Abstract
A novel nucleic acid amplification method, termed loop-mediated isothermal amplification (LAMP), which amplifies DNA with high specificity, efficiency, and rapidity under isothermal conditions, may be a valuable tool for the rapid detection of infectious agents. LAMP was developed for human herpesvirus 6 (HHV-6), and its reliability was evaluated in this study. Although LAMP products were detected in HHV-6 B and HHV-6 A DNA, they were not detected in HHV-7 and human cytomegalovirus DNA. The sensitivity of the original HHV-6 LAMP protocol was 50 copies/tube. In order to increase the method's sensitivity, HHV-6 LAMP was modified by increasing the primer concentration. As a result of the modification, sensitivity increased to 25 copies/tube. After these initial validation studies, 13 patients with fever were tested for HHV-6 by viral isolation, serological analysis, and HHV-6 LAMP. In three of the eight patients with primary HHV-6 infection, HHV-6 DNA was detected in whole blood by the original HHV-6 LAMP protocol in not only the acute phase but also the convalescent phase. HHV-6 DNA was detected by modified HHV-6 LAMP in all eight plasma samples collected in the acute phase; however, no HHV-6 DNA was detected in plasma samples collected in the convalescent phase. Although HHV-6 DNA was detected in both the acute and convalescent phases of whole-blood samples in patients with past HHV-6 infection, it was not detected in plasma samples that did not contain latent viral DNA. Thus, detection of HHV-6 DNA in plasma by using this modified HHV-6 LAMP protocol is appropriate for diagnosis of active HHV-6 infection.
Exanthem subitum (ES) is caused by primary human herpesvirus 6 (HHV-6) B infections (1, 24). Primary HHV-6 infection usually has a benign and self-limited clinical course (4), but it has been shown that the virus can cause several severe complications (2, 3, 11, 21, 28) in rare instances. Clinical diagnosis of the disease is difficult before appearance of the skin rash, because a characteristic clinical feature in the acute phase of the disease is a high fever without any specific signs or symptoms (4). Therefore, it is important to establish a reliable and rapid diagnostic procedure that can detect active HHV-6 infection during the febrile period of ES. Reactivation of the virus can also be manifest in several ways, such as acute graft-versus-host-disease-like illness (26, 29, 30), encephalitis (9, 20), bone marrow suppression (6, 8), and interstitial pneumonitis (5, 7) in organ transplant recipients. Thus, reliable and rapid diagnostic procedures are also important for management of organ transplant recipients. To date, however, rapid virological diagnosis has proven difficult. Isolation of the virus requires cocultivation with preactivated cord blood mononuclear cells, which is difficult to perform in commercial laboratories. Moreover, both viral isolation and serological testing require substantial amounts of time to obtain final results. Rapid diagnosis by using PCR may eventually become a valuable tool for bedside monitoring of active viral infection (14, 18); however, it has not yet become a common procedure in hospital laboratories because specific equipment (a thermal cycler) is needed.
Recently, Notomi et al. (17) have reported a novel nucleic acid amplification method, termed loop-mediated isothermal amplification (LAMP), that amplifies DNA with high specificity, efficiency, and speed under isothermal conditions. The LAMP reaction requires a DNA polymerase with strand displacement activity and a set of four specially designed primers, termed inner and outer primers, which improve specificity. As a first step, a stem-loop DNA structure, in which the sequences of both DNA ends are derived from the inner primer, is constructed as the starting material. Subsequently, one inner primer hybridizes to the loop on the product in the LAMP cycle and initiates strand displacement DNA synthesis, yielding the original stem-loop DNA and new stem-loop DNA with a stem twice as long. The final products are stem-loop DNAs with several inverted repeats of the target DNA and cauliflower-like structures with multiple loops, amplifying the amount to 109 copies of the target (17). The most significant advantage of the LAMP method is its ability to amplify specific sequences of DNA under isothermal conditions of between 63 and 65°C. As a result, it requires only simple and cost-effective reaction equipment that is amenable to use in hospital laboratories. A second characteristic of the LAMP method is that it has both high specificity and high amplification efficiency. Since the LAMP method uses four primers recognizing six distinct sequences on the target DNA, its specificity is extremely high. This method also exhibits extremely high amplification efficiency, due in part to its isothermal nature: there is no time lost due to changes in temperature, the reaction can be conducted at the optimal temperature for enzyme function, and the inhibition reaction that occurs at later stages of amplification, a typical problem with PCR, is less likely to take place. Thus, this method could potentially be a valuable tool for the rapid diagnosis of infectious diseases in either commercial or hospital laboratories. Our goal in the present study was to establish a LAMP-based HHV-6 DNA amplification method and to examine its reliability in the diagnosis of primary HHV-6 infection. We found this method to be highly sensitive and specific and sufficiently reliable in the diagnosis of primary HHV-6 infection.
