SUMMARY
The transcriptional activators Oct4, Sox2 and Nanog cooperate with a wide array of cofactors to orchestrate an embryonic stem (ES) cell-specific gene expression program that forms the molecular basis of pluripotency. Here we report using an unbiased in vitro transcription-biochemical complementation assay to discover a multi-subunit stem cell coactivator complex (SCC) that is selectively required for the synergistic activation of the Nanog gene by Oct4 and Sox2. Purification, identification and reconstitution of SCC revealed this coactivator to be the trimeric XPC-nucleotide excision repair complex. SCC interacts directly with Oct4 and Sox2 and is recruited to the Nanog and Oct4 promoters as well as a majority of genomic regions that are occupied by Oct4 and Sox2. Depletion of SCC/XPC compromised both pluripotency in ES cells and somatic cell reprogramming of fibroblasts to induced pluripotent stem (iPS) cells. This study identifies a transcriptional coactivator with diversified functions in maintaining ES cell pluripotency and safeguarding genome integrity.
INTRODUCTION
The molecular events leading to the maintenance of pluripotency in embryonic stem (ES) cells and re-acquisition of a stem-like state in induced pluripotent stem (iPS) cells during somatic reprogramming represent mechanistically distinct processes that however converge on a set of remarkably similar transcriptional events that underpin the pluripotent state. Both ES and iPS cells depend on fundamental transcription frameworks that are governed by a common set of “core” stem cell-specific transcription factors, namely Oct4, Sox2 and Nanog (Jaenisch and Young, 2008). These activators in turn collaborate with both ubiquitous and cell type-specific transcription factors to orchestrate complex gene expression programs that confer upon stem cells the unique ability to safeguard stemness while remaining poised to execute a broad range of developmental programs that drive lineage specification (Boyer et al., 2005; Chen et al., 2008; Kim et al., 2008; Marson et al., 2008).
Proper execution of these highly regulated processes by sequence-specific transcription factors often requires the coordinated recruitment of coactivator proteins to their cognate promoters. For example, transcriptional activators direct histone modifiers (e.g., CBP/p300) and chromatin remodelers (e.g., PBAF/BAF) to gene promoters to alter chromatin structure toward a state that is more permissive to transcriptional activation (Naar et al., 2001). Independent of chromatin, a variety of activators recruit other classes of coactivators, such as the multi-subunit Mediator, various TBP/TAF complexes, SRC, etc, via direct protein-protein interactions to execute specific transcriptional programs. This class of coactivators often serve as molecular “adaptors” by bridging activators to the general transcription machinery thereby mediating the synergistic response by these activators (Naar et al., 1999). Interestingly, subunits of Mediator have also been shown to interact with cohesin possibly to promote DNA looping and thereby facilitate long distance interactions between enhancers and core promoters in vivo (Kagey et al., 2010). Indeed, such coactivators are often multifunctional and can activate transcription through chromatin-dependent as well as independent mechanisms. Further expanding the transcriptional repertoire of coactivator complexes, their protein levels and subunit compositions are frequently modulated in a developmental stage and cell type-specific manner (Roeder, 2005; Taatjes et al., 2004). Additionally, these protein-protein driven coactivator-activator transactions are often critical nodes in various signal transduction pathways and can serve as molecular “sensors” by integrating cell intrinsic and extrinsic cues thereby coupling gene networks with specific cellular responses to produce complex biological programs of gene expression (Rosenfeld et al., 2006).
Totipotent ES cells employ these same sets of coactivators in conjunction with special activators such as Oct4 and Sox2 to regulate transcription of a large number of genes including Nanog that form the molecular basis of pluripotency (Gao et al., 2008; Kagey et al., 2010; Kidder et al., 2009; Tutter et al., 2009). The transcription of Nanog is exquisitely dependent on Oct4 and Sox2 (Kuroda et al., 2005; Rodda et al., 2005). However, co-expression of Oct4 and Sox2 failed to robustly activate a Nanog promoter reporter construct in differentiated cells like 293 or NIH3T3 cells, even though Mediator, p300/CBP and PBAF/BAF complexes remain abundantly expressed and active (Rodda et al., 2005). This led us to speculate that one or more as yet unidentified stem cell-specific cofactor may be required to activate the transcription of Nanog and other Oct4/Sox2-target genes in ES cells. Indeed, recent studies of germ cells and differentiated somatic cells revealed that even parts of the general transcriptional machinery may be radically altered in a tissue or cell-specific context (Goodrich and Tjian, 2010; Muller et al., 2010). Diversification of the transcriptional apparatus may therefore represent a fundamental strategy, particularly in ES cells, to cope with the multi-dimensional nature of transcription programs that must be precisely tuned to both maintain pluripotency and at the same time allow for lineage-specific programs of differentiation (Liu et al, Cell, in press).
The human Nanog promoter contains a prototypic composite oct-sox cis-acting regulatory element located immediately upstream of the transcription start site that is conserved across several mammalian species (Kuroda et al., 2005; Rodda et al., 2005). A Nanog promoter-GFP reporter construct containing a DNA fragment encompassing this promoter-proximal oct-sox element is sufficient to recapitulate the robust expression pattern of endogenous Nanog in ES cells in an Oct4-, Sox2-dependent manner (Kuroda et al., 2005; Rodda et al., 2005). Unbiased genome-wide motif searching analyses of Oct4 in both mouse and human ES cells identified an oct-sox composite consensus sequence element, confirming that Oct4 likely orchestrates an ES-specific gene expression program primarily through cooperation with Sox2 (Chen et al., 2008; Loh et al., 2006). Since the oct-sox cis-control element in the Nanog promoter represents a common configuration that is present in the promoters of many other Oct4 and Sox2-activated genes in ES cells, the well-characterized Nanog proximal promoter provided us with a useful model template for identifying potentially novel transcriptional cofactors required for Oct4 and Sox2-directed activation. Therefore, we took advantage of a fully reconstituted in vitro transcription system where one can unambiguously and systematically test and identify transcriptional cofactors that may be directly required to potentiate Oct4- and Sox2-dependent gene activation of Nanog. Here we report the biochemical purification and identification of a multi-subunit stem cell coactivator (SCC) that is required for the synergistic activation of Nanog by Oct4 and Sox2 in vitro. After extensive biochemical characterization, we surprisingly found that SCC is none other than the XPC-RAD23B-CETN2 (XPC) nucleotide excision repair (NER) complex. SCC/XPC interacts directly with Oct4 and Sox2 and co-occupies a majority of Oct4 and Sox2 targets genome-wide in mouse ES cells. Importantly, SCC/XPC is required for stem cell self-renewal and efficient somatic cell reprogramming. Thus, our findings unmask an unanticipated selective coactivator role of an NER complex in transcription in the context of ES cells and may provide a previously unknown molecular link that couples stem cell-specific transcription to DNA damage response with potential implications for enhanced ES cell genome stability.
RESULTS
Detection of an Oct4- and Sox2-dependent Coactivator Activity in EC and ES Cells
Having chosen the Nanog promoter as our model template, we next set out to develop an in vitro reconstituted transcription assay that could recapitulate the Oct4- and Sox2-dependent trans-activation at the Nanog promoter observed in vivo. To enhance the sensitivity of the assay, we inserted four copies of the Nanog oct-sox binding sites immediately upstream of the native oct-sox element found in the human Nanog promoter. Our basal in vitro transcription assay consisted of purified recombinant TFIIA, -B, -E and -F together with immuno-affinity purified native RNA polymerase II, TFIID and TFIIH (Figure S1A). When purified Oct4 and Sox2 were added to this reconstituted transcription system, only a very weak activation of the Nanog promoter was detected (Figure 1A, lanes 1 and 2). As a control, we could show that the same complement of general transcription factors (GTFs) was able to support strong Sp1-dependent activation from a GC box-containing “generic” transcription template (G3BCAT, Figure 1A, lanes 5 and 6). This initial result suggested that efficient activation of Nanog by Oct4 and Sox2 may require additional cofactors to potentiate a full activator-dependent response.
