Abstract
The lipid-lowering agent pravastatin and the antidepressant paroxetine are among the most widely prescribed drugs in the world. Unexpected interactions between them could have important public health implications. We mined the US Food and Drug Administration’s (FDA’s) Adverse Event Reporting System (AERS) for side-effect profiles involving glucose homeostasis and found a surprisingly strong signal for comedication with pravastatin and paroxetine. We retrospectively evaluated changes in blood glucose in 104 patients with diabetes and 135 without diabetes who had received comedication with these two drugs, using data in electronic medical record (EMR) systems of three geographically distinct sites. We assessed the mean random blood glucose levels before and after treatment with the drugs. We found that pravastatin and paroxetine, when administered together, had a synergistic effect on blood glucose. The average increase was 19 mg/dl (1.0 mmol/l) overall, and in those with diabetes it was 48 mg/dl (2.7 mmol/l). In contrast, neither drug administered singly was associated with such changes in glucose levels. An increase in glucose levels is not a general effect of combined therapy with selective serotonin reuptake inhibitors (SSRIs) and statins.
Drug interactions are common and usually result from shared pathways of metabolism or intersecting pathways of drug action. Although some interactions can be predicted through analysis of these pathways, others become apparent only empirically, often after the drugs are on the market. Interactions can lead to precautions in prescribing, absolute contraindications for combination use, or even drug withdrawal. In particular, understanding drug interactions between commonly prescribed drugs is of great clinical importance.
Depression and hypercholesterolemia are two of the most commonly treated conditions in the developed world; it is estimated that more than 30 million1 people have the former condition and more than 100 million2 have the latter condition in the United States alone. Over the past 15 years, selective serotonin reuptake inhibitors (SSRIs) have emerged as a first-line treatment for depression, and 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitors (statins) are the first-line treatment for hypercholesterolemia. In 2009 alone, there were an estimated 15 million prescriptions for paroxetine and 18 million prescriptions for pravastatin in the United States.3 There are undoubtedly many patients who are on both medications at the same time.
Type 2 diabetes mellitus (T2DM) is a well-recognized disease that has reached epidemic proportions in the developed world. The diagnostic criteria for T2DM rely fundamentally on blood glucose measurements, and these measurements are used to track the development of diabetes from insulin resistance.4 Therefore, it is important to detect drug interactions that may alter serum glucose levels.
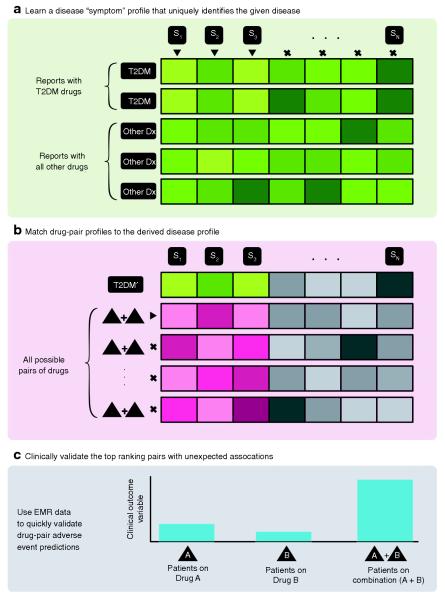
The challenge of discovering drug interactions is exacerbated because large clinical trials routinely focus on establishing the effects of single drugs. Unpredictable combinatorial effects can be identified only through postmarketing surveillance and signal detection.5 Spontaneous reporting systems such as the US Food and Drug Administration’s (FDA’s) Adverse Event Reporting System (AERS) provide an opportunity to study interaction effects. However, if the occurrence of the interaction-related adverse event is rare (or rarely reported), traditional signal detection algorithms5,6 may miss these interactions. These algorithms are limited in that they require the adverse events to have large effects and to be reported explicitly.7 We reasoned that algorithms that identify the presence of an adverse event through an analysis of aggregate side effects (rather than direct reporting) could be more successful. With this in mind, we developed a novel signal detection algorithm for the identification of drug–drug interactions from adverse event reports in spontaneous reporting systems, using an analysis of latent signals that indirectly provide evidence for an adverse event (Figure 1). We used this algorithm to study drug interactions with diabetes-related effects. We found an intriguing suggestion that pravastatin and paroxetine taken together may affect glucose homeostasis. Neither drug is typically associated with significant changes in glucose levels. We therefore studied this potential interaction in three independent cohorts of patients at academic medical centers with extensive electronic medical records (EMRs). In this retrospective observational study, all the patients were also on a variety of other medications.
Figure 1.
Methodological overview of latent signal detection algorithm. (a) Learning the side-effect profile of a disease. We assessed each adverse event reported in the Adverse Event Reporting System, Si, for significant enrichment on reports involving a type 2 diabetes mellitus (T2DM) drug (identified by the triangle indicators) vs. a background of all drugs and determined the significance by using a Fisher’s exact test. We call the result of this analysis the “disease’s side-effect profile.” (b) We then extracted reports for all possible pairs of drugs (~40,000) and scored each drug pair’s side-effect profile for its similarity to the disease’s profile. Note that we considered only the adverse events that were significantly correlated with the disease (colored boxes, i.e., S1, S2, and S3) and not others (gray boxes). We ranked each drug pair according to its similarity score, and (c) clinically validated the top-ranked pairs of drugs for interaction effects on a predetermined phenotype extracted from the electronic medical records (EMRs) (e.g., random blood glucose concentration). Dx, diagnosis.