MATERIALS AND METHODS
Study design.
HHV-6 B (Z29) DNA was used as a positive control for determining appropriate conditions for HHV-6 LAMP and to establish its baseline sensitivity and specificity. HHV-6 A (U1102), human cytomegalovirus (HCMV) (AD-169), and HHV-7 (RK) DNA was used for determining the specificity of HHV-6 LAMP, and a plasmid containing the target sequence of HHV-6 B was used for determining its sensitivity. Details of each experiment are described below.
A total of 13 children (eight male and five female, aged between 5 and 18 months) with fever, who attended an outpatient clinic in our university hospital or Showa hospital, were enrolled into a study assessing the reliability of our method in the diagnosis of primary HHV-6 infection. Informed consent was obtained from the parents of all children. Samples (2 ml) of heparinized blood were collected from the patients in the acute phase (febrile period) and in the convalescent phase (5 to 10 days after the onset of the illness). HHV-6 isolation from peripheral blood mononuclear cells (PBMCs), and serological analysis were carried out to confirm primary HHV-6 infection. Detection of HHV-6 DNA in either whole blood or plasma in each sample was tested by LAMP. The results obtained by virological examination and molecular analysis were compared to assess the reliability of LAMP as an indicator of active HHV-6 infection.
Isolation of HHV-6.
The procedures for isolation and identification of HHV-6 have been described elsewhere (1). Briefly, PBMCs were cocultured with cord blood mononuclear cells in culture medium. The presence of the virus was determined initially by observing morphological changes of cultured cells, which had characteristics of pleomorphic, balloon-like large cells, and was confirmed by specific immunofluorescence staining with monoclonal antibodies to HHV-6 (OHV-3; kindly provided by T. Okuno, Department of Microbiology, Hyogo Collage of Medicine, Hyogo, Japan).
Serological assay.
Immunoglobulin G antibody titers against HHV-6 were measured by an indirect immunofluorescence assay as described previously (25). A stain of HHV-6 variant B (FG-1), isolated from an ES patient, was used as the standard antigen. The antibody titer was defined as the reciprocal of the serum dilution showing specific fluorescence. A negative result was concluded if the antibody titer was <8.
DNA extraction.
For the experiment in which HHV-6 LAMP was being developed initially, viral DNA was extracted from HHV-6 A (U1102)-, HHV-6 B (Z29)-, HCMV (AD-169)-, and HHV-7 (RK)-infected cells by using a QIAamp blood kit (Qiagen, Chatsworth, Calif.). The same DNA extraction kit was used to extract DNA from 200 μl of whole blood, and plasma samples were collected from the patients. After extraction, DNA was eluted in 100 μl of buffer and stored at −20°C.
LAMP.