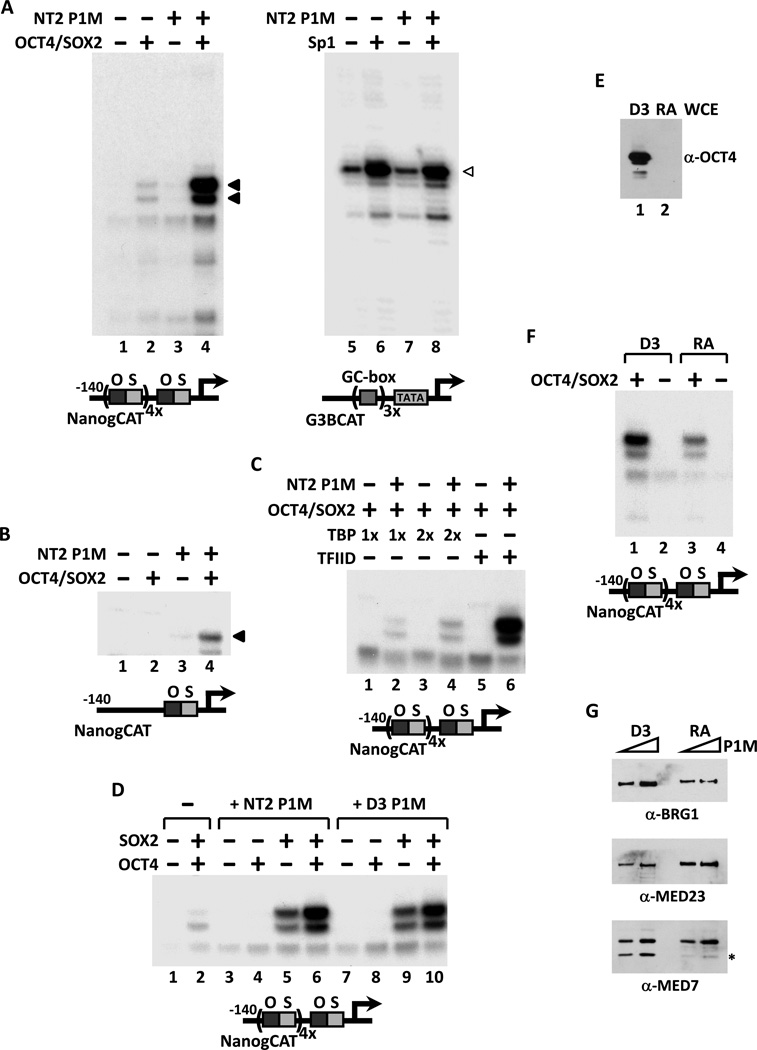
Figure 1. Transcriptional Activation of Nanog by Oct4 and Sox2 Requires a Stem Cell-Specific Cofactor.
(A) Reconstituted in vitro transcription reactions supplemented with Oct4 and Sox2 (lanes 2 and 4) or Sp1 (lanes 6 and 8), plus a phosphocellulose 1M KCl fraction derived from NT2 nuclear extracts (NT2 P1M, lanes 3, 4, 7 and 8) and programmed with either a Nanog template engineered with four extra copies of the oct-sox composite element (NanogCAT, lanes 1–4), or a GC box-containing template (G3BCAT, lanes 5–8). Oct4/Sox2, NT2 P1M-dependent transcripts are indicated by filled arrowheads and Sp1-dependent transcriptions by open arrowheads.
(B) Transcription of the native Nanog promoter requires Oct4, Sox2 and NT2 P1M fraction (lane 4).
(C) TFIID and NT2 P1M fraction are needed to potentiate Oct4/Sox2-dependent activation. Transcription reactions contain Oct4 and Sox2 (lanes 1–6), NT2 P1M fraction (lanes 2, 4 and 6), with increasing amounts of recombinant TBP (1x or 2x, lanes 1–4) or TFIID (lanes 5 and 6).
(D) Synergistic activation of Nanog by Oct4 and Sox2 requires P1M fractions prepared from NT2 or mouse ES cell line D3 nuclear extracts. In vitro transcription reactions contain equal amounts (~0.7 µg) of NT2 (lanes 3–6) or D3 P1M fractions (lanes 7–10), with Oct4 alone (lanes 4 and 8), Sox2 alone (lanes 5 and 9), or both activators (lanes 2, 6 and 10).
(E) Immunoblotting analysis of Oct4 levels in whole cell extracts (WCE) prepared from pluripotent D3 cells (D3, lane 1) and cells treated with retinoic acid for 6 days (RA, lane 2).
(F) P1M fractions prepared from pluripotent (D3, lanes 1 and 2) and differentiated (RA, lanes 3 and 4) D3 nuclear extracts were added to transcription reactions with or without Oct4 and Sox2.
(G) Western blots (2-fold titration) of P1M fractions prepared from pluripotent (D3) and differentiated (RA) D3 nuclear extracts using anti-BRG-1, anti-MED23 and anti-MED7 antibodies. Asterisk indicates a non-specific band or a breakdown product recognized by anti-MED7 antibody.
We reasoned that such a putative coactivator ought to be selectively active in pluripotent cell types that express Nanog under the control of Oct4 and Sox2. For example, NTERA-2 (NT2) is a pluripotent human embryonal carcinoma (EC) cell line that expresses Oct4, Sox2, Nanog and shares with ES cells core molecular mechanisms governing self-renewal (Pal and Ravindran, 2006). Detailed expression profiling of NT2 and bona fide human ES cell lines revealed many similarities, including robust expression of Nanog (Schwartz et al., 2005; Sperger et al., 2003). However, unlike human ES cells, NT2 cell culture can be more readily scaled up, a prerequisite to generating sufficient quantities of starting materials for the biochemical purification of putative Oct4/Sox2 coactivators. We therefore chose extracts derived from NT2 cells as our starting material in our efforts to develop a “biochemical complementation” assay to hunt for pluripotent stem cell selective cofactors.
We first fractionated NT2 nuclear extracts by conventional phosphocellulose ion exchange chromatography. Next, we supplemented our “basal” reconstituted transcription reactions with various salt-eluted fractions from the phosphocellulose column to see if there was any activity that could restore Oct4/Sox2-dependent activation of our Nanog promoter. This strategy allowed us to unmask an activity in the high salt phosphocellulose fraction (P1M) prepared from NT2 nuclear extracts (but not Hela extracts, Figure S1B) that strongly potentiated transcription of the Nanog promoter in an Oct4- and Sox2-dependent manner using either a naked (Figure 1A, lanes 3 and 4) or a Nanog chromatin template assembled with a crude Drosophila cytosolic extract (data not shown). This new cofactor activity is selectively required for transcription of Nanog as it had no effect on either basal or Sp1-activated transcription from a control G3BCAT template (Figure 1A, lanes 5–8). Importantly, this P1M fraction also stimulated the Oct4/Sox2-dependent transcription from a native Nanog promoter template (Figure 1B), as well as two other Oct4/Sox2-dependent templates derived from the mouse Fbxo15 promoter (Tokuzawa et al., 2003) (mFbxo15CAT, Figure S1C, lanes 1–4) and the human HESX1 promoter (Chakravarthy et al., 2008) (HESX1CAT, Figure S1C, lanes 5–8). Thus, our in vitro complementation assay programmed with naked DNA templates revealed at least one potential coactivator activity that directs Oct4/Sox2-dependent activation of Nanog. We decided to pursue characterization of this cofactor that does not appear to require chromatin-based functions. To the best of our knowledge, this finding also demonstrates for the first time a fully reconstituted, in vitro transcription system that can faithfully recapitulate stem cell-specific gene activation.
We next investigated the relative requirements for other cofactors in our assay system. Consistent with previous studies demonstrating that TAFs in the TFIID complex are often required for transcriptional activation by a variety of activators including nuclear receptors (Lemon et al., 2001), Sp1 (Ryu et al., 1999) and SREBP-1 (Naar et al., 1998), substituting holo-TFIID with recombinant human TBP resulted in a near complete loss of activation by Oct4 and Sox2 (Figure 1C). The very weak residual activation we see using TBP (Figure 1C, lanes 2 and 4) is most likely due to trace amounts of TFIID present in the NT2 P1M fraction (data not shown). These findings suggest that TAFs/holo-TFIID and the putative cofactor detected in the NT2 P1M fraction are both required for optimal transcription of Nanog elicited by Oct4 and Sox2. Interestingly, in this reconstituted system, the addition of CRSP/Mediator complex was not required to obtain robust Oct4/Sox2 activation at the Nanog promoter. However, it is likely that some CRSP/Mediator is present in the P1M fraction, and it remains possible that some other component of the reconstituted system (i.e., Pol II) may have some residual amount of CRSP/Mediator contamination (Naar et al., 2002). We found though that adding purified CRSP/Mediator instead of the NT2 P1M factor to these reactions completely failed to enhance Oct4/Sox2-dependent activation of Nanog transcription (Figure S1D). This finding indicates that the NT2 cofactor must be distinct from Mediator. Furthermore, addition of other transcriptional activators implicated in Nanog expression (i.e., Nanog, Sall4 (Zhang et al., 2006), Klf4 (Jiang et al., 2008) and Esrrb (van den Berg et al., 2008; Zhang et al., 2008)) also did not replace or enhance Oct4/Sox2-dependent transcription of Nanog in vitro (Figure S1E).
To confirm that this newly detected cofactor activity in NT2 cells is also present in bona fide ES cells, P1M fractions were prepared from the pluripotent D3 mouse ES cell line and assayed for transcription. We found that the D3 P1M fraction was as active as the NT2 P1M fraction in potentiating Oct4/Sox2-activated transcription of Nanog (Figure 1D, compare lane 2 to 6 and 10). Interestingly, the highest levels of trans-activation by the NT2 or D3 P1M fractions were observed only when both activators were added to the transcription reaction, whereas no activation was detected with Oct4 alone and a moderate level of activation was seen with Sox2 alone (Figure 1D, lanes 3–10). Apparently, this cofactor mediates the synergistic activation of Nanog by Oct4 and Sox2. If, as we postulated, this new coactivator functions selectively in pluripotent cells, one might expect that its presence or activity would need to be down-regulated upon differentiation, as is the case for Oct4. To investigate whether the cofactor activity is restricted to the pluripotent state of ES cells, D3 cells were induced to differentiate by removal of LIF and treatment with retinoic acid (RA). The extent of differentiation was monitored by the loss of Oct4 expression that was complete after 6 days (Figure 1E). Nuclear extracts and P1M fractions were then prepared from D3 cells before and after differentiation. When compared to pluripotent D3 P1M fractions, an equivalent amount of P1M fraction prepared from differentiated D3 nuclear extracts showed significantly decreased cofactor activity in our in vitro transcription assay (Figure 1F, compare lanes 1 and 3). This decrease is not due to a wholesale loss of transcription factors and other cofactors during stem cell differentiation because the levels of PBAF/BAF (BRG-1) and the Mediator complex (MED23 and MED7) were largely unchanged in the two extracts (Figure 1G).
