Abstract
A single-tube PCR method was developed for efficient identification of nontuberculous mycobacteria (NTM) and their environmental isolates in about 3 h without conventional DNA isolation. The following three steps were optimized or developed: (i) a simple, 6-min direct cell lysis protocol as a PCR prestep for generation of DNA-template, (ii) an improved Mycobacterium-specific PCR amplification protocol with a broader species specificity using newly designed primers targeting a 228-bp region of the 65-kDa heat shock protein (hsp) gene and optimal PCR amplification conditions, and (iii) a genus-specific restriction analysis of the PCR product for conclusive identification of the unknown NTM isolates.
Nontuberculous mycobacteria (NTM) are important causes of nosocomial infections and occupational illnesses. These organisms are commonly associated with natural ecosystems such as water supplies, aerosols, food, and soil (3, 4, 6, 7, 17). NTM that cause nosocomial infections are frequently associated with hospital water supplies and washing equipment. From an occupational health standpoint, NTM are considered causal agents for hypersensitivity pneumonitis, asthma, and bronchitis in machine workers exposed to metalworking fluids (MWF) and their aerosols, which are used in metalworking industries for cooling and lubrication (8, 10, 12, 14, 18). A method for early and reliable detection of mycobacteria from these environments might help minimize these illnesses. The existing practice of identification of mycobacteria from clinical and environmental sources includes isolation using enrichment and selective agar media, a method which often results in the collection of a large number of putative isolates for subsequent screening and confirmation by morphological, culture, and biochemical methods (20). Molecular methods such as PCR offer a significant alternative for rapid screening and identification of these bacteria (11). In practical terms, the major limiting steps of the PCR approach for the rapid screening are the extraction of amplifiable-quality genomic DNA and the availability of genus-specific primers with broad specificity for different species. Due to the complexity and rigidity of the cell walls of these acid-fast bacteria, several efforts have been reported for the rapid isolation of amplifiable genomic DNA by using physical, chemical, or enzymatic strategies or combinations thereof (1, 5, 9, 13, 16, 22). However, these cumbersome and/or time-consuming protocols failed to yield effective cell lysis, thereby preventing their use in routine screening and diagnosis of diverse environmental species of Mycobacterium. The two existing genus-specific PCR-protocols, one based on a 16S rRNA gene (15) and the other based on Mycobacterium-specific 65-kDa heat shock protein (hsp), are applied for identification of mycobacteria (16). The latter protocol has received wider acceptance. However, this protocol fails to reliably amplify the target hsp sequence in some environmental species such as Mycobacterium immunogenum, which is an important newly identified NTM species widely associated with both clinical infections and pseudo-outbreaks (19) and with MWF implicated in hypersensitivity pneumonitis (18). Considering the above limitations, we designed the present study to develop a simple and rapid cell lysis protocol that would be usable as a prestep in PCR; we also sought to develop an improved genus-specific hsp gene-based PCR protocol applicable to a broader range of Mycobacterium species coupled with a genus-specific restriction analysis of the amplicon for more reliable detection and identification.
Bacterial strains and isolates.
Four reference strains of the genus Mycobacterium, M. chelonae (ATCC 35752), M. smegmatis (ATCC 19420), M. immunogenum (ATCC 700506), and M. bovis BCG (ATCC 35741), originating from both clinical and environmental sources, and a total of eight isolates obtained from different types of used MWF originating from different industrial plants at diverse geographical locations were used for methods development and comparisons. In addition, Bacillus sp. (B-22), Escherichia coli (DH 5α), and Pseudomonas fluorescens (ATCC 13525) were included as negative controls. For isolation of mycobacteria, samples were plated on Middlebrook 7H10 agar supplemented with oleic acid-albumin-dextrose-catalase enrichment and Lowestein-Jensen agar slants and incubated at 37°C for up to 10 days. Putative Mycobacterium colonies selected based on growth rate, colony morphology, and acid-fast staining reactions were pursued further for confirmation by using the PCR method.
Cell lysis optimization.
