Abstract
A collection of 120 strains isolated from stool specimens collected from humans suffering from gastroenteritis and from environmental samples were analyzed by random amplified polymorphic DNA PCR (RAPD), repetitive extragenic palindromic PCR (REP-PCR), and enterobacterial repetitive intergenic consensus sequence PCR (ERIC-PCR). Species of Aeromonoas hydrophila, A. bestiarum, A. salmonicida, A. caviae, A. media, and A. veronii revealed clonal structure. There was no dominant clone causing gastroenteritis in humans. Moreover, there was no genetic similarity between clinical and environmental strains of Aeromonas sp. isolated from different geographical areas as well as from the same geographical area. Some clones colonized specific ecosystems, e.g., drinking water distribution systems. RAPD and ERIC-PCR methods had the same discriminatory power and proved to be useful for epidemiological investigation and population genetic analysis of Aeromonas spp., whereas REP-PCR was less effective for differentiating the isolates of Aeromonas spp.
Bacteria of Aeromonas sp. are gram-negative, straight cells (rod-shaped to coccoid) with rounded ends. They are oxidase and catalase positive, reduce nitrate to nitrite, and ferment d-glucose. These bacteria are widely spread in the environment, especially in surface water and sewage; they also occur in untreated and treated drinking water (1, 2, 4, 18). In humans, Aeromonas spp. are responsible for gastroenteritis, chronic diarrhea, wound infections, respiratory tract infections, peritonitis, urinary tract infections, and septicemia (2, 17). Among Aeromonas-associated infections of humans A. hydrophila, A. caviae, and A. veronii are the predominating species, whereas A. eucrenophila, A. popoffii, (2), and A. culicicola (28) have never been found in clinical samples. Some Aeromonas species are associated with a wide variety of diseases in cold- and warm-blooded animals, including fish, frogs, water buffaloes, reptiles, birds, and cattle (16, 28). A. veronii, A. hydrophila, and A. salmonicida are capable of causing septicemia in freshwater and marine fish (3, 9). Clinical and environmental Aeromonas sp. isolates secrete many extracellular products, such as hemolysins, enterotoxins, and proteases. Studies conducted by Kühn et al. (22) showed that some isolates of a given species produce virulence factors more frequently than others. These findings indicate that the virulence within the genus Aeromonas might be a clonal property and only some clones may be responsible for progressive disease. However, there have been no studies that would have determined clonal structure within Aeromonas spp. and the spread of specific clones in human population and in the environment. Our study was undertaken to recognize the clonal relatedness of strains derived from diarrheal stool specimens collected from humans living in different geographical areas. We also compared genetic similarities of clones recovered from stool and the environment. In addition, we attempted to determine the genetic relationship of Aeromonas strains isolated from healthy and dead fish of the species Rutilus rutilus. Moreover, we evaluated random amplified polymorphic DNA PCR (RAPD), repetitive extragenic palindromic sequence PCR (REP-PCR), and enterobacterial repetitive intergenic consensus sequence PCR (ERIC-PCR) PCR methods for fingerprinting of Aeromonas spp. isolates.
MATERIALS AND METHODS
Bacterial strains.
One hundred and twenty strains of Aeromonas spp. were used in this study. Their geographical origin and sources are listed in Tables 1 to 3. Forty type and reference strains representing all recognized Aeromonas species were included in the study. Identification of the strains was performed on the basis of their phenotypic properties and DNA-DNA hybridization according to the method of Kaznowski (19).
TABLE 1.
Strains of Aeromonas spp. isolated from humans
| Genospecies | HG | Strain no.a | Source of isolation |
|---|---|---|---|
| A. hydrophila | 1 | RK 70363, RK 226254, RK 217215 | Human stool, Hong Kong |
| SK 3, SK 6, SK 7, SK 12 | Human stool, Thailand | ||
| ATCC 49140 | Human | ||
| LMG 13656 | Feces, Switzerland | ||
| A. bestiarum | 2 | LMG 13448 | Feces, Switzerland |
| A. salmonicida | 3 | LMG 13450 | Feces, Switzerland |
| A. caviae | 4 | AK 375, AK 376, AK 377, AK 378, AK 379, AK 380, AK 383, AK 384, AK 385, AK 386, AK 388, AK 390, AK 393 | Human stool, Poland |
| RK 25447, RK 27611, RK 65541, RK 66942, RK 77620, RK 217455, RK 220132 | Human stool, Hong Kong | ||
| SK 4, SK 9, SK 11 | Human stool, Thailand | ||
| LMG 13454 | Human, Germany | ||
| A. media | 5A | LMG 13461 | Feces, Switzerland |
| A. veronii biotype sobria | 8/10 | AK 382, AK 387, AK 389, AK 391, AK 392 | Human stool, Poland |
| RK 43939, RK 66113, RK 77343 | Human stool, Hong Kong | ||
| SK 2, SK 14, SK 24 | Human stool, Thailand | ||
| A. veronii biotype veronii | 10/8 | ATCC 35624T | Sputum |
| A. jandaei | 9 | ATCC 49568T | Human stool |
| LMG 13065 | Human stool, Switzerland | ||
| Aeromonas sp. | 11 | ATCC 35941 | Abscess |
| A. schubertii | 12 | ATCC 43700T | Abscess |
| LMG 13473 | Human skin, United States | ||
| Aeromonas sp. | 13 | LMG 17321T | Human (leg wound), United States |
| A. trota | 14 | ATCC 49657T | Human stool |
| LMG 13081 | Human stool, Thailand | ||
| A. enteropelogenes | 14 | ATCC 49803T | Human stool |
| A. allosaccharophila | 15 | LMG 14021 | Human stool, United States |
| Aeromonas sp. | ND | RK 61871 | Human stool, Hong Kong |
Abbreviations: AK, Culture Collection of Department of Microbiology, A. Mickiewicz University, Poznán, Poland; RK, strains obtained from R. Kong, Hong Kong University; SK, strains obtained from S. Korbsrisate, Department of Immunology, Mahidol University, Bangkok, Thailand; ATCC, American Type Culture Collection, Manassas, Va.; LMG, Culture Collection, Laboratorium voor Microbiologie, Universiteit Gent, Ghent, Belgium; ND, isolate not included in any Aeromonas sp. HG.
