Abstract
Endogenous estrogens are important regulators of cardiovascular homeostasis in premenopausal women and interfere with the development of hypertension and coronary artery disease. These hormones act via three different estrogen receptors affecting both gene transcription and rapid signaling pathways in a complex interplay. In addition to the classical estrogen receptors ERα and ERβ, which are known mediators of estrogen-dependent vascular effects, a G protein-coupled estrogen receptor termed GPER that is expressed in the cardiovascular system has recently been identified. Endogenous human 17β-estradiol, selective estrogen receptor modulators (SERMs) including tamoxifen and raloxifene, and selective estrogen receptor downregulators (SERDs) such as ICI 182,780 are all agonists of GPER, which has been implicated in the regulation of vasomotor tone and protection from myocardial ischemia/reperfusion injury. As a result, understanding the individual role of ERα, ERβ, and GPER in cardiovascular function has become increasingly complex. With accumulating evidence that GPER is responsible for a variety of beneficial cardiovascular effects of estrogens, this receptor may represent a novel target to develop effective strategies for the treatment of cardiovascular diseases by tissue-specific, selective activation of estrogen-dependent molecular pathways devoid of side effects seen with conventional hormone therapy.
Keywords: Blood Pressure, Endothelium, Menopause, Nitric Oxide, Vasodilation
1. Estrogen Receptors and Vascular Estrogen Signaling
Hypertension affects one out of four women worldwide, and its prevalence is particularly high among females over 60 years of age (Kearney et al., 2005). Indeed, the first decade after menopause is accompanied by an increase in blood pressure (Burt et al., 1995), pointing to a blood pressure-lowering effect of endogenous estrogens (Barton and Meyer, 2009). Importantly, mild elevations in blood pressure can often been found in women shortly after menopause (Barton and Meyer, 2009) and have been associated with a higher risk of cardiovascular diseases such as myocardial infarction, stroke, and congestive heart failure (Vasan et al., 2001). In line with these epidemiological data, a variety of beneficial cardiovascular effects of endogenous estrogens are known based on numerous experimental studies, including vasodilator activity and improvement of ischemia/reperfusion-related myocardial injury (Deschamps et al., 2010; Meyer et al., 2006; Meyer and Barton, 2009). Conversely, randomized clinical trials using conjugated equine estrogens and medroxyprogesterone acetate for postmenopausal hormone therapy could not recapitulate these beneficial effects but reported increased cardiovascular risk instead (Hulley et al., 1998; Rossouw et al., 2002), indicating that this type of is not suitable for primary or secondary prevention of cardiovascular diseases.
The controversial outcome of these trials may partly result from the complexity of vascular estrogen signaling (Figure 1), which involves at least three different estrogen receptors. Estrogen receptor α (ERα) was identified in the 1960’s (then termed ER) (Soloff and Szego, 1969). In 1996, the less characterized estrogen receptor β (ERβ) was found (Kuiper et al., 1996). ERα and ERβ largely function as transcriptional regulators, but a small fraction may also be present at the plasma membrane, where estrogen binding activates intracellular signaling pathways that mediate acute effects of estrogens, such as rapid vasodilation (Meyer et al., 2006; Meyer et al., 2009). In 1997, a third ER, the seven-transmembrane G protein-coupled ER termed GPR30, was cloned from shear-stress exposed human endothelial cells among other sources (Takada et al., 1997). More recently, GPR30 has been shown to activate rapid signaling cascades, such as extracellular signal-related kinase (ERK) and phosphatidylinositol-3-kinase (PI3K), after estrogen binding (Filardo et al., 2000; Revankar et al., 2005; Thomas et al., 2005). After establishing GPR30 as a bona fide estrogen-binding receptor, it was renamed GPER by the International Union of Basic and Clinical Pharmacology (Alexander et al., 2008). Similar to ERα and ERβ (Meyer et al., 2006), GPER is expressed throughout the cardiovascular system in humans and animals of both sexes (Bopassa et al., 2010; Broughton et al., 2010; Deschamps and Murphy, 2009; Ding et al., 2009; Filice et al., 2009; Haas et al., 2007; Haas et al., 2009; Isensee et al., 2009; Lindsey et al., 2009; Ma et al., 2010; Takada et al., 1997). This suggests that in addition to ERα and ERβ, GPER also plays a physiological role in regulation of vascular and myocardial function. With the identification of three different receptors capable of mediating vascular estrogen signaling, the understanding of the cardiovascular effects of estrogen has become increasingly complex. This review addresses the individual role of the different ERs in cardiovascular function with a particular focus on its newest member, GPER.
Figure 1.
Proposed estrogen receptor (ER)-activated signaling pathways involved in vasodilation. 17β-Estradiol (E2) can activate a subpopulation of ER at the plasma membrane (mER) that interacts with adaptor proteins (adaptor) and signaling molecules such as c-Src (1), which is critical for down-stream ER-induced signaling via PI3K/Akt and MAPK pathways. E2 also binds to the G protein-coupled estrogen receptor GPER, which is primarily localized to the endoplasmic reticulum (2). GPER activates downstream effectors, such as adenylate cyclase (resulting in cAMP production), and c-Src. c-Src, in turn, activates matrix metalloproteinases (MMP), which cleave pro-heparin-bound-epidermal growth factor receptors (EGFR). EGFR leads to multiple downstream events, including activation of MAPK and PI3K. Once activated, PI3K can induce NO generation by NO synthase (NOS) in vascular endothelial (eNOS) or smooth muscle cells (nNOS). Membrane permeant NO, in turn, activates guanylate cyclase (resulting in cGMP production). The NO/cGMP pathway is involved in acute vasodilator mechanisms, such as protein kinase G (PKG)-dependent regulation of myosin light chain phosphorylation, and activation of large-conductance calcium-activated potassium (BKCa) channels. E2 also regulates cellular gene expression either via binding of ER dimers in the promotor region of target genes (3), through interaction of ER with other classes of transcription factors (TF) (4), or by regulation of TF phosphorylation (5). Target genes regulating vascular homeostasis include NOS and inflammatory cytokines. Adapted from Mol Cell Endocrinol, Vol. 308, Meyer, M.R., Haas, E., Prossnitz, E.R., Barton M, Non-genomic regulation of vascular cell function and growth by estrogen, Pages 9-16, ©2009, with permission from Elsevier.
2. Ligands of Vascular Estrogen Receptors
Although a variety of natural and synthetic estrogens are able to induce effects on the cardiovascular system, it is important to separate their ability to regulate ERα, ERβ, and GPER activity. 17β-Estradiol, the major human estrogen lost after menopause, is a combined activator of ERα, ERβ, and GPER, and mediates numerous beneficial vascular effects. Although 17β-estradiol is primarily synthesized by the ovaries, the enzyme aromatase converts androgens into 17β-estradiol and estrone at many sites throughout the body including the vascular wall, where they may have specialized local effects (Harada et al., 1999). Estrone and other endogenous estrogen-based steroids display a low binding affinity for ERα, ERβ, and GPER at physiological concentrations, and their physiological actions are less clear (Kuiper et al., 1998; Revankar et al., 2005; Thomas et al., 2005). On the other hand, humans are exposed to a variety of natural and man-made estrogenic compounds at low levels (Jacobs and Lewis, 2002). Natural environmental estrogens are synthesized by plants (phytoestrogens) and include coumestans and isoflavones, whereas synthetic estrogenic compounds (xenoestrogens, known as endocrine disruptors) comprise chemical detergents, pesticides, and plastic monomers. In fact, the isoflavone genistein is able to induce vasodilation (Martin et al., 2008), and has now been shown to not only activate ERα and ERβ, but also GPER (Thomas and Dong, 2006). Some xenoestrogens such as nonylphenol and DDT have also be implicated in regulation of vascular function (Hsieh et al., 2009; Ruehlmann et al., 1998), and these compounds weakly bind ERα/ERβ, and are also agonists of GPER (Thomas and Dong, 2006). Taken together, numerous endogenous and exogenous estrogenic compounds implicated in cardiovascular physiology are now known to activate all three known estrogen receptors.
Research to improve successful treatment of estrogen-sensitive tumors, breast cancer in particular, led to the development of drugs that oppose the action of estrogen. Referred to as selective estrogen receptor downregulators (SERDs), compounds such as ICI 182,780 (fulvestrant) abolish ERα/ERβ signaling in all tissues (Shanle and Xu, 2010). However, a group of compounds known as selective estrogen receptor modulators (SERMs) do not uniformly act as estrogen antagonists, but are generally regarded as ER agonists in the cardiovascular system, bone, and liver, and as ER antagonists in breast tissue (Katzenellenbogen and Katzenellenbogen, 2002). Importantly, the SERM tamoxifen as well as the SERD ICI 182,780 do not solely interact witch ERα and ERβ; they also display significant binding to GPER (Thomas et al., 2005) and induce GPER-dependent signaling in breast cancer cell lines (Filardo et al., 2000; Revankar et al., 2005). Their role as GPER agonists has been confirmed in several other cell types and tissues (Prossnitz and Barton, 2009), indicating that this receptor mediates estrogenic effects even with concomitant blockade of ERα and ERβ. This also indicates that older studies investigating cardiovascular effects of SERMs and SERDs may in fact point to GPER-dependent effects.