The LAMP reaction was conducted according to the original reports described by Notomi et al. (17) and Nagamine et al. (16). The LAMP method requires a set of four specially designed primers (B3, F3, BIP, and FIP) that recognize a total of six distinct sequences (B1, B2, B3, F1, F2, and F3) in the target DNA. Primers for HHV-6 LAMP were designed against the HHV-6 B U31 gene by using Primer Explorer V software. The location and sequence of each primer are shown in Fig. 1. BIP for the U31 gene of HHV-6 (H6U31BIP) consisted of the B1 direct sequence (22 nucleotides [nt]) and B2 complementary sequence (21 nt). Primer FIP for U31 of HHV-6 (H6U31FIP) consisted of F1 complementary sequence (21 nt) and F2 direct sequence (22 nt). Primers B3 (H6U31B3) and F3 (H6U31F3) for U31 of HHV-6 were located outside of the F2-B2 regions. Since it has been demonstrated that additional loop primers increase the amplification efficiency, loop primers for the HHV-6 U31 gene (H6U31LPB and H6U31LPF) were also synthesized. H6U31LPB consisted of the LPB sequence, and H6U31LPF consisted of LPF complementary sequence. The LAMP reaction was carried out in a 25-μl reaction containing 1.6 μM concentrations (each) of H6U31FIP and H6U31BIP, 0.2 μM concentrations (each) of outer primers (H6U31F3 and H6U31B3), 0.8 μM concentrations (each) of loop primers (H6U31LPF and H6U31LPB), a 1.4 mM concentration of each deoxynucleoside triphosphate, 0.8 M betaine (Sigma, St. Louis, Mo.), 20 mM Tris-HCl, 10 mM KCl, 10 mM (NH4)2SO4, 8 mM MgSO4, 0.2% Tween 20, 8 U of Bst DNA polymerase large fragment (New England Biolabs, Beverly, Mass.), and 5 μl of target DNA. The mixture was incubated at 63°C for 30 min by using a conventional heat block. LAMP products were subjected to electrophoresis on a 1.5% agarose gel and visualized under UV light after ethidium bromide staining. Care was taken to avoid contamination between samples: different rooms were used for DNA extraction, LAMP setup, and gel analysis, and all pipette tips had filters for aerosol protection.
FIG. 1.
Location and name of target sequences used as primers for HHV-6 LAMP on the HHV-6 U31 gene (A) and name and sequence of each primer for HHV-6 LAMP (B). B2c, sequence complementary to B2; F1c, sequence complementary to F1.
In order to increase the sensitivity of the LAMP (modified HHV-6 LAMP), primer concentrations were raised to 2.4 μM (each) of H6U31FIP and H6U31BIP, 0.4 μM (each) of the outer primers (H6U31F3 and H6U31B3), and 1.2 μM (each) of the loop primers (H6U31LPF and H6U31LPB). Concentrations of other reagents were the same as for the original HHV-6 LAMP protocol.
Cloning of HHV-6 DNA.
In order to determine the sensitivity of the HHV-6 LAMP method, a plasmid containing the target DNA sequence was constructed. First, upstream (H6S1; 5′-CAACATTCTGCACAGAAGATG-3′) and downstream (H6S2; 5′-AGCGTGTGCGTATTTAGGAC-3′) primers spanning the sequences between the F3 and B3 primers were synthesized. HHV-6 DNA (Z29) was amplified with these two primers by conventional PCR as described elsewhere (22). The PCR product was cloned into a pGEM-T vector by using pGEM-T Vector System II (Promega, Madison, Wis.) according to the manufacturer's instructions. The plasmid (pGEMH6S12) constructed by the system was used to make standard dilutions for the evaluation of the lower detection limit of the LAMP protocol.
RESULTS
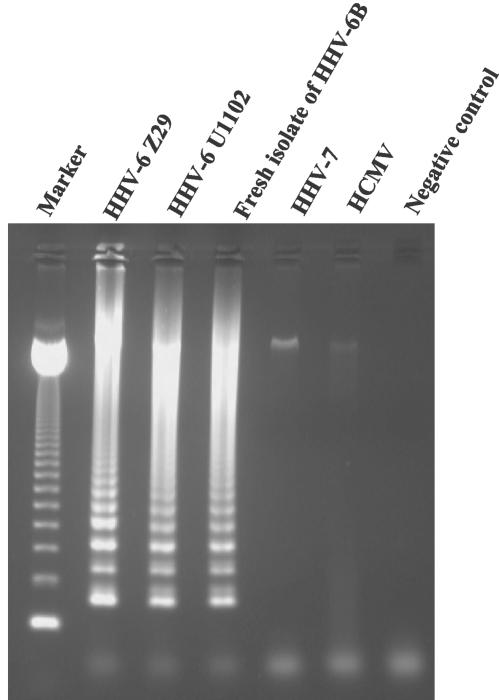
During assay development, the specificity of the HHV-6 primers was evaluated first. HHV-6 LAMP was performed on DNA extracted from HHV-6 A (U1102)-, HHV-6 B (Z29)-, freshly isolated HHV-6 B-, HHV-7 (RK)-, and HCMV (AD-169)-infected cells. Since the LAMP products consisted of several inverted-repeat structures, positive samples demonstrate many bands of different sizes upon agarose gel electrophoresis. Although amplified HHV-6 B and HHV-6 A DNA demonstrated typical ladder patterns as shown in Fig. 2, no LAMP product was detected in reactions performed with HHV-7 and HCMV DNA.
