questions that remain to be addressed. One of the most imperative is the nature of the regulatory mechanisms that control DGKα and DGKζ enzymatic activity and their spatiotemporal subcellular localization in response to TCR stimulation. Studies in T cells that ectopically express the muscarinic type I receptor showed that each isoform responds with different translocation kinetics as the result of their distinct activation mechanisms (Sanjuan et al., 2001; Santos et al., 2002). DGKα relocates to the membrane with rapid and transient kinetics that require tyrosine kinase activation and Ca2+ elevation (Sanjuan et al., 2001). DGKζ on the other hand shows sustained translocation kinetics regulated by PKC-mediated phosphorylation of its myristoylated alanine-rich C kinase substrate (MARCKS) domain (Santos et al., 2002). Enzyme activity is dispensable for DGKζ membrane relocation, whereas DGKα inactivation promotes sustained membrane localization of the inactive enzyme (Sanjuan et al., 2001; Santos et al., 2002). The relevance of these mechanisms in the regulation of DGKα and DGKζ localization and activity during IS formation has not been explored. There are no data showing direct DGK recruitment to the TCR–coreceptor complex, although signaling protein recruitment to scaffolds and adaptor proteins in close proximity to the TCR is the immediate consequence of T cell–APC interaction. Finally, whether the DGKs contribute to DAG metabolism outside the IS as a mechanism to promote polarization of DAG-mediated signals or whether they consume the DAG generated at the T cell–APC contact site is still debated.
Here we evaluated DGKα and DGKζ recruitment to the TCR complex using affinity purification of active TCR. We then analyzed DGK activity at the TCR complex and used RNA interference (RNAi)–mediated gene silencing to determine the contribution of each isozyme to PA generation. We used GFP-fused DGKα and DGKζ constructs to assess translocation dynamics during IS formation and a fluorescent DAG sensor to evaluate the effect of DGK translocation on DAG metabolism at the cell–cell contact site. Our data showed recruitment of both isoforms to the TCR complex, where DGKζ is the major DAG regulatory isozyme. Videomicroscopy analysis indicated rapid, sustained DGKζ mobilization to the T cell plasma membrane (PM) after APC encounter and confirms that this translocation correlates with rapid consumption of DAG at the IS. We propose a positive feedback mechanism by which IS formation promotes membrane recruitment of DGKζ that is further activated at this site by DAG-regulated mechanisms. Our results suggest that this complex mechanism of DGKζ regulation in response to TCR triggering contributes to ensure accurate DAG:PA balance during IS formation.
RESULTS
Isolation of activated TCR complexes using anti-CD3/CD28–coated beads
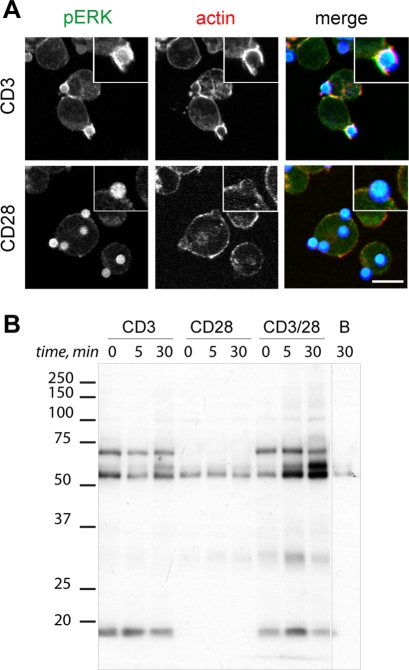
The TCR complex is composed of eight transmembrane chains—the TCR α/β heterodimer and the CD3 εγ, εδ, and ζζ dimers. Each CD3 cytosolic chain bears an immunoreceptor tyrosine kinase cytosolic motif (ITAM) rich in tyrosine residues, which serve as a platform to recruit protein tyrosine kinases and adaptor molecules. To isolate the protein complex recruited to TCR after T cell activation, we used magnetic beads coated with anti-CD3 and/or anti-CD28 antibodies to act as surrogate APC and partially mimic IS-mediated actin and PM reorganization (Viola et al., 1999). We tested bead recognition by immunofluorescence (Figure 1A). Anti-CD3–coated beads showed phospho-ERK (pERK) induction and accumulation around the bead; we also observed formation of an actin cup that was not seen with anti-CD28 alone, which was subsequently used to discriminate TCR-activated clusters. Cells in complex with antibody-coated beads were lysed in a mild nonionic detergent, Brij 96V (2-[(Z)-octadec-9-enoxy]ethanol), to preserve noncovalent interactions between TCR and signaling components (Beyers et al., 1992). We monitored tyrosine-phosphorylated proteins recruited to beads alone or coupled to anti-CD3, anti-CD28, or anti-CD3/CD28 (Figure 1B). As predicted, full stimulation with anti-CD3/CD28 induced greater tyrosine kinase activation than did anti-CD3, whereas anti-CD28 beads or uncoated beads did not (Figure 1B). These optimum stimulation and affinity purification conditions were used for specific isolation of the active TCR complex.
FIGURE 1:
Isolation of TCR immune complexes using anti-CD3/CD28–coated beads. (A) Jurkat T cells were stimulated with sheep anti–mouse IgG Dynabeads coated with human anti-CD3 or anti-CD28 (10 min). Immunostaining is shown for activation (pERK) and actin dynamics markers (phalloidin–rhodamine). Insets, zoomed area. Bar, 10 μm. (B) Jurkat T cells (107 cells/ml) serum starved in DMEM, 0.5% BSA (2 h), were incubated with anti-CD3, anti-CD28, or anti-CD3/CD28 on ice, followed by Dynabeads (37°C) for indicated times. Time 0 shows cells incubated with antibody and Dynabeads on ice. Associated protein complexes were resolved by SDS–PAGE and tyrosine-phosphorylated proteins developed in WB. Left, molecular weight markers. Control immunoprecipitation was performed with Dynabeads alone (lane B).
DGKs are recruited to activated TCR complexes
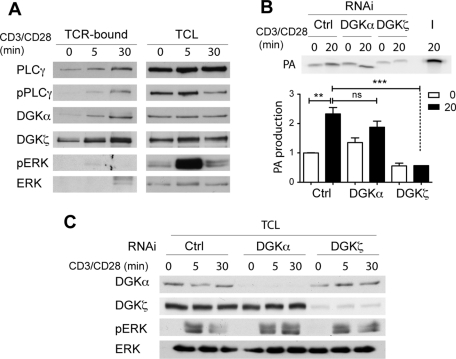
Using our model for isolation of activated TCR, we tested whether the two main DGKs in T cells were recruited directly to TCR complexes. DGKα and DGKζ segregated with the TCR immune complex after activation in a manner comparable to phospho-PLCγ, as early as the first minutes of stimulation (Figure 2A). In contrast, ERK1/2 was activated by anti-CD3/CD28 beads but was not enriched at the TCR complex, indicating that beads do not precipitate nonspecific proteins stimulated by, but not associated to, TCR complexes.