RESULTS
Data mining and clinical screening of patients without diabetes
We examined 12,627 AERs for 37 drugs with diabetes-related effects that had a minimum of 10 AERs each. We used these reports to derive an adverse-event profile for diabetes-related effects (see Supplementary Figure S2a online). We applied this model to pairs of drugs, seeking pairs that together created side effects reminiscent of diabetes; the top four pairs that most closely matched this profile were mefloquine and sertraline, allopurinol and bendamustine, acetaminophen and trastuzumab, and paroxetine and pravastatin. We focused on paroxetine and pravastatin because the other three pairs are used together only infrequently. From the EMRs at Stanford University Hospital (see Supplementary Methods online), we extracted glucose measurements, both at baseline and at random time points during treatment, for 374 diabetes-free patients on paroxetine, 449 on pravastatin, and 8 on a comedication regimen involving both paroxetine and pravastatin. We conducted an analysis of covariance (ANCOVA) on the changes in blood glucose levels between baseline and values at random time points during treatment in three patient cohorts: (i) patients on paroxetine but not pravastatin, (ii) patients on pravastatin but not paroxetine, and (iii) patients on both pravastatin and paroxetine. We found that the treatment condition was a significant covariate (F = 6.8, P = 0.001) in the model, with patients on the combination treatment having the greatest increase, 22.6 mg/dl (1.3 mmol/l), followed by those on pravastatin without paroxetine, 3.3 mg/dl (0.2 mmol/l), and, finally, those on paroxetine without pravastatin, 0.8 mg/dl (0.0 mmol/l). The combination treatment cohort showed a significantly greater change in blood glucose in post hoc tests as compared with either the pravastatin cohort (P = 0.008) or the paroxetine cohort (P = 0.036). We investigated this potential interaction further at two separate clinical sites with EMRs.
Cohort characteristics
Patient demographic data are provided in Table 1. Some biases in the patient demographic data exist. For example, the group receiving paroxetine-only treatment had a slightly higher percentage of women than the pravastatin-only group (59% vs. 54%) and patients taking pravastatin alone were slightly older than those taking paroxetine alone. We included all available demographic data in the ANCOVA model to account for their potential effects on blood glucose levels.
Table 1. Demographic and clinical characteristics of cohorts.
| Variable | site 1 | site 2 | site 3 |
|---|---|---|---|
| Combination | |||
| N | 8 | 18 | 109 |
| Demographic | |||
| gAe (mean ± SD) | 63.1 ± 10.1 | 55.1 ± 9.4 | 73.9 ± 13.1 |
| Sex (% female) | 88 | 78 | 66 |
| Race (% of group) | |||
| White | 63 | 94 | 93 |
| African American | 0 | 6 | 2 |
| Other/unknown | 38 | 0 | 5 |
| Paroxetine only | |||
| N | 374 | 449 | 780 |
| Demographic | |||
| Age (mean ± SD) | 59.0 ± 17.3 | 48.7 ± 18.0 | 67.9 ± 14.5 |
| Sex (% female) | 59 | 57 | 49 |
| Race (% of group) | |||
| White | 62 | 86 | 90 |
| African American | 3 | 9 | 2 |
| Other/unknown | 35 | 5 | 8 |
| Pravastatin only | |||
| N | 449 | 982 | 632 |
| Demographic | |||
| Age (mean ± SD) | 63.8 ± 16.2 | 55.3 ± 16.3 | 67.9 ± 14.1 |
| Sex (% female) | 54 | 40 | 48 |
| Race (% of group) | |||
| White | 63 | 80 | 90 |
| African American | 5 | 15 | 2 |
| Other/unknown | 33 | 5 | 8 |
Replication sites
We extracted blood glucose measurements, both at baseline and at random time points during treatment, for 449 patients on pravastatin, 982 on paroxetine, and 18 on a combination regimen of paroxetine and pravastatin from the EMR data at Vanderbilt Hospital (see Supplementary Methods online). We did the same for 632 patients on pravastatin, 780 on paroxetine, and 109 on the combination regimen from the EMR data at Partners HealthCare (see Supplementary Methods online). For each data set, we conducted an ANCOVA analysis to compare the effects on random blood glucose levels among the three treatment groups. We found that treatment group was a significant covariate with regard to the data from both Vanderbilt (F = 6.8, P = 0.001) and Partners (F = 38.7, P < 2.2 × 10−16). We observed increases of 26.1 mg/dl (1.4 mmol/l) and 17.0 mg/dl (0.9 mmol/l) in the average glucose levels in the combination regimen cohort from the data from Vanderbilt and at Partners, respectively. In the paroxetine-only cohort, the corresponding glucose level changes were −1.5 mg/dl (−0.1 mmol/l) and −6.3 mg/dl (−0.3 mmol/l), and in the pravastatin-only cohort, −1.3 mg/dl (−0.1 mmol/l) and −3.2 mg/dl (−0.2 mmol/l) for the data from Vanderbilt and Partners, respectively. In post hoc tests the combination treatment had a significantly greater effect on random blood glucose as compared with either paroxetine alone (P = 0.009 and P < × 10−10) or pravastatin alone (P = 0.002 and P < 1 × 10−10) in the two data sets. Table 2 and Figure 2 summarize these results.
Table 2. Baseline and treatment blood glucose levels and ANCOVA results.