FIG. 2.
DNA extracted from Betaherpesvirinae-infected cells was amplified by using the original HHV-6 LAMP protocol to determine the specificity of the method. A fresh isolate of HHV-6 B was prepared from a patient with ES in our institute. Marker, 123-bp DNA ladder marker.
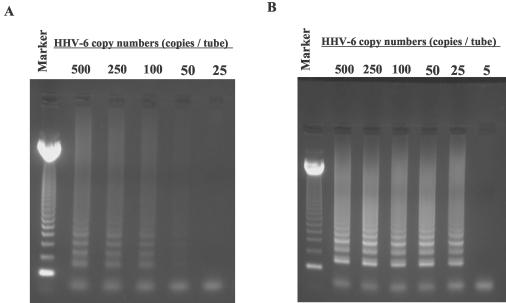
The sensitivity of this method was also determined. Serial dilutions of pGEMH6S12 plasmid were used to determine the detection limit. The sensitivity of the original HHV-6 LAMP was 50 copies/tube (Fig. 3A). In order to increase this sensitivity, the protocol was modified by raising the concentration of each primer, resulting in a twofold increase of the sensitivity, to 25 copies/tube (Fig. 3B).
FIG. 3.
Serial dilutions of pGEMH6S12 plasmid DNA were amplified by the original (A) and modified HHV-6 LAMP (B) protocols to determine the respective sensitivities of each assay. Marker, 123-bp DNA ladder marker.
After these initial validation studies, 13 patients with fever were tested for HHV-6 by viral isolation, serological analysis, and HHV-6 LAMP. Whole blood and plasma were tested for LAMP expansion of HHV-6 DNA. As shown in Table 1, HHV-6 was isolated from peripheral blood, and significant increases in HHV-6 antibody titers were demonstrated in eight patients (cases 1 to 8), indicating that these patients received a primary HHV-6 infection. In three (cases 1, 3, and 4) of the eight patients, HHV-6 DNA was detected in blood by the original HHV-6 LAMP protocol during not only the acute phase but also the convalescent phase of the infection. Although HHV-6 DNA was not detected by the original HHV-6 LAMP protocol in any of the plasma samples, it was detected by modified HHV-6 LAMP in all eight plasma samples collected in the acute phase; however, no HHV-6 DNA was detected in any of the plasma samples collected in the convalescent phase. HHV-6 was not isolated from the remaining five patients (cases 9 to 13). Three patients without HHV-6 antibody (cases 9 to 11) were considered to be HHV-6 uninfected patients, and the remaining two patients that were positive for HHV-6 antibody (cases 12 and 13) were patients with a past viral infection. All of the whole blood and plasma samples collected from the HHV-6-uninfected patients (cases 9 to 11) were negative for HHV-6 DNA. Although HHV-6 DNA was detected in whole blood samples collected in both the acute and the convalescent phases from a patient with a past HHV-6 infection (case 12), it was not detected in the plasma samples.
TABLE 1.
Patient characteristics and results of HHV-6 LAMP and virological examinations
| Case | Age (mo) | Gendera | Sampling periodb | Isolation of HHV-6 | HHV-6 antibody titer | HHV-6 LAMP (original), whole blood | HHV-6 LAMP (modified), plasma |
|---|---|---|---|---|---|---|---|
| 1 | 15 | F | A | + | 16 | + | + |
| C | − | >256 | + | − | |||
| 2 | 13 | M | A | + | <8 | + | + |
| C | − | >256 | − | − | |||
| 3 | 8 | F | A | + | <8 | + | + |
| C | − | 128 | + | − | |||
| 4 | 7 | F | A | + | <8 | + | + |
| C | − | >256 | + | − | |||
| 5 | 7 | M | A | + | <8 | ND | + |
| C | − | 32 | ND | − | |||
| 6 | 6 | M | A | + | <8 | ND | + |
| C | − | >256 | ND | − | |||
| 7 | 8 | M | A | + | <8 | ND | + |
| C | − | >256 | ND | − | |||
| 8 | 9 | F | A | + | <8 | ND | + |
| C | − | >256 | ND | − | |||
| 9 | 9 | F | A | − | <8 | − | − |
| C | − | <8 | − | − | |||
| 10 | 5 | M | A | − | <8 | − | − |
| C | − | <8 | − | − | |||
| 11 | 6 | M | A | − | <8 | − | − |
| C | NDc | <8 | ND | − | |||
| 12 | 18 | M | A | − | >256 | + | − |
| C | − | >256 | + | − | |||
| 13 | 5 | M | A | − | 128 | − | − |
| C | ND | 128 | ND | − |
F, female; M, male.