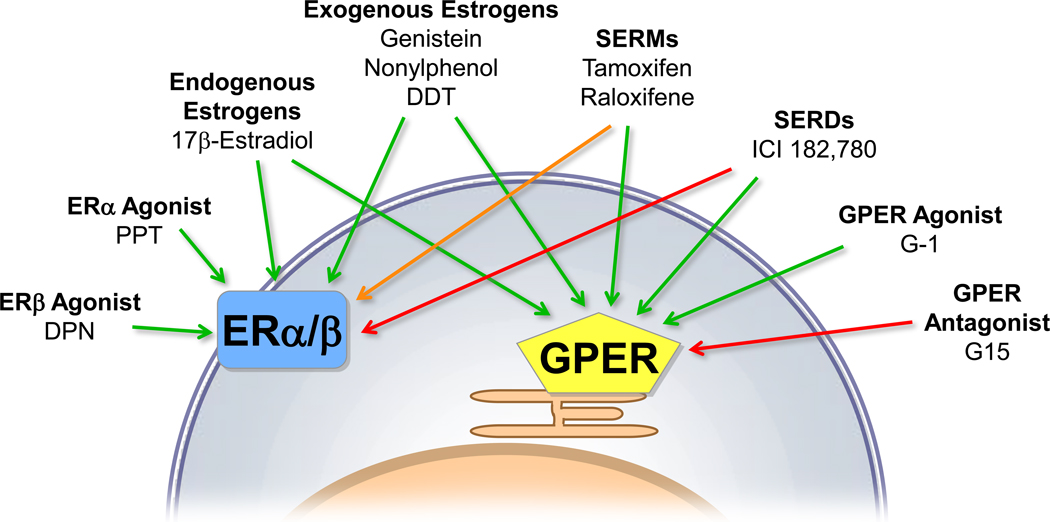
As GPER is known to bind many of the same ligands as ERα and ERβ (Figure 2), selective agonists/antagonists were needed to unravel the functional roles of the individual receptors, particularly GPER. This led to the identification of the first GPER-specific agonist G-1 (Bologa et al., 2006) and the first GPER-selective antagonist G15 (Dennis et al., 2009). Both compounds share a tetrahydro-3H-cyclopenta-[c]quinoline scaffold domain and show an extremely high selectivity for GPER over ERα and ERβ. In addition, agonists for ERα (PPT) and ERβ (DPN) (Harrington et al., 2003) have been used in the past years. The availability of these compounds has provided the opportunity to better characterize the role of individual ERs, and GPER in particular, in cardiovascular physiology.
Figure 2.
Agonists and antagonists of GPER and plasma membrane-associated subpopulations of ERα and ERβ involved in rapid vascular estrogen signaling. Green arrows indicate activation, red arrows indicate inhibition, and the orange arrow indicates tissue-dependent activation or inhibition. SERM, selective estrogen receptor modulator; SERD, selective estrogen receptor downregulator.
3. Mechanisms of Estrogen-Dependent Vasodilation
17β-Estradiol is a powerful vasodilator of human blood vessels (Mügge et al., 1993; Silva de Sa, Meirelles, 1977), and acute effects on vasomotor tone have been observed in arteries of both women and men (Haas et al., 2007; Mügge et al., 1993). Shortly after the seminal observation that endothelial cells release a relaxing factor in response to acetylcholine (Furchgott and Zawadski, 1980) and its subsequent identification as nitric oxide (NO) (Furchgott, 1988; Ignarro et al., 1987), enhanced endothelium-dependent responses to acetylcholine were reported in estrogen-treated ovariectomized animals (Gisclard et al., 1988). Similarly, flow-mediated vasodilation and NO production correlate with high estrogen levels during the menstrual cycle and increase after estrogen therapy in postmenopausal women (Miller and Duckles, 2008). These findings support the notion that estrogen acutely and chronically enhances endothelium-/NO-dependent relaxation of human arteries, including human coronary arteries (Barton et al., 1998; Miller, Duckles, 2008). Estrogen-stimulated endothelium-dependent NO release is the result of rapid PI3K/Akt activation and subsequent phosphorylation of endothelial (type III) NO synthase (eNOS) (Haynes et al., 2000; Simoncini et al., 2000), although other mechanisms have also been proposed (Chow et al., 2010). This effect is believed to be mediated by subpopulations of ERα and possibly ERβ that localize to vesicular invaginations of the plasma membrane termed caveolae (Figure 1) (Chambliss et al., 2000; Chambliss et al., 2002; Simoncini et al., 2000). However, endothelium-dependent relaxation in response to estrogens does not involve NO exclusively, but is also mediated by endothelium-derived hyperpolarizing factors (EDHFs), including epoxyeicosatrienoic acids (EETs), H2O2, potassium, and endothelial gap junction communications (Huang et al., 2004; Luksha et al., 2006; Traupe et al., 2007). In addition, estrogens can act directly on vascular smooth muscle cells to regulate vascular function (White, 2002). These endothelium-independent effects include estrogen-dependent regulation of calcium and potassium fluxes (White, 2002), mainly through the activity of large-conductance calcium- and voltage-activated potassium (BKCa) channels (Figure 1) (Han et al., 2007; White et al., 1995). Indeed, 17β-estradiol-dependent vasodilator responses in human coronary arteries remain effective after removal of endothelial cells (Mügge et al., 1993). Moreover, estrogen also indirectly reduces vasomotor tone by inhibiting the activity of vasoconstrictors, such as prostanoids, endothelin-1, and angiotensin II (Barton and Meyer, 2009; Mügge et al., 1997).
In addition to 17β-estradiol, an agonist of ERα, ERβ, and GPER, there has been great interest in defining the role of SERMs in the regulation of vascular tone. Tamoxifen and raloxifene, which are agonists of GPER (Prossnitz and Barton, 2009), activate BKCa channels and evoke acute endothelium-independent vasodilation (Darkow et al., 1997; Hutchison et al., 2001; Leung et al., 2007b). They also induce NO-mediated endothelium-dependent relaxation and increase eNOS phosphorylation in porcine coronary arteries and other vascular beds (Chan et al., 2010; Figtree et al., 1999; Leung et al., 2006; Leung et al., 2007a). Moreover, raloxifene activates eNOS via the PI3K/Akt-pathway in endothelial cells (Simoncini et al., 2002). Recently, the SERM estrogen-dendrimer conjugate was found to activate eNOS and stimulate growth of endothelial cells (Chambliss et al., 2010). On the other hand, the endogenous SERM and cholesterol metabolite 27-hydroxycholesterol decreases vascular NOS expression and activity (Umetani et al., 2007). Thus, both 17β-estradiol and several synthetically generated estrogen-like substances affect vascular function in distinct ways, although it is often unclear which ER mediates these responses. Defining the role of individual ERs in response to activation by SERMs may help to identify and selectively target ERs that mediate the beneficial vascular effects of estrogens.
4. Role of ERα and ERβ for Vascular Function
Estrogens acutely reduce vascular tone in various arteries of different species, yet the molecular mechanisms and the role of individual estrogen receptors in this response are less clear. Long-term 17β-estradiol treatment increases basal NO production in thoracic aorta of ERβ-, but not of ERα-knockout mice, indicating that ERα mediates NO-dependent vasodilation (Darblade et al., 2002). On the other hand, ERβ-knockout mice develop elevated blood pressure as they age, also suggesting a role for ERβ (Zhu et al., 2002). Indeed, disruption of ERβ abrogates the effect of estrogen to attenuate vasoconstriction (Zhu et al., 2002). Conversely, 17β-estradiol lacks vasodilatory effects in perfused carotid or femoral arteries of either ERα- or ERβ-knockout mice (Guo et al., 2005). In addition, 17β-estradiol stimulates neither ERK nor PI3K activity in carotid arteries of ERα- or ERβ-deficient animals (Guo et al., 2005). This is not only compatible with the notion that both ERα and ERβ are required for estrogen-dependent vasodilation, but also that other mechanisms independent of ERα/ERβ may be involved in these responses. Moreover, it is likely that a functional crosstalk between ERs defines the vasodilator effect of estrogens (Matthews and Gustafsson, 2003). In porcine coronary arteries, selective activation of ERα by PPT evokes a rapid, endothelium- and NO-dependent relaxant response within minutes, followed by a more sustained NO-independent relaxing effect (Traupe et al., 2007). In contrast, non-selective ER activation by 17β-estradiol and selective activation of ERβ by DPN causes a sustained relaxation only (Traupe et al., 2007). This points to an inhibitory role of ERβ on ERα-dependent, NO-mediated acute vasodilation. A similar effect has also been suggested in murine femoral arteries, where 17β-estradiol and PPT induce NO-dependent vasodilation only in animals lacking ERβ, but not in wild-type mice (Cruz et al., 2006). Of note, PPT- and DPN-dependent vasodilator effects differ between distinct vascular beds and species (Al Zubair et al., 2005; Bolego et al., 2005). Taken together, functional interactions of both ERα and ERβ are likely to regulate vasomotor tone depending on anatomical localization of vascular beds in different species.
Conversely, a number of investigators found that the ERα/ERβ-antagonist (but GPER-agonist (Filardo et al., 2000; Thomas et al., 2005)) ICI 182,780 does not block estrogen-dependent vasodilator effects in several vascular beds of different species (Bracamonte et al., 2002; Scott et al., 2007; Shaw et al., 2000; Sudhir et al., 1995; Teoh et al., 1999). Moreover, we recently demonstrated that ICI 182,780 alone causes a rapid dilation in epicardial porcine coronary arteries, which represent a good model of the human coronary vasculature with regard to size and mediators of vascular function (Figure 2B) (Meyer et al., 2010). These findings suggest that this vasodilator response does not depend on the activity of ERα and ERβ, but that these responses are mediated by GPER instead (Meyer et al., 2010). In line with these findings, ICI 182,780 causes a rapid, NO-dependent dilation of pressurized carotid (but not femoral) arteries of ovariectomized mice (Guo et al., 2005), and acts as a vasodilator in rat superior mesenteric arteries (Keung et al., 2011). Interestingly, vasodilator effects in response to ICI 182,780 are absent in carotid arteries of mice lacking ERα or ERβ (Guo et al., 2005), which would be compatible with crosstalk between GPER, ERα and ERβ recently suggested (Albanito et al., 2007; Gao et al., 2011). From a clinical point of view, vasodilator effects of ICI 182,780 (fulvestrant, Faslodex®) via GPER may provide the first mechanistic explanation for symptomatic hypotension, a common side effect of this agent when used as endocrine treatment for advanced breast cancer (Vergote and Abram, 2006).