Isolation of DNA.
Colonies of Aeromonas spp. grown on Trypticase soy agar at 37°C for 24 h were scraped off and suspended in 0.85% NaCl. After centrifugation, the pellet was resuspended in a lysis buffer and incubated after adding protease K. The DNA was extracted using the NucleoSpin C + T kit (Macherey-Nagel, Düren, Germany) according to the procedure established by the manufacturer. The quality and quantity of the DNA were determined spectrophotometrically at 260 nm.
RAPD typing.
The RAPD method involves the use of short random sequence primers, usually 9 to 10 nucleotides long, and low-stringency primer annealing conditions to amplify arbitrary fragments of template DNA. The single primer anneals anywhere on the genome where a near-complementary sequence exists, and if two priming sites are sufficiently close, PCR then amplifies the fragment between them (27). The following primers were used for RAPD typing: OPB-1 5′-GTTTCGCTCC-3′ (27), OPB-6 5′-TGCTCTGCCC-3′ (27), OPB-7 5′-GGTGACGCAG-3′ (27), AP3 5′-TCACGATGCA-3′ (35), AP5 5′-TCACGCTGCG-3′ (35), AK1 5′-ATCACTATGA-3′, AK2 5′-GATCCTGCAG-3′, and AK3 5′-TAAGGTTCGG-3′. RAPD PCR mixtures were prepared as previously described (27). The reaction mixture consisted of 4 μl of 10× reaction buffer [750 mM Tris-HCl (pH 8.8), 200 mM (NH4)2SO4, 0.1% Tween 20], a 250 μM concentration of each deoxynucleoside triphosphate (dNTP), 50 pmol of a primer, 3.75 mM MgCl2, 35 ng of template DNA, and 2 U of Taq polymerase (MBI Fermentas) made up to 40 μl with sterile distilled water. The reaction mixture was denatured for 1 min at 94°C and then subjected to 25 cycles of denaturation for 1 min at 94°C, annealing for 1 min at 36°C, and extension for 2 min at 72°C and 15 cycles of denaturation for 1 min at 94°C, annealing for 1 min at 36°C, and extension for 3 min at 72°C with a final extension for 2 min at 72°C.
REP-PCR typing.
The REP-PCR method uses primers complementary to REP elements of bacterial genomic DNA (36). PCR amplification of template genomic DNA results in products of different sizes, presumably reflecting the distance and orientation of endogenous repeats. The following primers were used for REP-PCR: Rep1R-I 5′-(inosine)(inosine)(inosine)(ino-sine)CG(inosine)CG(inosine)CATC(inosine)GGC-3′, and Rep2-I 5′-(inosine)CG(inosine)CTTATC(inosine)GGCCTAC-3′ (36). REP-PCR analysis was performed in accordance with previous description (36). The reaction mixture consisted of 4 μl of 10× reaction buffer [750 mM Tris-HCl (pH 8.8), 200 mM (NH4)2SO4, 0.1% Tween 20], a 250 μM concentration of each dNTP, 50 pmol of primer Rep1R-I, 50 pmol of primer Rep2-I, 3.75 mM MgCl2, 100 ng of template DNA, and 2 U of Taq polymerase (MBI Fermentas) made up to 25 μl with sterile distilled water. The reaction mixture was denatured for 7 min at 95°C and then subjected to 30 cycles of denaturation for 30 s at 90°C, annealing for 1 min 40°C, extension for 8 min at 65°C, and a final extension for 16 min at 65°C.
ERIC-PCR typing.
The ERIC-PCR method utilizes primers complementary to ERIC sequences of bacterial genomic DNA (36). The following primers were used for ERIC-PCR: ERIC 1R (5′-ATG TAA GCT CCT GGG GAT TCA C-3′) and ERIC 2 (5′-AAG TAA GTG ACT GGG GTG AGC G-3′) (36). ERIC-PCR was performed as described previously (36). The reaction mixture consisted of 4 μl of 10× reaction buffer [750 mM Tris-HCl (pH 8.8), 200 mM (NH4)2SO4, 0.1% Tween 20], a 250 μM concentration of each dNTP, 50 pmol of primer ERIC 1R, 50 pmol of primer ERIC 2, 3.75 mM MgCl2, 100 ng of template DNA, and 2 U of Taq polymerase (MBI Fermentas) made up to 25 μl with sterile distilled water. The reaction mixture was denatured for 7 min at 95°C and then subjected to 30 cycles of denaturation for 30 s at 90°C, annealing for 1 min at 52°C, extension for 8 min at 65°C, and a final extension for 16 min at 65°C.