| Variable | Combination | paroxetine only | pravastatin only | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Site 1 | |||||||||
| N | 8 | 374 | 449 | ||||||
| Glucose (mg/dl mean, 95% CI) | |||||||||
| Baseline (base) | 112.6 | (101.7, 123.6) | 117.1 | (113.9, 120.3) | 120.2 | (117.3, 123.1) | |||
| mmol/l | 6.2 | (5.6, 6.9) | 6.5 | (6.3, 6.7) | 6.7 | (6.5, 6.8) | |||
| After treatment(s) (post) | 135.2 | (121.4, 149.0) | 117.9 | (115.0, 120.8) | 123.5 | (120.8, 126.2) | |||
| mmol/l | 7.5 | (6.7, 8.3) | 6.5 | (6.4, 6.7) | 6.9 | (6.7, 7.0) | |||
| Change (post-base) | 22.6 | (6.1, 39.1) | 0.8 | (−1.9, 3.5) | 3.3 | (1.0, 5.6) | |||
| mmol/l | 1.3 | (0.3, 2.2) | 0.0 | (−0.1, 0.2) | 0.2 | (0.1, 0.3) | |||
| ANCOVA F-test P value | F = 6.8, P = 0.001 | ||||||||
| Post hoc test vs. combination P value | – | 0.008 | 0.036 | ||||||
| Site 2 | |||||||||
| N | 18 | 449 | 982 | ||||||
| Glucose (mg/dl mean, 95% CI) | |||||||||
| Baseline (base) | 98.8 | (89.3, 108.3) | 108.8 | (106.1, 111.5) | 105.0 | (103.6, 106.4) | |||
| mmol/l | 5.5 | (5.0, 6.0) | 6.0 | (5.9, 6.2) | 5.8 | (5.7, 5.9) | |||
| After treatment(s) (post) | 124.9 | (101.8, 148.0) | 107.4 | (104.4, 110.4) | 103.8 | (102.2, 105.4) | |||
| mmol/l | 6.9 | (5.6, 8.2) | 6.0 | (5.8, 6.1) | 5.8 | (5.7, 5.8) | |||
| Change (post-base) | 26.1 | (5.4, 46.8) | −1.5 | (−4.9, 1.9) | −1.3 | (−3.1, 0.5) | |||
| mmol/l | 1.4 | (0.3, 2.6) | −0.1 | (−0.3, 0.1) | −0.1 | (−0.2, 0.0) | |||
| ANCOVA F-test P value | F = 6.8, P = 0.001 | ||||||||
| Post hoc test vs. combination P value | – | 0.009 | 0.002 | ||||||
| Site 3 | |||||||||
| N | 109 | 780 | 632 | ||||||
| Glucose (mg/dl mean, 95% CI) | |||||||||
| Baseline (base) | 111.7 | (106.9, 116.5) | 108.5 | (106.9, 110.1) | 106.2 | (104.5, 107.9) | |||
| mmol/l | 6.2 | (5.9, 6.5) | 6.0 | (5.9, 6.1) | 5.9 | (5.8, 6.0) | |||
| After treatment(s) (post) | 128.7 | (116.7, 140.7) | 102.2 | (100.9, 103.5) | 103.0 | (101.2, 104.8) | |||
| mmol/l | 7.1 | (6.5, 7.8) | 5.7 | (5.6, 5.7) | 5.7 | (5.6, 5.8) | |||
| Change (post-base) | 17.0 | (6.1, 27.9) | −6.3 | (−7.9, -4.7) | −3.2 | (−5.3, -1.1) | |||
| mmol/l | 0.9 | (0.3, 1.5) | −0.3 | (−0.4, -0.3) | −0.2 | (−0.3, -0.1) | |||
| ANCOVA F-test P value | F = 38.7, P < 2.2 × 10−16 | ||||||||
| Post hoc test vs. combination P value | – | <1 × 10−10 | <1 × 10−10 | ||||||
ANCOVA, analysis of covariance; CI, confidence interval.
Figure 2.
Combination treatment with paroxetine and pravastatin is associated with increase in blood glucose levels. Mean baseline and post-treatment blood glucose concentrations with 95% confidence intervals at each of the three independent replication sites are shown. Each site contained three patient cohorts, pravastatin-only (diamonds), paroxetine-only (circles), and pravastatin plus paroxetine (triangles). Site 3 contained the largest cohort of patients on combined pravastatin and paroxetine therapy (N = 109), followed by site 2 (N = 18), and site 1 (N = 8). The treatment condition was significant at each site per ANCOVA. In post hoc tests the paroxetine plus pravastatin combination cohorts at each site showed significantly greater increases in mean blood glucose levels relative to the cohorts of paroxetine-only (P = 0.008, P = 0.009, and P < 1 × 10−10) and pravastatin-only (P = 0.036, P = 0.002, and P < 1 × 10−10). The mean change in blood glucose in patients on the combined treatment was 23 mg/dl (1.3 mmol/l), 26 mg/dl (1.4 mmol/l), and 17 mg/dl (0.9 mmol/l) at sites 1, 2, and 3, respectively. The pravastatin-only cohorts had changes of 3.3 mg/dl (0.2 mmol/l), −1.3 mg/dl (−0.1 mmol/l), and −3.2 mg/dl (−0.2 mmol/l) at the three sites, respectively. The paroxetine-only cohorts had changes of 0.8 mg/dl (0.04 mmol/l), −1.5 mg/dl (−0.1 mmol/l), and −6.3 mg/dl (−0.3 mmol/l) at the three sites, respectively.
Three-site combined analysis
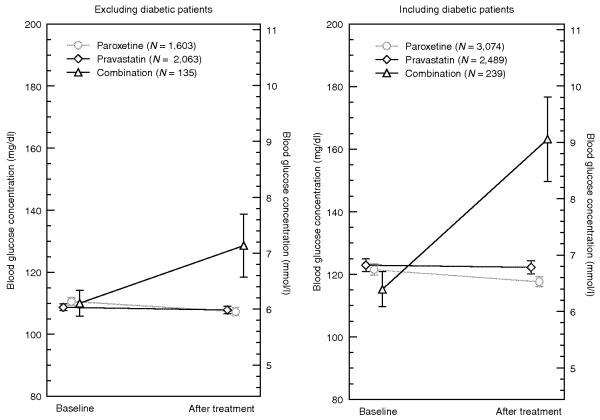
We combined the data across all three sites to increase statistical power and conducted a paired t-test analysis to evaluate the overall presence of an interaction effect. We found that patients on the combination regimen showed much larger increases in random blood glucose levels (18.5 mg/dl, 1.0 mmol/l, P = 1.5 × 10−4, N = 135) as compared with the groups on paroxetine alone (−3.3 mg/dl, −0.2 mmol/l, P = 3.3 × 10−6, N = 1,603) and pravastatin alone (−0.9 mg/dl, 0.0 mmol/l, P = 0.14, N = 2,063) (Figure 3, left panel). Institutional review board restrictions at each site did not allow for an ANCOVA of the combined data.
Figure 3.
Mean baseline and post-treatment blood glucose concentrations with 95% confidence intervals for a combined analysis of data from the three independent sites. The left panel shows the analysis excluding data relating to patients with diabetes, and the right panel shows the analysis including such data. Both combination cohorts (triangles) had significantly increased random blood glucose levels: 18.5 mg/dl (1.0 mmol/l), P < 0.001 after excluding data from patients with diabetes, and 48 mg/dl (2.7 mmol/l), P < 0.001 after including these data. No significant changes in glucose levels were observed in the pravastatinonly (diamonds) cohorts. A slight decrease in glucose levels was observed in the paroxetine-only (circles) cohorts in both analyses: −3.3 mg/dl (−0.2 mmol/l), P < 0.001, after excluding data from patients with diabetes, and −4 mg/dl (−0.2 mmol/l), P < 0.001 after including these data (Table 3).