FIGURE 2:
DGK recruitment to active TCR complex; DGKζ is necessary for PA production. (A) Jurkat T cells were presented to anti-CD3/CD28–coated Dynabeads for various times. Cell treatment and lysate precipitation were as in Figure 1B. TCR immune complexes were eluted (TCR bound), resolved by SDS–PAGE, and probed in WB with DGK- and activation marker–specific antibodies. Unprecipitated samples were used as activation controls (total cell lysate [TCL]). (B, C) DGKζ is the main PA-generating enzyme recruited to the TCR complex. RNAi was used to silence DGKα and DGKζ; at 72 h posttreatment, cells (107 cells/ml) were starved as in Figure 1B and stimulated with anti-CD3/CD28 beads for various times. TCR-bound samples or TCL (input, I) were used as an enzyme source for the DAG kinase assay (type I; see Materials and Methods); a representative experiment is shown (B). PA production is shown as the mean ± SEM (n = 4). A fraction of the samples used in B was analyzed by WB to confirm RNAi efficiency and activation (C).
DGK are activated downstream of the TCR, and DGK activity is induced by T cell activation (Sanjuan et al., 2003); however, there are no studies of this lipid kinase activity associated to the TCR complex. To determine whether DGK recruitment contributes to DAG consumption at the TCR complex, we measured TCR complex–associated DGK activity using exogenous DAG as substrate and monitored PA production by [γ32P]ATP incorporation. After 20 min of stimulation with anti-CD3/CD28 beads, DGK activity increased at the TCR complex, correlating with DGKζ and DGKα recruitment (Figure 2B). To determine the contribution of each DGK isozyme to PA production, we analyzed the effect of DGKα or DGKζ RNAi on DGK activity. Isozyme-specific depletion showed that DGKα RNAi had little effect on DGK activity, whereas activity was significantly reduced when DGKζ was silenced (Figure 2B). To exclude any potential disadvantage for DGKα, we performed additional DGK assays in the presence of phosphatidylserine and Ca2+, the two cofactors that maximize type I DGK activation. Under these conditions, we observed similar results (Supplemental Figure S1). As reported in KO mice (Zhong et al., 2003; Olenchock et al., 2006), attenuation of DGKα or DGKζ resulted in sustained ERK phosphorylation kinetics (Figure 2C). We conclude that DGKζ is the main isozyme controlling DAG metabolism at the TCR immune complex after engagement.
Regulation of DGKζ at the TCR complex
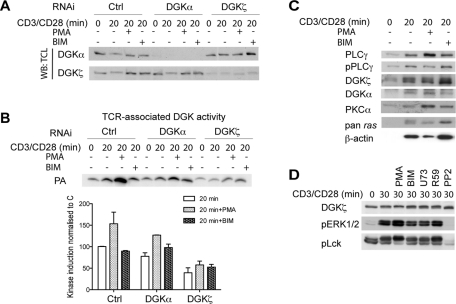
Several studies proposed a role for PKCα in the regulation of DGKζ membrane recruitment and activation (Santos et al., 2002; Abramovici et al., 2003; Luo et al., 2003). We monitored DGK activity at the TCR complex after cotreatment with phorbol-12-myristate-13-acetate (PMA), a potent PKCα activator that is not consumed by DGK, and we observed a notable increase in DGK activity in response to PMA treatment (Figure 3, A and B). We performed RNAi gene silencing of both DGKα and DGKζ in Jurkat T cells (Figure 3A) and observed that this increase in activity was dependent on DGKζ but not DGKα expression (Figure 3, A and B). We used bisindolylmaleimide II (BIM), a broad PKC inhibitor with a lower IC50 for PKCα, to determine PKC activity input on DGK activity following TCR signaling. PKC inhibition apparently did not alter TCR-recruited DGK activity (Figure 3B).
FIGURE 3:
PMA promotes DGKζ activity at the TCR complex. (A, B) DGKα and DGKζ were silenced and DAG kinase activity measured at the TCR complex. Where indicated, Jurkat T cells were pretreated with 200 ng/ml PMA or 50 nM BIM (15 min). (A) DGKα and DGKζ expression levels from total cell lysates (TCL) for each condition. (B) DGK assay measured at the TCR complex from cells treated (as in A). A representative kinase assay (PA) is shown (top), percentage PA production after TCR induction, normalized to controls, is represented based on three individual experiments (bottom). Data show mean ± SD; n = 3. (C) Jurkat T cells, untreated or pretreated with PMA or BIM (as in A), were stimulated with anti-CD3/CD28 beads and TCR immune complexes collected at times 0 and 20 min poststimulation. Eluted complexes were resolved by SDS–PAGE and processed in WB with antibodies to endogenous proteins. (D) Jurkat T cells were stimulated with anti-CD3/CD28 beads, untreated or pretreated as in (A) with 200 ng/ml PMA, 50 nM BIM, 1 μM U73122, 30 μM R59949, or 30 μM PP2, and whole-cell extract analyzed by WB using specific antibodies. (C, D) Representative WB images from at least three individual experiments are shown.
To evaluate whether the increased DGK activity correlated with enhanced enzyme recruitment, we analyzed TCR-associated proteins after PMA or BIM cotreatment (Figure 3C). We observed no major changes in DGKζ recruitment to the TCR after either condition, although BIM treatment resulted in greater TCR recruitment of a faster-migrating DGKζ band. Our data thus suggest that the increased DGK activity observed after PMA treatment does not correspond to greater enzyme recruitment but could reflect posttranslational regulation. In fact, we also detected PKCα recruited to the activated TCR complex, with greater mobilization after PMA treatment (Figure 3C). PKC function is related to the control of actin dynamics in T cells (Sims et al., 2007; Beal et al., 2008), so we assessed Ras and actin recruitment under the different conditions as a measure of TCR clustering and a marker of cytoskeletal reorganization. Actin recruitment was markedly blocked with PMA cotreatment and enhanced following PKCα inhibition, further confirming that this isoform contributes to the regulation of TCR signals.