5. GPER: A Novel Regulator of Vascular Tone and Blood Pressure
GPER is an intracellular transmembrane G protein-coupled receptor that mediates rapid estrogen signaling (Filardo et al., 2000; Revankar et al., 2005; Thomas et al., 2005). The potential relevance of GPER in cardiovascular physiology was perhaps first suggested by one of the approaches used to clone cDNA encoding GPER in human endothelial cells exposed to fluid shear stress (Takada et al., 1997). To better define the role of GPER in estrogen-dependent vasodilation, the selective GPER-agonist G-1 (Bologa et al., 2006) as well as genetically modified animals have been used. G-1 acutely dilates human internal mammary and procine coronary arteries, rat aorta and mesenteric arteries, as well as rat and murine carotid arteries from males and females, with the effect being less potent in larger conduit arteries (Figure 3) (Broughton et al., 2010; Haas et al., 2009; Lindsey et al., 2009; Lindsey et al., 2011; Meyer et al., 2010). In human internal mammary and murine carotid arteries, the G-1-dependent effect was more pronounced than that of 17β-estradiol, again suggesting that estrogen-dependent vasodilation involves complex functional crosstalk between GPER, ERα, and ERβ (Figure 3A) (Haas et al., 2009). G-1 also inhibits endothelin- (Meyer et al., 2010), angiotensin II- (Lindsey et al., 2009), and serotonin-dependent (Haas et al., 2009) contractions in certain vascular beds. Interestingly, G-1 regulates calcium flux in vascular smooth muscle cells, an effect that is inhibited by GPER siRNA treatment (Haas et al., 2009). In fact, GPER activation by G-1 abrogates calcium flux induced by the vasoconstrictor serotonin indicating calcium-antagonistic or desensitizing effects, with intracellular application being much more rapid than extracellular application, indicative of the intracellular function of GPER (Haas et al., 2009).
Figure 3.
GPER-dependent regulation of vascular tone. In precontracted carotid arteries from mice, the selective GPER-agonist G-1 (3 µM) causes acute dilation, which is even stronger than that of 17β-estradiol (E2, 3 µM, A). Dilatory effects of G-1 (3 µM) in carotid arteries of wild-type mice (GPER +/+) are absent in GPER-knockout animals (GPER −/−, B). Acute exposure to G-1 (3 µM) and the GPER agonist and ERα/ERβ antagonist ICI 182,780 (ICI, 3 µM) for 60 minutes evokes similar vasodilator responses of porcine epicardial coronary arteries (C). Intravenous injection of G-1 at increasing concentrations (4.12 ng/kg, 41.2 ng/kg, 412 ng/kg, and 20.6 µg/kg) acutely reduces mean arterial blood pressure (MAP) in normotensive rats. For comparison, the response to achetylcholine (ACh, 30 ng/kg) is shown (D). CTL, solvent control (ethanol 0.3%). Panels A, B, and D are reproduced from Haas, E., Bhattacharya, I., Brailoiu, E., Damjanovic, M., Brailoiu, G.C., Gao, X., Mueller-Guerre, L., Marjon, N.A., Gut, A., Minotti, R., Meyer, M.R., Amann, K., Ammann, E., Perez-Dominguez, A., Genoni, M., Clegg, D.J., Dun, N.J., Resta, T.C., Prossnitz, E.R., Barton, M., Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity, Circ Res, 104(3), 288-291, ©2009 by the American Heart Association, with permission of the publisher. Panel C is adapted from Meyer, M.R., Baretella, O., Prossnitz, E.R., Barton, M., Dilation of epicardial coronary arteries by the G protein-coupled estrogen receptor agonists G-1 and ICI 182,780, Pharmacology, 86(1), 58-64, ©2010, with permission from S. Karger AG, Basel, Switzerland.
Acute vasodilation in response to G-1 is abolished in animals lacking the GPER gene (Haas et al., 2009) or after pretreatment with the GPER-selective antagonist G15 (Lindsey et al., 2011), providing further evidence for a role of GPER for control of vasomotor tone (Figure 3B). In GPER-deficient mice, development of hypertension in females at 9 months of age has been claimed; however, this assumption was based on blood pressure differences in wild-type mice, and mean blood pressure values were essentially normotensive in all groups investigated (Martensson et al., 2009). On the other hand, intravenous injection of G-1 in normotensive rats acutely reduces mean arterial blood pressure (Figure 3D) (Haas et al., 2009). Moreover, treatment with G-1 for 2 weeks lowers blood pressure only in ovariectomized but not in ovary-intact female or in male mRen2.Lewis rats, an estrogen-sensitive model of hypertension (Lindsey et al., 2009). In these animals, angiotensin II type 1 receptor and angiotensin-converting enzyme gene expression was reduced by G-1 treatment, suggesting that GPER activation lowers blood pressure in part by attenuating vascular angiotensin II signaling (Lindsey et al., 2009). In line with G-1-dependent effects on the renin-angiotensin-aldosterone system is a recent report suggesting that vascular signaling of aldosterone may involve GPER-dependent pathways (Gros et al., 2011). Taken together, these studies clearly indicate a role for GPER activation mediating acute and chronic vasodilation in response to estrogen (Table 1).
Table 1.
GPER-Mediated Effects on Cardiovascular Function.
| Function | Organ/Tissue | Species | Reference |
|---|---|---|---|
| Vasodilation | Aorta | Mouse | (Haas et al., 2009) |
| Rat | (Lindsey et al., 2009; Lindsey et al., 2011) | ||
| Carotid Artery | Mouse | (Haas et al., 2009) | |
| Rat | (Broughton et al., 2010) | ||
| Coronary Artery | Pig | (Meyer et al., 2010) | |
| Mesenteric Artery | Rat | (Haas et al., 2009; Lindsey et al., 2011) | |
| Internal Mammary Artery | Human | (Haas et al., 2009) | |
| Inhibition of Vasoconstriction | Aorta | Mouse | (Haas et al., 2009) |
| Rat (OVX) | (Lindsey et al., 2009) | ||
| Carotid Artery | Mouse | (Haas et al., 2009) | |
| Coronary Artery | Pig | (Meyer et al., 2010) | |
| Blood Pressure | |||
| Acute Reduction | Rat | (Haas et al., 2009) | |
| Chronic Reduction | Rat (OVX) | (Lindsey et al., 2009) | |
| No Chronic Effect | Rat (Female) | (Lindsey et al., 2009) (Jessup et al., 2010) | |
| Anti-Ischemic Effects | Heart | Mouse | (Bopassa et al., 2010) |
| Rat | (Deschamps, Murphy, 2009; Filice et al., 2009; Patel et al., 2010; Weil et al., 2010) | ||
| Cardiomyocytes | Mouse | (Recchia et al., 2011) | |
| Anti-Inflammatory Effects | Heart | Rat | (Weil et al., 2010) |
| Preservation of LV Structure/Function | Heart | Mouse (Male) | (Delbeck et al., 2011) |
| Rat | (Jessup et al., 2010) |
LV, left-ventricular; OVX, ovariectomized.
6. Mechanisms of GPER-Dependent Vasodilation
While several investigators have reported vasodilator effects of G-1, the mechanisms involved are still scarcely understood. In rat aorta, carotid and mesenteric as well as in porcine coronary arteries, G-1-mediated relaxation depends at least partly on the presence of an intact endothelium and is prevented by the NOS inhibitor L-NAME (Broughton et al., 2010; Lindsey et al., 2011; Meyer et al., 2010). This suggests that release of endothelium-derived NO is involved in GPER-dependent vasodilation in certain vascular beds. However, the exact functional and molecular interactions of GPER with the NO pathway, although suggested in previous studies (Broughton et al., 2010; Filice et al., 2009; Lindsey et al., 2011; Meyer et al., 2010), still need to be substantiated. Of note, although G-1-dependent vasodilation is endothelium-dependent, G-1 treatment of ovariectomized mRen2.Lewis rats alters neither aortic eNOS gene expression nor endothelium- and NO-dependent relaxation to acetylcholine (Lindsey et al., 2009), both of which unexpectedly remain intact despite severe hypertension and ovariectomy in this model. On the other hand, preliminary evidence indicates that GPER activation by G-1 and ICI 182,780 also evokes endothelium-independent coronary vasodilation by regulating potassium efflux via BKCa channels, possibly mediated by ERK and PI3K/Akt signaling pathways (Han et al., 2009; Han et al., 2010).
Although there is now evidence that endothelium-derived NO is an important mediator of GPER-dependent vasodilation (Broughton et al., 2010; Lindsey et al., 2011; Meyer et al., 2010), it is unknown whether GPER activation also induces estrogen-dependent vasodilator responses through release of EDHFs, which has previously been shown for ERβ (Luksha et al., 2006; Traupe et al., 2007). EDHFs are important mediators of endothelium-dependent, NO-independent vasodilation in small resistance arteries, and estrogen therapy as well as a phytoestrogen-rich soy diet improve the EDHF response in mesenteric and uterine arteries of rodents (Burger et al., 2009; Knock et al., 2006). Based on the fact that G-1 dilates resistance arteries (Haas et al., 2009; Lindsey et al., 2011), it is plausible that GPER activation improves EDHF-dependent vasodilation in these small vessels, which may contribute to the observed blood pressure-lowering effect of G-1 (Haas et al., 2009; Lindsey et al., 2009).