A, acute; C; convalescent.
ND, not done.
DISCUSSION
Because the majority of cases of primary HHV-6 infection occur within the first year of life (25), for some infants, ES may represent the first febrile episode after birth. In Japan, since parental concern about ES is generally high, most cases are seen in outpatient clinics. Meanwhile, in the United States, it has been reported that primary HHV-6 infection accounts for 10% of visits to emergency departments for acute febrile illness among children under 2 years old (10). In order to differentiate ES (primary HHV-6 infection), which is generally a benign and self-limited disease, from other severe febrile illness such as bacterial meningitis, it is important to establish a rapid method for diagnosing HHV-6 infection. Furthermore, since HHV-6 does on rare occasions cause severe clinical manifestations (2, 3, 11, 21, 28), rapid diagnosis is very important in order to begin administration of effective antiviral treatment as soon as possible. In addition to the manifestations of HHV-6 as a primary infection, it has been demonstrated that reactivation of the virus in immunocompromised hosts is associated with several severe clinical presentations (5-8, 20, 26, 29, 30). Thus, a rapid method of detecting viral infection would likely benefit in the management of these patients as well.
PCR detection of HHV-6 DNA in PBMCs is now the most common procedure for monitoring active viral infection in organ transplant recipients (14, 18). After primary HHV-6 infection, latent infection of PBMCs persists throughout life (13, 22), thus requiring quantitative PCR to differentiate active viral infection from latent infection. Because of its accuracy and rapidity, quantification of HHV-6 load by using real-time PCR has recently become a popular method for monitoring active viral infection (12, 23, 31). However, its routine implementation in hospital laboratories has been impeded by the need for expensive equipment. In contrast, a recently introduced nucleic acid amplification method, the LAMP method, has been shown to promote the amplification of DNA under isothermal conditions with a high specificity and efficiency comparable to that of traditional PCR (17). Therefore, we attempted to develop HHV-6 LAMP as a more cost-effective alternative to quantitative PCR for future use in hospital laboratories. Although HHV-6 LAMP amplified both HHV-6 A and HHV-6 B DNA, no cross-reactivity was observed between other Betaherpesvirinae (HHV-7 and HCMV), as shown in Fig. 2. The detection limits of the original and modified HHV-6 LAMP protocols for HHV-6 B DNA were 50 and 25 copies/tube, respectively. It seems that sensitivity of modified HHV-6 LAMP is at least two times as high as the ginal HHV-6 LAMP. These findings demonstrate that HHV-6 LAMP has high specificity and high efficiency in the amplification of viral DNA. Moreover, all amplification steps are completed in a conventional heat block and require only 30 min to perform. These two features are major advantages for hospital laboratory use. In order to detect amplification products, gel electrophoresis was performed in the present study. Mori et al. (15) have reported that the formation of LAMP products can be detected as an increase in solution turbidity due to magnesium pyrophosphate formation. Turbidity measurement of LAMP products will allow a reduction in operation time, obviating the need for gel electrophoresis, and reduce contamination risk. Moreover, quantitative analysis of LAMP products is also possible by using this method. In order to better optimize the HHV-6 DNA detection system for hospital laboratory use, establishment of semiquantitative HHV-6 LAMP is now under way.