DGKζ migrated as a doublet, indicative of putative posttranslational regulation, and showed a molecular weight shift following stimulation with anti-CD3/CD28 beads in total cell lysates (Figure 3D). This shift was greater after PMA cotreatment and was not observed in the presence of the src kinase inhibitor PP2. Taken together, these data suggest that endogenous DGKζ is recruited to the TCR complex, where it might be activated in response to DAG-mediated signals in a feedback loop in which DAG-regulated signals contribute to its consumption.
DGKζ translocation dynamics during immune synapse formation
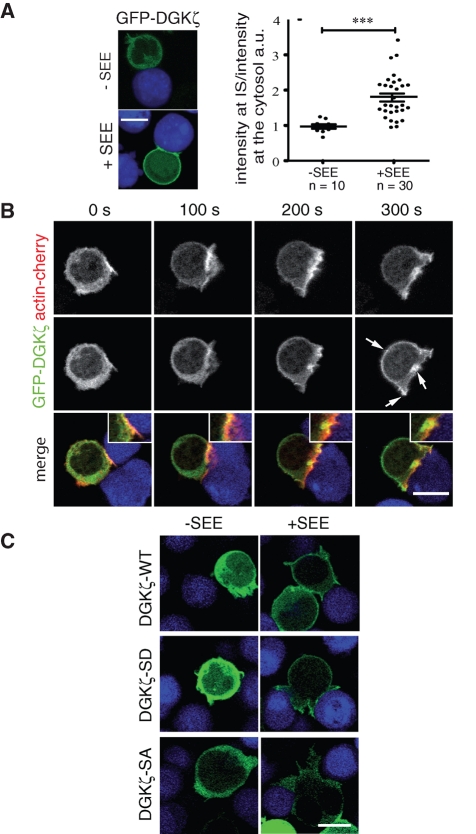
We used the green fluorescent protein (GFP)–DGKζ fusion protein to examine DGKζ translocation dynamics to TCR complexes in live T cells. Our anti-CD3/CD28 complex isolation strategy showed that the chimera was recruited to the TCR complex without altering recruitment of endogenous DGKζ (Supplemental Figure S2A). Microscopy analysis showed that GFP-DGKζ was recruited to the bead-cell contact area as soon as the bead contacted the cell (Supplemental Figure S2B). We stimulated Jurkat T cells using Raji B cells loaded with the staphylococcal enterotoxin E (SEE) superantigen as APC (Fraser et al., 2000; Friedl and Storim, 2004). Time-lapse fluorescence microscopy showed that, as in the case of antibody-coated beads, GFP-DGKζ redistributed to the PM within minutes of cell–cell contact (Figure 4A, Supplemental Figure S2C, and Supplemental Movie 1), and this localization was sustained for up to 30 min after T/B cell encounter. This suggests that DAG level control is important from early signal transduction stages and precedes IS maturation. Quantification of GFP-DGKζ fluorescence density showed significant DGKζ accumulation at the IS compared with cytosol (Figure 4A), correlating with biochemical estimates. To visualize simultaneous changes in T cell morphology and DGKζ translocation after antigen presentation, we coexpressed GFP-DGKζ with actin-Cherry, followed by double fluorescence time-lapse microscopy. DGKζ shuttled to the PM in the initial T lymphocyte contact with the APC, and accumulated in an F-actin–enriched fraction (Figure 4B and Supplemental Movie 2).
FIGURE 4:
GFP-DGKζ translocates to the PM in response to APC-driven antigen presentation. (A, B) Jurkat T cells transfected with GFP-DGKζ alone (A) or cotransfected with actin-Cherry (B) were collected after 24 h in HBSS, 2% FBS in poly-l-lysine–coated chambers, and placed on a warmed microscope stage (37°C) for image acquisition; Raji B cells, alone or pulsed with 1 μg/ml SEE, were prestained with the fluorescent marker CMAC (blue) and added in droplets. (A) Representative images (left). Quantitative image analysis of GFP-DGKζ accumulated at the IS (right; see Materials and Methods). Each dot shows the synapse:cytosol intensity ratio for accumulated GFP-DGKζ constructs in a T cell after APC encounter. Bars, mean ± SEM (***p < 0.001; Kolmogorov–Smirnov test). (B) Dynamic translocation of GFP-DGKζ (green) with actin-Cherry (red) in the presence of SEE-pulsed APC (blue). Arrows, DGKζ accumulation; time 0 represents first cell–cell contact. Bar, 10 μm. (C) Translocation of DGKζ wild type (WT) vs. MARCKS-domain mutants (SD, SA) after antigen presentation. At 48 h posttransfection, Jurkat T cells were collected and incubated with APC; representative confocal images of live cells are shown.
PKC-dependent phosphorylation of DGKζ at the MARCKS region (Supplemental Figure S3A) is necessary for enzyme localization to the PM in response to G protein–coupled receptor stimulation in both lymphoid and nonlymphoid cell lines (Santos et al., 2002; Abramovici et al., 2003). We examined translocation of DGKζ mutated in the MARCKS domain (Ser-to-Asp [SD] or Ser-to-Ala [SA], which mimicked or impaired phosphorylation, respectively) in response to T cell–APC contact. The SA mutant was unresponsive to antigen presentation and remained diffuse in cytosol, whereas the SD mutant behaved like the wild-type form after stimulation (Figure 4C). MARCKS-domain phosphorylation is believed to release the negative restriction imposed by the DGKζ C-terminal domain, which bears ankyrin repeat sequences and a PDZ-binding motif (Santos et al., 2002). Accordingly, the SA mutant recovered PM localization in response to T cell activation following truncation of its C-terminal domain (SA-ΔAnk; Supplemental Figure S3B). As shown for endogenous DGKζ, BIM treatment did not affect GFP-DGKζ translocation to the PM, although some intracellular vesicular staining was detected. GFP-DGKζ dynamics was also unaffected by rottlerin, an inhibitor of novel PKC (Supplemental Figure S3C). These findings further demonstrate that PM relocation of DGKζ is independent of PKC activity and suggest that phosphorylation of the DGKζ MARCKS region could take place prior to T cell activation, possibly as a priming step.
DGKζ regulates DAG at the immunological synapse
Videomicroscopy analysis showed that T cell–APC contact results in rapid GFP-DGKζ recruitment to the entire PM and not only to the IS. To examine the DGKζ contribution to the regulation of DAG generated at the IS, we used the C1ab tandem domain of PKCθ previously characterized as a DAG sensor in living T cells (Carrasco and Merida, 2004). Nonstimulated cells expressing the PKCθ C1ab domain fused to the red fluorescent protein Cherry (Cherry-C1ab) showed fluorescence accumulation mainly in internal membranes, as reported for this domain when fused to GFP (Carrasco and Merida, 2004; Supplemental Figure S4). Following APC contact, the DAG sensor shuttled rapidly to the cell–cell contact zone and also labeled the Golgi-positive pool that polarized to the IS (Figure 5A and Supplemental Movie 3).