GPER may also reduce vascular tone by interfering with constrictor reactive oxygen species (ROS), since G-1 has antioxidative properties inhibiting ROS production (Broughton et al., 2010). Importantly, an excess amount of ROS impairs endothelial function partly by reducing NO bioavailability (Ushio-Fukai, 2009). Moreover, ROS function as second messengers regulating signaling of G protein-coupled receptors such as the angiotensin II type 1 receptor, resulting in enhanced vasoconstriction (Ushio-Fukai, 2009). This may represent an additional mechanism whereby GPER beneficially affect vascular tone, particularly in the presence of impaired endothelial function due to cardiovascular diseases, such as hypertension or atherosclerosis.
In summary, current evidence suggests that ERα, ERβ, and GPER all contribute to estrogen-induced vasodilation, with a role for GPER best described in endothelium-/NO-dependent vasodilation. Selective GPER activation is also likely to induce NO-independent vasodilation and to reduce vascular tone via indirect effects on vasoconstrictors that are yet not fully understood. These responses depend on anatomical localization of vascular beds in different species and the time-course of estrogen administration. In line with its beneficial vasodilator effects, GPER also regulates cell growth and apoptosis of vascular smooth muscle cells (Ding et al., 2009; Haas et al., 2009), pointing towards a possible atheroprotective role of this receptor.
7. Protective Effects of GPER on Myocardial Function
In view of the estrogen-dependent vasodilator effects and low incidence of coronary artery disease in premenopausal women (Meyer et al., 2006), a role for ER in protection from ischemic heart disease has been suggested (Deschamps et al., 2010). Endogenous estrogens convey protection in models of acute myocardial infarction and also have antiarrhythmic activity mimicking ischemic preconditioning, although the exact mechanisms involved are still unclear (Das and Sarkar, 2006; Deschamps et al., 2010). The ability of estrogen to stimulate NO generation has been of particular interest given that NO-dependent vasodilation may increase myocardial perfusion and thus contractility, alongside several other NO-mediated cardioprotective effects (Jones and Bolli, 2006). Of note, NO-mediated cholinergic vasodilation induced by coronary acetylcholine infusion is absent in patients with advanced coronary atherosclerosis, where infusion results in paradoxical vasoconstriction (Ludmer et al., 1986). In line with beneficial effects of estrogen on myocardial function, acute treatment with the non-selective ER agonist 17β-estradiol before temporary coronary artery occlusion reduces infarct size in female and male animals (Delyani et al., 1996; Hale et al., 1996; Hale et al., 1997). In addition, selective activation of ERβ provides cardioprotection that requires the availability of NO (Lin et al., 2009). Acute and chronic treatment of dogs or rodents with the SERM raloxifene also improves ischemia/reperfusion-related myocardial injury, and again partly involves NO-dependent mechanisms mediated by the PI3K/Akt-pathway (Chung et al., 2010; Nemcsik et al., 2004; Ogita et al., 2002; Ogita et al., 2004). A number of experimental studies on ischemia/reperfusion injury subsequently found beneficial effects mediated by both ERα and ERβ, yet some controversies exist whether both receptors provide acute and chronic cardioprotection (Deschamps et al., 2010). Interestingly, functional crosstalk between ERα and ERβ determining the estrogen-dependent cardioprotective effects has also been suggested (Babiker et al., 2007).
Although these studies clearly demonstrate a role for both ERα and ERβ in cardiac pathophysiology, it is unclear whether effects are mediated by nuclear or membrane populations of these receptors. Also, several estrogen-dependent effects such as inhibition of cardiomyocyte cell growth and contraction involve mechanisms independent of ERα and ERβ (Mercier et al., 2003; Ullrich et al., 2008). More recently, impaired left-ventricular contractility and relaxation capacity consistent with left-ventricular dysfunction were noted in male, but not in female GPER-deficient mice (Delbeck et al., 2011). Moreover, G-1 ameliorates diastolic dysfunction and reduces left-ventricular hypertrophy in a model of salt-induced hypertensive cardiomyopathy independent of blood pressure (Jessup et al., 2010). In addition, involvement of estrogen signaling via GPER in ischemic heart disease has been suggested based on the observation of increased GPER expression mediated by hypoxia-inducible factor HIF-1α in a murine cardiomyocyte-like cell line in response to mimicking hypoxia (Recchia et al., 2011). These studies clearly indicate a role for GPER in preservation of cardiac structure and function.
Using a model of cardiac ischemia/reperfusion injury, several groups reported additional GPER-dependent cardioprotective effects (Table 1). In rat hearts, G-1 treatment reduces infarct size and postischemic contractile dysfunction independent of sex (Figure 4) (Deschamps and Murphy, 2009; Filice et al., 2009; Patel et al., 2010; Weil et al., 2010), possibly involving a PI3K kinase/Akt-dependent mechanism (Deschamps and Murphy, 2009). Similarly, the same treatment reduces infarct size and improves functional recovery in male mice by inhibiting the mitochondria permeability transition pore opening (Bopassa et al., 2010). In these animals, blocking of ERK signaling prevents the G-1-induced improvement in heart function and infarct size (Bopassa et al., 2010). In rats, ERK activation by G-1 also mediates negative inotropic effects and induces the phosphorylation of eNOS (Filice et al., 2009), while no involvement of ERK signaling on acute cardioprotection was observed in a second report (Deschamps and Murphy, 2009). Interestingly, improved heart function in response to G-1 has been linked to a reduced production of proinflammatory cytokines including TNF-α, IL-1β, and IL-6 (Weil et al., 2010).
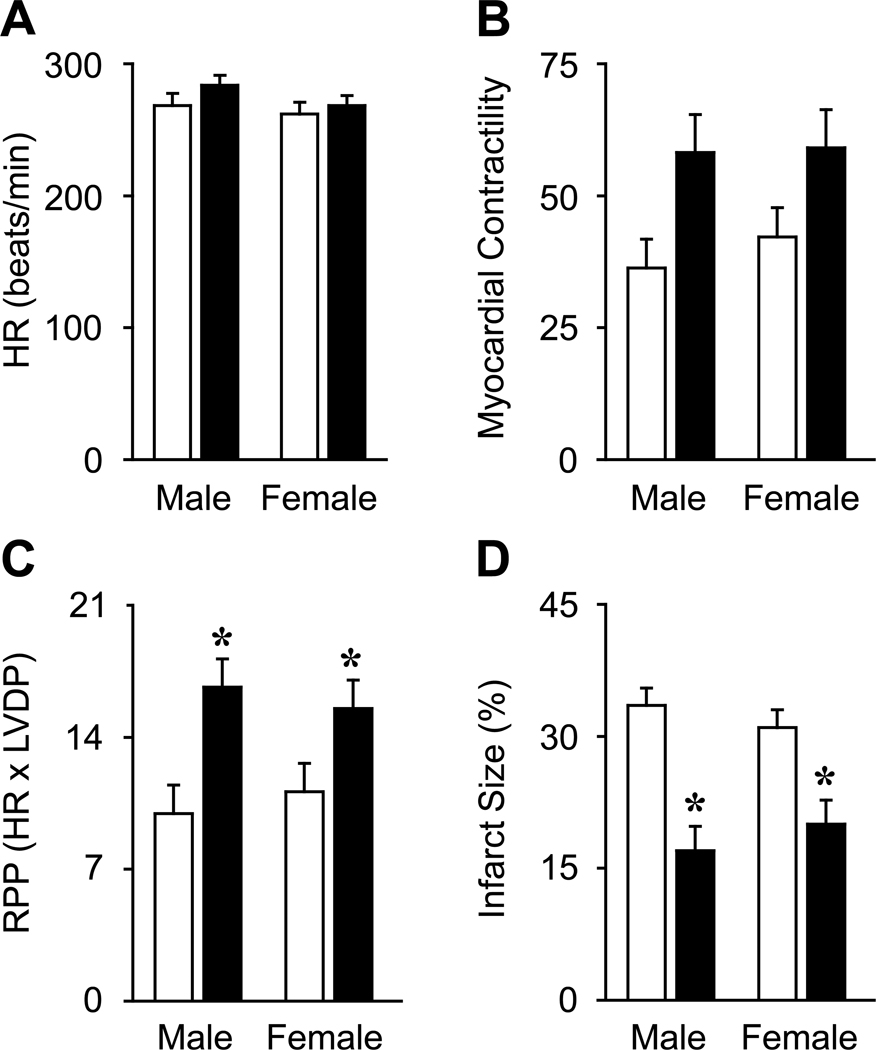
Figure 4.
Sex-independent cardioprotective effects of GPER activation. Selective GPER activation by G-1 (110 nM, black bars) before global cardiac ischemia does not significantly alter heart rate (HR, A) and myocardial contractility (measured as left ventricular developed pressure, B) after 120 minutes of reperfusion in male and female rats compared to untreated controls (open bars). In contrast, G-1 treatment reduces postischemic contractive dysfunction (measured as rate pressure product, RPP, C) and infarct size (measured as percentage of the entire ventricular area, D). *P < 0.05 vs. same gender control. Adapted from Deschamps, A.M., Murphy, E, Am J Physiol Heart Circ Physiol, ©2009 by the American Physiological Society, used with permission.
Taken together and consistent with its dilator effects, GPER activation improves functional recovery and infarct size after myocardial ischemia although the precise mechanisms mediating this protection remain to be characterized. Moreover, functional crosstalk between ERα, ERβ, and GPER may also determine the cardiac effects of estrogen as suggested by experimental studies using ICI 182,780 (Filice et al., 2009). In line with its beneficial effects on the heart, GPER may also be protective in scenarios involving acute hypoxic injury of other organs, such as ischemic stroke (Zhang et al., 2010) and liver injury (Hsieh et al., 2007).