Because HHV-6 can persist in a latent state in PBMCs after primary infection (13, 22), it has been argued that detection of viral DNA in PBMCs by sensitive PCR cannot differentiate between active infection and latent infection in organ transplant recipients. In the present study, three of the four whole blood samples (cases 1 to 3) collected from patients in the convalescent phase of primary HHV-6 infection were positive for HHV-6 LAMP, demonstrating the detection of latently resident viral DNA. Moreover, whole blood from patients in both the acute and the convalescent phases of infection were positive for HHV-6 LAMP in one patient (case 12) with a past history of HHV-6 infection. These results suggest that the original HHV-6 LAMP protocol, since it cannot differentiate between active and latent infection in whole blood samples, is too sensitive to be used for the monitoring of active viral infection. It has been proposed, however, that detection of viral DNA in plasma correlates well with active viral infection (19, 27). Although the original HHV-6 LAMP protocol was not sufficiently sensitive to detect viral DNA in plasma, the more-sensitive modified HHV-6 LAMP method was able to detect viral DNA in plasma. Indeed, all eight acute-phase plasma samples collected from patients with primary HHV-6 infection were positive for HHV-6 LAMP. Furthermore, in all eight patients, convalescent-phase plasma samples were negative for HHV-6 LAMP, and all plasma samples collected from either uninfected patients or patients with past infection were also negative. These results suggest that detection of HHV-6 DNA in plasma by HHV-6 LAMP may be a valuable tool for the rapid diagnosis of active viral infection. Since the number of evaluated cases in the present study was limited, a large number of cases should be analyzed to confirm the reliability of this method.
In summary, an HHV-6 DNA detection system using a novel nucleoside amplification method, LAMP, was developed. This method is highly specific and highly sensitive. Advantages of HHV-6 LAMP for use in hospital laboratories are its limited requirements of simple and cost-effective equipment and a very short time (30 min) to complete amplification. Although these are only preliminary results, they suggest that HHV-6 LAMP may be good tool for rapid diagnosis of primary HHV-6 infection.
Acknowledgments
We thank Takeda Chemical Industries, Ltd., Osaka, Japan, for supplying recombinant human interleukin-2 and gratefully acknowledge Eiken Chemical for their contribution to carry out this work. We also thank Akiko Yoshikawa and Maki Sawamura for technical assistance.
This study was supported in part by grants from Fujita Health University and the Japan Society for the Promotion of Science (JSPS-RFTF97L00703) and by a grant-in-aid for scientific research from the Ministry of Education, Science, and Culture of Japan.
REFERENCES
- 1.Asano, Y., T. Yoshikawa, S. Suga, T. Yazaki, T. Hata, T. Nagai, Y. Kajita, T. Ozaki, and S. Yoshida. 1989. Viremia and neutralizing antibody response in infants with exanthem subitum. J. Pediatr. 114:535-539. [DOI] [PubMed] [Google Scholar]
- 2.Asano, Y., T. Yoshikawa, S. Suga, T. Yazaki, K. Kondo, and K. Yamanishi. 1990. Fatal fulminant hepatitis in an infant with human herpesvirus-6 infection. Lancet 335:862-863. [DOI] [PubMed] [Google Scholar]
- 3.Asano, Y., T. Yoshikawa, Y. Kajita, R. Ogura, S. Suga, T. Yazaki, T. Nakashima, A. Yamada, and T. Kurata. 1992. Fatal encephalitis/encephalopathy in primary human herpesvirus-6 infection. Arch. Dis. Child. 67:1484-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asano, Y., T. Yoshikawa, S. Suga, I. Kobayashi, T. Nakashima, T. Yazaki, Y. Kajita, and T. Ozaki. 1994. Clinical features of infants with primary human herpesvirus 6 infection (exanthem subitum, roseola infantum). Pediatrics 93:104-108. [PubMed] [Google Scholar]