Five different cell lysis reagents were prepared using various concentrations of sodium dodecyl sulfate (SDS) and Triton X-100 (Sigma, St. Louis, Mo.) in Tris-EDTA buffer (pH 8.0) as presented in Table 1. Initially, a six-step thermal regime involving different heating-cooling temperatures and incubation times was used in combination with each of the formulated lysis reagents using the GeneAmp PCR system model 9700 (Applied Biosystems, Foster City, Calif.). In order to develop a simpler thermal regime, four other combinations involving shorter heating-cooling regimes were examined, as shown in Table 2. By using a sterile autopipette tip, we transferred an isolated mycobacterial colony to an amplification tube (0.2 ml) containing 5 μl of a selected lysis reagent and resuspended by gentle mixing. The contents were subjected to lysis under the appropriate combinations of chemical lysis reagents and thermal regimes listed in Tables 1 and 2 by using the GeneAmp PCR system model 9700. The tube containing the crude DNA-containing lysate was used directly for the subsequent PCR.
TABLE 1.
Different lysis reagents used for cell lysis
| Reagent type | Composition (%) of:
|
|
|---|---|---|
| SDS | Triton X-100 | |
| 1 | 10 | 10 |
| 2 | 10 | 1 |
| 3 | 5 | 2 |
| 4 | 2 | 5 |
| 5 | 2 | 10 |
TABLE 2.
Different thermal regimes used for cell lysis
| Step no. | Parameters for thermal regimea:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I
|
II
|
III
|
IV
|
V
|
||||||
| H | T | H | T | H | T | H | T | H | T | |
| 1 | 80 | 1 | 80 | 1 | 80 | 1 | 95 | 1 | 98 | 5 |
| 2 | 8 | 0.5 | 4 | 0.5 | 4 | 0.5 | 4 | 0.5 | 4 | 1 |
| 3 | 95 | 5 | 95 | 8 | 95 | 5 | 95 | 5 | ||
| 4 | 8 | 1 | 4 | 1 | 4 | 1 | 4 | 1 | ||
| 5 | 95 | 3 | ||||||||
| 6 | 4 | 1 | ||||||||
H, heating-cooling temperature (°C); T, time (minutes).
Genus-specific PCR.
We evaluated two distinct protocols for their applicability to diverse mycobacterial species. The first was an existing genus-specific PCR protocol based on the hsp gene and involving amplification of a 439-bp region using the recommended primer pair (16); the second was its modified version which uses newly designed broad-spectrum genus-specific PCR primers and optimal amplification conditions (Table 3). Direct cell lysis was used to generate the DNA template. The PCR amplification reaction with either of the primer pairs was performed by using Ex-Taq DNA polymerase and the compatible PCR reagents (Panvera, Madison, Wis.) in the GeneAmp PCR system model 9700. The reaction mixture (50 μl) consisted of 5 μl of DNA template (cell lysate), 1× Ex-Taq buffer with MgCl2, 200 μM of each of the four deoxynucleoside triphosphates, 100 ng of both the forward and reverse primers, and 1.25 U of ex-Taq DNA polymerase. The presence of PCR products was determined by electrophoresing 10 μl of the reaction product on a 1% Trevigel gel matrix (Trevigen, Gaithersburg, Md.) with 1× Tris-acetate-EDTA buffer containing ethidium bromide (0.5 μg/ml) and using 5 μl of a 100-bp DNA size marker (PGC Scientifics [Frederick, Md.] and Invitrogen [Carlsbad, Calif.]). Each PCR product was further quantitated and photographed by using the Kodak EDAS 290 gel documentation system (Kodak, Rochester, N.Y.).
TABLE 3.
Oligonucleotide primers and conditions used for hsp-based PCRs for mycobacteria
| Amplicon length (bp) | Direction and sequence of primer
|
PCR program | |
|---|---|---|---|
| Forward (5′-3′) | Reverse (5′-3′) | ||
| 439 | ACCAACGATGGTGTGTCCAT | CTTGTCGAACCGCATACCCT | Aa |
| 228 | CTGGTCAAGGAAGGTCTGCG | GATGACACCCTCGTTGCCAAC | Bb |
Program A consisted of 35 cycles of 94°C for 60 s, 60°C for 60 s, and 72°C for 60 s.