8. Clinical Implications and Need for Further Research
Recent research has shown that GPER mediates numerous beneficial effects of estrogens on vascular and myocardial function. With the presence of three different cellular estrogen targets, predicting the cardiovascular response to estrogen has become increasingly complex due to nuclear and extranuclear localization and functional crosstalk between ERα, ERβ, and GPER, resulting in activation of multiple genomic and non-genomic signaling pathways (Figure 1) (Meyer et al., 2009). Moreover, the identification of SERMs such as tamoxifen and raloxifene and SERDs such as ICI 182,780 as GPER agonists (Abdelhamid et al., 2011; Filardo et al., 2000; Revankar et al., 2005; Thomas et al., 2005) suggests that many previous studies using these compounds as “pure” agonists/antagonists of ERα and ERβ must be reconciled. Evidence for the role of GPER in the cardiovascular system is mainly based on studies using G-1 as a selective agonist. With the availability of GPER-knockout animals (Prossnitz and Barton, 2009) and the GPER antagonist G15 (Dennis et al., 2009), new experimental tools are available for more detailed mechanistic studies to better characterize the individual roles of GPER, ERα, and ERβ in the cardiovascular system (Meyer and Barton, 2009).
The complexity of estrogen signaling might in part also explain why clinical trials using conjugated equine estrogens and medroxyprogesterone acetate for postmenopausal hormone therapy failed to prove a therapeutic benefit on cardiovascular outcomes (Hulley et al., 1998; Rossouw et al., 2002). In addition, there were significant unfavorable effects mainly related to the development of breast cancer and venous thromboembolic events (Hulley et al., 1998; Rossouw et al., 2002). The scientific question therefore remains how to pharmacologically separate the beneficial vascular signaling pathways of estrogens from their harmful actions, which can possibly be achieved by tissue-specific, selective ER targeting devoid of the side effects seen with other estrogens. Interestingly, the SERMs raloxifene and lasofoxifene have recently been reported to reduce the risk of cardiovascular events and breast cancer at least in younger postmenopausal women (Barrett-Connor et al., 2006; Collins et al., 2009; Cummings et al., 2010; Ensrud et al., 2010). On the other hand, raloxifene does not affect blood pressure of normo- and hypertensive postmenopausal women (Cagnacci et al., 2003; Collins et al., 2009; Morgante et al., 2006; Sumino et al., 2010). Compared to other SERMs currently available for postmenopausal women, lasofoxifene appears to have the most favorable cardiovascular benefit-to-risk profile, although it was primarily found to be effective when used for the treatment of osteoporosis and breast cancer (Ensrud et al., 2010). Therefore, randomized clinical trials using lasofoxifene in postmenopausal women who are at increased cardiovascular risk are warranted. This also indicates that the cardiovascular effects of distinct SERMs are likely to differ. Thus, future basic research should further address the question how ERα, ERβ and GPER are involved in the beneficial cardiovascular effects observed after treatment with different SERMs. In addition, designing synthetic ligands that selectively mimic the beneficial vascular effects of 17β-estradiol but lack its deleterious effects at pharmacological doses represents an exciting area of research (Chow et al., 2010). Based on the current evidence, G-1 might be a candidate for such a compound. In fact, with the accumulating evidence that GPER mediates a variety of beneficial cardiovascular effects, this receptor may represent a novel target to develop effective strategies to treat cardiovascular diseases based on the clear-cut evidence of beneficial effects of premenopausal estrogens on the cardiovascular system.
Acknowledgements
Supported by Swiss National Science Foundation (SNSF) grants 3200-108528/1 and K-33KO-122504/1 (to M.B.) and PBZHP3-135874 (to M.R.M.), and National Institutes of Health (NIH) grants CA116662, CA118743 and CA127731 (to E.R.P.).
Abbreviations
- BKCa channels
Large-conductance calcium- and voltage-activated potassium channels
- EDHF
Endothelium-derived hyperpolarizing factors
- eNOS
Endothelial nitric oxide synthase
- ER
Estrogen receptor
- ERK
Extracellular signal-related kinase
- GPER
G protein-coupled estrogen receptor
- NO
Nitric oxide
- PI3K
Phosphatidylinositol-3-kinase
- ROS
Reactive oxygen species
- SERD
Selective estrogen receptor downregulator
- SERM
Selective estrogen receptor modulator
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
E.R.P. is an inventor on United States Patent Number 7,875,721.
References
- Abdelhamid R, Luo J, VandeVrede L, Kundu I, Michalsen B, Litosh VA, Schiefer IT, Gherezghiher T, Yao P, Qin Z, Thatcher GRJ. Benzothiophene selective estrogen receptor modulators provide neuroprotection by a novel GPR30-dependent mechanism. ACS Chem Neurosci. 2011;2:256–268. doi: 10.1021/cn100106a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Zubair K, Razak A, Bexis S, Docherty JR. Relaxations to oestrogen receptor subtype selective agonists in rat and mouse arteries. Eur J Pharmacol. 2005;513:101–108. doi: 10.1016/j.ejphar.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, Carpino A, Oprea TI, Prossnitz ER, Musti AM, Ando S, Maggiolini M. G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17beta-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Res. 2007;67:1859–1866. doi: 10.1158/0008-5472.CAN-06-2909. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153 Suppl 2:S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiker FA, Lips DJ, Delvaux E, Zandberg P, Janssen BJ, Prinzen F, van Eys G, Grohe C, Doevendans PA. Oestrogen modulates cardiac ischaemic remodelling through oestrogen receptor-specific mechanisms. Acta Physiol (Oxf) 2007;189:23–31. doi: 10.1111/j.1748-1716.2006.01633.x. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Mosca L, Collins P, Geiger MJ, Grady D, Kornitzer M, McNabb MA, Wenger NK. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125–137. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]
- Barton M, Cremer J, Mügge A. 17Beta-estradiol acutely improves endothelium-dependent relaxation to bradykinin in isolated human coronary arteries. Eur J Pharmacol. 1998;362:73–76. doi: 10.1016/s0014-2999(98)00787-0. [DOI] [PubMed] [Google Scholar]
- Barton M, Meyer MR. Postmenopausal hypertension: mechanisms and therapy. Hypertension. 2009;54:11–18. doi: 10.1161/HYPERTENSIONAHA.108.120022. [DOI] [PubMed] [Google Scholar]
- Bolego C, Cignarella A, Sanvito P, Pelosi V, Pellegatta F, Puglisi L, Pinna C. The acute estrogenic dilation of rat aorta is mediated solely by selective estrogen receptor-alpha agonists and is abolished by estrogen deprivation. J Pharmacol Exp Ther. 2005;313:1203–1208. doi: 10.1124/jpet.104.082867. [DOI] [PubMed] [Google Scholar]
- Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2:207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- Bopassa JC, Eghbali M, Toro L, Stefani E. A novel estrogen receptor GPER inhibits mitochondria permeability transition pore opening and protects the heart against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2010;298:H16–H23. doi: 10.1152/ajpheart.00588.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracamonte MP, Jayachandran M, Rud KS, Miller VM. Acute effects of 17beta -estradiol on femoral veins from adult gonadally intact and ovariectomized female pigs. Am J Physiol Heart Circ Physiol. 2002;283:H2389–H2396. doi: 10.1152/ajpheart.00184.2002. [DOI] [PubMed] [Google Scholar]
- Broughton BR, Miller AA, Sobey CG. Endothelium-dependent relaxation by G protein-coupled receptor 30 agonists in rat carotid arteries. Am J Physiol Heart Circ Physiol. 2010;298:H1055–H1061. doi: 10.1152/ajpheart.00878.2009. [DOI] [PubMed] [Google Scholar]
- Burger NZ, Kuzina OY, Osol G, Gokina NI. Estrogen replacement enhances EDHF-mediated vasodilation of mesenteric and uterine resistance arteries: role of endothelial cell Ca2+ Am J Physiol Endocrinol Metab. 2009;296:E503–E512. doi: 10.1152/ajpendo.90517.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- Cagnacci A, Zanni AL, Volpe A. Administration of raloxifene does not influence 24-hour ambulatory blood pressure of postmenopausal women with osteopenia: a double-blind placebo-controlled study. Am J Obstet Gynecol. 2003;188:1278–1282. doi: 10.1067/mob.2003.299. [DOI] [PubMed] [Google Scholar]
- Chambliss KL, Yuhanna IS, Mineo C, Liu P, German Z, Sherman TS, Mendelsohn ME, Anderson RG, Shaul PW. Estrogen receptor alpha and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circ Res. 2000;87:E44–E52. doi: 10.1161/01.res.87.11.e44. [DOI] [PubMed] [Google Scholar]