- 5.Carrigan, D. R., W. R. Drobyski, S. K. Russler, M. A. Tapper, K. K. Knox, and R. C. Ash. 1991. Interstitial pneumonitis associated with human herpesvirus-6 infection after marrow transplantation. Lancet 338:147-149. [DOI] [PubMed] [Google Scholar]
- 6.Carrigan, D. R., and K. K. Knox. 1994. Human herpesvirus 6 (HHV-6) isolation from bone marrow: HHV-6-associated bone marrow suppression in bone marrow transplant patients. Blood 84:3307-3310. [PubMed] [Google Scholar]
- 7.Cone, R. W., R. C. Hackman, M. L. Huang, R. A. Bowden, J. D. Meyers, M. Metcalf, J. Zeh, R. Ashley, and L. Corey. 1993. Human herpesvirus 6 in lung tissue from patients with pneumonitis after bone marrow transplantation. N. Engl. J. Med. 329:156-161. [DOI] [PubMed] [Google Scholar]
- 8.Drobyski, W. R., W. M. Dunne, E. M. Burd, K. K. Knox, R. C. Ash, M. M. Horowitz, N. Flomenberg, and D. R. Carrigan. 1993. Human herpesvirus-6 (HHV-6) infection in allogeneic bone marrow transplant recipients: evidence of a marrow-suppressive role for HHV-6 in vivo. J. Infect. Dis. 167:735-739. [DOI] [PubMed] [Google Scholar]
- 9.Drobyski, W. R., K. K. Knox, D. Majewski, and D. R. Carrigan. 1994. fatal encephalitis due to variant B human herpesvirus-6 infection in a bone marrow-transplant recipient. N. Engl. J. Med. 330:1356-1360. [DOI] [PubMed] [Google Scholar]
- 10.Hall, C. B., C. E. Long, K. C. Schnabel, M. T. Caserta, K. M. McIntyre, M. A. Costanzo, A. Knott, S. Dewhurst, R. A. Insel, and L. G. Epstein. 1994. Human herpesvirus-6 infection in children. A prospective study of complications and reactivation. N. Engl. J. Med. 331:432-438. [DOI] [PubMed] [Google Scholar]
- 11.Huang, L. M., C. Y. Lee, K. H. Lin, W. M. Chuu, P. I. Lee, R. L. Chen, J. M. Chen, and D. T. Lin. 1990. Human herpesvirus-6 associated with fatal haemophagocytic syndrome. Lancet 336:60-61. [DOI] [PubMed] [Google Scholar]
- 12.Ihira, M., T. Yoshikawa, K. Suzuki, M. Ohashi, S. Suga, K. Horibe, N. Tanaka, H. Kimura, S. Kojima, K. Kato, T. Matsuyama, Y. Nishiyama, and Y. Asano. 2002. Monitoring of active HHV-6 infection in bone marrow transplant recipients by real-time PCR; comparison to detection of viral DNA in plasma by qualitative PCR. Microbiol. Immunol. 46:701-705. [DOI] [PubMed] [Google Scholar]
- 13.Kondo, K., T. Kondo, T. Okuno, M. Takahashi, and K. Yamanishi. 1991. Latent human herpesvirus 6 infection of human monocytes/macrophages. J. Gen. Virol. 72:1401-1408. [DOI] [PubMed] [Google Scholar]
- 14.Ljungman, P., F. Z. Wang, D. A. Clark, V. C. Emery, M. Remberger, O. Ringden, and A. Linde. 2000. High levels of human herpesvirus 6 DNA in peripheral blood leucocytes are correlated to platelet engraftment and disease in allogeneic stem cell transplant patients. Br. J. Haematol. 111:774-781. [PubMed] [Google Scholar]
- 15.Mori, Y., K. Nagamine, N. Tomita, and T. Notomi. 2002. Detection of Loop-Mediated Isothermal Amplification Reaction by Turbidity Derived from Magnesium Pyrophosphate Formation. Biochem. Biophys. Res. Commun. 289:150-154. [DOI] [PubMed] [Google Scholar]
- 16.Nagamine, K., T. Hase, and T. Notomi. 2002. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol. Cell. Probes 16:223-229. [DOI] [PubMed] [Google Scholar]
- 17.Notomi, T., H. Okayama, H. Masubuchi, T. Yonekawa, K. Watanabe, N. Amino, and T. Hase. 2000. Loop mediated isothermal amplification of DNA. Nucleic Acids Res. 28:e63. [DOI] [PMC free article] [PubMed]