Program B consisted of 30 cycles of 95°C for 15 s, 58°C for 15 s, and 72°C for 30 s.
Amplicon identification by restriction analysis and DNA sequencing.
The mycobacterial origin of the PCR products was confirmed by restriction analysis. A protocol was optimized based on the unique NarI restriction site in these amplicons as determined by aligning the available hsp gene sequences for this region. A randomly selected PCR amplicon (corresponding to the isolate M-JY3) was also sequenced at the University of Cincinnati's DNA core facility for confirmation. When analyzed with a BLAST search against available gene database, the sequence showed homology with hsp gene sequences for mycobacteria. The sequence showed closest homology (99%) with the M. chelonae hsp sequence.
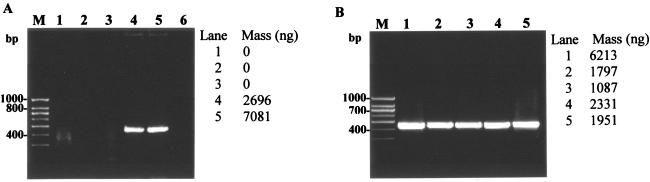
Of the five lysis reagents tested with a six-step heating regime (Tables 1 and 2), only reagent 4 (containing 2% SDS and 5% Triton X-100) and reagent 5 (2% SDS and 10% Triton X-100) yielded an effective cell lysis result as a prestep to PCR amplification (Fig. 1A). However, for some mycobacterial isolates such as M-JY4, lysis reagent 4 was not as effective as lysis reagent 5 (data not shown). The results showed that the proportional ratio of the two chemicals (SDS and Triton X-100) is important for achieving optimal cell lysis without interfering with the subsequent amplification reaction step. Hence, reagent 5 was selected as the optimum lysis reagent and was used for subsequent thermal regime optimizations. The six-step thermal regime was further modified and shortened by examining four other heating-cooling regimes (Fig. 1B). The five regimes tested gave nearly comparable cell lysis results for all reference strains and culture isolates when used with the optimal lysis reagent 5. Hence, the shortest thermal regime (V) involving heating at 98°C for 5 min followed by cooling at 4°C for 1 min was selected for the optimized protocol. This shows that the heating regime is not as critical as the chemical composition of the lysis reagent in achieving the desired cell lysis. Due to the relatively resistant cell walls of mycobacteria, various combinations of chemicals (such as SDS and Triton X-100), mechanical devices, heat, and solvents are often applied for the conventional extraction and purification of DNA from mycobacteria (1, 2, 15). However, these protocols are cumbersome and often time-consuming, and they fail to yield PCR-quality DNA; they also increase the chances of contamination or exposure during analysis. Our developed lysis protocol involves direct single-tube cell lysis in the PCR tube using a one-step heating cycle and has the potential for high-throughput applications as well as offering a safer alternative to conventional protocols. Single-tube cell lysis also makes the protocol ideal for use in quantitative PCR applications. The direct cell lysis procedure was also found to be equally effective against other bacteria, including both gram-negative and gram-positive bacteria (data not shown).
FIG. 1.
Optimization of cell lysis protocol for direct PCR-based detection of mycobacteria. (A) Optimization of chemical reagent for cell lysis as a prestep in hsp-based PCR amplification of M. smegmatis. Lanes 1 to 5, comparison of five different lysis reagents (reagents 1 through 5) containing various concentrations of SDS and Triton X-100 in Tris-EDTA buffer used in combination with thermal regime I (Table 1); lane 6, negative control (no template DNA). (B) Optimization of heating regimes for cell lysis of M. smegmatis using lysis reagent 5 (Table 1) in the hsp-based PCR. Lanes 1 to 5, comparison of thermal regimes 1 through 5 (Table 2); PCR amplification was based on mycobacterium-specific hsp gene with an expected 439-bp amplicon as described in the text. Lane M, 100-bp DNA size marker (PGC Scientifics).