Effect of baseline glucose, sex, age, and race
The ANCOVA revealed that none of the covariates (age, sex, and race) was significant in the model at Stanford; however, age was significant at both Vanderbilt (P = 1.8 × 10−8) and Partners (P = 0.01), whereas sex and race were not. We found a significant effect of baseline glucose on the change in glucose levels (P < 2.2 × 10−16 at all three sites).
Effect of order of treatment, time of day, and concomitant medications
We evaluated additional possible covariates in a univariate analysis in the Stanford cohort data. In the statistical covariate model, we included the order in which the drugs were administered if treatment began on different days, so as to account for the potential effects of individual drugs. The order in which the drugs were prescribed was not found to be a significant covariate (P = 0.42). The time of day at which the random glucose measurements were taken did not deviate significantly between baseline and treatment measurements (P = 0.62). In addition, we found no significant correlation between any of the concomitant medications and the changes in random blood glucose levels.
Other combinations of SSRIs and statins
We used a paired t-test to evaluate 13 other combinations of SSRIs and statins for their interaction effects on random glucose levels in patient cohorts without diabetes, from data extracted from the EMR at Stanford (see Supplementary Figure S3 online). Despite the adequate power (see Supplementary Table S1 online) provided by the sample size, only two drug combinations—fluoxetine plus atorvastatin and sertraline plus rosuvastatin—had significant effects on random blood glucose levels (6.6 mg/dl, 0.4 mmol/l, P = 0.008, and 12.2 mg/dl, 0.7 mmol/l, P = 0.04, respectively). However, a follow-up ANCOVA with post hoc testing revealed no significant effect from either of the drug pairs when covariates were included.
We grouped 324 patients who were receiving any SSRI other than paroxetine, and any statin other than pravastatin, and performed a paired t-test. We found a modest, yet significant, effect on blood glucose levels (1.7 mg/dl, 0.1 mmol/l, P = 0.02). We repeated the paired t-test analysis for 115 patients receiving paroxetine plus any statin and found no significant effect on blood glucose levels. Our analysis did reveal a significant effect in 38 patients who were on a combination of pravastatin plus any other SSRI (9.5 mg/dl, 0.5 mmol/l, P = 0.008). A follow-up ANCOVA with post hoc testing revealed no significant difference in the outcomes of the combination cohorts vs. the single-agent cohorts. These results are summarized in Supplementary Table S1 online and Supplementary Figure S2b online.
Extension of analysis to include diabetic patients
Using the same methods we had used for identification and extraction of the data pertaining to patients without diabetes, we identified 2,001 patients who had been diagnosed with diabetes and for whom there were sufficient data with regard to glucose measurements and drug exposures. When we included patients with and without diabetes in the cohorts, we observed larger increases in random blood glucose levels in the combination treatment cohort (48.1 mg/dl, 2.7 mmol/l, P = 5.5 × 10−12, N = 239) and corresponding changes in the single-agent therapy cohorts receiving pravastatin (−1 mg/dl, 0.0 mmol/l, P = 7.8 × 10−6, N = 3,074) and paroxetine (−3.3 mg/dl, −0.2 mmol/l, P = 0.48, N = 2,489). Institutional review board restrictions at each site did not allow for an ANCOVA of the combined data.
DISCUSSION
Doctors have observed for centuries that patients on multiple medications can have varied and unexpected adverse events. Some of these can be linked to the recognized adverse effects of an individual drug. Others can be traced to pairs of drugs that share a common pathway of metabolism (e.g., both are metabolized by a particular isoform of cytochrome P450), thereby rendering the “effective dose” very different from the intended one. Still others may result from two drugs interacting with redundant biological pathways, such that the effects are not additive but nonlinear or synergistic. This may happen, for example, when there are two parallel pathways that perform the same function. If one is blocked, the other can still perform the function and maintain homeostasis. However, if both are blocked, there is a sudden and dramatic decrease in the ability of the cell to perform the function. These kinds of interactions have been demonstrated clearly through synthetic genetic knockout experiments.8 Our results are consistent with the hypothesis that pravastatin and paroxetine may interact in this manner with biological pathways that are critical for glucose metabolism. Our results highlight not only the possibility of such interactions but the potential clinical significance of these interactions.
Paroxetine and pravastatin show evidence of a synergistic interaction
An analysis of the prescriptions at the three sites from which we drew data revealed that ~6% of the patients on pravastatin were also on paroxetine and ~4% of patients on paroxetine were also on pravastatin. Given that there were ~18 million prescriptions for pravastatin and ~15 million for paroxetine in 2009,3 we estimate that between 550,000 and 715,000 individuals received prescriptions for combined therapy with these two drugs in the United States that year (see Supplementary Table S2 online). In this retrospective observational study, a majority of patients showed increases in random blood glucose levels when paroxetine was added to a drug regimen that included pravastatin (and vice versa). We stress that the clinical significance of our finding is not clear. However, it is important to evaluate whether this effect extends to fasting blood glucose levels and whether it can potentially push glucose-intolerant patients into frank T2DM. Patients on pravastatin are already at increased risk for T2DM (as part of the metabolic syndrome9), and therefore changes in blood glucose could complicate both the management of these patients and decisions regarding the diagnosis of T2DM.10
The mechanism of the interaction between pravastatin and paroxetine is not clear. The literature is contentious regarding the effect of pravastatin on glucose and on diabetes,11,13-19 but hyperglycemia is not a well-recognized clinical side effect. Paroxetine has played a role in two reports of hyperglycemia involving multiple drug interactions.20,21 Paroxetine is known to be associated with diabetes,22 which could explain an associated increase in blood glucose. We controlled for this in several ways. First, we found no significant change in blood glucose in patients receiving paroxetine alone; second, we included the sequence in which the drugs were taken as a covariate and did not find a significant association. There are molecular connections linking pravastatin and paroxetine to diabetes-related pathways. The pleiotropic effects of pravastatin are mediated by inhibition of Rac1,23 which also plays a crucial role in the translocation of a glucose transporter (GLUT4) to the plasma membrane.24 Paroxetine targets the serotonin transporter protein SLC6A4, and this interaction leads to inhibition of serotonin reuptake.25-27 In β-islet cells, serotonin is involved in collocating insulin to the plasma membrane before it is secreted. Interestingly, both Rac1 (ref. 28) and SLC6A4 (ref. 29) interact with syntaxin 1A, which is associated with impaired glucose regulation30 and promotes insulin secretion.31 These proteins provide a clear set of pathways that require careful study in order to elucidate a possible mechanism for synergy between these two drugs.