- 18.Sashihara, J., K. Tanaka-Taya, S. Tanaka, K. Amo, H. Miyagawa, G Hosoi, T. Taniguchi, T. Fukui, N. Kasuga, T. Aono, M. Sako, J. Hara, K. Yamanishi, and S. Okada. 2002. High incidence of human herpesvirus 6 infection with a high viral load in cord blood stem cell transplant recipients. Blood 100:2005-2011. [PubMed] [Google Scholar]
- 19.Secchiero, P., D. R. Carrigan, Y. Asano, L. Benedetti, R. W. Crowley, A. L. Komaroff, R. C. Gallo, and P. Lusso. 1995. Detection of human herpesvirus 6 in plasma of children with primary infection and immunosuppressed patient by polymerase chain reaction. J. Infect. Dis. 171:273-280. [DOI] [PubMed] [Google Scholar]
- 20.Singh, N., and D. L. Paterson. 2000. Encephalitis caused by human herpesvirus-6 in transplant recipients: relevance of a novel neurotropic virus. Transplantation 69:2474-2479. [DOI] [PubMed] [Google Scholar]
- 21.Suga, S., T. Yoshikawa, Y. Asano, T. Kozawa, T. Nakashima, I. Kobayashi, T. Yazaki, H. Yamamoto, Y. Kajita, and T. Ozaki. 1993. Clinical and virological analyses of 21 infants with exanthem subitum (roseola infantum) and central nervous system complications. Ann. Neurol. 33:597-603. [DOI] [PubMed] [Google Scholar]
- 22.Suga, S., T. Yoshikawa, Y. Kajita, T. Ozaki, and Y. Asano. 1998. Prospective study of persistence and excretion of human herpesvirus 6 in patients with exanthem subitum and their parents. Pediatrics 102:900-904. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka, N., H. Kimura, Y. Hoshino, K. Kato, T. Yoshikawa, Y. Asano, K. Horibe, S. Kojima, and T. Morishima. 2000. Monitoring four herpesviruses in unrelated cord blood transplantation. Bone Marrow Transplant 26:1193-1197. [DOI] [PubMed] [Google Scholar]
- 24.Yamanishi, K., T. Okuno, K. Shiraki, M. Takahashi, T. Kondo, Y. Asano, and T. Kurata. 1988. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet i:1065-1067. [DOI] [PubMed]
- 25.Yoshikawa, T., S. Suga, Y. Asano, T. Yazaki, H. Kodama, and T. Ozaki. 1989. Distribution of antibodies to a causative agent of exanthem subitum (human herpesvirus 6) in healthy individuals. Pediatrics 84:675-677. [PubMed] [Google Scholar]
- 26.Yoshikawa, T., S. Suga, Y. Asano, T. Nakashima, T. Yazaki, R. Sobue, M. Hirano, M. Fukuda, S. Kojima, and T. Matsuyama. 1991. Human herpesvirus-6 infection in bone marrow transplantation. Blood 78:1381-1384. [PubMed] [Google Scholar]
- 27.Yoshikawa, T., M. Ihira, K. Suzuki, S. Suga, K. Iida, Y. Saito, K. Asonuma, K. Tanaka, and Y. Asano. 2000. Human herpesvirus 6 infection after living related liver transplantation. J. Med. Virol. 62:52-59. [PubMed] [Google Scholar]
- 28.Yoshikawa, T., M. Ihira, K. Suzuki, S. Suga, H. Kito, T. Iwasaki, T. Kurata, T. Tanaka, Y. Saito, and Y. Asano. 2001. Fatal acute myocarditis in an infant with human herpesvirus 6 infection. J. Clin. Pathol. 54:792-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshikawa, T., M. Ihira, M. Ohashi, S. Suga, Y. Asano, H. Miyazaki, M. Hirano, K. Suzuki, K. Matsunaga, K. Horibe, S. Kojima, K. Kudo, K. Kato, T. Matsuyama, and Y. Nishiyama. 2001. Correlation between HHV-6 infection and skin rash after allogeneic bone marrow transplantation. Bone Marrow Transplant. 28:77-81. [DOI] [PubMed] [Google Scholar]
- 30.Yoshikawa, T., Y. Asano, M. Ihira, K. Suzuki, M. Ohashi, S. Suga, K. Kudo, K. Horibe, S. Kojima, K. Kato, T. Matsuyama, and Y. Nishiyama. 2002. Human herpesvirus 6 viremia in bone marrow transplant recipients: clinical features and risk factors. J. Infect. Dis. 185:847-853. [DOI] [PubMed] [Google Scholar]
- 31.Yoshikawa, T., M. Ihira, Y. Asano, A. Tomitaka, K. Suzuki, K. Matsunaga, Y. Kato, S. Hiramitsu, T. Nagai, N. Tanaka, H. Kimura, and Y. Nishiyama. 2002. Fatal adult case of severe lymphocytopenia associated with reactivation of human herpesvirus 6. J. Med. Virol. 66:82-85. [DOI] [PubMed] [Google Scholar]