FIGURE 5:
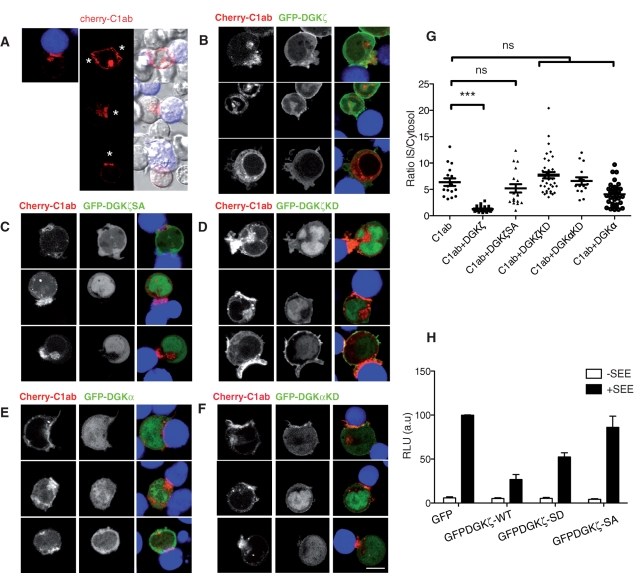
DGK localization modulates local DAG pools. (A–F) Jurkat T cells were transfected with the tandem C1 domain of PKCθ fused to Cherry (Cherry-C1ab) alone (A) or cotransfected with GFP-tagged constructs of DGKα or ζ wild type (WT) (B and E, respectively) or mutated DGKζSA, DGKζKD, or DGKαKD (C, D, and F, respectively), to visualize DAG generation by videomicroscopy during antigen presentation. Raji B cells were treated as in Figure 4; asterisks, Raji B cell position. Representative images from different fields during in vivo imaging are shown (A–F). Bar, 5 μm. (G) Quantitative analysis of Cherry-C1ab accumulation at the IS in cells transfected with Cherry-C1ab alone or with GFP-DGK constructs taken from three individual experiments. Each dot represents the synapse:cytosol intensity ratio of Cherry C1ab after APC encounter. Bars, mean ± SEM, n ≥ 15 (***p < 0.001; Kolmogorov–Smirnov test). (H) Reporter assay for the IL-2 promoter. Jurkat T cells were cotransfected with 15 μg of IL-2 luciferase reporter/10 ng Renilla and with GFP alone or with GFP-DGKζ WT or mutant (SD or SA). At 48 h posttransfection, cells were stimulated at a 1:1 ratio with APC alone or SEE loaded (24 h). Data show mean ± SD; n = 3 from individual assays.
Coexpression of GFP-DGKζ did not alter the subcellular distribution of Cherry-C1ab in resting cells (Supplemental Figure S4), but during APC contact the sensor failed to accumulate as efficiently at the IS, although internal staining was observed (Figure 5B and Supplemental Movie 4). Translocation of Cherry-C1ab was quantified as the ratio of signal intensity at the IS compared with cytosol (Figure 5G). To confirm that membrane localization of DGKζ is necessary for DAG consumption, we performed a similar experiment with the DGKζ SA mutant. Enzymatic activity of this mutant is similar to that of the wild-type enzyme (Santos et al., 2002), but the mutant did not translocate to the PM in response to antigen presentation (Figure 4C). GFP-DGKζSA and Cherry-C1ab cotransfection did not alter DAG generation at the IS, as determined by Cherry-C1ab construct accumulation (Figure 5, C and G). To evaluate the requirement for enzymatic activity, we next used a GFP-DGKζ construct with a mutation in the conserved GGDG motif that impairs catalytic activity (Santos et al., 2002). Cotransfection of this GFP-DGKζKD and Cherry-C1ab did not affect the rapid accumulation of the DAG sensor at the IS (Figure 5, D and G). Image analysis showed partial colocalization of the DAG sensor with the GFP-DGKζKD at the IS, whereas the sensor was excluded from regions enriched in WT and SA forms (Figure 5D). Taken together, our data confirm the key role of DGKζ as a modulator of DAG levels at the IS and link DGKζ subcellular dynamics with the control of DAG-dependent signals.
We also evaluated translocation dynamics of a GFP-DGKα construct, as well as its effect on DAG sensor localization. Tests of GFP-DGKα expression on Cherry-C1ab accumulation at the IS showed neither PM accumulation of this isoform nor significant changes on C1ab synapse accumulation (Figure 5, E and G). A kinase-inactive GFP-DGKαKD mutant showed enhanced membrane localization, in accordance with previous findings (Sanjuan et al., 2001), but it did not alter DAG generation at the IS (Figure 5, F and G).
To evaluate the functional impact of GFP-DGKζ on DAG consumption during IS formation, we monitored interleukin-2 (IL-2) promoter induction in reporter luciferase assay using cells expressing the distinct DGKζ constructs (Figure 5H). Expression of either DGKζ-WT or the SD mutant reduced IL-2 promoter induction by 50% compared with controls, whereas the SA mutant had no effect. These findings were not due to altered kinase activity, which is similar in both mutants (Santos et al., 2002; unpublished results). These results further demonstrate that membrane localization of DGKζ is required for its negative function in T cell signaling.
DISCUSSION
DGK enzymes regulate the balance between DAG and PA, and impairment of their function is linked to the onset of various diseases, including diabetes and bipolar disorders (Yakubchyk et al., 2005; Chibalin et al., 2008; Kakefuda et al., 2010). Recent evidence shows the importance of DGKα and DGKζ in T cell activation and tolerance and places them at the front line as important brakes to immune functions (Zhong et al., 2008). Nonetheless, their regulation downstream of TCR signaling is not well characterized and, although their relocalization to the TCR activation site might appear conceptually trivial, no direct proof of this dynamic regulation has been reported.
Here we developed a biochemical tool using CD3- and CD28-coated beads with which to study DGK in activated TCR complexes. In addition, we used mild nonionic detergent to partially solubilize membrane structures while preserving proximal TCR complexes. Beads are used successfully for biochemical studies that analyze TCR supramolecular complexes (Viola et al., 1999; Harder and Kuhn, 2001; de Wet et al., 2011). In our studies, both DGKα and DGKζ were recruited to the TCR complex, which correlated with an increase in associated DGK activity. DGKζ was the main isozyme controlling PA generation at this site. This, however, does not rule out DGKα as a player in TCR signaling but points out that its activity at the TCR complex can possibly be compensated by DGKζ. In addition, DGKα could contribute on a different time scale, which warrants further study, and probably correlates with the milder phenotype of DGKα compared with that of DGKζ-KO mice (Zhong et al., 2003; Olenchock et al., 2006).