Purification and Identification of a Stem Cell Coactivator (SCC)
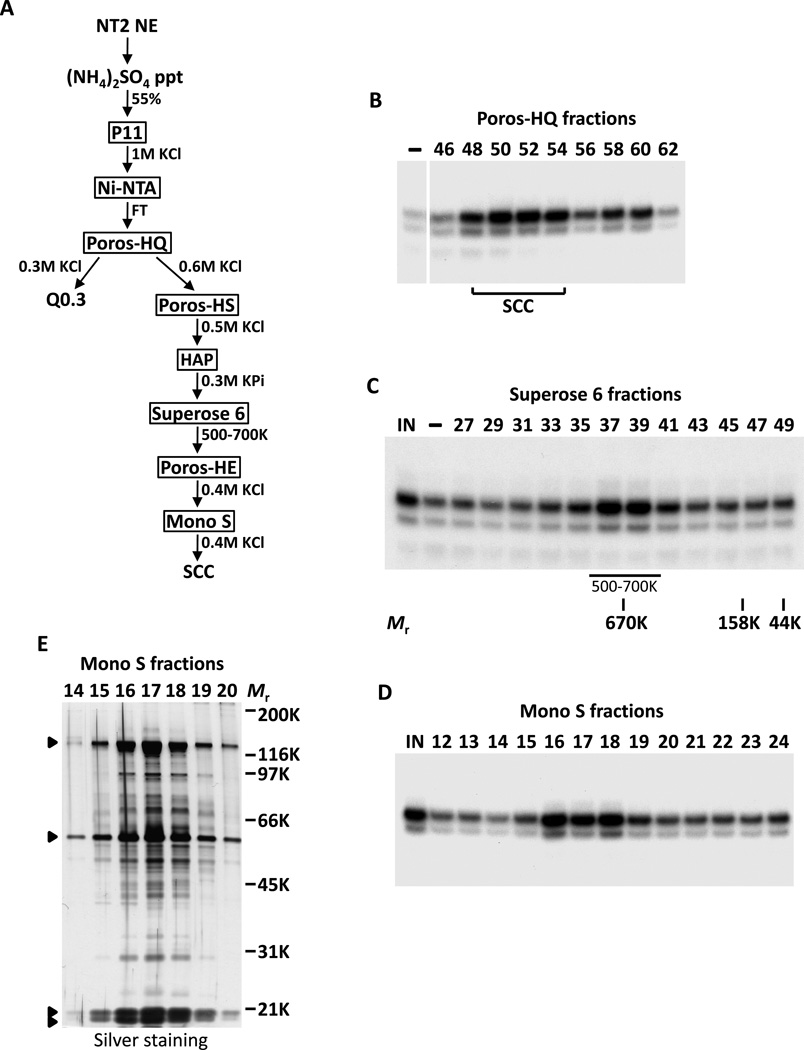
Starting with 200–400 L of NT2 cells, we were able to separate the cofactor activity into two distinct chromatographic fractions. One cofactor activity eluted from an anion exchanger (Poros-HQ) at ~0.3M KCl (Q0.3, data not shown) while a second distinct activity eluted at ~0.6M KCl (SCC, Figures 2A and 2B). Full synergistic Oct4/Sox2-dependant activation of Nanog required both fractions in our in vitro reconstituted transcription reactions (Figure S2). Using this biochemical complementation system, we sequentially purified the more robust activity, SCC, over eight chromatographic columns, resulting in >50,000-fold increase in specific activity (Figure 2A). Since SCC activity migrated with an apparent native molecular mass (Mr) of ~600kDa during size-exclusion chromatography (Figure 2C), it seemed likely that this coactivator was a multi-protein complex. Accordingly, SDS-polyacrylamide gel electrophoresis (SDS-PAGE) of the most purified Mono S fractions revealed a distinct pattern of four major polypeptides (along with multiple breakdown products) that consistently co-purified with the SCC activity (Figures 2D and 2E). For the remainder of this report, we focus on the identification and functional characterization of SCC in vitro and in vivo.
Figure 2. Purification of Stem Cell Coactivator (SCC).
(A) Chromatography scheme for partial purification of Q0.3 and purification of SCC from NT2 nuclear extracts (NT2 NE). NT2 NE is first subjected to ammonium sulfate precipitation (55% saturation) followed by a series of chromatographic columns as indicated.
(B) Buffer (−) and fractions containing SCC eluted from a Poros-HQ anion exchanger (top) assayed in the presence of Oct4 and Sox2 in in vitro transcription assays.
(C) Coactivator SCC migrates as a large complex. Input (IN), buffer (−), and Superose 6 fractions (top) assayed as in (B) except all reactions are supplemented with Q0.3 (Figure 2A). Mobilities of peak activity (500–700K) and gel filtration protein standards are shown (bottom).
(D) Transcription profile of SCC activity after the final Mono S chromatography step. Reactions contain input (IN), Mono S fractions (top) and are assayed as in (C).
(E) Silver-stained SDS-PAGE gel of the active Mono S fractions. Filled arrowheads indicate polypeptides that co-migrate with SCC activity.
To identify polypeptides comprising the SCC complex, peak Mono S-purified fractions were pooled and separated by SDS-PAGE. Surprisingly, tryptic digests of excised gel bands followed by high sensitivity mass spectrometry revealed all detectable constituents of SCC to be none other than the Xeroderma pigmentosum group C (XPC)-RAD23B-Centrin 2 (CETN2) nucleotide excision repair (NER) complex (Araki et al., 2001) (Figure 3A). We next carried out western blot analysis with antibodies specific to XPC, RAD23B and CETN2 to confirm the identities of the purified SCC subunits (Figure 3B). As expected, these three polypeptides were highly enriched in the purified SCC Mono S peak fractions when compared to the crude NT2 P1M fraction (Figure 3B). Because identification of SCC as being identical to the XPC-NER complex was so unexpected, particularly as this repair complex has not been associated with any cell type-specific function nor linked to stem cell transcription, we next wanted to compare the relative amounts of this factor in different cell types. Consistent with the notion that SCC may be functioning in an unusual way in pluripotent stem cells, we found that these three proteins are highly enriched in ES and EC cells. For example, the levels of XPC, RAD23B and CETN2 in the NT2 P1M fraction are much higher than in an equivalent amount of P1M fraction prepared from HeLa nuclear extracts (Figure 3B). Accordingly, in in vitro transcription reactions, Oct4/Sox2-dependent activation of Nanog by HeLa P1M fraction is much lower than that of NT2 P1M fraction (Figure S1B). XPC and RAD23B were rapidly down-regulated upon RA-induced differentiation of mouse D3 ES cells, whereas CETN2, components of the basal transcription machinery (TBP and TFIIE) and other NER factors (XPA and XPB) decreased only slightly while the loading control β-actin remained unchanged (Figure 3C). This finding is consistent with our previous observation that the D3 P1M fraction from differentiated cells is significantly less active than the pluripotent D3 P1M fraction in potentiating Nanog transcription (Figure 1F).
Figure 3. SCC is the XPC-RAD23B-CETN2 Nucleotide Excision Repair Complex.
(A) Mass spectrometry analysis of Mono S peak activity fractions (16–18) in Figure 2E with protein identities indicated.
(B) SCC is highly enriched in NT2 P1M fraction. Comparative western blot analysis of HeLa and NT2 P1M fractions (1.5 µg each), and purified Mono S SCC fraction (Purif, ~30 ng) using anti-XPC, anti-RAD23B and anti-CETN2 antibodies.
(C) Down-regulation of XPC and RAD23B upon RA-induced differentiation of mouse D3 ES cells. Western blot analysis of whole cell extracts prepared from D3 cells (D3 WCE) collected at indicated days post RA treatment using antibodies against XPC, RAD23B, CETN2, OCT4, XPB, XPA, TFIIEβ, TBP and loading control β-actin (ACTB).