- Chambliss KL, Yuhanna IS, Anderson RG, Mendelsohn ME, Shaul PW. ERbeta has nongenomic action in caveolae. Mol Endocrinol. 2002;16:938–946. doi: 10.1210/mend.16.5.0827. [DOI] [PubMed] [Google Scholar]
- Chambliss KL, Wu Q, Oltmann S, Konaniah ES, Umetani M, Korach KS, Thomas GD, Mineo C, Yuhanna IS, Kim SH, Madak-Erdogan Z, Maggi A, Dineen SP, Roland CL, Hui DY, Brekken RA, Katzenellenbogen JA, Katzenellenbogen BS, Shaul PW. Non-nuclear estrogen receptor alpha signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. J Clin Invest. 2010;120:2319–2330. doi: 10.1172/JCI38291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YC, Leung FP, Wong WT, Tian XY, Yung LM, Lau CW, Tsang SY, Yao X, Chen ZY, Huang Y. Therapeutically relevant concentrations of raloxifene dilate pressurized rat resistance arteries via calcium-dependent endothelial nitric oxide synthase activation. Arterioscler Thromb Vasc Biol. 2010;30:992–999. doi: 10.1161/ATVBAHA.110.203935. [DOI] [PubMed] [Google Scholar]
- Chow RW, Handelsman DJ, Ng MK. Minireview: rapid actions of sex steroids in the endothelium. Endocrinology. 2010;151:2411–2422. doi: 10.1210/en.2009-1456. [DOI] [PubMed] [Google Scholar]
- Chung MT, Cheng PY, Lam KK, Chen SY, Ting YF, Yen MH, Lee YM. Cardioprotective effects of long-term treatment with raloxifene, a selective estrogen receptor modulator, on myocardial ischemia/reperfusion injury in ovariectomized rats. Menopause. 2010;17:127–134. doi: 10.1097/gme.0b013e3181b4c4ac. [DOI] [PubMed] [Google Scholar]
- Collins P, Mosca L, Geiger MJ, Grady D, Kornitzer M, Amewou-Atisso MG, Effron MB, Dowsett SA, Barrett-Connor E, Wenger NK. Effects of the selective estrogen receptor modulator raloxifene on coronary outcomes in the Raloxifene Use for The Heart trial: results of subgroup analyses by age and other factors. Circulation. 2009;119:922–930. doi: 10.1161/CIRCULATIONAHA.108.817577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz MN, Douglas G, Gustafsson JA, Poston L, Kublickiene K. Dilatory responses to estrogenic compounds in small femoral arteries of male and female estrogen receptor-beta knockout mice. Am J Physiol Heart Circ Physiol. 2006;290:H823–H829. doi: 10.1152/ajpheart.00815.2005. [DOI] [PubMed] [Google Scholar]
- Cummings SR, Ensrud K, Delmas PD, LaCroix AZ, Vukicevic S, Reid DM, Goldstein S, Sriram U, Lee A, Thompson J, Armstrong RA, Thompson DD, Powles T, Zanchetta J, Kendler D, Neven P, Eastell R. Lasofoxifene in postmenopausal women with osteoporosis. N Engl J Med. 2010;362:686–696. doi: 10.1056/NEJMoa0808692. [DOI] [PubMed] [Google Scholar]
- Darblade B, Pendaries C, Krust A, Dupont S, Fouque MJ, Rami J, Chambon P, Bayard F, Arnal JF. Estradiol alters nitric oxide production in the mouse aorta through the alpha-, but not beta-, estrogen receptor. Circ Res. 2002;90:413–419. doi: 10.1161/hh0402.105096. [DOI] [PubMed] [Google Scholar]
- Darkow DJ, Lu L, White RE. Estrogen relaxation of coronary artery smooth muscle is mediated by nitric oxide and cGMP. Am J Physiol. 1997;272:H2765–H2773. doi: 10.1152/ajpheart.1997.272.6.H2765. [DOI] [PubMed] [Google Scholar]
- Das B, Sarkar C. Similarities between ischemic preconditioning and 17beta-estradiol mediated cardiomyocyte KATP channel activation leading to cardioprotective and antiarrhythmic effects during ischemia/reperfusion in the intact rabbit heart. J Cardiovasc Pharmacol. 2006;47:277–286. doi: 10.1097/01.fjc.0000202563.54043.d6. [DOI] [PubMed] [Google Scholar]
- Delbeck M, Golz S, Vonk R, Janssen W, Hucho T, Isensee J, Schäfer S, Otto C. Impaired left-ventricular cardiac function in male GPR30-deficient mice. Mol Med Rep. 2011;4:37–40. doi: 10.3892/mmr.2010.402. [DOI] [PubMed] [Google Scholar]
- Delyani JA, Murohara T, Nossuli TO, Lefer AM. Protection from myocardial reperfusion injury by acute administration of 17 beta-estradiol. J Mol Cell Cardiol. 1996;28:1001–1008. doi: 10.1006/jmcc.1996.0093. [DOI] [PubMed] [Google Scholar]
- Dennis MK, Burai R, Ramesh C, Petrie WK, Alcon SN, Nayak TK, Bologa CG, Leitao A, Brailoiu E, Deliu E, Dun NJ, Sklar LA, Hathaway HJ, Arterburn JB, Oprea TI, Prossnitz ER. In vivo effects of a GPR30 antagonist. Nat Chem Biol. 2009;5:421–427. doi: 10.1038/nchembio.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschamps AM, Murphy E. Activation of a novel estrogen receptor, GPER, is cardioprotective in male and female rats. Am J Physiol Heart Circ Physiol. 2009;297:H1806–H1813. doi: 10.1152/ajpheart.00283.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschamps AM, Murphy E, Sun J. Estrogen receptor activation and cardioprotection in ischemia reperfusion injury. Trends Cardiovasc Med. 2010;20:73–78. doi: 10.1016/j.tcm.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Gros R, Limbird LE, Chorazyczewski J, Feldman RD. Estradiol-mediated ERK phosphorylation and apoptosis in vascular smooth muscle cells requires GPR 30. Am J Physiol Cell Physiol. 2009;297:C1178–C1187. doi: 10.1152/ajpcell.00185.2009. [DOI] [PubMed] [Google Scholar]
- Ensrud K, LaCroix A, Thompson JR, Thompson DD, Eastell R, Reid DM, Vukicevic S, Cauley J, Barrett-Connor E, Armstrong R, Welty F, Cummings S. Lasofoxifene and cardiovascular events in postmenopausal women with osteoporosis: Five-year results from the Postmenopausal Evaluation and Risk Reduction with Lasofoxifene (PEARL) trial. Circulation. 2010;122:1716–1724. doi: 10.1161/CIRCULATIONAHA.109.924571. [DOI] [PubMed] [Google Scholar]
- Figtree GA, Lu Y, Webb CM, Collins P. Raloxifene acutely relaxes rabbit coronary arteries in vitro by an estrogen receptor-dependent and nitric oxide-dependent mechanism. Circulation. 1999;100:1095–1101. doi: 10.1161/01.cir.100.10.1095. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- Filice E, Recchia AG, Pellegrino D, Angelone T, Maggiolini M, Cerra MC. A new membrane G protein-coupled receptor (GPR30) is involved in the cardiac effects of 17beta-estradiol in the male rat. J Physiol Pharmacol. 2009;60:3–10. [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Furchgott RF. Studies on relaxation of rabbit aorta by sodium nitrite: the basis for the proposal that acid-activable inhibitory factor from bovine retractor penis is inorganic nitrite and the endothelium-derived relaxing factor is nitric oxide. In: Vanhoutte PM, editor. Vasodilation: Vascular Smooth Muscle, Peptides, Autonomic Nerves and Endothelium. New York: Raven Press; 1988. pp. 401–414. [Google Scholar]
- Gao F, Ma X, Ostmann AB, Das SK. GPR30 activation opposes estrogen-dependent uterine growth via inhibition of stromal ERK1/2 and estrogen receptor alpha (ERalpha) phosphorylation signals. Endocrinology. 2011;152:1434–1447. doi: 10.1210/en.2010-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisclard V, Miller VM, Vanhoutte PM. Effect of 17 beta-estradiol on endothelium-dependent responses in the rabbit. J Pharmacol Exp Ther. 1988;244:19–22. [PubMed] [Google Scholar]
- Gros R, Ding Q, Sklar LA, Prossnitz ER, Arterburn JB, Chorazyczewski J, Feldman RD. GPR30 expression is required for the mineralocorticoid receptor-independent rapid vascular effects of aldosterone. Hypertension. 2011:57. doi: 10.1161/HYPERTENSIONAHA.110.161653. published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Razandi M, Pedram A, Kassab G, Levin ER. Estrogen induces vascular wall dilation: mediation through kinase signaling to nitric oxide and estrogen receptors alpha and beta. J Biol Chem. 2005;280:19704–19710. doi: 10.1074/jbc.M501244200. [DOI] [PubMed] [Google Scholar]
- Haas E, Meyer MR, Schurr U, Bhattacharya I, Minotti R, Nguyen HH, Heigl A, Lachat M, Genoni M, Barton M. Differential effects of 17beta-estradiol on function and expression of estrogen receptor alpha, estrogen receptor beta, and GPR30 in arteries and veins of patients with atherosclerosis. Hypertension. 2007;49:1358–1363. doi: 10.1161/HYPERTENSIONAHA.107.089995. [DOI] [PubMed] [Google Scholar]
- Haas E, Bhattacharya I, Brailoiu E, Damjanovic M, Brailoiu GC, Gao X, Mueller-Guerre L, Marjon NA, Gut A, Minotti R, Meyer MR, Amann K, Ammann E, Perez-Dominguez A, Genoni M, Clegg DJ, Dun NJ, Resta TC, Prossnitz ER, Barton M. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ Res. 2009;104:288–291. doi: 10.1161/CIRCRESAHA.108.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale SL, Birnbaum Y, Kloner RA. beta-Estradiol, but not alpha-estradiol, reduced myocardial necrosis in rabbits after ischemia and reperfusion. Am Heart J. 1996;132:258–262. doi: 10.1016/s0002-8703(96)90419-6. [DOI] [PubMed] [Google Scholar]
- Hale SL, Birnbaum Y, Kloner RA. Estradiol, administered acutely, protects ischemic myocardium in both female and male rabbits. J Cardiovasc Pharmacol Ther. 1997;2:47–52. doi: 10.1177/107424849700200106. [DOI] [PubMed] [Google Scholar]