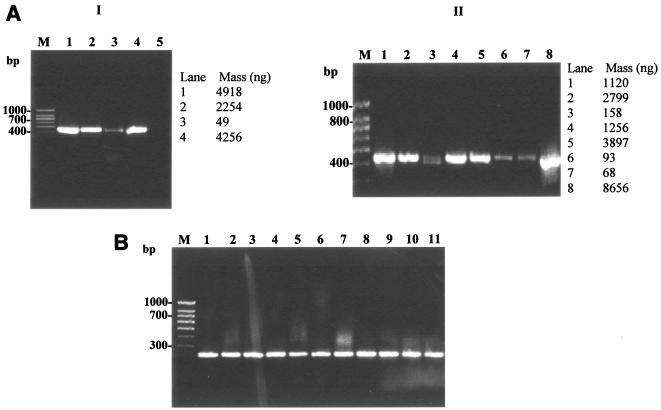
A 439-bp region of the hsp gene has been used as a PCR target for the identification and species differentiation in mycobacteria by restriction enzyme analysis or nucleotide sequencing (16, 18, 19, 21). In the preceding amplifications using this existing hsp-based PCR method, one of the four reference strains and two of the eight isolates yielded weak amplification signals (Fig. 2A [I and II]). Quantification of the amplified PCR products using the Kodak gel documentation system showed a range of DNA concentrations (Fig. 2A [I and II]). Amplicon signal variability among the reference strains (Fig. 1 and 2A) was traced to nucleotide sequence variation in the primer binding regions based on multiple alignment of their hsp sequences using MegAlign 5.0 software (DNASTAR, Inc., Madison, Wis.). Hence, in order to achieve unambiguous PCR amplification for all NTM species, including the three strains showing weak amplification, a new genus-specific primer pair based on the sequence alignment of the available hsp gene sequences was designed. We selected modified PCR amplification conditions compatible with the melting point values for the new primers as determined by using Gene Runner software. When the optimized lysis protocol was used, the modified protocol yielded a 228-bp PCR product of comparable intensity and concentration for all tested isolates and strains (Fig. 2B). All control strains of various nonmycobacterial genera were negative, thus confirming the genus-specific nature of the developed protocol. Moreover, the modified PCR conditions optimized for the new primers resulted in a relatively rapid amplification compared to that of the existing method (Table 3).
FIG. 2.
Evaluation of the optimized protocols for cell lysis and hsp-based PCR for mycobacteria. (A) Evaluation of the optimized cell lysis protocol (lysis reagent 5, thermal regime V) for hsp-based PCR amplification (439 bp) of mycobacterial reference strains and MWF isolates. Panel I: lanes 1 to 4, M. smegmatis, M. chelonae, M. immunogenum, and M. bovis; lane 5, no-template control. Panel II: lanes 1 to 8, M. smegmatis, M. chelonae, M. immunogenum, and Mycobacterium isolates M-JY1, M-JY2, M-JY3, M-JY4 and M-JY5; lane M, 100-bp DNA size marker (PGC Scientifics). PCR primers and conditions were the same as described in the legend of Fig. 1. (B) Evaluation of new hsp-based PCR primers and modified PCR conditions for genus-specific PCR amplification (228 bp) of mycobacteria. Lanes 1 to 3, M. smegmatis, M. chelonae, and M. immunogenum; lanes 4 to 11, Mycobacterium isolates M-JY1, M-JY2, M-JY3, M-JY4, M-JY5, M-JY6, M-JY7 and M-JY8; lane M, 100-bp DNA size marker (PGC Scientifics). Cells were lysed using the optimized direct cell lysis method as described for panel A, and the lysates were amplified using new PCR primers and conditions described in the text for amplification of a 228-bp PCR product of the hsp gene.
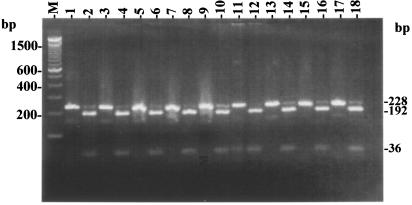
The NarI digestion of the amplicons, performed using recommended digestion conditions (New England Biolabs, Beverly, Mass.), yielded two fragments (192 and 36 bp) for the reference strains and the eight isolates of mycobacteria (Fig. 3). The developed genus-specific protocol for restriction pattern analysis of the amplicons enabled rapid confirmation of the mycobacterial identity of the environmental isolates.