Reconstitution and Mechanism of Coactivation by SCC
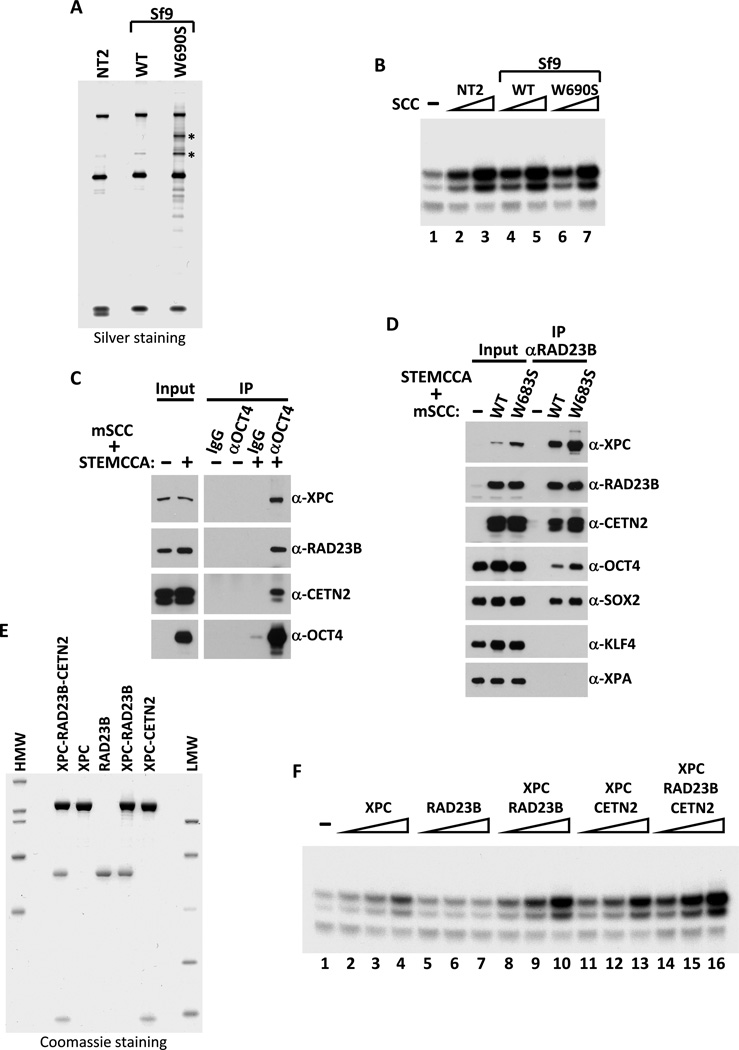
While we were in the process of further characterizing the role of the XPC-RAD23B-CETN2 complex in transcription, Le May et al reported that XPC and other components of the NER apparatus can be recruited to a gene promoter (e.g., RARβ2) upon nuclear hormone induction (Le May et al., 2010). Although the mechanism by which XPC and other NER factors mediate gene activation remains unclear, these recent studies and our new findings have unmasked a hitherto unknown and potentially important role for XPC that is directly linked to transcription. In our case, the most striking finding was the direct requirement for the SCC/XPC complex in selectively potentiating the transcriptional activation of Nanog by Oct4 and Sox2 in ES cell extracts. However, to more firmly establish this exciting new connection, we first needed to eliminate the possibility that trace amounts of contaminants present in our purified SCC fraction were responsible for the coactivator activity detected in our in vitro transcription assays. Therefore, we set about to reconstitute the heterotrimeric XPC-RAD23B-CETN2 complex from recombinant gene products expressed in insect (Sf9) cells following co-infection with baculoviruses expressing His-tagged XPC, FLAG-tagged RAD23B and untagged CETN2. Using an efficient two-step affinity purification procedure, we were able to purify the recombinant heterotrimeric complex to near homogeneity (Figure 4A). Our ability to generate pure polypeptide subunits, as well as various combinations of dimeric and trimeric complexes, allowed us to address a number of important questions, such as whether known functional domains of XPC required for NER are also necessary for the cofactor activity. It is well established that XPC’s ability to interact non-specifically with DNA is essential for its NER function. Indeed, a single point mutation in the DNA binding domain (W690S) of XPC, identified in an XP patient (XP13PV), abolishes binding to damaged (and undamaged) DNA and is defective in repair in vivo and in vitro (Maillard et al., 2007; Yasuda et al., 2007). To address whether XPC’s non-specific DNA binding activity is also important for its coactivator function, a mutant DNA-binding defective XPC (W690S) complex (that had been independently confirmed to be compromised for DNA binding in vitro, Figures S3A and S3B) was reconstituted in Sf9 cells and tested along with the wild type complex for their ability to support Oct4/Sox2-dependent transcriptional activation of Nanog in vitro. Surprisingly, both the recombinant wild-type and mutant complexes exhibited specific activities for coactivation comparable to that observed for purified native endogenous SCC from NT2 cells (Figure 4B). Taken together, these results confirm that the XPC-RAD23B-CETN2 complex is indeed SCC, and suggest that its DNA binding (and repair) activity is dispensable and functionally separable from its transcriptional cofactor activity at least in vitro. It has also been reported that XPC can interact directly with TFIIH (Uchida et al., 2002) and thus might provide a DNA-independent mechanism by which SCC can be recruited to gene promoters. To test this possibility, a C-terminal truncation of XPC that abolishes TFIIH (and CETN2) but retains RAD23B binding (amino acids 1 to 813, C814St (Bernardes de Jesus et al., 2008)) was used in our in vitro assay and found to have no adverse affect on the ability of a XPC (C814St)-RAD23B heterodimer to mediate Oct4/Sox2-activated transcription of Nanog (Figures S3C and S3D). We therefore speculate that SCC/XPC is most likely targeted to its cognate promoters via potential interactions with specific activators such as Oct4 and Sox2.
Figure 4. Reconstitution of Recombinant SCC Complexes.
(A) Silver-stained SDS-PAGE gel of purified NT2 SCC (NT2), recombinant wild-type (WT) and DNA-binding defective mutant (W690S) XPC-containing SCC complexes reconstituted in insect Sf9 cells by co-infection with baculoviruses expressing His-tagged XPC, FLAG-tagged RAD23B and untagged-CETN2. Major proteolytic fragments of mutant XPC are indicated by asterisks.
(B) Recombinant SCC complex enhances Oct4/Sox2-activated transcription of Nanog independent of DNA binding. Buffer (−), NT2 (Mono S peak activity fractions; lanes 2 and 3), recombinant WT (lanes 4 and 5) and W690S mutant (lanes 6 and 7) SCC complexes are assayed (over a three-fold concentration range). All transcription reactions contain Oct4, Sox2 and Q0.3 (lanes 1–7).
(C) Oct4 interacts with SCC. Western blot analysis of input lysates (2%) and co-immunoprecipitated proteins from extracts of 293T cells transfected with a polycistronic expression plasmid encoding all three subunits of mouse SCC (mSCC) with or without a polycistronic plasmid expressing mouse Oct4, Sox2, Klf4 and c-Myc (STEMCCA) using normal IgG or anti-Oct4 antibody.
(D) SCC-B interacts directly with Oct4 and Sox2 independent of DNA binding. Control vector (−), plasmids expressing wild-type (WT) or mutant (W683S) XPC-containing mSCC complexes were co-transfected with STEMCCA into 293T cells and immunoprecipitated with anti-RAD23B antibody. Input lysates (2%) and RAD23B-bound proteins were detected by immunoblotting.
(E) Coomassie-stained SDS-PAGE gel of purified recombinant XPC, RAD23B, dimeric (XPC-RAD23B and XPC-CETN2) and holo-SCC (XPC-RAD23B-CETN2) complexes.
(F) Titrations (over a four-fold concentration range) of XPC (lanes 2–4), RAD23B (lanes 5–7), XPC-RAD23B (lanes 8–10), XPC-CETN2 (lanes 11–13) and XPC-RAD23B-CETN2 (lanes 14–16) in in vitro transcription reactions supplemented with Q0.3 (lanes 1–16) and assayed as in (B).
To probe for a potential direct interaction between SCC and Oct4 and/or Sox2, mouse SCC subunits were over-expressed with Oct4, Sox2, Klf4 and c-Myc (STEMCCA (Sommer et al., 2009)) in 293T cells. SCC co-immunoprecipitated with Oct4 but not with control IgG (Figure 4C). To examine whether the DNA-binding property of SCC is required for its interaction with Oct4 and other activators, both the wild-type (WT) and DNA-binding defective (W683S in mouse) XPC/SCC complexes were co-expressed with STEMCCA. Immunoprecipitation of WT and mutant SCC complexes using an anti-RAD23B antibody pulled down both Oct4 and Sox2 but not Klf4 or XPA (Figure 4D). These data indicate a direct and specific protein-protein binding between SCC and select activators thus providing a mechanism by which SCC may serve as a transcriptional coactivator for Oct4 and Sox2 (but not Klf4, see Figure S1E) in potentiating Nanog transcription. These findings may also explain why the DNA-binding activity of the XPC subunit of SCC is dispensable for transcription in vitro. We were however unable to reproducibly detect a stable interaction between SCC and Oct4/Sox2 in D3 ES cell extracts. It is worth noting, though, that other coactivators implicated in Oct4/Sox2-directed transcriptional activation (e.g., Mediator and p300/CBP) have not been identified in recent “interactome” studies on Oct4, Sox2 or Nanog-associating factors (Engelen et al., 2011; van den Berg et al., 2010; Wang et al., 2006), supporting the idea that functional coactivator-activator interactions can often be weak and transient.
The ability to reconstitute active SCC from purified recombinant subunits also provided us with a unique opportunity to examine the contribution of individual subunits, as well as different dimeric combinations in supporting Oct4/Sox2 transcriptional activation. Purified individual subunits (XPC or RAD23B), partial dimeric complexes (XPC-RAD23B or XPC-CETN2), and holo-SCC complexes (Figure 4E) were assayed over a four-fold dose response range in our fully reconstituted in vitro transcription reactions containing Oct4, Sox2 and a partially purified Q0.3 fraction (Figure 4F). The large XPC subunit alone only slightly activated transcription above background at the highest concentrations tested (Figure 4F, compare lanes 1 and 4) while RAD23B alone was essentially inactive. The XPC-CETN2 dimer was slightly more active than XPC alone. By contrast, a marked gain in specific activity was observed with the XPC-RAD23B dimeric complex that was nearly as active as the holo-complex (Figure 4F). These results suggest that the minimal active complex likely consists of XPC and RAD23B, while CETN2 may enhance the activity of the complex by providing structural support or stability.