Electrophoresis and computer analysis.
The amplification products were electrophoresed in 1.5% agarose gel in Tris-borate buffer (0.089 M Tris, 0.089 M H3BO3, 0.002 M EDTA). Gene Ruler 100-bp DNA Ladder Plus (MBI Fermentas) was used as a molecular size standard. The gels were stained with ethidium bromide, visualized on a UV light transilluminator, and documented with V.99 Bio-Print system (Vilber Lourmat, Torcy, France). Computer analyses was carried out using GelCompar II (version 3.0; Applied Maths, Kortrijk, Belgium) software. Similarity between fingerprints was calculated with the Dice coefficient. Cluster analysis was performed using the unweighted pair-group method with average linkages (UPGMA).
RESULTS
RAPD fingerprinting.
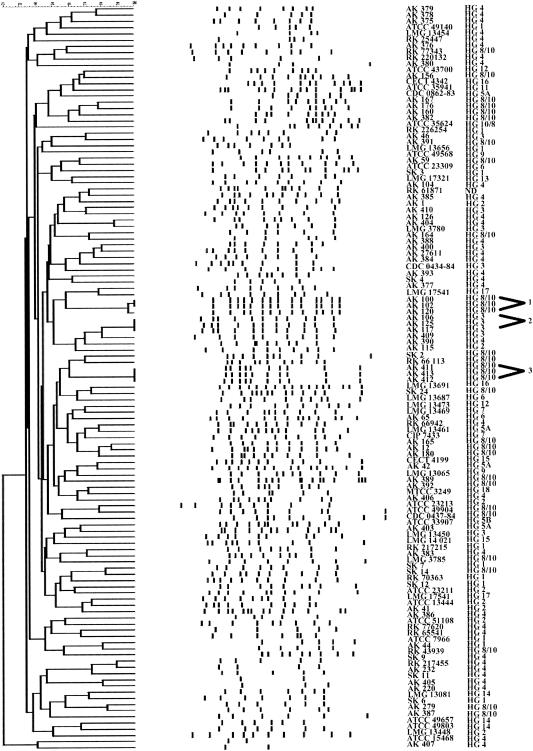
Eight primers were used for RAPD fingerprinting of 30 Aeromonas sp. strains representing all hybridization groups. PCR with some primers did not result in amplification products for all isolates. The results obtained with primers AK1 and AK2 revealed poor reproducibility. The best results were achieved with the AP5 primer (34). The AP5 primer was used for the typing of 120 Aeromonas sp. strains. The fingerprints of these isolates consisted of 2 to 17 bands ranging from 100 to 3,500 bp (Fig. 1). The bands in each of the patterns produced by the RAPD method were analyzed by applying the Dice coefficient. The similarities of the same strains from different agarose gels ranged from 98 to 100%.
FIG. 1.
Dendrogram showing genetic relatedness of 120 strains of Aeromonas sp. determined by analysis of RAPD fingerprint patterns using Dice similarity coefficient and UPGMA cluster method.
Numerical analysis of RAPD profiles revealed three clusters at the 90% similarity level. Strains within these clusters were considered to be genetically related. The first cluster consisted of three isolates of A. veronii biotype sobria (AK 100, AK 102, and AK 120) cultured from drinking water collected from a local industrial water distribution system in Konin, Poland. These isolates shared 98% similarity. The isolates AK 100 and AK 102 appeared to be identical; they shared the following bands: 3,500, 2,440, 2,000, 1,770, 1,580, 1,320, 1,180, 1,060, 840, 740, 580, 540, 450, and 390 bp. The RAPD profile of AK 120 was identical with those of AK 100 and AK 102 except for a single faint band at 2,000 bp. The second cluster was composed of three isolates of A. salmonicida, AK 106, AK 117, and AK 125, isolated from aluminum rolling emulsion. The same RAPD pattern was obtained for each of these isolates, showing expansion of a single clone colonizing aluminum cooling fluid. PCR products of these isolates showed fragments with common bands of 3,600, 3,500, 3,100, 2,100, 1,570, 1,330, 1,090, 910, 710, 600, and 440 bp. The third cluster comprised three isolates of A. veronii biotype sobria (AK 411, AK 413, AK 412) that originated from drinking water from a city distribution system. The RAPD patterns of these isolates were identical; isolates had fragments with common bands of 2,650, 2,350, 1,970, 1,750, 1,590, 1,220, 1,030, 900, 500, and 240 bp.