FIG. 3.
Confirmation of the mycobacterial origin of PCR amplicons (228 bp) based on NarI restriction patterns (192 and 36 bp). Shown are gel patterns for M. chelonae reference strain (lane 1 and 2) and for NTM field isolates M-JY1 (lanes 3 and 4), M-JY2 (lanes 5 and 6), M-JY3 (lanes 7 and 8), M-JY4 (lanes 9 and 10), M-JY5 (lanes 11 and 12), M-JY6 (lanes 13 and 14), M-JY7 (lanes 15 and 16), and M-JY8 (lanes 17 and 18); lane M, 100-bp DNA size marker (Invitrogen). For each isolate, the two lanes represent the original amplicon (8 μl) and its NarI restriction digestion product, respectively.
In conclusion, the developed direct cell lysis-based PCR protocol requires less than 2 h for identification of a putative mycobacterial isolate, involving a 6-min lysis step followed by a 50-min amplification and a 30- to 60-min gel analysis. The single-tube protocol is potentially adaptable as a diagnostic tool in routine analytical and clinical laboratories for rapid or high-throughput screening with minimized risks of contamination or exposure. Confirmation of the mycobacterial origin of the PCR product may require an additional hour for restriction analysis. The detection time (2.5 to 3.0 h) could be further shortened in situations in which the real-time format for PCR is available, as the use of such a format would allow early detection of the amplicon as well as confirmation of the mycobacterial origin of the amplicon based on melting-curve analysis.
Nucleotide sequence accession number.
The sequence described herein has been submitted to GenBank (accession number AY322157).
Acknowledgments
The study was supported by a Centers for Disease Control and Prevention National Institute of Occupational Safety and Health grant (number 1R01OH007364-01A1 to J.S.Y.).
We thank Milacron management for providing the used MWF samples for isolation of mycobacteria.
REFERENCES
- 1.Armand, M. O., M. Mestdagh, and F. Porteals. 2001. DNA isolation from chloroform/methanol-treated mycobacterial cells without lysozyme and proteinase K. BioTechniques 30:272-274. [DOI] [PubMed] [Google Scholar]
- 2.Bollet, C., M. J. Gevaudan, X. de Lamballerie, C. Zandotti, and P. D. Micco. 1991. A simple method for the isolation of chromosomal DNA from gram positive or acid-fast bacteria. Nucleic Acids Res. 19:1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Covert, T. C., M. R. Rodgers, A. L. Reyes, and G. N. Stelma, Jr. 1999. Occurrence of nontuberculous mycobacteria in environmental samples. Appl. Environ. Microbiol. 65:2492-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dailloux, M., C. Laurain, M. Weber, and P. Hartemann. 1999. Water and nontuberculosis mycobacteria. Water Res. 33:2219-2228. [Google Scholar]
- 5.De Baere, T., R. de Mendonca, G. Claeys, G. Verschraegen, W. Mijs, R. Verhelst, S. Rottiers, L. Van Simaey, C. De Ganck, and M. Vaneechoutte. 2002. Evaluation of amplified rDNA restriction analysis (ARDRA) for the identification of cultured mycobacteria in a diagnostic laboratory. BMC Microbiol. 2:4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falkinham, J. O., III. 2002. Nontuberculous mycobacteria in the environment. Clin. Chest Med. 23:529-551. [DOI] [PubMed] [Google Scholar]
- 7.Fraser, V., and R. J. Wallace, Jr. 1996. Nontuberculous mycobacteria, p. 1224-1338. In C. Glen. Mayhall (ed.), Hospital epidemiology and infection controls. Williams & Wilkins, Baltimore, Md.