SCC Coactivator Function in ES Cell Self-renewal and Somatic Cell Reprogramming
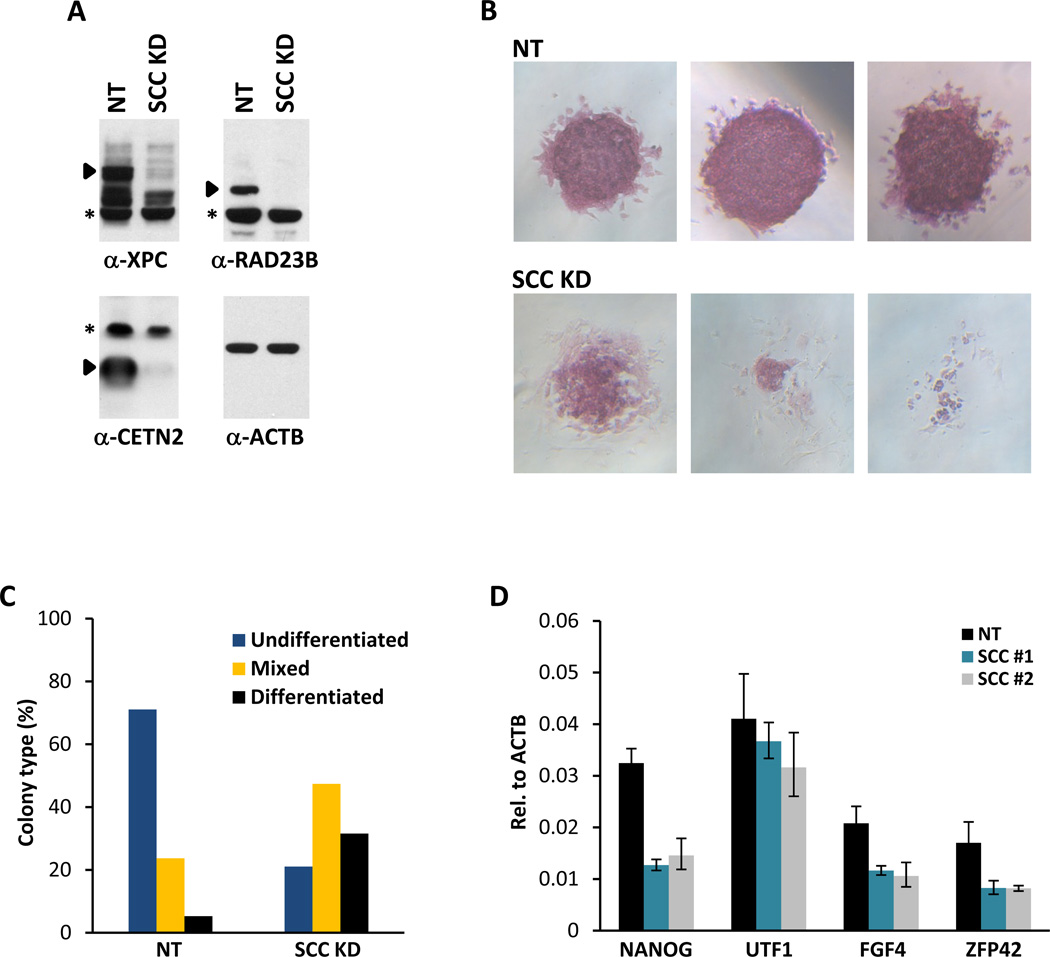
We next set out to determine the role of the SCC/XPC complex on gene expression and Nanog transcription by loss-of-function studies in ES cells. Lentiviruses containing two independent short hairpin RNAs (shRNAs) specifically targeting XPC, RAD23B and CETN2 were used to infect mouse D3 ES cells to selectively deplete SCC (Figures 5A, S4A and S4B). Knockdown of SCC subunits resulted in pronounced cellular morphological abnormalities and decreased alkaline phosphatase (AP) activity (Figures 5B and S4C). These knockdown cells also showed reduced proliferation rates when compared to control ES cells infected with non-target viruses, indicating that the self-renewal capacity of ES cells depleted of SCC may also be compromised (data not shown). Indeed, prolonged depletion of SCC resulted in the apoptosis of flattened, fibroblastic AP-negative cells surrounding the collapsing ES cell colonies (Figure 5B and data not shown). Therefore, knockdown of SCC in ES cells likely promotes differentiation followed by rapid apoptosis, two processes that are often coupled. Quantification of colony assays revealed that ES cells depleted of SCC formed fewer undifferentiated colonies with a corresponding increase in partially and fully differentiated colonies (Figure 5C). Consistent with the observed morphological changes associated with compromised stem cell identity, double and triple knockdown of XPC, RAD23B and CETN2 resulted in a 2–3-fold reduction in the mRNA level of Nanog (Figures 5D and S4D) as well as several other stem cell markers (Fgf4, Zfp42 and Utf1) (Figure 5D). Knockdown of individual subunits of SCC resulted in only mild effects on Nanog expression (Figure S4D). Accordingly, we did not observe overt defects in self-renewal in these single subunit knockdown ES cells (data not shown).
Figure 5. SCC is Required for ES Cell Maintenance.
(A) Efficiency of shRNA-mediated depletion of SCC in mouse ES cell line D3. Whole cell extracts of mouse D3 cells infected with non-target (NT) lentiviruses (MOI of 300) or with an equal mixture of three lentiviruses (MOI of 100 each) targeting XPC, RAD23B and CETN2 (SCC KD) are analyzed by western blotting. Specific bands recognized by their respective antibodies are indicated by filled arrowheads. Asterisks denote non-specific signals.
(B) ES cell colony morphology and alkaline phosphatase (AP) activity (red) are maintained in control D3 cells (NT, top panels) but are compromised in SCC-depleted D3 cells (SCC KD, bottom panels) (See also Figure S4C).
(C) Clonal assays on SCC-depleted D3 ES cells. Stable non-target (NT) and SCC-depleted (SCC KD) D3 cell pools were plated at 300 cells per well in 6-well plates, emerging colonies were stained for AP activity and scored according to differentiation status after 6 days.
(D) Two non-overlapping sets of shRNAs targeting SCC (SCC #1 and SCC #2) are used to deplete SCC. Quantification of Nanog, Utf1, Fgf4 and Zfp42 mRNA levels are analyzed by real time quantitative PCR (qPCR) and normalized to Actb. Data from representative experiments are shown; error bars represent standard deviations (n=3).
To further probe the molecular mechanism underpinning the function of SCC as a transcriptional coactivator for Oct4 and Sox2 in vivo, we investigated whether regulatory regions of Nanog and Oct4 might serve as direct SCC targets by performing chromatin immunoprecipitation (ChIP) assays in D3 cells using a RAD23B antibody. ChIP-qPCR analysis revealed that RAD23B (and presumably XPC/SCC) occupancy sites coincide with those of Oct4 (Boyer et al., 2005; Chen et al., 2008; Kim et al., 2008) and Sox2 (Figures 6A and S5A). By contrast, we failed to detect any significant enrichment of RAD23B at housekeeping genes (β-actin (Actb, Figure 6A) and dihydrofolate reductase (Dhfr, Figure S5B), or an intergenic region on chromosome 1 (Figure S5B).
Figure 6. SCC is Recruited to the Nanog and Oct4 Promoters and Genomic Regions Occupied by Oct4 and Sox2.
(A) Co-occupancy of SCC, Oct4 and Sox2 on the promoters of Nanog and Oct4. ChIP analysis of RAD23B occupancy on distal enhancers (enh), proximal promoter (transcription start site, TSS), and upstream (positions indicated by numbers) and downstream intronic regions of the Nanog (left), Oct4 (middle), and Actb (right) gene loci. Representative data (n>5) showing the enrichment of RAD23B (black bars) compared to normal IgGs (white bars) are analyzed by qPCR and expressed as percentage of input chromatin. Schematic diagrams of Oct4 and Sox2 binding sites on the Nanog and Oct4 regulatory regions (TSS and enhancers; see also Figure S5A) are indicated at the bottom. Error bars represent standard deviations (n=3).
(B) Percent peak overlap between RAD23B and control IgG ChIP-seq data relative to published Oct4/Sox2 and Nanog/Tcf3 peak data.
(C) Percent base-pair overlap between RAD23B and control IgG ChIP-seq data relative to Oct4/Sox2 and Nanog/Tcf3 ChIP-seq data sets.
(D) The distribution of distance (in base pair) of RAD23B and control IgG peaks from Oct4/Sox2 and Nanog/Tcf3 peaks.