- Han G, Ma H, Chintala R, Miyake K, Fulton DJ, Barman SA, White RE. Nongenomic, endothelium-independent effects of estrogen on human coronary smooth muscle are mediated by type I (neuronal) NOS and PI3-kinase-Akt signaling. Am J Physiol Heart Circ Physiol. 2007;293:H314–H321. doi: 10.1152/ajpheart.01342.2006. [DOI] [PubMed] [Google Scholar]
- Han G, Barman SA, White RE. Rapid estrogen signaling via GPR30 in coronary artery smooth muscle. Faseb J. 2009:23. doi: 10.1124/jpet.108.149112. (Meeting Abstract Supplement), abstract 968.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han G, Ma H, Barman SA, Sellers M, Yu X, Stallone JN, White RE. Rapid estrogen signaling via GPER in human coronary artery smooth muscle. Faseb J. 2010:24. (Meeting Abstract Supplement), abstract 957.1. [Google Scholar]
- Harada N, Sasano H, Murakami H, Ohkuma T, Nagura H, Takagi Y. Localized expression of aromatase in human vascular tissues. Circ Res. 1999;84:1285–1291. doi: 10.1161/01.res.84.11.1285. [DOI] [PubMed] [Google Scholar]
- Harrington WR, Sheng S, Barnett DH, Petz LN, Katzenellenbogen JA, Katzenellenbogen BS. Activities of estrogen receptor alpha- and beta-selective ligands at diverse estrogen responsive gene sites mediating transactivation or transrepression. Mol Cell Endocrinol. 2003;206:13–22. doi: 10.1016/s0303-7207(03)00255-7. [DOI] [PubMed] [Google Scholar]
- Haynes MP, Sinha D, Russell KS, Collinge M, Fulton D, Morales-Ruiz M, Sessa WC, Bender JR. Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ Res. 2000;87:677–682. doi: 10.1161/01.res.87.8.677. [DOI] [PubMed] [Google Scholar]
- Hsieh CY, Miaw CL, Hsieh CC, Tseng HC, Yang YH, Yen CH. Effects of chronic 4-nnonylphenol treatment on aortic vasoconstriction and vasorelaxation in rats. Arch Toxicol. 2009;83:941–946. doi: 10.1007/s00204-009-0447-6. [DOI] [PubMed] [Google Scholar]
- Hsieh YC, Yu HP, Frink M, Suzuki T, Choudhry MA, Schwacha MG, Chaudry IH. G protein-coupled receptor 30-dependent protein kinase A pathway is critical in nongenomic effects of estrogen in attenuating liver injury after trauma-hemorrhage. Am J Pathol. 2007;170:1210–1218. doi: 10.2353/ajpath.2007.060883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A, Sun D, Wu Z, Yan C, Carroll MA, Jiang H, Falck JR, Kaley G. Estrogen elicits cytochrome P450--mediated flow-induced dilation of arterioles in NO deficiency: role of PI3K-Akt phosphorylation in genomic regulation. Circ Res. 2004;94:245–252. doi: 10.1161/01.RES.0000111525.96232.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- Hutchison SJ, Chou TM, Chatterjee K, Sudhir K. Tamoxifen is an acute, estrogen-like, coronary vasodilator of porcine coronary arteries in vitro. J Cardiovasc Pharmacol. 2001;38:657–665. doi: 10.1097/00005344-200111000-00002. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isensee J, Meoli L, Zazzu V, Nabzdyk C, Witt H, Soewarto D, Effertz K, Fuchs H, Gailus-Durner V, Busch D, Adler T, de Angelis MH, Irgang M, Otto C, Noppinger PR. Expression pattern of G protein-coupled receptor 30 in LacZ reporter mice. Endocrinology. 2009;150:1722–1730. doi: 10.1210/en.2008-1488. [DOI] [PubMed] [Google Scholar]
- Jacobs MN, Lewis DF. Steroid hormone receptors and dietary ligands: a selected review. Proc Nutr Soc. 2002;61:105–122. doi: 10.1079/pns2001140. [DOI] [PubMed] [Google Scholar]
- Jessup JA, Lindsey SH, Wang H, Chappell MC, Groban L. Attenuation of salt-induced cardiac remodeling and diastolic dysfunction by the GPER agonist G-1 in female mRen2.Lewis rats. PLoS One. 2010;5:15433. doi: 10.1371/journal.pone.0015433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SP, Bolli R. The ubiquitous role of nitric oxide in cardioprotection. J Mol Cell Cardiol. 2006;40:16–23. doi: 10.1016/j.yjmcc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen BS, Katzenellenbogen JA. Defining the "S" in SERMs. Science. 2002;295:2380–2381. doi: 10.1126/science.1070442. [DOI] [PubMed] [Google Scholar]
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- Keung W, Chan ML, Ho EY, Vanhoutte PM, Man RY. Non-genomic activation of adenylyl cyclase and protein kinase G by 17beta-estradiol in vascular smooth muscle of the rat superior mesenteric artery. Pharmacol Res. 2011 doi: 10.1016/j.phrs.2011.05.010. published online. [DOI] [PubMed] [Google Scholar]
- Knock GA, Mahn K, Mann GE, Ward JP, Aaronson PI. Dietary soy modulates endothelium-dependent relaxation in aged male rats: Increased agonist-induced endothelium-derived hyperpolarising factor and basal nitric oxide activity. Free Radic Biol Med. 2006;41:731–739. doi: 10.1016/j.freeradbiomed.2006.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Leung FP, Yung LM, Leung HS, Au CL, Yao X, Vanhoutte PM, Laher I, Huang Y. Therapeutic concentrations of raloxifene augment nitric oxide-dependent coronary artery dilatation in vitro. Br J Pharmacol. 2007a;152:223–229. doi: 10.1038/sj.bjp.0707387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung HS, Yung LM, Leung FP, Yao X, Chen ZY, Ko WH, Laher I, Huang Y. Tamoxifen dilates porcine coronary arteries: roles for nitric oxide and ouabain-sensitive mechanisms. Br J Pharmacol. 2006;149:703–711. doi: 10.1038/sj.bjp.0706921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung HS, Seto SW, Kwan YW, Leung FP, Au AL, Yung LM, Yao X, Huang Y. Endothelium-independent relaxation to raloxifene in porcine coronary artery. Eur J Pharmacol. 2007b;555:178–184. doi: 10.1016/j.ejphar.2006.10.035. [DOI] [PubMed] [Google Scholar]
- Lin J, Steenbergen C, Murphy E, Sun J. Estrogen receptor-beta activation results in S-nitrosylation of proteins involved in cardioprotection. Circulation. 2009;120:245–254. doi: 10.1161/CIRCULATIONAHA.109.868729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey SH, Cohen JA, Brosnihan KB, Gallagher PE, Chappell MC. Chronic treatment with the G protein-coupled receptor 30 agonist G-1 decreases blood pressure in ovariectomized mRen2.Lewis rats. Endocrinology. 2009;150:3753–3758. doi: 10.1210/en.2008-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey SH, Carver KA, Prossnitz ER, Chappell MC. Vasodilation in response to the GPR30 agonist G-1 is not different from estradiol in the mRen2.Lewis female rat. J Cardiovasc Pharmacol. 2011 doi: 10.1097/FJC.0b013e3182135f1c. published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- Luksha L, Poston L, Gustafsson JA, Hultenby K, Kublickiene K. The oestrogen receptor beta contributes to sex related differences in endothelial function of murine small arteries via EDHF. J Physiol. 2006;577:945–955. doi: 10.1113/jphysiol.2006.121939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Qiao X, Falone AE, Reslan OM, Sheppard SJ, Khalil RA. Gender-specific reduction in contraction is associated with increased estrogen receptor expression in single vascular smooth muscle cells of female rat. Cell Physiol Biochem. 2010;26:457–470. doi: 10.1159/000320569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martensson UE, Salehi SA, Windahl S, Gomez MF, Sward K, Daszkiewicz-Nilsson J, Wendt A, Andersson N, Hellstrand P, Grande PO, Owman C, Rosen CJ, Adamo ML, Lundquist I, Rorsman P, Nilsson BO, Ohlsson C, Olde B, Leeb-Lundberg LM. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology. 2009;150:687–698. doi: 10.1210/en.2008-0623. [DOI] [PubMed] [Google Scholar]
- Martin D, Song J, Mark C, Eyster K. Understanding the cardiovascular actions of soy isoflavones: potential novel targets for antihypertensive drug development. Cardiovasc Hematol Disord Drug Targets. 2008;8:297–312. doi: 10.2174/187152908786786214. [DOI] [PubMed] [Google Scholar]
- Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol Interv. 2003;3:281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- Mercier I, Mader S, Calderone A. Tamoxifen and ICI 182,780 negatively influenced cardiac cell growth via an estrogen receptor-independent mechanism. Cardiovasc Res. 2003;59:883–892. doi: 10.1016/s0008-6363(03)00517-0. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Haas E, Barton M. Gender differences of cardiovascular disease: new perspectives for estrogen receptor signaling. Hypertension. 2006;47:1019–1026. doi: 10.1161/01.HYP.0000223064.62762.0b. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Barton M. ERalpha, ERbeta, and gpER: novel aspects of oestrogen receptor signalling in atherosclerosis. Cardiovasc Res. 2009;83:605–610. doi: 10.1093/cvr/cvp187. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Haas E, Prossnitz ER, Barton M. Non-genomic regulation of vascular cell function and growth by estrogen. Mol Cell Endocrinol. 2009;308:9–16. doi: 10.1016/j.mce.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MR, Baretella O, Prossnitz ER, Barton M. Dilation of epicardial coronary arteries by the G protein-coupled estrogen receptor agonists G-1 and ICI 182,780. Pharmacology. 2010;86:58–64. doi: 10.1159/000315497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev. 2008;60:210–241. doi: 10.1124/pr.107.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgante G, Delia A, Musacchio MC, Severi FM, Petraglia F, De Leo V. Effects of raloxifene therapy on plasma renin and aldosterone levels and blood pressure in postmenopausal women. Gynecol Endocrinol. 2006;22:376–380. doi: 10.1080/09513590600850300. [DOI] [PubMed] [Google Scholar]