- 8.Freeman, A., J. Lockey, P. Hawley, P. Biddinger, and D. Trout. 1998. Hypersensitivity pneumonitis in a machinist. Am. J. Ind. Med. 34:387-392. [DOI] [PubMed] [Google Scholar]
- 9.Kirschner, P., B. Springer, U. Vogel, A. Meier, A. Wrede, M. Kiekenbeck, F. C. Bange, and E. C. Bottger. 1993. Genotypic identification of mycobacteria by nucleic acid sequence determination: report of a 2-year experience in a clinical laboratory. J. Clin. Microbiol. 31:2882-2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lummus, Z. L., J. E. Lockey, and I. L. Bernstein. 1998. Microbial flora of metalworking fluids associated with occupational respiratory disorders. J. Allergy Clin. Immunol. 1:166. [Google Scholar]
- 11.Miyazaki, Y., H. Koga, S. Kohno, and M. Kaku. 1993. Predictive value of PCR applied to clinical samples for Mycobacterium tuberculosis detection. J. Clin. Microbiol. 31:2228-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muilenberg, M. L., H. A. Berge, and T. Sweet. 1993. Hypersensitivity pneumonitis and exposure to acid-fast bacilli in coolant aerosols. J. Allergy Clin. Immunol. 91:311. [Google Scholar]
- 13.Reischl, U., M. Pulz, W. Ehret, and H. Wolf. 1994. PCR-based detection of Mycobacterium in sputum samples using a simple and reliable DNA extraction protocol. BioTechniques 17:844-845. [PubMed] [Google Scholar]
- 14.Shelton, G. B., W. D. Flanders, and K. G. Morris. 1999. Mycobacterium sp. as a possible cause of hypersensitivity pneumonitis in machine workers. Emerg. Infect. Dis. 5:270-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talaat, A. M., R. Reimschuessel, and M. Trucksis. 1997. Identification of mycobacteria infecting fish to the species level using polymerase chain reaction and restriction enzyme analysis. Vet. Microbiol. 58:229-237. [DOI] [PubMed] [Google Scholar]
- 16.Telenti, A., F. Marchesi, M. Balz, F. Bally, E. C. Bottger, and T. Bodmer. 1993. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J. Clin. Microbiol. 31:175-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallace, R. J., Jr., V. A. Silcox, M. Tsukamura, B. A. Brown, J. O. Kilburn, W. R. Butler, and G. Onyi. 1993. Clinical significance, biochemical features, and susceptibility patterns of sporadic isolates of the Mycobacterium chelonae-like organism. J. Clin. Microbiol. 31:3231-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallace, R. J., Jr., Y. Zhang, R. W. Wilson, L. Mann, and H. Rossmoore. 2002. Presence of a single genotype of the newly described species Mycobacterium immunogenum in industrial metalworking fluids associated with hypersensitivity pneumonitis. Appl. Environ. Microbiol. 68:5580-5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson, R. W., V. A. Steingrube, E. C. Bottger, B. Springer, B. A. Brown-Elliott, V. Vincent, K. C. Jost, Jr., Y. Zhang, M. J. Garcia, S. H. Chiu, G. O. Onyi, H. Rossmoore, D. R. Nash, and R. J. Wallace, Jr. 2001. Mycobacterium immunogenum sp. nov., a novel species related to Mycobacterium abscessus and associated with clinical disease, pseudo-outbreaks and contaminated metalworking fluids: an international cooperative study on mycobacterial taxonomy. Int. J. Syst. E vol. Microbiol. 51:1751-1764. [DOI] [PubMed] [Google Scholar]
- 20.Yadav, J. S., I. U. H. Khan, F. Fakhari, and M. B. Soellner. 2003. DNA-based methodologies for rapid detection, quantification, and species- or strain-level identification of respiratory pathogens (Mycobacteria and Pseudomonads) in metalworking fluids. Appl. Occup. Environ. Hyg. 18:966-975. [DOI] [PubMed] [Google Scholar]
- 21.Yakrus, M. A., S. M. Hernandez, M. M. Floyd, D. Sikes, W. R. Butler, and B. Metchock. 2001. Comparison of methods for identification of Mycobacterium abscessus and M. chelonae isolates. J. Clin. Microbiol. 39:4103-4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoder, S., C. Argueta, A. Holtzman, T. Aronson, O. G. Berlin, P. Tomasek, N. Glover, S. Froman, and G. Stelma, Jr. 1999. PCR comparison of Mycobacterium avium isolates obtained from patients and foods. Appl. Environ. Microbiol. 65:2650-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]