A model of antigen presentation with SEE-pulsed APC demonstrated rapid DGKζ translocation to the PM, concomitant with initial actin recruitment. This concurs with reports in other cell systems, in which DGKζ colocalized with filamentous actin to modulate lipid signals for correct actin organization and cell migration (Abramovici et al., 2003; Yakubchyk et al., 2005). The regulatory mechanism observed in our study closely resembles that described for the carbachol-triggered muscarinic type I receptor (Santos et al., 2002) or in C2C12 myoblasts (Abramovici et al., 2003); in both cases, phosphorylation of the DGKζ MARCKS region was critical for localization to PM. Whereas the DGKζ SD mutant (which mimics phosphorylation) showed PM translocation when T cells were in contact with SEE-loaded APC, the SA mutant (which impaired MARCKS-domain phosphorylation) failed to do so.
Although eukaryotic DGKζ has not yet been characterized structurally, mutational analysis suggests that its C-terminal region has a negative regulatory function that maintains the protein in a closed conformation. MARCKS-domain phosphorylation appears to facilitate an open conformation, exposing the C1 domain and the catalytic region (Santos et al., 2002; Nelson et al., 2007); this would facilitate membrane–protein interactions to further control DGKζ subcellular localization, as well as regulation of restricted DAG pools. In accordance with this hypothesis, the SA mutant lacking the C-terminal region translocated to the PM during antigen presentation.
Several studies identified PKCα as the kinase responsible for DGKζ phosphorylation at the MARCKS domain (Luo et al., 2003). Our data showed direct recruitment of these two enzymes to the TCR complex. There were nonetheless striking differences in DGKζ dynamics during IS formation compared with other models in which PKCα strictly controls GFP-DGKζ translocation. Indeed, pharmacological inhibition of PKC activity under the conditions tested blocked neither GFP-DGKζ membrane localization nor recruitment of the endogenous enzyme to the TCR complex. This could suggest that kinases other than PKC can activate DGKζ, possibly by releasing the negative restriction imposed by the C-terminal region.
PMA cotreatment does not promote further recruitment to the TCR but activates recruited DGKζ, suggesting further DAG-mediated regulation at this site. Changes in DGK activity did not correlate with enhanced DGKζ recruitment, indicating that this activation was due to posttranslational modifications and/or protein–protein interactions at the IS. This regulatory mechanism would enhance DGKζ activation at the synapse as a direct result of local DAG changes and warrants further analysis. PMA activates critical DAG-dependent targets, including PKC and RasGRP1 (Geiger et al., 2003). PKCθ is the main isoform recruited to the T cell synapse (Isakov and Altman, 2002), although our data show that endogenous PKCα is also recruited to the TCR complex. While BIM treatment alters the DGKζ migration pattern, suggesting an altered phosphorylation status, it does not block activity. Other DAG-regulated kinases, such as ERK or novel PKC, should also be considered, since DGKζ has consensus sites for ERK and PKC phosphorylation that could contribute to the changes seen in enzyme activity (Abramovici et al., 2003). An alternative possibility to be considered, based on studies of non-T cells, is that recruited PKCα associates with DGKζ and alters its activation status (Luo et al., 2003). PMA would release this association, maintaining PKCα and DGKζ in active states, whereas BIM would promote association of inactive PKCα with DGKζ. All of these are positive feedback mechanisms, which would guarantee adequate DGKζ activation at the site of DAG generation.
Localized DAG production not only acts as a signaling platform to recruit DAG effectors, but it also assures polarization of the microtubule-organizing center (MTOC; Quann et al., 2009). Pharmacological inhibition of DGK in primary T cells affects DAG gradient formation and MTOC reorientation, suggesting that DGK activity helps to prevent DAG diffusion across the PM (Quann et al., 2009). DGKζ distribution to the entire PM following T cell–APC contact hinted at a role for this isoform in the control of DAG production outside the IS. Use of a DAG sensor enabled direct evaluation of the functional effect of DGKζ on DAG levels during TCR activation. Our results demonstrate that although DGKζ relocates to the entire PM, DAG is consumed at IS, which correlates with recruitment of active DGKζ to the TCR complex. Indeed, the kinase-inactive DGKζ did not disrupt DAG pool formation at the IS, thus confirming that enzyme activity is needed to control DAG levels at the IS. In addition, membrane localization was required both for DAG consumption and for DGKζ to exert its negative control over T cell signal as detected by TCR-dependent IL-2 induction.
In summary, our results indicate that DGKζ recruitment to the membrane is important for maintaining negative control of DAG accumulation at the IS. On the basis of our data, we propose a model in which mature DGKζ in cytosol relocates to the PM in response to T cell–APC encounter; DAG production at the IS catalyzes its enzymatic activity to restrict local DAG levels. These findings correlate with the proposed role of DGKζ in mouse models and suggest that isoform-specific inhibitors would be beneficial in modulating the amplitude of the immune response.
MATERIALS AND METHODS
Reagents and antibodies
Leupeptin and aprotinin were purchased from Roche (Indianapolis, IN). Orthovanadate, phenylmethylsulfonyl fluoride, poly-d-lysine, paraformaldehyde, Igepal CA-630 (NP40), PMA, and BIM were from Sigma-Aldrich (St. Louis, MO). U73122, R59949. and PP2 were all from Calbiochem (La Jolla, CA). Purified mouse anti–human CD28 (CD28.2) and mouse anti–human CD3 (HIT3a) were from BD PharMingen (San Diego, CA). Anti-mouse immunoglobulin G (IgG)–coated Dynabeads and anti-GFP antibody were from Invitrogen (Carlsbad, CA). Antibody to endogenous DGKζ and DGKα were kind gifts of Matthew Topham (Huntsman Cancer Institute, Salt Lake City, UT) and Wim J. Van Blitterswijk (Netherlands Cancer Institute, Amsterdam, Netherlands), respectively; anti-PKCα was from Santa Cruz Biotechnology (Santa Cruz, CA), anti-pERK1/2, anti-pZAP70 (Y319), and anti-psrc (Y416) were from Cell Signaling Technology (Beverly, MA). Horseradish peroxidase–conjugated goat anti–mouse and rat IgG were from Dako (Glostrup, Denmark). Lipids for DGK assays were from Avanti Polar Lipids (Alabaster, AL).