No evidence of class effects between SSRIs and statins
Our data do not suggest that the interaction of pravastatin and paroxetine is a general class effect between all statins and all SSRIs. Although we do see a small effect on glucose levels when pravastatin is taken in combination with any of the SSRIs, no significant interaction effect was observed in our statistical model. Of note, the FDA-AERS data mining procedure did not identify other specific statin–SSRI pairs as being disruptive of glucose hemostasis. In fact, only atorvastatin plus fluoxetine and rosuvastatin plus sertraline demonstrated a significant increase in glucose values relative to baseline in the paired t-test analysis. However, after correcting for covariates in the ANCOVA, the effects on glucose levels were no longer significant with respect to either of these drug combinations.
Clinical implications for diabetic patients receiving combination therapy
We designed the initial clinical analysis to exclude diabetic patients to mitigate confounding factors. For example, a diabetic patient may alter his or her medications based on self-assessment of glucose levels and thereby negate the putative effect of the paroxetine-pravastatin interaction. On the other hand, diabetics who observe large changes in their glucose levels may be more likely to come in for treatment at the hospital and have their blood glucose measured, thereby introducing selection bias into our patient cohorts. Nevertheless, an analysis of the effect of combined therapy with paroxetine and pravastatin in patients with diabetes revealed a large increase in random blood glucose levels after the start of therapy (48 mg/dl, 2.7 mmol/l, Figure 3). This finding indicates that these drugs may complicate the treatment and management of diabetes and could lead to a high incidence of adverse events. Further study is required to establish the clinical significance of these interactions and to indicate whether physicians should consider alternatives to combination treatment involving paroxetine and pravastatin in patients with diabetes.
Limitations of retrospective observation study
Our observations have several limitations revolving around potential covariates. A traditional covariate analysis was not possible in this study. Our retrospective observational data do not allow for controlling many potential covariates that a traditional prospective study offers. It is therefore impractical to enumerate, much less measure, all the covariates from the EMR data. Previous work in surveillance studies has identified these limitations and highlighted the benefit and utility of simple associations to identify avenues of further study.32 Nonetheless, we identified potentially significant variables and examined their individual effects.
First, random blood glucose measurements are known to be highly variable within individuals from hour to hour. However, we found no significant differences in the time of day at which glucose levels were measured in patients, both before and after combined treatment with pravastatin and paroxetine. Second, we assumed that patients who were prescribed pravastatin and/or paroxetine were taking the drugs; however, our databases do not have verified data regarding patient compliance (however, this limitation would reduce, not increase, the signal). Third, many of the patients were receiving not only pravastatin and paroxetine but also many other medications. However, we found no significant correlation between the presence of other concurrent medications and the change in blood glucose levels. Finally, we do not know why glucose measurements were ordered for the patients in our cohorts; however, we controlled for this uncertainty by also assessing glucose level changes in patients on either drug alone and in a set of control analyses of patients receiving similar treatment regimens. The vast majority of glucose measurements were random and not carried out with the patient in a fasting condition.
We are not aware of any literature, guidelines, or conventional wisdom suggesting that pravastatin and paroxetine should be used preferentially in pre-diabetes patients, and our evaluation of other combinations of statins and SSRIs suggests that there are no differences in the baseline characteristics of patients on these two drugs as compared with other statin/SSRI combinations. If there had been a preexisting clinical concern about a prediabetic state in patients who were prescribed combination treatment with these two drugs, we should have seen more frequent measurements of fasting glucose in these patients; however, we observed that the blood glucose measurements were almost exclusively random, suggesting that these measurements were taken as part of a routine screening panel and not out of specific concern about glucose levels. We cannot rule out other potential correlated variables that we did not include in the statistical model, but the adverse-event data mining, the triple-replicated observation in three hospitals, and the initial analysis of the molecular links between the two drugs point to a causative role for the combination treatment. Many of these limitations could be addressed in a prospective clinical trial in which fasting blood glucose, insulin secretion, and insulin action are evaluated before and after the start of pravastatin and paroxetine combination therapy. As a first step, we are following this up with in vivo studies in mice. Figure 4 shows preliminary data supporting our observations.
Figure 4.
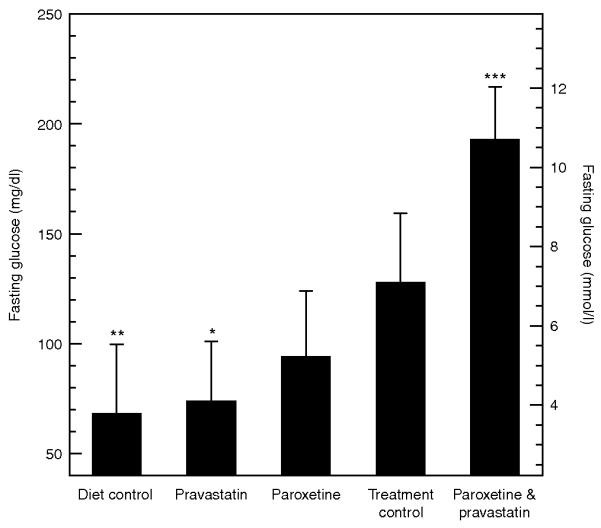
Preliminary analysis of mean and standard error of fasting blood glucose concentrations in five groups of mice (n = 10/group). Asterisks indicate significance in a multivariate linear model with covariates (***P < 0.001, **P < 0.01, *P < 0.05). (Diet control, DC) Normal diet and no treatment. (Treatment control, TC) High fat, high sucrose diet (HFSD) plus saline vehicle. (Pravastatin, PR) HFSD plus pravastatin at 1.25 mg/kg/day. (Paroxetine, PA) HFSD plus paroxetine at 1.25 mg/kg/day. (Paroxetine and pravastatin, CO) HFSD plus paroxetine and pravastatin at 1.25 mg/kg/day for each drug. Animals were kept on their respective diets for 3 months prior to drug exposure. We measured 4-hour fasting blood glucose concentration after 3 weeks of drug treatment. Fasting glucoses were 68.5 ± 31.3 mg/dl (3.8 ± 1.7 mmol/l), 74.2 ± 26.7 mg/dl (4.1 ± 1.5 mmol/l), 94.4 ± 29.6 mg/dl (5.2 ± 1.6 mmol/l), 128.1 ± 31.3 mg/dl (7.1 ± 1.7 mmol/l), 193.1 ± 23.8 mg/dl (10.7 ± 1.3) for DC, PR, PA, TC, CO groups, respectively. We tested the interaction effect of pravastatin and paroxetine using an analysis of variance with covariates and interaction terms on the log transform of the fasting glucose measurements. We included diet as a covariate in addition to the pravastatin and paroxetine exposures and the interaction term. Our preliminary analysis shows a significant effect from diet (β = −0.19 ± 0.13, P = 0.004), pravastatin (β = −0.15 ± 0.13, P = 0.02), and the strongest effect from the interaction of paroxetine and pravastatin (β = 0.33 ± 0.18, P = 5.0 × 10−4). We assessed significance using an F test.