The RAPD patterns of all remaining strains were diverse, with similarities below 90%, and the strains were considered genetically unrelated. The 13 strains of A. caviae isolated from stool specimens collected from Polish children generated 13 clearly distinct patterns. The highest degree of similarity (77%) was obtained for AK 379 and AK 378. Among five isolates of A. veronii biotype sobria that originated from stool specimens collected from Polish children, a great diversity of fingerprints was also observed, and the strains did not form a separate cluster. The six strains isolated from specimens from patients living in other European countries had six different patterns and did not constitute a group. Strains isolated from stool specimens collected from people living in Asia showed similarities below 90%. The highest similarity (88%) was obtained for two isolates of A. caviae: SK 9 and RK 217455. All remaining strains showed similarities below 80%. The RAPD patterns of strains isolated from clinical sources were found to be distinct from those of the strains isolated from environmental sources. It is clear from the dendrogram that there was genetic diversity among strains isolated from healthy and dead fish (R. rutilus). The highest degree of similarity (76%) was found between two strains of A. veronii biotype sobria: AK 167 and AK 176.
REP-PCR fingerprinting.
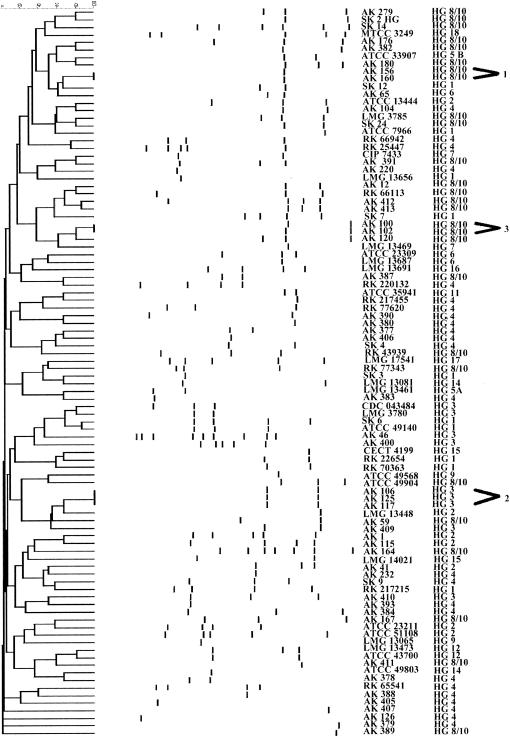
Among the 120 isolates of Aeromonas sp., 25 were not typeable by REP-PCR. This analysis yielded one to nine bands depending on the isolate; the size of the DNA fragments ranged from 100 to 3,500 bp. The majority of the isolates exhibited patterns with small number of REP-PCR products (Fig. 2). The similarities of the DNA patterns of the same strains from different agarose gels ranged from 98 to 100%.
FIG. 2.
Dendrogram showing genetic relatedness of 95 strains of Aeromonas sp. determined by analysis of REP-PCR fingerprint patterns using Dice similarity coefficient and UPGMA cluster method.
Three clusters were identified among the 95 strains of Aeromonas spp. The first cluster was composed of two A. veronii biotype sobria isolates (AK 156 and AK 160) isolated from two dead fish (R. rutilus) collected from the same lake. The isolates appeared to be identical; they had one fragment of 400 bp. Second group included three isolates of A. salmonicida: AK 106, AK 117, and AK 125. They shared two common fragments of 530 and 240 bp. The third cluster consisted of two isolates, AK 100 and AK 102, of A. veronii biotype sobria. The REP-PCR patterns of these isolates were identical and consisted of fragments of 390 and 140 bp. The REP-PCR patterns of all remaining strains showed similarities below 90%. Note that none of the clinical strains had the same pattern; however, we did not obtain fingerprints for 13 strains isolated from humans.
ERIC PCR fingerprinting.
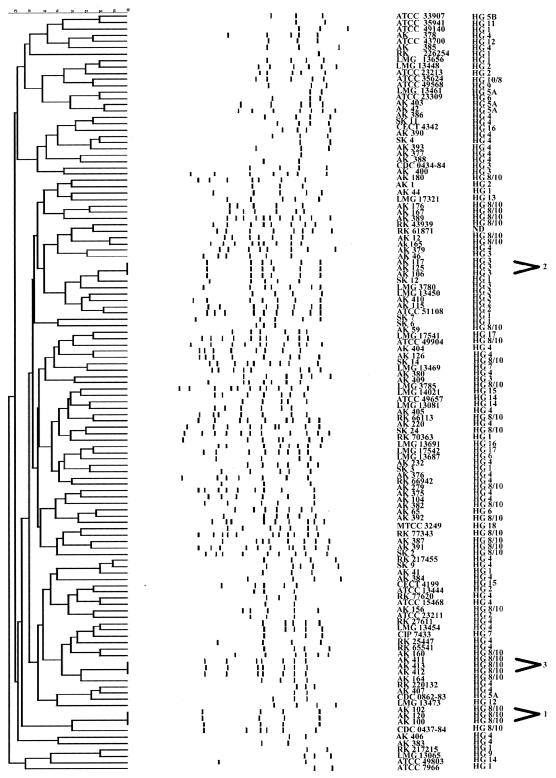
The fingerprints of Aeromonas sp. isolates consisted of 1 to 11 amplification bands, ranging in size from 100 to 3,500 bp (Fig. 3). All strains were typeable by ERIC-PCR. Reproducibility of the DNA patterns from different gels was in the range of 98 to 100%.
FIG. 3.
Dendrogram showing genetic relatedness of 120 strains of Aeromonas sp. determined by analysis of ERIC-PCR fingerprint patterns using Dice similarity coefficient and UPGMA cluster method.