Isolation of TCR immune complexes
Jurkat T cells in exponential growth were collected at 10–20 × 106 cells/ml and serum-starved in DMEM with 0.1% bovine serum albumin (BSA; 2 h). Anti-CD3 and anti-CD28 (5 μg/ml) were added and incubated (10 min, on ice). Anti–mouse IgG–coated Dynabeads (40 μl) were mixed and incubated (10 min, on ice). Bead–cell complexes were allowed to activate (37°C, 0–30 min or as indicated) and reactions terminated by washing in ice-cold phosphate-buffered saline (PBS). Where stated, cells were preincubated with specific inhibitor (37°C, 30 min). Cell–bead complexes were lysed in Brij lysis buffer on ice (0.33% Brij 96V, 20 mM Tris-HCl, pH 8, 150 mM NaCl, 2 mM EDTA, 1 mM MgCl2, 1 mM NaF, containing inhibitor cocktail [10 μg/ml aprotinin, 10 μg/ml leupeptin, 2 mM sodium orthovanadate, and 10 mM β-glycerophosphate]) and washed three times in Brij lysis buffer and once in 50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), pH 7.4. For in vitro kinase assays, TCR-associated complexes were used as an enzyme source; for Western blot, TCR-bound proteins were eluted in Laemmli sample buffer with boiling. Protein complexes were analyzed by Western blot using protein-specific antibodies.
DGK activity assay
Lipid kinase activity was measured at the TCR complex using two conditions. The first one was unbiased and permits the measurement of broad DGK activity, as previously described, with minor modifications (Flores et al., 1996). Briefly, TCR immune complexes were incubated with DAG micelles (0.2 mg/ml 1,2-dioctanoyl-sn-glycerol [C8:0]) in 50 mM HEPES, pH 7.4, and sonicated for 15 min. In addition, we further determined DGKα contribution by measuring DGK activity as in Sakane et al. (1991). DAG micelles were formed in octylglucoside (1.2 mg/ml 1,2-dioleoyl-sn-glycerol [C18:1]) and 1.6 mg/ml l-α-phosphatidylserine) and dried; lipids were then resuspended in 0.16 M octylglucoside (50 μM final concentration in the assay) and incubated (15 min, 37°C). Kinase reactions (50 μl final volume) were initiated by addition of the kinase reaction mixture (assay I was carried out with 20 μM ATP, 10 mM MgCl2, 10 μCi [γ32P]ATP, 50 mM HEPES, pH 7.4; assay II was performed with 1 mM ATP, 10 mM MgCl2, 100 mM NaCl, 1 mM dithiothreitol, 10 μCi of [γ32P]ATP, 50 mM HEPES, pH 7.4, and 30 μM CaCl2). Reactions continued for 15 min at room temperature and were terminated with 50 μl of 1 M HCl and 100 μl of MeOH. Lipids were extracted in CHCl3 phase and washed once in 1:1 (vol/vol) HCl/MeOH to remove free [γ32P]ATP. Lipids were dried in a SpeedVac, resuspended in 1:1 (vol/vol) MeOH/CHCl3, and resolved by TLC using silica gel plates in 9:7:2 CHCl3/MeOH/4 M NH4OH solvent. PA generation was detected in a phosphorimager (Bio-Rad, Hercules, CA) and quantified using the Quantity One program. Statistical analyses show measurements from at least three independent experiments, normalized to time point 0, corresponding to control cells.
Cell transfection and short hairpin RNA
Jurkat T cells were maintained in DMEM supplemented with 10% fetal bovine serum (FBS) and 2 mM l‑glutamine (both from Sigma-Aldrich) at 37°C, 5% CO2. Cells were collected in exponential growth (1.2 × 107 cells in 400 μl) and transfected by electroporation with 20 μg (for protein expression) or 30 μg (for short hairpin RNA) of plasmid DNA using a Gene Pulser (975 μF, 270 mV; Bio-Rad), as described (Rincon et al., 2007). Plasmids used for overexpression studies, encoding wild-type DGKα and DGKζ fused to GFP, or bearing specific mutations, were reported elsewhere (Sanjuan et al., 2001; Santos et al., 2002). The PKCθ C1ab region was excised from the PKCθ C1ab-GFP construct described elsewhere (Carrasco and Merida, 2004); actin fused to Cherry was a kind gift of Ann Wheeler (Queen Mary University, London, United Kingdom). At 24 h posttransfection, cells were washed and used, or allowed to expand for 24 h. For knockdown analysis of DGKα or DGKζ, we used specific RNAi to human forms and a negative control that targets murine DGKα (Rincon et al., 2007; A. Avila-Flores and I. Merida, unpublished data), which were cloned into a p-Super Retro plasmid. For maximal down-modulation of target proteins, cells were assayed at 72 or 96 h posttransfection.
Immunofluorescence
Jurkat T cells were preincubated with antibody-coated beads on poly-d-lysine–coated coverslips, fixed in 4% paraformaldehyde (20 min), and permeabilized in 0.2% Triton X100/PBS (5 min, on ice). After washing, fixed cells were blocked in 2% BSA (15 min), incubated with primary antibody (1:50 in 3% FBS/PBS, 30 min, 37°C in a humidified chamber), washed in PBS, and incubated with Alexa-conjugated secondary antibody (1:500, 30 min, 37°C). F-actin was detected with phalloidin-rhodamine (Molecular Probes, Invitrogen (15 min, 37°C) and washed in PBS prior to mounting. Images were captured using a Zeiss (Thornwood, NY) confocal microscope.
Luciferase reporter assay
Jurkat T cells were transiently cotransfected with 15 μg of GFP-tagged constructs (GFP, GFP-DGKζ-WT, GFP-DGKζ-SD, GFP-DGKζ-SA) and 15 μg of IL-2-luciferase reporter construct/10 ng Renilla luciferase as internal standard. At 48 h posttransfection, cells were incubated with APC nonpulsed or pulsed with 1 μg/ml SEE at a 1:1 ratio (total 2 × 106 cells) for 24 h. Each reading was normalized internally to Renilla and the percentage induction represented; GFP cells incubated with SEE-loaded APC were considered 100%.
Time-lapse fluorescence microscopy
Chambers were precoated with poly-d-lysine and Jurkat T cells prepared at 0.5 × 106 cells/ml in HBSS buffer (25 mM HEPES-KOH, pH 7.4, 1 mM MgCl2, 1 mM CaCl2, 132 mM NaCl, 0.1% BSA) containing 1% FBS (15 min), then placed on a microscope stage at 37°C. Raji B cells stained with 7-amino-4-chloromethylcoumarin (CMAC) were preincubated with 1 μg/ml SEE superantigen or left untreated and were added at a 1:1 ratio to adhered Jurkat T cells. Videos were captured on an FV1000 confocal laser-scanning microscope (Olympus, Center Valley, PA) and images processed using ImageJ, version 1.43u (National Institutes of Health, Bethesda, MD), and Adobe Photoshop (San Jose, CA). Still images were captured after 15 min of incubation of Jurkat T cells with pretreated APC.