Data mining and hypothesis generation
The use of data mining to generate the hypothesis motivating our investigation provides an excellent example of the power of sharing large data sets. The noise in these large data sets is justifiably used as a reason to be suspicious of results obtained from them. However, the results reported here suggest that these data sets allow detection of potentially important clinical signals. The increasing availability of clinical data repositories, suitably consented and de-identified to support translational research, is a key element in the ability to generate hypotheses and test them via discovery-oriented data mining.33 Indeed, the mature EMR systems at three institutions enabled us to discover this putative drug interaction very quickly.
In conclusion, we present a novel method for discovering putative drug interactions from the FDA’s AERS. We validated the generated hypothesis with a retrospective observational study of patients on combined therapy with paroxetine and pravastatin. To determine the significance of an interaction effect, we analyzed three independent data sets and also performed a combined analysis. Across the data sets from all three sites, we found that patients on pravastatin and paroxetine showed a surprisingly large increase in random blood glucose levels relative to baseline values (19 mg/dl, 1.0 mmol/l).
METHODS
Derivation of a diabetes-related adverse-event profile and hypothesis generation
The FDA’s AERS contains ~2.7 million adverse event reports, including 675,372 related to single-drug therapy and 201,816 referencing two drugs. For 37 single-drug therapy drugs with diabetes-related effects, there were 12,627 reports. We used these reports to create an adverse-event profile for glucose-modulating drugs. To build this profile, we examined 2,228 adverse events and scored each for its correlation with the 37 diabetes-related drugs. We included all adverse events having significant correlations, whether positive or negative, with the therapy. We weighted the contribution of each adverse event to the profile by fitting a logistic regression to the data. This allows for both positively and negatively correlated adverse events to be integrated into a simple linear equation. We used this equation to assign scores to 4,239 pairs of drugs for similarity to this profile. Of the top 30 pairs of drugs identified, 24 contained drugs already associated with glucose homeostasis, leaving six pairs of drugs to be investigated. Of these six, we focused on the pair consisting of pravastatin and paroxetine because of their high prevalence of use.
Defining clinical cohorts from the EMR
Three cohorts of patients without diabetes were constructed from the EMR system at each institution: (i) a cohort on paroxetine and pravastatin concurrently, (ii) a cohort on paroxetine but not pravastatin, and (iii) a cohort on pravastatin but not paroxetine. One of the criteria for inclusion was that the patient’s data show at least one random glucose measurement made within 36 days before and another within 36 days after, the start of therapy (see Supplementary Figure S1 online). We restricted the analysis to just ±36 days relative to the treatment initiation date so as to limit any potential confounding effect from other interventions the patient may have undergone. We kept the follow-up period short to minimize the chance of new confounding events or diagnoses; also, because many follow-up visits are scheduled in units of weeks, we included 5 weeks plus 1 day for lab orders to be executed. Although this decision was arbitrary, we do not believe it has materially affected our results. Slight differences with respect to patient cohort identification exist between replication sites because of the institution-specific capabilities (see Supplementary Methods online). Initially we excluded patients with diabetes from the study so as to mitigate the effect of unknown covariates (e.g., self-administered treatments). The “baseline glucose level” is defined as the mean of the glucose measurements made prior to the start of treatment, and the “treatment glucose level” is the mean of the measurements made after treatment was started. We used ANCOVA with post hoc testing to establish the effect on the treatment groups.
We repeated this method of EMR data extraction relating to patient cohorts to assess the effects of other combinations of SSRIs and statins (see Supplementary Table S1 online). We log-transformed the glucose measurements because they are known to be right-skewed. Each of these combinations was then tested using a simple paired t-test. We performed a subsequent ANCOVA on any combination that showed a significant change from baseline as measured by the paired t-test.
Combined analysis and extension of the analysis to include diabetic patients
We performed a pooled analysis combining each of the cohorts (pravastatin-only, paroxetine-only, and combination) across all three sites (see Supplementary Methods online for full details). We also included data for an additional 2,001 patients diagnosed with diabetes (defined as an International Classification of Diseases, ninth revision, code of 250.*) before the start of treatment with paroxetine, pravastatin, or the combination. For the pooled analysis we compared the main effects of the treatment groups using a t-test (Figure 3). Institutional review board restrictions did not allow for an ANCOVA.
Statistical methods
Arithmetic mean (SD) values were used to describe the patient characteristics, both at baseline and during treatment. To assess the presence of an interaction effect between paroxetine and pravastatin, we first used a paired t-test analysis to test the difference between baseline and treatment log-transformed glucose levels. We followed the t-test with an ANCOVA to compare the treatment groups as well as test the significance of the covariates. For the analysis of class effects, simple paired t-tests were used to test the change in random glucose levels, and, when significant, this analysis was also followed up with an ANCOVA. An F-test was performed to determine the significance of the model fit with and without the inclusion of the treatment group. If significant, post hoc tests were performed to compare the treatment groups directly. These analytical methods are consistent with those previously used for this type of observational study.32 R was used for all the analysis at site 1 and the ANCOVAs at all sites. Site-specific software was used for the other analyses (see Supplementary Methods online).