Among the 120 strains of Aeromonas spp. examined, three clusters were identified. The first group included three isolates of A. veronii biotype sobria (AK 100, AK 102, and AK 120), which had shown the same DNA profile, consisting of fragments of 1,940, 690, 470, and 220 bp. The second cluster was composed of three A. salmonicida isolates: AK 106, AK 117, and AK 125. They shared fragments of 1,760, 780, 650, 550, 340, and 210 bp. The third cluster included three strains of A. veronii biotype sobria: AK 411, AK 413, and AK 412. These isolates appeared to be identical and shared the following common bands: 1,800, 1,200, 720, 650, 470, 390, and 300 bp. Figure 3 shows that 18 strains isolated from stool specimens collected from Polish children were distinct, and the highest similarity between two strains was 72% as indicated by the Dice coefficient. The ERIC-PCR patterns of six strains isolated from people living in other European countries were clearly different. Strains isolated from stool specimens collected from people living in Asia exhibited genetic variability as well. The clinical strains could be clearly distinguished from each other and from environmental isolates. No specific profile was obtained for strains isolated from healthy and dead fish (R. rutilus). The highest similarity (72%) was obtained for two strains A. veronii biotype sobria, AK 176 and AK 167.
DISCUSSION
Several studies have provided strong evidence that some bacterial epidemic clones can circulate in an infected population for several years and are responsible for outbreaks (5, 15, 30, 32). There have been no studies that would have investigated the relationship among the clones of the pathogenic Aeromonas spp. isolated from humans living in different parts of the world. We compared strains isolated from stool specimens collected from humans suffering from gastroenteritis by using RAPD, REP-PCR, and ERIC-PCR methods. The DNA profiles of 18 pathogenic strains isolated from stool specimens collected from Polish children, who were hospitalized in one unit of a hospital in Poznán, Poland, did not form a separate group of related strains. This indicated that the children were not infected with clonally related strains. Our data suggested that the hospital environment was not the source of Aeromonas sp. infections of hospitalized patients. This study also revealed that the Aeromonas sp. strains recovered from patients in Poland and from different parts of Europe were different clones. We found high genetic diversity among the strains isolated from stool specimens collected from humans living in Asia. None of the clones isolated in Poland showed relatedness to the clones originating from Hong Kong, Thailand, and other areas. Previously, Moyer et al. (26) reported that two A. hydrophila and four A. caviae strains isolated from stool specimens collected from people living in the same city exhibited genetic variability. Hänninen et al. (13) indicated that strains within A. veronii biotype sobria, A. caviae, and A. hydrophila had a unique ribotype. Those strains were isolated from stool specimens collected from people who had been on the same trip in Morocco. Our experimental results and previous observations provide strong evidence that there have not been predominant clones responsible for Aeromonas-associated gastroenteritis.
Several authors suggested the possibility of waterborne Aeromonas sp. infection in humans (8, 20, 21, 22, 24, 29, 34). Therefore, one of the purposes of the study was to investigate whether the same clones exist in the environment and in the stool specimens collected from patients with diarrhea. We found that there was no genetic similarity between clinical and environmental clones of Aeromonas spp. Previously, Moyer et al. (26) also showed no genetic relationship between clinical and environmental strains. Davin-Regli et al. (7) found that the water sampled in the hospital was not the source of infections of patients at the University Hospital in Marseille, France. The present study revealed the existence of a single clone of A. veronii biotype sobria in a city water distribution system for several months. Domination of some clones in the water distribution system could be a result of being a component of biological membranes (6, 11). We identified three other isolates of A. salmonicida which showed the expansion of a single clone in a specific environment, i.e., the rolling emulsion. However, we did not note Aeromonas-associated infections among humans working in the emulsion's aerosol.
Another area of interest in our study was determination of genetic similarity among strains isolated from healthy and dead fish (R. rutilus) that were collected from the same lake. We found that the isolates of A. veronii biotype sobria were different clones. Previously, Garcia et al. (10) found genetic heterogeneity among strains of A. salmonicida subsp. salmonicida isolated from different species of fish. However, they also observed a predominant clone that was responsible for most of the outbreaks of furunculosis in fish. Some authors indicated that water contaminated with Aeromonas sp. could be a source of fish disease (2). However, our results revealed that clones of Aeromonas sp. involved in fish infections did not show a close genetic similarity with clones isolated from water samples from the lake. The present study revealed the coexistence of many A. veronii biotype sobria clones in the water of this recreational lake.
Over the last few years several authors have used molecular typing methods for determination of the clonal structure of several species, e.g., Neisseria meningitidis (5), Corynebacterium diphtheriae (12, 33), Streptococcus pneumoniae (15, 32), Salmonella enterica (14), S. enterica serovar Enteritidis (23), Clostridium difficile (25). We found that species of A. hydrophila (HG 1), A. bestiarum (HG 2), A. salmonicida (HG 3), A. caviae (HG 4), A. media (HG 5), and A. veronii biotype sobria (HG 8/10) had clonal structures. In addition, none of the A. eucrenophila (HG 6), A. sobria (HG 7), A. jandaei (HG 9), A. schubertii (HG 12), A. trota (HG 14), A. allosaccharophila (HG 15), A. encheleia (HG 16), and A. popoffii (HG 17) strains had similar DNA profiles. Probably these species also possessed a clonal structure, but the number of strains in this study was too low to make a clear conclusion.