Analysis of protein accumulation at the T cell–APC contact area
To quantify the amount of GFP-DGKζ or C1-GFP accumulated at the IS compared with cytosol, we used an ImageJ plugin (Rincon et al., 2011) that measures average intensity value of the image in a small circular area; we monitored background (Bg), cytosol of the T cell (T), and the synapse (S) when the fluorescent protein was expressed by only one of the two cells. Average pixel value was computed for each measurement (Bg, T, S). From these observed values, we separated the contribution of each component (background, T cell, and T cell at the synapse). Finally, we computed the ratio between fluorescence at the synapse and in cytosol. Ratio values were represented as dot plots, with each dot representing an individual cell.
Statistical analysis
To analyze the fluorescence intensity of GFP-DGKζ or of Cherry-C1ab at the IS, we used a two-sample Kolmogorov–Smirnov test to compare pairs of distribution ratios (IS/T) from different cells. The Bonferroni correction was applied to confidence thresholds to correct for multiple comparisons. Differences were considered nonsignificant (ns) when p > 0.05, significant (*) when p < 0.05, very significant (**) when p < 0.01, and extremely significant (***) when p < 0.001.
Supplementary Material
Acknowledgments
We thank colleagues who generously provided reagents; group members; J. Millán and M. Valés-Gómez for input on the manuscript; R. Arcos for excellent technical support; and C. Mark for editorial assistance. S.G. holds a JAE-DOC fellowship from the Consejo Superior de Investigaciones Científicas. A.A.F. is an Asociación Española Contra el Cáncer Fellow. M.A. and E.R. are recipients of Madrid Regional Government fellowships. P.T.A. is a Spanish Ministry of Education Fellow. This work was supported in part by Grants RD067002071035 from the Spanish Ministry of Health (Instituto de Salud Carlos III), BFU2010-21138 from the Spanish Ministry of Education, and S-SAL-0311 from Comunidad de Madrid to I.M.
Abbreviations used:
- APC
antigen-presenting cell
- DAG
diacylglycerol
- DGK
diacylglycerol kinase
- IS
immunological synapse
- PA
phosphatidic acid
- PKC
protein kinase C
- PM
plasma membrane
- PMA
phorbol-12-myristate-13-acetate
- TCR
T cell receptor
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-03-0247) on September 21, 2011.
REFERENCES
- Abramovici H, Hogan A, Obagi C, Topham M, Gee S. Diacylglycerol kinase-zeta localization in skeletal muscle is regulated by phosphorylation and interaction with syntrophins. Mol Biol Cell. 2003;14:4499–4511. doi: 10.1091/mbc.E03-03-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal AM, Anikeeva N, Varma R, Cameron TO, Norris PJ, Dustin ML, Sykulev Y. Protein kinase C theta regulates stability of the peripheral adhesion ring junction and contributes to the sensitivity of target cell lysis by CTL. J Immunol. 2008;181:4815–4824. doi: 10.4049/jimmunol.181.7.4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyers AD, Spruyt LL, Williams AF. Molecular associations between the T-lymphocyte antigen receptor complex and the surface antigens CD2, CD4, or CD8 and CD5. Proc Natl Acad Sci USA. 1992;89:2945–2949. doi: 10.1073/pnas.89.7.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco S, Merida I. Diacylglycerol-dependent binding recruits PKCtheta and RasGRP1 C1 domains to specific subcellular localizations in living T lymphocytes. Mol Biol Cell. 2004;15:2932–2942. doi: 10.1091/mbc.E03-11-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco S, Merida I. Diacylglycerol, when simplicity becomes complex. Trends Biochem Sci. 2007;32:27–36. doi: 10.1016/j.tibs.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Chibalin AV, et al. Downregulation of diacylglycerol kinase delta contributes to hyperglycemia-induced insulin resistance. Cell. 2008;132:375–386. doi: 10.1016/j.cell.2007.12.035. [DOI] [PubMed] [Google Scholar]
- de Wet B, Zech T, Salek M, Acuto O, Harder T. Proteomic characterization of plasma membrane-proximal T cell activation responses. J Biol Chem. 2011;286:4072–4080. doi: 10.1074/jbc.M110.165415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebinu JO, Stang SL, Teixeira C, Bottorff DA, Hooton J, Blumberg PM, Barry M, Bleakley RC, Ostergaard HL, Stone JC. RasGRP links T-cell receptor signaling to Ras. Blood. 2000;95:3199–3203. [PubMed] [Google Scholar]
- Flores I, Casaseca T, Martinez AC, Kanoh H, Merida I. Phosphatidic acid generation through interleukin 2 (IL-2)-induced alpha-diacylglycerol kinase activation is an essential step in IL-2-mediated lymphocyte proliferation. J Biol Chem. 1996;271:10334–10340. doi: 10.1074/jbc.271.17.10334. [DOI] [PubMed] [Google Scholar]
- Fooksman DR, Vardhana S, Vasiliver-Shamis G, Liese J, Blair DA, Waite J, Sacristan C, Victora GD, Zanin-Zhorov A, Dustin ML. Functional anatomy of T cell activation and synapse formation. Annu Rev Immunol. 2010;28:79–105. doi: 10.1146/annurev-immunol-030409-101308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser J, Arcus V, Kong P, Baker E, Proft T. Superantigens—powerful modifiers of the immune system. Mol Med Today. 2000;6:125–132. doi: 10.1016/s1357-4310(99)01657-3. [DOI] [PubMed] [Google Scholar]
- Friedl P, Storim J. Diversity in immune-cell interactions: states and functions of the immunological synapse. Trends Cell Biol. 2004;14:557–567. doi: 10.1016/j.tcb.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Geiger M, Wrulich OA, Jenny M, Schwaiger W, Grunicke HH, Uberall F. Defining the human targets of phorbol ester and diacylglycerol. Curr Opin Mol Ther. 2003;5:631–641. [PubMed] [Google Scholar]