Supplementary Material
Table 3. Combined analysis of the three sites.
| Combination | paroxetine only | pravastatin only | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Excluding diabetic patients | |||||||||
| N | 135 | 1,603 | 2,063 | ||||||
| Glucose (mg/dl mean 95% CI) | |||||||||
| Baseline (base) | 110.0 | (105.8, 114.2) | 110.6 | (109.1, 112.1) | 108.7 | (107.6, 109.8) | |||
| mmol/l | 6.1 | (5.9, 6.3) | 6.1 | (6.1, 6.2) | 6.0 | (6.0, 6.1) | |||
| After treatment(s) (post) | 128.6 | (118.4, 138.8) | 107.3 | (106.0, 108.6) | 107.8 | (106.5, 109.1) | |||
| mmol/l | 7.1 | (6.6, 7.7) | 6.0 | (5.9, 6.0) | 6.0 | (5.9, 6.1) | |||
| Change (post-base) | 18.5 | (9.2, 27.8) | −3.3 | (−4.7, -1.9) | −0.9 | (−2.1, 0.3) | |||
| mmol/l | 1.0 | (0.5, 1.5) | −0.2 | (−0.3, -0.1) | 0.0 | (−0.1, 0.0) | |||
| Change t-test P value | 1.50 × 10−4 | 3.30 × 10−6 | 0.14 | ||||||
| t-test vs. combination | – | 1.20 × 10−5 | 8.50 × 10−5 | ||||||
| Including diabetic patients | |||||||||
| N | 239 | 3,074 | 2,489 | ||||||
| Glucose (mg/dl mean, 95% CI) | |||||||||
| Baseline (base) | 115.2 | (109.6, 120.8) | 121.5 | (119.7, 123.3) | 122.9 | (120.9, 124.9) | |||
| mmol/l | 6.4 | (6.1, 6.7) | 6.7 | (6.6, 6.8) | 6.8 | (6.7, 6.9) | |||
| After treatment(s) (post) | 163.2 | (149.7, 176.7) | 117.6 | (116.0, 119.2) | 122.2 | (120.1, 124.3) | |||
| mmol/l | 9.1 | (8.3, 9.8) | 6.5 | (6.4, 6.6) | 6.8 | (6.7, 6.9) | |||
| Change (post-base) | 48.1 | (35.1, 61.1) | −3.9 | (−5.6, -2.2) | −0.7 | (−2.8, 1.4) | |||
| mmol/l | 2.7 | (1.9, 3.4) | −0.2 | (−0.3, -0.1) | −0.04 | (−0.2, 0.1) | |||
| Change t-test P value | 5.50 × 10−12 | 7.80 × 10−6 | 0.48 | ||||||
| t-test vs. combination | – | 1.70 × 10−13 | 3.90 × 10−12 | ||||||
ACKNOWLEDGMENTS
The authors thank the statistics reviewers at Clinical Pharmacology & Therapeutics for their valuable insights. N.P.T. and G.H.F. are supported by a training grant from the National Library of Medicine (NIH LM007033). N.P.T. is supported by an award from DOE SCGF. J.C.D. and D.M.R. are supported in part by the NHGRI Electronic Medical Records and Genomics network (U01 HG04603) and the NIH/NIGMS Pharmacogenetics Research Network (U01 HL65962). S.N.M. and I.K. are supported by the NIH Roadmap for Medical Research grant (NIH U54LM008758). S.N.M. is supported by FNIH MURPHY09OMOP0. V.C. is supported by NIH R01MH085542. R.B.A. is supported by NIH/NIGMS PharmGKB resource (NIH R24GM61374).
Footnotes
J.C.D. and S.N.M. contributed equally to this article and are the contact persons for the replication sites.
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/cpt
CONFLICT OF INTEREST
The authors declared no conflict of interest.
References
- 1.Strine TW, et al. Depression and anxiety in the United States: findings from the 2006 Behavioral Risk Factor Surveillance System. Psychiatr. Serv. 2008;59:1383–1390. doi: 10.1176/ps.2008.59.12.1383. [DOI] [PubMed] [Google Scholar]
- 2.Ford ES, Mokdad AH, Giles WH, Mensah GA. Serum total cholesterol concentrations and awareness, treatment, and control of hypercholesterolemia among US adults: findings from the National Health and Nutrition Examination Survey, 1999 to 2000. Circulation. 2003;107:2185–2189. doi: 10.1161/01.CIR.0000066320.27195.B4. [DOI] [PubMed] [Google Scholar]
- 3. [Accessed 2 November 2010];2008 Top 200 generic drugs by total prescriptions. Drug Topics. 2009 < http://drugtopics.modernmedicine.com/drugtopics/data/articlestandard//drugtopics/222009/599844/article.pdf>.
- 4.National Institute of Diabetes and Digestive and Kidney Diseases [Accessed 18 April 2010];Insulin resistance and pre-diabetes. No. 09–4893. 2008 < http://diabetes.niddk.nih.gov/dm/pubs/insulinresistance/>.