- Mügge A, Riedel M, Barton M, Kuhn M, Lichtlen PR. Endothelium independent relaxation of human coronary arteries by 17 beta-oestradiol in vitro. Cardiovasc Res. 1993;27:1939–1942. doi: 10.1093/cvr/27.11.1939. [DOI] [PubMed] [Google Scholar]
- Mügge A, Barton M, Fieguth HG, Riedel M. Contractile responses to histamine, serotonin, and angiotensin II are impaired by 17 beta-estradiol in human internal mammary arteries in vitro. Pharmacology. 1997;54:162–168. doi: 10.1159/000139483. [DOI] [PubMed] [Google Scholar]
- Nemcsik J, Morschl E, Egresits J, Kordas K, Laszlo F, Laszlo FA, Pavo I. Raloxifene lowers ischaemia susceptibility by increasing nitric oxide generation in the heart of ovariectomized rats in vivo. Eur J Pharmacol. 2004;495:179–184. doi: 10.1016/j.ejphar.2004.05.039. [DOI] [PubMed] [Google Scholar]
- Ogita H, Node K, Asanuma H, Sanada S, Liao Y, Takashima S, Asakura M, Mori H, Shinozaki Y, Hori M, Kitakaze M. Amelioration of ischemia- and reperfusion-induced myocardial injury by the selective estrogen receptor modulator, raloxifene, in the canine heart. J Am Coll Cardiol. 2002;40:998–1005. doi: 10.1016/s0735-1097(02)02056-9. [DOI] [PubMed] [Google Scholar]
- Ogita H, Node K, Asanuma H, Sanada S, Kim J, Takashima S, Minamino T, Hori M, Kitakaze M. Raloxifene improves coronary perfusion, cardiac contractility, and myocardial metabolism in the ischemic heart: role of phosphatidylinositol 3-kinase/Akt pathway. J Cardiovasc Pharmacol. 2004;43:821–829. doi: 10.1097/00005344-200406000-00012. [DOI] [PubMed] [Google Scholar]
- Patel VH, Chen J, Ramanjaneya M, Karteris E, Zachariades E, Thomas P, Been M, Randeva HS. G-protein coupled estrogen receptor 1 expression in rat and human heart: Protective role during ischaemic stress. Int J Mol Med. 2010;26:193–199. doi: 10.3892/ijmm_00000452. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Barton M. Signaling, physiological functions and clinical relevance of the G proteincoupled estrogen receptor GPER. Prostaglandins Other Lipid Mediat. 2009;89:89–97. doi: 10.1016/j.prostaglandins.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recchia AG, De Francesco EM, Vivacqua A, Sisci D, Panno ML, Ando S, Maggiolini M. The G protein-coupled receptor 30 is up-regulated by hypoxia inducible factor-1{alpha} (HIF-1{alpha}) in breast cancer cells and cardiomyocytes. J Biol Chem. 2011;286:10773, 10782. doi: 10.1074/jbc.M110.172247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Ruehlmann DO, Steinert JR, Valverde MA, Jacob R, Mann GE. Environmental estrogenic pollutants induce acute vascular relaxation by inhibiting L-type Ca2+ channels in smooth muscle cells. FASEB J. 1998;12:613–619. doi: 10.1096/fasebj.12.7.613. [DOI] [PubMed] [Google Scholar]
- Scott PA, Tremblay A, Brochu M, St-Louis J. Vasorelaxant action of 17 -estradiol in rat uterine arteries: role of nitric oxide synthases and estrogen receptors. Am J Physiol Heart Circ Physiol. 2007;293:H3713–H3719. doi: 10.1152/ajpheart.00736.2007. [DOI] [PubMed] [Google Scholar]
- Shanle EK, Xu W. Selectively targeting estrogen receptors for cancer treatment. Adv Drug Deliv Rev. 2010;62:1265–1276. doi: 10.1016/j.addr.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw L, Taggart MJ, Austin C. Mechanisms of 17 beta-oestradiol induced vasodilatation in isolated pressurized rat small arteries. Br J Pharmacol. 2000;129:555–565. doi: 10.1038/sj.bjp.0703084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva de Sa MF, Meirelles RS. Vasodilating effect of estrogen on the human umbilical artery. Gynecol Invest. 1977;8:307–313. doi: 10.1159/000301109. [DOI] [PubMed] [Google Scholar]
- Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoncini T, Genazzani AR, Liao JK. Nongenomic mechanisms of endothelial nitric oxide synthase activation by the selective estrogen receptor modulator raloxifene. Circulation. 2002;105:1368–1373. doi: 10.1161/hc1102.105267. [DOI] [PubMed] [Google Scholar]
- Soloff MS, Szego CM. Purification of estradiol receptor from rat uterus and blockade of its estrogen-binding function by specific antibody. Biochem Biophys Res Commun. 1969;34:141–147. doi: 10.1016/0006-291x(69)90540-3. [DOI] [PubMed] [Google Scholar]
- Sudhir K, Chou TM, Mullen WL, Hausmann D, Collins P, Yock PG, Chatterjee K. Mechanisms of estrogen-induced vasodilation: in vivo studies in canine coronary conductance and resistance arteries. J Am Coll Cardiol. 1995;26:807–814. doi: 10.1016/0735-1097(95)00248-3. [DOI] [PubMed] [Google Scholar]
- Sumino H, Ichikawa S, Kasama S, Takahashi T, Kumakura H, Takayama Y, Kanda T, Murakami M, Kurabayashi M. Effects of raloxifene on the renin-angiotensin-aldosterone system and blood pressure in hypertensive and normotensive osteoporotic postmenopausal women. Geriatr Gerontol Int. 2010;10:70–77. doi: 10.1111/j.1447-0594.2009.00562.x. [DOI] [PubMed] [Google Scholar]
- Takada Y, Kato C, Kondo S, Korenaga R, Ando J. Cloning of cDNAs encoding G protein-coupled receptor expressed in human endothelial cells exposed to fluid shear stress. Biochem Biophys Res Commun. 1997;240:737–741. doi: 10.1006/bbrc.1997.7734. [DOI] [PubMed] [Google Scholar]
- Teoh H, Leung SW, Man RY. Short-term exposure to physiological levels of 17 beta-estradiol enhances endothelium-independent relaxation in porcine coronary artery. Cardiovasc Res. 1999;42:224–231. doi: 10.1016/s0008-6363(98)00265-x. [DOI] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- Thomas P, Dong J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J Steroid Biochem Mol Biol. 2006;102:175–179. doi: 10.1016/j.jsbmb.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Traupe T, Stettler CD, Li H, Haas E, Bhattacharya I, Minotti R, Barton M. Distinct roles of estrogen receptors alpha and beta mediating acute vasodilation of epicardial coronary arteries. Hypertension. 2007;49:1364–1370. doi: 10.1161/HYPERTENSIONAHA.106.081554. [DOI] [PubMed] [Google Scholar]
- Ullrich ND, Krust A, Collins P, MacLeod KT. Genomic deletion of estrogen receptors ERalpha and ERbeta does not alter estrogen-mediated inhibition of Ca2+ influx and contraction in murine cardiomyocytes. Am J Physiol Heart Circ Physiol. 2008;294:H2421–H2427. doi: 10.1152/ajpheart.01225.2007. [DOI] [PubMed] [Google Scholar]
- Umetani M, Domoto H, Gormley AK, Yuhanna IS, Cummins CL, Javitt NB, Korach KS, Shaul PW, Mangelsdorf DJ. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat Med. 2007;13:1185–1192. doi: 10.1038/nm1641. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M. Vascular signaling through G protein-coupled receptors: new concepts. Curr Opin Nephrol Hypertens. 2009;18:153–159. doi: 10.1097/MNH.0b013e3283252efe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasan RS, Larson MG, Leip EP, Evans JC, O'Donnell CJ, Kannel WB, Levy D. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–1297. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- Vergote I, Abram P. Fulvestrant, a new treatment option for advanced breast cancer: tolerability versus existing agents. Ann Oncol. 2006;17:200–204. doi: 10.1093/annonc/mdj047. [DOI] [PubMed] [Google Scholar]
- Weil BR, Manukyan MC, Herrmann JL, Wang Y, Abarbanell AM, Poynter JA, Meldrum DR. Signaling via GPR30 protects the myocardium from ischemia/reperfusion injury. Surgery. 2010;148:436–443. doi: 10.1016/j.surg.2010.03.011. [DOI] [PubMed] [Google Scholar]
- White RE, Darkow DJ, Lang JL. Estrogen relaxes coronary arteries by opening BKCa channels through a cGMP-dependent mechanism. Circ Res. 1995;77:936–942. doi: 10.1161/01.res.77.5.936. [DOI] [PubMed] [Google Scholar]
- White RE. Estrogen and vascular function. Vascul Pharmacol. 2002;38:73–80. doi: 10.1016/s0306-3623(02)00129-5. [DOI] [PubMed] [Google Scholar]
- Zhang B, Subramanian S, Dziennis S, Jia J, Uchida M, Akiyoshi K, Migliati E, Lewis AD, Vandenbark AA, Offner H, Hurn PD. Estradiol and G1 reduce infarct size and improve immunosuppression after experimental stroke. J Immunol. 2010;184:4087–4094. doi: 10.4049/jimmunol.0902339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, Hodgin J, Shaul PW, Thoren P, Smithies O, Gustafsson JA, Mendelsohn ME. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science. 2002;295:505–508. doi: 10.1126/science.1065250. [DOI] [PubMed] [Google Scholar]