- Guo R, Wan CK, Carpenter JH, Mousallem T, Boustany RM, Kuan CT, Burks AW, Zhong XP. Synergistic control of T cell development and tumor suppression by diacylglycerol kinase alpha and zeta. Proc Natl Acad Sci USA. 2008;105:11909–11914. doi: 10.1073/pnas.0711856105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder T, Kuhn M. Immunoisolation of TCR signaling complexes from Jurkat T leukemic cells. Sci STKE. 2001;2001:pl1. doi: 10.1126/stke.2001.71.pl1. [DOI] [PubMed] [Google Scholar]
- Isakov N, Altman A. Protein kinase C(theta) in T cell activation. Annu Rev Immunol. 2002;20:761–794. doi: 10.1146/annurev.immunol.20.100301.064807. [DOI] [PubMed] [Google Scholar]
- Kakefuda K, et al. Diacylglycerol kinase beta knockout mice exhibit lithium-sensitive behavioral abnormalities. PLoS One. 2010;5:e13447. doi: 10.1371/journal.pone.0013447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Weiss A. T cell receptor signalling. J Cell Sci. 2001;114:243–244. doi: 10.1242/jcs.114.2.243. [DOI] [PubMed] [Google Scholar]
- Luo B, Prescott S, Topham M. Protein kinase C alpha phosphorylates and negatively regulates diacylglycerol kinase zeta. J Biol Chem. 2003;278:39542–39547. doi: 10.1074/jbc.M307153200. [DOI] [PubMed] [Google Scholar]
- Merida I, Avila-Flores A, Merino E. Diacylglycerol kinases: at the hub of cell signalling. Biochem J. 2008;409:1–18. doi: 10.1042/BJ20071040. [DOI] [PubMed] [Google Scholar]
- Nelson CD, Perry SJ, Regier DS, Prescott SM, Topham MK, Lefkowitz RJ. Targeting of diacylglycerol degradation to M1 muscarinic receptors by beta-arrestins. Science. 2007;315:663–666. doi: 10.1126/science.1134562. [DOI] [PubMed] [Google Scholar]
- Olenchock BA, Guo R, Carpenter JH, Jordan M, Topham MK, Koretzky GA, Zhong XP. Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nat Immunol. 2006;7:1174–1181. doi: 10.1038/ni1400. [DOI] [PubMed] [Google Scholar]
- Quann EJ, Liu X, Altan-Bonnet G, Huse M. A cascade of protein kinase C isozymes promotes cytoskeletal polarization in T cells. Nat Immunol. 2011;12:647–654. doi: 10.1038/ni.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quann EJ, Merino E, Furuta T, Huse M. Localized diacylglycerol drives the polarization of the microtubule-organizing center in T cells. Nat Immunol. 2009;10:627–635. doi: 10.1038/ni.1734. [DOI] [PubMed] [Google Scholar]
- Rincon E, Saez de Guinoa J, Gharbi SI, Sorzano CO, Carrasco YR, Merida I. Translocation dynamics of sorting nexin 27 in activated T cells. J Cell Sci. 2011;124:776–788. doi: 10.1242/jcs.072447. [DOI] [PubMed] [Google Scholar]
- Rincon E, Santos T, Avila-Flores A, Albar JP, Lalioti V, Lei C, Hong W, Merida I. Proteomics identification of sorting nexin 27 as a diacylglycerol kinase zeta-associated protein: new diacylglycerol kinase roles in endocytic recycling. Mol Cell Proteomics. 2007;6:1073–1087. doi: 10.1074/mcp.M700047-MCP200. [DOI] [PubMed] [Google Scholar]
- Sakane F, Yamada K, Imai S, Kanoh H. Porcine 80-kDa diacylglycerol kinase is a calcium-binding and calcium/phospholipid-dependent enzyme and undergoes calcium-dependent translocation. J Biol Chem. 1991;266:7096–7100. [PubMed] [Google Scholar]
- Sanjuan MA, Jones DR, Izquierdo M, Merida I. Role of diacylglycerol kinase alpha in the attenuation of receptor signaling. J Cell Biol. 2001;153:207–220. doi: 10.1083/jcb.153.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjuan MA, Pradet-Balade B, Jones DR, Martinez AC, Stone JC, Garcia-Sanz JA, Merida I. T cell activation in vivo targets diacylglycerol kinase alpha to the membrane: a novel mechanism for Ras attenuation. J Immunol. 2003;170:2877–2883. doi: 10.4049/jimmunol.170.6.2877. [DOI] [PubMed] [Google Scholar]
- Santos T, Carrasco S, Jones DR, Merida I, Eguinoa A. Dynamics of diacylglycerol kinase zeta translocation in living T-cells. Study of the structural domain requirements for translocation and activity. J Biol Chem. 2002;277:30300–30309. doi: 10.1074/jbc.M200999200. [DOI] [PubMed] [Google Scholar]
- Siliceo M, Merida I. T cell receptor-dependent tyrosine phosphorylation of beta2-chimaerin modulates its Rac-GAP function in T cells. J Biol Chem. 2009;284:11354–11363. doi: 10.1074/jbc.M806098200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims TN, et al. Opposing effects of PKCtheta and WASp on symmetry breaking and relocation of the immunological synapse. Cell. 2007;129:773–785. doi: 10.1016/j.cell.2007.03.037. [DOI] [PubMed] [Google Scholar]
- Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitaler M, Emslie E, Wood CD, Cantrell D. Diacylglycerol and protein kinase D localization during T lymphocyte activation. Immunity. 2006;24:535–546. doi: 10.1016/j.immuni.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Viola A, Schroeder S, Sakakibara Y, Lanzavecchia A. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 1999;283:680–682. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- Yakubchyk Y, Abramovici H, Maillet JC, Daher E, Obagi C, Parks RJ, Topham MK, Gee SH. Regulation of neurite outgrowth in N1E-115 cells through PDZ-mediated recruitment of diacylglycerol kinase zeta. Mol Cell Biol. 2005;25:7289–7302. doi: 10.1128/MCB.25.16.7289-7302.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong XP, Guo R, Zhou H, Liu C, Wan CK. Diacylglycerol kinases in immune cell function and self-tolerance. Immunol Rev. 2008;224:249–264. doi: 10.1111/j.1600-065X.2008.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong XP, Hainey EA, Olenchock BA, Jordan MS, Maltzman JS, Nichols KE, Shen H, Koretzky GA. Enhanced T cell responses due to diacylglycerol kinase zeta deficiency. Nat Immunol. 2003;4:882–890. doi: 10.1038/ni958. [DOI] [PubMed] [Google Scholar]
- Zhong XP, Hainey EA, Olenchock BA, Zhao H, Topham MK, Koretzky GA. Regulation of T cell receptor-induced activation of the Ras-ERK pathway by diacylglycerol kinase zeta. J Biol Chem. 2002;277:31089–31098. doi: 10.1074/jbc.M203818200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.