- 5.Norén G, Sundberg R, Bate A. A statistical methodology for drug-drug interaction surveillance. Stat. Med. 2008;27:3057–3070. doi: 10.1002/sim.3247. [DOI] [PubMed] [Google Scholar]
- 6.DuMouchel W, Pregibon D. Empirical Bayes screening for multi-item associations; Proceedings of the Seventh ACM SIGKDD International Conference on Knowledge Discovery and Data Mining; 2001.pp. 67–76. [Google Scholar]
- 7.Bate A, Evans SJ. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol. Drug Saf. 2009;18:427–436. doi: 10.1002/pds.1742. [DOI] [PubMed] [Google Scholar]
- 8.Costanzo M, et al. The genetic landscape of a cell. Science. 2010;327:425–431. doi: 10.1126/science.1180823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones PH. Expert perspective: reducing cardiovascular risk in metabolic syndrome and type 2 diabetes mellitus beyond low-density lipoprotein cholesterol lowering. Am. J. Cardiol. 2008;102:41L–47L. doi: 10.1016/j.amjcard.2008.09.074. [DOI] [PubMed] [Google Scholar]
- 10.Selvin E, Zhu H, Brancati FL. Elevated A1C in adults without a history of diabetes in the U.S. Diabetes Care. 2009;32:828–833. doi: 10.2337/dc08-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koh KK, et al. Differential metabolic effects of pravastatin and simvastatin in hypercholesterolemic patients. Atherosclerosis. 2009;204:483–490. doi: 10.1016/j.atherosclerosis.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajpathak SN, Kumbhani DJ, Crandall J, Barzilai N, Alderman M, Ridker PM. Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes Care. 2009;32:1924–1929. doi: 10.2337/dc09-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Araki K, Masaki T, Katsuragi I, Kakuma T, Yoshimatsu H. Effects of pravastatin on obesity, diabetes, and adiponectin in diet-induced obese mice. Obesity (Silver Spring) 2008;16:2068–2073. doi: 10.1038/oby.2008.323. [DOI] [PubMed] [Google Scholar]
- 14.Gannagé-Yared MH, et al. Pravastatin does not affect insulin sensitivity and adipocytokines levels in healthy nondiabetic patients. Metab. Clin. Exp. 2005;54:947–951. doi: 10.1016/j.metabol.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Sugiyama S, et al. Pravastatin improved glucose metabolism associated with increasing plasma adiponectin in patients with impaired glucose tolerance and coronary artery disease. Atherosclerosis. 2007;194:e43–e51. doi: 10.1016/j.atherosclerosis.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 16.Güçlü F, Ozmen B, Hekimsoy Z, Kirmaz C. Effects of a statin group drug, pravastatin, on the insulin resistance in patients with metabolic syndrome. Biomed. Pharmacother. 2004;58:614–618. doi: 10.1016/j.biopha.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Chan P, Tomlinson B, Lee CB, Pan WH, Lee YS. Beneficial effects of pravastatin on fasting hyperinsulinemia in elderly hypertensive hypercholesterolemic subjects. Hypertension. 1996;28:647–651. doi: 10.1161/01.hyp.28.4.647. [DOI] [PubMed] [Google Scholar]
- 18.Freeman DJ, et al. Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation. 2001;103:357–362. doi: 10.1161/01.cir.103.3.357. [DOI] [PubMed] [Google Scholar]
- 19.Baker WL, Talati R, White CM, Coleman CI. Differing effect of statins on insulin sensitivity in non-diabetics: a systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2010;87:98–107. doi: 10.1016/j.diabres.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Petty KJ. Hyperglycemia associated with paroxetine. Ann. Intern. Med. 1996;125:782. doi: 10.7326/0003-4819-125-9-199611010-00031. [DOI] [PubMed] [Google Scholar]
- 21.Vucicevic Z, Degoricija V, Alfirevic Z, Vukicevic-Badouin D. Fatal hyponatremia and other metabolic disturbances associated with psychotropic drug polypharmacy. Int. J. Clin. Pharmacol. Ther. 2007;45:289–292. doi: 10.5414/cpp45289. [DOI] [PubMed] [Google Scholar]
- 22.Raeder MB, Bjelland I, Emil Vollset S, Steen VM. Obesity, dyslipidemia, and diabetes with selective serotonin reuptake inhibitors: the Hordaland Health Study. J. Clin. Psychiatry. 2006;67:1974–1982. doi: 10.4088/jcp.v67n1219. [DOI] [PubMed] [Google Scholar]
- 23.Rashid M, Tawara S, Fukumoto Y, Seto M, Yano K, Shimokawa H. Importance of Rac1 signaling pathway inhibition in the pleiotropic effects of HMG-CoA reductase inhibitors. Circ. J. 2009;73:361–370. doi: 10.1253/circj.cj-08-0817. [DOI] [PubMed] [Google Scholar]
- 24.Ueda S, et al. Crucial role of the small GTPase Rac1 in insulin-stimulated translocation of glucose transporter 4 to the mouse skeletal muscle sarcolemma. FASEB J. 2010;24:2254–2261. doi: 10.1096/fj.09-137380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nemeroff CB, Owens MJ. Neuropharmacology of paroxetine. Psychopharmacol. Bull. 2003;37(suppl. 1):8–18. [PubMed] [Google Scholar]
- 26.Iordanidou M, et al. The serotonin transporter promoter polymorphism (5-HTTLPR) is associated with type 2 diabetes. Clin. Chim. Acta. 2010;411:167–171. doi: 10.1016/j.cca.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 27.Yamakawa M, Fukushima A, Sakuma K, Yanagisawa Y, Kagawa Y. Serotonin transporter polymorphisms affect human blood glucose control. Biochem. Biophys. Res. Commun. 2005;334:1165–1171. doi: 10.1016/j.bbrc.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 28.Lage K, et al. A human phenome-interactome network of protein complexes implicated in genetic disorders. Nat. Biotechnol. 2007;25:309–316. doi: 10.1038/nbt1295. [DOI] [PubMed] [Google Scholar]
- 29.Haase J, Killian AM, Magnani F, Williams C. Regulation of the serotonin transporter by interacting proteins. Biochem. Soc. Trans. 2001;29:722–728. doi: 10.1042/0300-5127:0290722. [DOI] [PubMed] [Google Scholar]
- 30.Romeo S, et al. Search for genetic variants of the SYNTAXIN 1A (STX1A) gene: the −352 A>T variant in the STX1A promoter associates with impaired glucose metabolism in an Italian obese population. Int. J. Obes. (Lond) 2008;32:413–420. doi: 10.1038/sj.ijo.0803743. [DOI] [PubMed] [Google Scholar]
- 31.Chen HC, et al. Synthesis and photoirradiation of isomeric ethylchrysenes by UVA light leading to lipid peroxidation. Int. J. Environ. Res. Public Health. 2007;4:145–152. doi: 10.3390/ijerph2007040009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stang PE, et al. Advancing the science for active surveillance: rationale and design for the Observational Medical Outcomes Partnership. Ann. Intern. Med. 2010;153:600–606. doi: 10.7326/0003-4819-153-9-201011020-00010. [DOI] [PubMed] [Google Scholar]
- 33.Ritchie MD, et al. Robust replication of genotype-phenotype associations across multiple diseases in an electronic medical record. Am. J. Hum. Genet. 2010;86:560–572. doi: 10.1016/j.ajhg.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.