Despite the fact that Aeromonas species had a clonal structure, we did not find pathogenic clones which circulated among humans living in different geographic areas. In addition we did not determine the reservoir of pathogenic strains in the environment. It is probable that the pathovars of Aeromonas spp. do not represent a single evolutionary line but rather that they are composed of several lines. A similar phenomenon was reported for other bacterial species, e.g., most pathogenic Escherichia coli strains capable of causing the same disease do not constitute a monophyletic group and do not have a single evolutionary origin (31).
An excellent correlation was obtained between the results of RAPD and ERIC-PCR analyses. All strains with the same RAPD type were found to have the same ERIC type. Our observations demonstrate that RAPD and ERIC-PCR have a good discriminatory power. These methods are useful for distinguishing Aeromonas sp. clones and for epidemiological investigation. We do not recommend the use of the REP-PCR analysis for typing of Aeromonas sp. strains, because not all strains could be typed by this method. Moreover, the REP-PCR analysis yields a relatively small number of products. This suggests that the repetitive extragenic palindromic sequence may not be widely distributed in Aeromonas sp. genome.
TABLE 2.
Strains of Aeromonas spp. isolated from animals
| Genospecies | HG | Strain no.a | Source of isolation |
|---|---|---|---|
| A. bestiarum | 2 | ATCC 51108T | Diseased fish |
| A. salmonicida | 3 | LMG 3780T | Atlantic salmon (Salmo salar) |
| A. caviae | 4 | ATCC 15468T | Guinea pig |
| A. media | 5A | CDC 0862-83 | Fish |
| A. sobria | 7 | CIP 7433T, LMG 13469 | Fish |
| A. veronii biotype sobria | 8/10 | AK 156, AK 160, AK 164, AK 180 | Dead fish (Rutilus rutilus) |
| AK 165, AK 167, AK 176 | Healthy fish (Rutilus rutilus) | ||
| CDC 0437-84 | Fish | ||
| LMG 3785 | Frog | ||
| A. allosaccharophila | 15 | CECT 4199T | Diseased eel |
| A. encheleia | 16 | CECT 4342T | Fish |
| A. culicicola | 18 | MTCC 3249T | Midgut of Culex quinquefasciatus, India |
Abbreviations: AK, Culture Collection of Department of Microbiology, A. Mickiewicz University, Poznán, Poland; ATCC, American Type Culture Collection, Manassas, Va.; LMG, Culture Collection, Laboratorium voor Microbiologie Universiteit, Gent, Ghent, Belgium; CECT, Coleccion Espanola de Cultivos Tipo, Universitad de Valencia, Valencia, Spain; CIP, Collection bacterienne de l'Institut Pasteur, Paris, France; CDC, Centers for Disease Control and Prevention, Atlanta, Ga.; MTCC, Microbial Type Culture Collection, Institute of Microbial Technology, Chandigarh, India.
TABLE 3.
Strains of Aeromonas spp. isolated from environmental sources
| Genospecies | HG | Strain no.a | Source of isolation |
|---|---|---|---|
| A. hydrophila | 1 | AK 44 | Lake water |
| ATCC 7966T | Canned milk | ||
| A. bestiarum | 2 | AK 1, AK 41 | Lake water |
| AK 115 | Drinking water, aluminum rolling mill in Konin, Poland | ||
| ATCC 23213 | River water | ||
| ATCC 23211 | Drinking water distribution system | ||
| ATCC 13444 | Surface water | ||
| A. salmonicida | 3 | AK 46 | Lake water |
| AK 106, AK 117, AK 125 | Rolling mill emulsion | ||
| AK 400 | Sea water | ||
| AK 409, AK 410 | Drinking water, Poznán | ||
| CDC 0434-84 | Freshwater | ||
| A. caviae | 4 | AK 104, AK 126 | Drinking water, aluminum rolling mill in Konin, Poland |
| AK 220, AK 232 | Rolling mill emulsion | ||
| AK 404, AK 405 | River water | ||
| AK 406, AK 407 | Sea water | ||
| A. media | 5A | AK 42 | Lake water |
| AK 403 | Sea water | ||
| 5B | ATCC 33907T | Fresh water | |
| A. eucrenophila | 6 | AK 65 | Lake water |
| ATCC 23309T | Freshwater | ||
| LMG 13687 | Urban well, Germany | ||
| A. veronii biotype sobria | 8/10 | AK 12, AK 59 | Lake water |
| AK 100, AK 102, AK 120 | Drinking water, aluminum rolling mill in Konin, Poland | ||
| AK 279 | Sewage | ||
| AK 411, AK 412, AK 413 | Drinking water, Poznán | ||
| A. ichthiosmia | 8/10 | ATCC 49904 | Surface water |
| A. encheleia | 16 | LMG 13691 | Infiltration well, Germany |
| A. popoffii | 17 | LMG 17541T | Drinking water, Belgium |
| LMG 17542 | Drinking water production plant, Belgium |
Abbreviations: AK, Culture Collection of Department of Microbiology, A. Mickiewicz University, Poznán, Poland; ATCC, American Type Culture Collection, Manassas, Va.; LMG, Culture Collection, Laboratorium voor Microbiologie, Universiteit Gent, Ghent, Belgium; CDC, Centers for Disease Control and Prevention, Atlanta, Ga.