To evaluate the extent to which Oct4 and Sox2 target sites overlap those of RAD23B on a genome-wide scale, we performed RAD23B ChIP assays followed by high-throughput sequencing (ChIP-seq) to identify an entire range of RAD23B/SCC-bound genomic regions in D3 cells. RAD23B ChIP-seq results were then compared with published Oct4 and Sox2 ChIP-seq data along with those of Nanog and Tcf3 (Marson et al., 2008) to assess any potential bias in RAD23B occupancy in relation to these transcription factors. This analysis revealed a striking binding preference of RAD23B/SCC to genomic sites that are also co-occupied by Oct4 and Sox2, but not Nanog or Tcf3 only (~70% versus ~28%, p <10−15, ANOVA). This strong bias is maintained whether the ChIP-seq data sets are analyzed by the degree of peak overlap (defined by any two peaks with at least one nucleotide of overlap, Figure 6B) or base-pair coverage (Figure 6C), indicating that the majority of RAD23B/SCC binding sites align with those of Oct4 and Sox2. Importantly, the same analyses performed on ChIP-seq samples obtained from control IgG immunoprecipitations yielded only background correlation (between 4–8%), confirming the specificity of the RAD23B/SCC association. We further validated the co-localization among RAD23B/SCC, Oct4 and Sox2 by measuring the distance between overlapping RAD23B/SCC and Oct4/Sox2 peaks (See Extended Experimental Procedures). The majority of them (76%) lie within close proximity (≤ 200 base pairs) of each other (Figure 6D). Even though most of RAD23B/SCC-bound regions overlap poorly with those bound by Nanog/Tcf3 (~28%), those that do are still largely (64%) positioned within 200 base pairs from each other but with a noticeably different distribution pattern than that of Oct4/Sox2 (p <10−15, ANOVA, Figure 6D). However, upon a closer look at the Nanog/Tcf3 “only” genomic coordinates that overlap with RAD23B-bound sites we found that many of them (~40%) could in fact contain Oct4 and/or Sox2 when an alternative peak calling strategy (MACS) was used. Taken together, these data strongly suggest a classical coactivator function rather than a purely NER function of SCC both in vitro with naked DNA and in the context of chromatin in ES cells as XPC/RAD23B-mediated DNA damage repair generally involves transient interactions with DNA (Camenisch et al., 2009) that would not show either sequence or promoter specificity.
Given the importance of SCC in stem cell maintenance, we next asked whether it might also play a role in the re-acquisition of pluripotency during somatic cell reprogramming. Down-regulation of either XPC or RAD23B in Oct4-GFP mouse embryonic fibroblasts (MEFs), which express some SCC albeit at significantly lower levels than ES cells, led to a dramatic reduction in the reprogramming efficiency. We observed a significant decrease in the number of AP-positive colonies, as well as a marked reduction in the percentage of partially (SSEA-1+, GFP−) and fully (SSEA-1+, GFP+) reprogrammed cells, as determined by FACS sorting (Figures 7A, 7B and S6A). Consistent with our in vitro reconstitution result showing that the CETN2 subunit may not be essential for the transcriptional activity of SCC (Figure 4F), knockdown of CETN2 had minor effects on iPS cell derivation efficiency. As expected, reprogramming efficiency using MEFs derived from XPC and RAD23B knockout (KO) mice (Ng et al., 2003) was also highly compromised. Surprisingly, RAD23A KO MEFs were nearly as efficient as wild-type or RAD23A and B double heterozygous MEFs in generating AP-positive colonies upon iPS cell induction (Figures 7C and S6B). This result may point to a non-redundant function of RAD23B in somatic reprogramming independent of its role in DNA repair as RAD23B KO (and RAD23A KO) MEFs are NER proficient (Ng et al., 2003). Importantly, depletion of XPC (knockdown and knockout) and CETN2 in MEFs did not affect proliferation rates when compared to non-target or Oct4 knockdown MEFs. However, RAD23B-depleted MEFs displayed noticeable changes in growth rates, which may partially account for the marked reduction in reprogramming efficiency (data not shown). These data suggest that efficient reprogramming may require SCC/XPC in conjunction with Oct4 and Sox2 to re-establish ES-specific gene expression programs.
Figure 7. SCC is Required for Efficient Somatic Cell Reprogramming.
(A) Depletion of SCC blocks somatic cell reprogramming. Oct4-GFP mouse embryonic fibroblasts infected with lentiviruses expressing STEMCCA and rtTA together with non-target shRNA (NT), shRNAs against Oct4, individual subunits of SCC, or all three subunits simultaneously at low or high multiplicity of infection (SCC LO or HI) are plated in 6 well plates for colony counting and FACS, or in 24 well plates for AP staining. AP-positive (red) cells are stained and counted 17 days (14 days + dox, 3 days − dox) post induction (dpi). Results from two separate experiments are shown.
(B) Single cell suspensions of 17 dpi Oct4-GFP MEFs as described in (A) are stained with anti-mouse SSEA-1 antibodies and analyzed by FACS.
(C) Wild-type (WT), RAD23A and B double-heterozygous (23A/B d-Het) MEFs, together with XPC, RAD23A and RAD23B knockout (KO) MEFs, are induced with STEMCCA. AP-positive colonies are stained and counted as in (A).
DISCUSSION
Establishment of ground state pluripotency in embryonic stem cells represents one of the most remarkable events in development. Stem cells have evolved a subset of cell type-specific activators among a constellation of previously identified transcription factors and cofactors to resolve the dichotomy between self-renewal versus differentiation. Our de novo purification of the SCC/XPC complex as a potent coactivator for Oct4 and Sox2 was unanticipated but may in part reflect the need for stem cells to robustly expand and diversify their transcriptional repertoire while also maintaining genome integrity. Indeed, other NER factors have been shown to participate in transcriptional regulation both at the basal and activated levels. For instance, the general transcription factor TFIIH is a classic example with established roles in both transcription initiation and NER (Schaeffer et al., 1993). Interestingly, it has recently been reported that, in HeLa cells, the entire NER complex can be assembled onto promoters of activated genes in an XPC-dependent manner. However, XPC alone is not sufficient, as other NER components appear to be responsible for RA-activated transcription (Le May et al., 2010). This finding in Hela cells is distinct from our observation that the XPC-NER (SCC) complex plays a direct and critical role in Nanog transcription in vitro and in ES cells. In our studies, optimal activation of Nanog by Oct4/Sox2 potentiated by SCC requires a second activity present in the Q0.3 fraction. However, preliminary mass spectrometry analyses of the partially purified Q0.3 fraction failed to detect any other XP or NER factors, or factors previously identified to co-purify with Nanog or Oct4 in ES cells (van den Berg et al., 2010; Wang et al., 2006) (data not shown). Therefore, the SCC/XPC complex can potentiate Nanog transcription and likely other Oct4/Sox2-directed promoters in the absence of additional XP and NER factors in vitro. Taken together, these results suggest that the mechanism by which the SCC/XPC complex coactivates transcription in ES cells may be distinct from its function in HeLa cells.
Although XPC plays a critical role in DNA lesion recognition, XPC is not universally required for NER as certain types of bulky DNA lesions (e.g., cholesterol-DNA adducts) can be repaired without XPC (Mu et al., 1996). Intriguingly, even though XPC is recruited to gene promoters irrespective of DNA damage signals (Le May et al., 2010), the XPC-NER complex is the only factor in the XP family that is dispensable for transcription-coupled repair (TCR) (Venema et al., 1990). Indeed, our findings suggest that the coactivator and NER duties carried out by SCC are mechanistically distinct processes as SCC can function as part of the transcriptional cofactor apparatus via a direct interaction with Oct4 and Sox2 without requiring either DNA or TFIIH binding mediated by XPC.
It is worth noting that the effect of single knockdown of XPC or RAD23B was much more pronounced in the reprogramming of MEFs than in the maintenance of ES cells. We surmise that perhaps other redundant regulatory mechanisms in established ES cells can partially compensate for the loss of SCC. Such robust regulatory circuitries are likely to be less developed during the early phase of reprogramming in MEFs and are thus more susceptible to perturbation by SCC depletion. It is conceivable that SCC/XPC may also contribute to the process of chromatin reorganization and facilitate changes in the epigenetic landscape that are conducive to iPS conversion (Le May et al., 2010).
Also in agreement with our in vitro and cell-based studies, a mouse double KO of RAD23B and its homolog RAD23A was found to be early embryonic lethal (Ng et al., 2003). This previously puzzling phenotype can now be more readily rationalized in light of the functional role of XPC in transcriptional coactivation revealed here. These results, taken together, strongly suggest that loss of the SCC/XPC complex may indeed compromise the transcriptional integrity of pluripotent stem cells, as well as the ability of somatic cells to re-establish pluripotency. However, XPC KO mice are UV-sensitive but otherwise normal with no obvious developmental defects (Sands et al., 1995). It has been shown that RAD23B is in vast excess relative to XPC (Sugasawa et al., 1996), suggesting that RAD23B may exist in other complexes independent of XPC that functionally replace SCC.
Embryonic stem cells are thought to be under strong selective pressure to maintain genome fidelity since accumulation and propagation of DNA errors to progenitor cells would be lethal during development, therefore, DNA damage response factors and pathways are often up-regulated in ES cells (e.g., XPC, RAD23B, ERCC5, etc) (Cervantes et al., 2002; Ramalho-Santos et al., 2002). Should DNA repair fail, UV-damaged ES cells can be eliminated first by repressing Nanog expression through p53 up-regulation, which in turn promotes spontaneous differentiation and efficient apoptosis (Lin et al., 2005). It is interesting to note that upon UV-induced DNA damage in HeLa cells, recruitment of XPC to and expression of non-UV inducible genes are dramatically delayed (Le May et al., 2010). This suggests that some sort of redistribution mechanism may redirect XPC from transcription duty at promoter targets to the NER pathway in response to DNA damage. In light of these observations, it is tempting to speculate that redistribution of XPC-RAD23B-CETN2 from Nanog and presumably other Oct4/Sox2-regulated promoters to DNA damage sites may provide an efficient sensing mechanism to perturb stem cell-specific gene expression programs and thus provide a window of opportunity for ES cells to either repair the lesions or commit to differentiation and apoptosis. The SCC/XPC complex may therefore act as a molecular link to couple stem cell-specific gene expression programs and genome surveillance in ES cells.