REFERENCES
- 1.Altwegg, M. 1996. Subtyping methods for Aeromonas species, p. 109-126. In B. Austin, M. Altwegg, P. J. Gosling, and S. W. Joseph (ed.), The genus Aeromonas. J. Wiley & Sons, Chichester, United Kingdom.
- 2.Altwegg, M. 1999. Aeromonas and Plesiomonas, p. 507-516. In P. Murray, E. Baron, M. Pfaller, F. Tenover, and R. Yolken (ed.), Manual of clinical microbiology. ASM Press, Washington, D.C.
- 3.Austin, B., and C. Adams. 1996. Fish pathogens, p. 197-229, In B. Austin, M. Altwegg, P. J. Gosling, and S. W. Joseph (ed.), The genus Aeromonas. J. Wiley & Sons, Chichester, United Kingdom.
- 4.Brandi, G., M. Sisti, G. F. Schiavano, L. Salvaggio, and A. Albano. 1996. Survival of Aeromonas hydrophila, Aeromonas caviae and Aeromonas sobria in soil. J. Appl. Bacteriol. 81:439-444. [Google Scholar]
- 5.Caugant, D. A. 1998. Population genetics and molecular epidemiology of Neisseria meningitidis. APMIS 106:505-525. [PubMed] [Google Scholar]
- 6.Daly, B., W. B. Betts, A. P. Brown, and J. G. O'Neill. 1998. Bacterial loss from biofilms exposed to free chlorine. Microbios 96:7-21. [PubMed] [Google Scholar]
- 7.Davin-Regli, A., C. Bollet, E. Chamorey, V. Colonna D'Istria, and A. Cremieux. 1998. A cluster of cases of infections due to Aeromonas hydrophila revealed by combined RAPD and ERIC-PCR. J. Med. Microbiol. 47:499-504. [DOI] [PubMed] [Google Scholar]
- 8.Demarta, A., M. Tonolla, A.-P. Caminada, M. Beretta, and R. Peduzzi. 2000. Epidemiological relationships between Aeromonas strains isolated from symptomatic children and household environments as determined by ribotyping. Eur. J. Epidemiol. 16:447-453. [DOI] [PubMed] [Google Scholar]
- 9.Esteve, C., C. Amaro, E. Garay, Y. Santos, and A. E. Toranzo. 1995. Pathogenicity of live bacteria and extracellular products of motile Aeromonas isolated from eels. J. Appl. Bacteriol. 78:555-562. [Google Scholar]
- 10.Garcia, J. A., J. L. Larsen, I. Dalsgaard, and K. Pedersen. 2000. Pulsed-field gel electrophoresis analysis of Aeromonas salmonicida ssp. salmonicida. FEMS Microbiol. Lett. 190:163-166. [DOI] [PubMed] [Google Scholar]
- 11.Gavriel, A. A., J. P. B. Landre, and A. J. Lamb. 1988. Incidence of mesophilic Aeromonas within a public drinking water supply in north-east Scotland. J. Appl. Microbiol. 84:383-392. [DOI] [PubMed] [Google Scholar]
- 12.Gubler, J., C. Huber-Schneider, E. Gruner, and M. Altwegg. 1998. An outbreak of nontoxigenic Corynebacterium diphtheriae infection: single bacterial clone causing invasive infection among Swiss drug users. Clin. Infect. Dis. 27:1295-1298. [DOI] [PubMed] [Google Scholar]
- 13.Hänninen, M. L., S. Salmi, L. Mattila, R. Taipalinen, and A. Siitonen. 1995. Association of Aeromonas spp. with travellers' diarrhoea in Finland. J. Med. Microbiol. 42:26-31. [DOI] [PubMed] [Google Scholar]
- 14.Hilton, A. C., and C. W. Penn. 1998. Comparison of ribotyping and arbitrarily primed PCR for molecular typing of Salmonella enterica and relationships between strains on the basis of these molecular markers. J. Appl. Microbiol. 85:933-940. [DOI] [PubMed] [Google Scholar]
- 15.Hsueh, P.-R., L.-J. Teng, L.-N. Lee, P.-C. Yang, S.-W. Ho, and K.-T. Luh. 1999. Dissemination of high-level penicillin-, extended-spectrum cephalosporin-, and erythromycin-resistant Streptococcus pneumoniae clones in Taiwan. J. Clin. Microbiol. 37:221-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janda, J. M. 1991. Recent advances in the study of the taxonomy, pathogenicity, and infectious syndromes associated with the genus Aeromonas. Clin. Microbiol. Rev. 4:397-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janda, J. M., and S. L. Abbott. 1996. Human pathogens, p. 151-170. B. Austin, M. Altwegg, P. J. Gosling, and S. W. Joseph (ed.), The genus Aeromonas. J. Wiley & Sons, Chichester, United Kingdom.