EXPERIMENTAL PROCEDURES
DNA Constructs, Cell Lines and Cell Culture
Construction of in vitro transcription templates and protein expression plasmids are described in Extended Experimental Procedures. HeLa, 293T, NTERA-2 (NT2) and mouse ES cell line D3 were maintained in standard conditions. Differentiation of D3 ES cells was carried out by LIF removal followed by retinoic acid treatment (5–10 µM, Sigma).
Purification and Identification of SCC
Nuclear extracts from ~400 L of NT2 cells were purified over eight chromatographic steps to homogeneity. Methods for purification and mass spectrometry analyses of SCC are detailed in Extended Experimental Procedures.
Western Blotting, Immunoprecipitation, and Affinity Purification
Antibodies used are described in Extended Experimental Procedures. Transcriptional activators were purified from transiently transfected HeLa cells followed by affinity purification using anti-FLAG (M2) agarose (Sigma) as described in Extended Experimental Procedures. Recombinant SCC complexes were purified from Sf9 cells infected with baculoviruses (BAC-to-BAC system, Invitrogen) expressing N-terminal His6-tagged or FLAG-tagged XPC, N-FLAG-tagged RAD23B and untagged CETN2. Sf9 cells were harvested 48 hr after infection and protein complexes were purified by incubating cell lysates with Ni-NTA resin (Qiagen), anti-FLAG (M2) agarose (Sigma), and elution by the FLAG peptides.
shRNA-mediated Knockdown of SCC by Lentiviral Infection
Control non-target and pLKO shRNA plasmids targeting XPC, RAD23B and CETN2 (Sigma) were transfected with packaging vectors into 293T cells using FuGENE 6 (Roche). Supernatants were concentrated by ultracentrifugation and resuspended in PBS. Viral titer was determined by a QuickTiter Lentivirus Titer Kit (Cell Biolabs). SCC knockdown was performed by incubating lentiviral concentrates with D3 cells in the presence of 8 µg/ml polybrene followed by puromycin selection (1.5 µg/ml).
Gene Expression Analysis and ChIP
Total RNA from shRNA-mediated knockdown D3 ES cells was isolated using RNeasy Plus Kit (Qiagen) and analyzed by qRT-PCR. Chromatin immunoprecipitation (ChIP) assays were performed in D3 cells as described in Extended Experimental Procedures. Precipitated DNA was measured by qPCR, or sequenced using an Illumina HiSeq 2000 sequencing platform. Methods for gene expression and ChIP analyses are detailed in Extended Experimental Procedures.
Somatic Cell Reprogramming
Oct4-GFP MEFs (The Jackson Laboratory) were infected with lentiviruses containing STEMCCA and rtTA, followed by infection with pLKO shRNA lentiviral supernatants targeting SCC. Oct4, Sox2, Klf4 and c-Myc expressions were induced by doxycycline and SCC knockdown MEFs were selected with puromycin. Reprogrammed cells were either detected by alkaline phosphatase activity or were stained with anti-SSEA-1 antibodies conjugated to Alexa Fluor 647 (BioLegends) and analyzed by FACS. XPC, RAD23A and B knockout MEFs were generous gifts from Dr. Hoeijmakers (Rotterdam, The Netherlands).
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to thank A. Fischer and M. Richner at the Tissue Culture Facility (University of California at Berkeley), S. Zheng, G. Dailey, M. Haggart and E. Bourbon for technical assistance; S. Chen at National Institute of Biological Science (Beijing, China) for mass spectrometry analysis; S. Zhou for initial mass spectrometry analysis; J. Hoeijmakers for knockout MEFs; G. Mostoslavsky for STEMCCA; O. Puig for pGL3-CAT plasmid; S. Ryu for purified rTBP and G3BCAT plasmid; K. Hochedlinger, M. Stadtfeld, J. de Wit and M. Holmes for technical advice; M. Botchan, M. Holmes, M. Levine, S. Martin, M. Rape, D. Rio, and all members of our laboratory for critical reading of the manuscript. Y. Fong was a California Institute for Regenerative Medicine scholar. T. Yamaguchi was supported by the Swiss National Science Foundation, the Siebel Stem Cell Institute, and is a Jane Coffin Childs fellow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Araki M, Masutani C, Takemura M, Uchida A, Sugasawa K, Kondoh J, Ohkuma Y, Hanaoka F. Centrosome protein centrin 2/caltractin 1 is part of the xeroderma pigmentosum group C complex that initiates global genome nucleotide excision repair. J. Biol. Chem. 2001;276:18665–18672. doi: 10.1074/jbc.M100855200. [DOI] [PubMed] [Google Scholar]
- Bernardes de Jesus BM, Bjoras M, Coin F, Egly JM. Dissection of the molecular defects caused by pathogenic mutations in the DNA repair factor XPC. Mol. Cell. Biol. 2008;28:7225–7235. doi: 10.1128/MCB.00781-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camenisch U, Trautlein D, Clement FC, Fei J, Leitenstorfer A, Ferrando-May E, Naegeli H. Two-stage dynamic DNA quality check by xeroderma pigmentosum group C protein. EMBO J. 2009;28:2387–2399. doi: 10.1038/emboj.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes RB, Stringer JR, Shao C, Tischfield JA, Stambrook PJ. Embryonic stem cells and somatic cells differ in mutation frequency and type. Proc. Natl. Acad. Sci. USA. 2002;99:3586–3590. doi: 10.1073/pnas.062527199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthy H, Boer B, Desler M, Mallanna SK, McKeithan TW, Rizzino A. Identification of DPPA4 and other genes as putative Sox2:Oct-3/4 target genes using a combination of in silico analysis and transcription-based assays. J. Cell. Physiol. 2008;216:651–662. doi: 10.1002/jcp.21440. [DOI] [PubMed] [Google Scholar]
- Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB, Wong E, Orlov YL, Zhang W, Jiang J, et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133:1106–1117. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- Engelen E, Akinci U, Bryne JC, Hou J, Gontan C, Moen M, Szumska D, Kockx C, van Ijcken W, Dekkers DH, et al. Sox2 cooperates with Chd7 to regulate genes that are mutated in human syndromes. Nat. Genet. 2011;43:607–611. doi: 10.1038/ng.825. [DOI] [PubMed] [Google Scholar]
- Gao X, Tate P, Hu P, Tjian R, Skarnes WC, Wang Z. ES cell pluripotency and germ-layer formation require the SWI/SNF chromatin remodeling component BAF250a. Proc. Natl. Acad. Sci. USA. 2008;105:6656–6661. doi: 10.1073/pnas.0801802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich JA, Tjian R. Unexpected roles for core promoter recognition factors in cell-type-specific transcription and gene regulation. Nat. Rev. Genet. 2010;11:549–558. doi: 10.1038/nrg2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R, Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008;132:567–582. doi: 10.1016/j.cell.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Chan YS, Loh YH, Cai J, Tong GQ, Lim CA, Robson P, Zhong S, Ng HH. A core Klf circuitry regulates self-renewal of embryonic stem cells. Nat. Cell Biol. 2008;10:353–360. doi: 10.1038/ncb1698. [DOI] [PubMed] [Google Scholar]
- Kagey MH, Newman JJ, Bilodeau S, Zhan Y, Orlando DA, van Berkum NL, Ebmeier CC, Goossens J, Rahl PB, Levine SS, et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature. 2010;467:430–435. doi: 10.1038/nature09380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidder BL, Palmer S, Knott JG. SWI/SNF-Brg1 regulates self-renewal and occupies core pluripotency-related genes in embryonic stem cells. Stem Cells. 2009;27:317–328. doi: 10.1634/stemcells.2008-0710. [DOI] [PubMed] [Google Scholar]
- Kim J, Chu J, Shen X, Wang J, Orkin SH. An extended transcriptional network for pluripotency of embryonic stem cells. Cell. 2008;132:1049–1061. doi: 10.1016/j.cell.2008.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda T, Tada M, Kubota H, Kimura H, Hatano SY, Suemori H, Nakatsuji N, Tada T. Octamer and Sox elements are required for transcriptional cis regulation of Nanog gene expression. Mol. Cell. Biol. 2005;25:2475–2485. doi: 10.1128/MCB.25.6.2475-2485.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le May N, Mota-Fernandes D, Velez-Cruz R, Iltis I, Biard D, Egly JM. NER factors are recruited to active promoters and facilitate chromatin modification for transcription in the absence of exogenous genotoxic attack. Mol. Cell. 2010;38:54–66. doi: 10.1016/j.molcel.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Lemon B, Inouye C, King DS, Tjian R. Selectivity of chromatin-remodelling cofactors for ligand-activated transcription. Nature. 2001;414:924–928. doi: 10.1038/414924a. [DOI] [PubMed] [Google Scholar]
- Lin T, Chao C, Saito S, Mazur SJ, Murphy ME, Appella E, Xu Y. p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat. Cell Biol. 2005;7:165–171. doi: 10.1038/ncb1211. [DOI] [PubMed] [Google Scholar]
- Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, Bourque G, George J, Leong B, Liu J, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat. Genet. 2006;38:431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- Maillard O, Solyom S, Naegeli H. An aromatic sensor with aversion to damaged strands confers versatility to DNA repair. PLoS Biol. 2007;5:e79. doi: 10.1371/journal.pbio.0050079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marson A, Levine SS, Cole MF, Frampton GM, Brambrink T, Johnstone S, Guenther MG, Johnston WK, Wernig M, Newman J, et al. Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell. 2008;134:521–533. doi: 10.1016/j.cell.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu D, Hsu DS, Sancar A. Reaction mechanism of human DNA repair excision nuclease. J. Biol. Chem. 1996;271:8285–8294. doi: 10.1074/jbc.271.14.8285. [DOI] [PubMed] [Google Scholar]
- Muller F, Zaucker A, Tora L. Developmental regulation of transcription initiation: more than just changing the actors. Curr. Opin. Genet. Dev. 2010;20:533–540. doi: 10.1016/j.gde.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Naar AM, Beaurang PA, Robinson KM, Oliner JD, Avizonis D, Scheek S, Zwicker J, Kadonaga JT, Tjian R. Chromatin, TAFs, and a novel multiprotein coactivator are required for synergistic activation by Sp1 and SREBP-1a in vitro. Genes Dev. 1998;12:3020–3031. doi: 10.1101/gad.12.19.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naar AM, Beaurang PA, Zhou S, Abraham S, Solomon W, Tjian R. Composite co-activator ARC mediates chromatin-directed transcriptional activation. Nature. 1999;398:828–832. doi: 10.1038/19789. [DOI] [PubMed] [Google Scholar]
- Naar AM, Lemon BD, Tjian R. Transcriptional coactivator complexes. Annu. Rev. Biochem. 2001;70:475–501. doi: 10.1146/annurev.biochem.70.1.475. [DOI] [PubMed] [Google Scholar]
- Naar AM, Taatjes DJ, Zhai W, Nogales E, Tjian R. Human CRSP interacts with RNA polymerase II CTD and adopts a specific CTD-bound conformation. Genes Dev. 2002;16:1339–1344. doi: 10.1101/gad.987602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng JM, Vermeulen W, van der Horst GT, Bergink S, Sugasawa K, Vrieling H, Hoeijmakers JH. A novel regulation mechanism of DNA repair by damage-induced and RAD23-dependent stabilization of xeroderma pigmentosum group C protein. Genes Dev. 2003;17:1630–1645. doi: 10.1101/gad.260003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R, Ravindran G. Assessment of pluripotency and multilineage differentiation potential of NTERA-2 cells as a model for studying human embryonic stem cells. Cell Prolif. 2006;39:585–598. doi: 10.1111/j.1365-2184.2006.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA. "Stemness": transcriptional profiling of embryonic and adult stem cells. Science. 2002;298:597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- Rodda DJ, Chew JL, Lim LH, Loh YH, Wang B, Ng HH, Robson P. Transcriptional regulation of nanog by OCT4 and SOX2. J. Biol. Chem. 2005;280:24731–24737. doi: 10.1074/jbc.M502573200. [DOI] [PubMed] [Google Scholar]
- Roeder RG. Transcriptional regulation and the role of diverse coactivators in animal cells. FEBS Lett. 2005;579:909–915. doi: 10.1016/j.febslet.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Rosenfeld MG, Lunyak VV, Glass CK. Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev. 2006;20:1405–1428. doi: 10.1101/gad.1424806. [DOI] [PubMed] [Google Scholar]
- Ryu S, Zhou S, Ladurner AG, Tjian R. The transcriptional cofactor complex CRSP is required for activity of the enhancer-binding protein Sp1. Nature. 1999;397:446–450. doi: 10.1038/17141. [DOI] [PubMed] [Google Scholar]
- Sands AT, Abuin A, Sanchez A, Conti CJ, Bradley A. High susceptibility to ultraviolet-induced carcinogenesis in mice lacking XPC. Nature. 1995;377:162–165. doi: 10.1038/377162a0. [DOI] [PubMed] [Google Scholar]
- Schaeffer L, Roy R, Humbert S, Moncollin V, Vermeulen W, Hoeijmakers JH, Chambon P, Egly JM. DNA repair helicase: a component of BTF2 (TFIIH) basic transcription factor. Science. 1993;260:58–63. doi: 10.1126/science.8465201. [DOI] [PubMed] [Google Scholar]
- Schwartz CM, Spivak CE, Baker SC, McDaniel TK, Loring JF, Nguyen C, Chrest FJ, Wersto R, Arenas E, Zeng X, et al. NTera2: a model system to study dopaminergic differentiation of human embryonic stem cells. Stem Cells Dev. 2005;14:517–534. doi: 10.1089/scd.2005.14.517. [DOI] [PubMed] [Google Scholar]
- Sommer CA, Stadtfeld M, Murphy GJ, Hochedlinger K, Kotton DN, Mostoslavsky G. Induced pluripotent stem cell generation using a single lentiviral stem cell cassette. Stem Cells. 2009;27:543–549. doi: 10.1634/stemcells.2008-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperger JM, Chen X, Draper JS, Antosiewicz JE, Chon CH, Jones SB, Brooks JD, Andrews PW, Brown PO, Thomson JA. Gene expression patterns in human embryonic stem cells and human pluripotent germ cell tumors. Proc. Natl. Acad. Sci. USA. 2003;100:13350–13355. doi: 10.1073/pnas.2235735100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugasawa K, Masutani C, Uchida A, Maekawa T, van der Spek PJ, Bootsma D, Hoeijmakers JH, Hanaoka F. HHR23B, a human Rad23 homolog, stimulates XPC protein in nucleotide excision repair in vitro. Mol. Cell. Biol. 1996;16:4852–4861. doi: 10.1128/mcb.16.9.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taatjes DJ, Marr MT, Tjian R. Regulatory diversity among metazoan co-activator complexes. Nat. Rev. Mol. Cell Biol. 2004;5:403–410. doi: 10.1038/nrm1369. [DOI] [PubMed] [Google Scholar]
- Tokuzawa Y, Kaiho E, Maruyama M, Takahashi K, Mitsui K, Maeda M, Niwa H, Yamanaka S. Fbx15 is a novel target of Oct3/4 but is dispensable for embryonic stem cell self-renewal and mouse development. Mol. Cell. Biol. 2003;23:2699–2708. doi: 10.1128/MCB.23.8.2699-2708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutter AV, Kowalski MP, Baltus GA, Iourgenko V, Labow M, Li E, Kadam S. Role for Med12 in regulation of Nanog and Nanog target genes. J. Biol. Chem. 2009;284:3709–3718. doi: 10.1074/jbc.M805677200. [DOI] [PubMed] [Google Scholar]
- Uchida A, Sugasawa K, Masutani C, Dohmae N, Araki M, Yokoi M, Ohkuma Y, Hanaoka F. The carboxy-terminal domain of the XPC protein plays a crucial role in nucleotide excision repair through interactions with transcription factor IIH. DNA Repair (Amst.) 2002;1:449–461. doi: 10.1016/s1568-7864(02)00031-9. [DOI] [PubMed] [Google Scholar]
- van den Berg DL, Snoek T, Mullin NP, Yates A, Bezstarosti K, Demmers J, Chambers I, Poot RA. An Oct4-centered protein interaction network in embryonic stem cells. Cell Stem Cell. 2010;6:369–381. doi: 10.1016/j.stem.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg DL, Zhang W, Yates A, Engelen E, Takacs K, Bezstarosti K, Demmers J, Chambers I, Poot RA. Estrogen-related receptor beta interacts with Oct4 to positively regulate Nanog gene expression. Mol. Cell. Biol. 2008;28:5986–5995. doi: 10.1128/MCB.00301-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venema J, van Hoffen A, Natarajan AT, van Zeeland AA, Mullenders LH. The residual repair capacity of xeroderma pigmentosum complementation group C fibroblasts is highly specific for transcriptionally active DNA. Nucleic Acids Res. 1990;18:443–448. doi: 10.1093/nar/18.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Rao S, Chu J, Shen X, Levasseur DN, Theunissen TW, Orkin SH. A protein interaction network for pluripotency of embryonic stem cells. Nature. 2006;444:364–368. doi: 10.1038/nature05284. [DOI] [PubMed] [Google Scholar]
- Yasuda G, Nishi R, Watanabe E, Mori T, Iwai S, Orioli D, Stefanini M, Hanaoka F, Sugasawa K. In vivo destabilization and functional defects of the xeroderma pigmentosum C protein caused by a pathogenic missense mutation. Mol. Cell. Biol. 2007;27:6606–6614. doi: 10.1128/MCB.02166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Tam WL, Tong GQ, Wu Q, Chan HY, Soh BS, Lou Y, Yang J, Ma Y, Chai L, et al. Sall4 modulates embryonic stem cell pluripotency and early embryonic development by the transcriptional regulation of Pou5f1. Nat. Cell Biol. 2006;8:1114–1123. doi: 10.1038/ncb1481. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang J, Wang T, Esteban MA, Pei D. Esrrb activates Oct4 transcription and sustains self-renewal and pluripotency in embryonic stem cells. J. Biol. Chem. 2008;283:35825–35833. doi: 10.1074/jbc.M803481200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.