- 18.Janda, J. M., and S. L. Abbott. 1998. Evolving concepts regarding the genus Aeromonas: an expanding panorama of species, disease presentations, and unanswered questions. Clin. Infect. Dis. 27:332-344. [DOI] [PubMed] [Google Scholar]
- 19.Kaznowski, A. 1998. Identification of Aeromonas strains of different origin to the genomic species level. J. Appl. Microbiol. 84:423-430. [DOI] [PubMed] [Google Scholar]
- 20.Krovacek, K., V. Pasquale, S. B. Baloda, V. Soprano, M. Conte, and S. Dumontet. 1994. Comparison of putative virulence factors in Aeromonas hydrophila strains isolated from the marine environment and human diarrheal cases in southern Italy. Appl. Environ. Microbiol. 60:1379-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kü hn, I., M. J. Albert, M. Ansaruzzaman, N. A. Bhuiyan, S. A. Alabi, M. S. Islam, P. K. B. Neogi, G. Huys, P. Janssen, K. Kersters, and R. Möllby. 1997. Characterization of Aeromonas spp. isolated from humans with diarrhea, from healthy controls, and from surface water in Bangladesh. J. Clin. Microbiol. 35:369-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kühn, I., G. Allestam, G. Huys, P. Janssen, K. Kersters, K. Krovacek, and T. Stenström. 1997. Diversity, persistence, and virulence of Aeromonas strains isolated from drinking water distribution system in Sweden. Appl. Environ. Microbiol. 63:2708-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin, A. W., M. A. Usera, T. J. Barrett, and R. A. Goldsby. 1996. Application of random amplified polymorphic DNA analysis to differentiate strains of Salmonella enteritidis. J. Clin. Microbiol. 34:870-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinetti Lucchini, G. 1993. Typing methods as epidemiological tools in the genus Aeromonas. Med. Microbiol. Lett. 2:226-230. [Google Scholar]
- 25.Martirosian, G., S. Kuipers, H. Verbrugh, A. van Belkum, and F. Meisel-Mikołajczyk. 1995. PCR ribotyping and arbitrarily primed PCR for typing strains of Clostridium difficile from a Polish maternity hospital. J. Clin. Microbiol. 33:2016-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moyer, N. P., G. Martinetti Luccini, L. A. Holcomb, N. H. Hall, and M. Altwegg. 1992. Application of ribotyping for differentiating aeromonads isolated from clinical and environmental sources. Appl. Environ. Microbiol. 58:1940-1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oakey, H., J. J. T. Ellis, and L. F. Gibson. 1996. Differentiation of Aeromonas genomospecies using random amplified polymorphic DNA polymerase chain reaction (RAPD-PCR). J. Appl. Bacteriol. 80:402-410. [DOI] [PubMed] [Google Scholar]
- 28.Pidiyar, V., A. Kaznowski., N. B. Narayan, M. Patole, and S. Shouche. 2002. Aeromonas culicicola sp. nov., from the midgut of Culex quinquefasciatus. Int. J. Syst. Evol. Microbiol. 52:1723-1728. [DOI] [PubMed] [Google Scholar]
- 29.Pin, C., M. L. Marin, D. Selgas, M. L. Garcia, J. Tormo, and C. Casas. 1995. Differences in production of several extracellular virulence factors in clinical and food Aeromonas spp. strains. J. Appl. Bacteriol. 78:175-179. [DOI] [PubMed] [Google Scholar]
- 30.Poh, C. L., V. Ramachandran, and J. W. Tapsall. 1996. Genetic diversity of Neisseria gonorrhoeae IB-2 and IB-6 isolates revealed by whole-cell repetitive element sequence-based PCR. J. Clin. Microbiol. 34:292-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pupo, G. M., D. K. Karaolis, R. Lan, and P. R. Reeves. 1997. Evolutionary relationships among pathogenic and nonpathogenic Escherichia coli strains inferred from multilocus enzyme electrophoresis and mdh sequence studies. Infect. Immun. 65:2685-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson, D. A., S. K. Hollingshead, J. M. Musser, A. J. Parkinson, D. E. Briles, and M. J. Crain. 1998. The IS1167 insertion sequence is a phylogenetically informative marker among isolates of serotype 6B Streptococcus pneumoniae. J. Mol. Evol. 47:222-229. [DOI] [PubMed] [Google Scholar]
- 33.Sulakvelidze, A., M. Kekelidze, T. Gomelauri, Y. Deng, N. Khetsuriani, K. Kobaidze, A. de Zoysa, A. Efstratiou, J. G. Morris, J. R., and P. Imnadze. 1999. Diphtheria in the Republic of Georgia: use of molecular typing techniques for characterization of Corynebacterium diphtheriae strains. J. Clin. Microbiol. 37:3265-3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talon, D., M. J. Dupont, J. Lesne, M. Thouverez, and Y. Michel-Briand. 1996. Pulsed-field gel electrophoresis as an epidemiological tool for clonal identification of Aeromonas hydrophila. J. Appl. Bacteriol. 80:277-282. [DOI] [PubMed] [Google Scholar]
- 35.Talon, D., B. Mulin, and M. Thouverez. 1998. Clonal identification of Aeromonas hydrophila strains using randomly amplified polymorphic DNA analysis. Eur. J. Epidemiol. 14:305-310. [DOI] [PubMed] [Google Scholar]
- 36.Versalovic, J., T. Koeuth, and J. R. Lupski. 1991. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 19:6823-6831. [DOI] [PMC free article] [PubMed] [Google Scholar]