Abstract
Streptococcus mutans organisms are occasionally isolated from the blood of patients with infective endocarditis, though the mechanisms of invasion and survival remain to be elucidated. Two of four blood isolates from patients with bacteremia or infective endocarditis (strains TW295 and TW871) were serologically untypeable by immunodiffusion testing, which was due to a lack of the glucose side chain of the serotype-specific polysaccharide antigen of S. mutans. Immunodiffusion analyses using antisera against these strains demonstrated that 2 of 100 isolates from 100 subjects showed a positive reaction, while further analysis of 2,500 isolates from 50 subjects revealed that all 50 isolates from a single subject were not reactive with anti-c, -e, and -f antisera, though they were reactive with anti-TW295 and -TW871 antisera. The oral isolates showed biological properties similar to those of the reference S. mutans strain MT8148, including high levels of sucrose-dependent adhesion and cellular hydrophobicity, along with expression of glucosyltransferases and a protein antigen, PA. We designated these organisms serotype k. A glucose side chain-defective mutant strain was then constructed by insertional inactivation of the gluA gene of strain MT8148, which showed biological properties similar to those of serotype k of S. mutans. Serotype k oral isolates were less susceptible to phagocytosis, as were the gluA-inactivated mutant of strain MT8148 and blood isolates. These results indicate that S. mutans serotype k strains are present in the oral cavity in humans and may be able to survive longer in blood owing to their low susceptibility to phagocytosis.
Based on differences in their compositions and the linkages of cell wall polysaccharides, mutans streptococci are classified into eight serotypes as follows; Streptococcus mutans (serotypes c, e, and f), Streptococcus sobrinus (serotypes d and g), Streptococcus cricetus (serotype a), Streptococcus rattus (serotype b), Streptococcus ferus (serotype c), Streptococcus macacae (serotype c), and Streptococcus downei (serotype h) (3). Among these, S. mutans is known to be a major causative bacterium of dental caries in humans and is occasionally isolated from the blood of patients with infective endocarditis (19, 20). In a previous study, four streptococcal strains isolated from patients with infective endocarditis or bacteremia following a tooth extraction procedure were specified as S. mutans based on their biological properties and 16S rRNA alignments (4). However, two of these strains (TW295 and TW871) were shown to be serologically untypeable, while the others belonged to serotype e or f.
The serotype-specific polysaccharides of S. mutans are composed of rhamnose-glucose polymers, with a backbone of rhamnose and side chains of α- or β-linked glucosidic residues (9). The serologically untypeable properties of strains TW295 and TW871 have been shown to be derived from the lack of a glucose side chain in the serotype-specific polysaccharide (4). Furthermore, the polysaccharides of these blood isolates show similarities to those of the mutant strain with inactivation of the gluA gene, which encodes the enzyme that catalyzes the production of the immediate precursor of the glucose side chain donor, in that they all have low glucose contents (21).
The serologically untypeable strains were shown to have hydrophobicity and sucrose-dependent adhesion levels as high as those of the oral isolates (4) and were less susceptible to phagocytosis in a preliminary study. These findings imply that S. mutans organisms without a glucose side chain of serotype c-, e-, or f-specific polysaccharide may be present in the oral cavity in humans because of their high cellular hydrophobicity and sucrose-dependent adherence levels. Furthermore, they may be able to survive in blood longer owing to their low susceptibility to phagocytosis during the invasion of blood. In the present study, we surveyed the occurrence of S. mutans organisms from human oral cavities that had serological properties similar to those of untypeable blood isolates.
MATERIALS AND METHODS
S. mutans strains.
Blood isolates TW295 (untypeable), TW871 (untypeable), TW964 (f), and TW1378 (e) (4) and the orally isolated strains MT8148 (c), MT4245 (e), and MT4251 (f) were selected from the stock culture collection in our laboratory (14). Features of these strains are presented in Table 1.
TABLE 1.
Bacterial strains used in the present study
| Strain | Serotype | Features | Reference |
|---|---|---|---|
| TW295 | k | Blood isolate from a 59-year-old male with bacteremia following a tooth extraction procedure | 4 |
| TW871 | k | Blood isolate from a 45-year-old female with infective endocarditis complicated by subarachnoid hemorrhage | 4 |
| TW964 | f | Blood isolate from a 72-year-old male with infective endocarditis | 4 |
| TW1378 | e | Blood isolate from a 59-year-old male with infective endocarditis | 4 |
| MT8148 | c | Oral isolate from a healthy child | 14 |
| MT4245 | e | Oral isolate from a healthy child | 14 |
| MT4251 | f | Oral isolate from a healthy child | 14 |
| NN2001 | c | Oral isolate from a healthy 6-year-old boy | This study |
| NN2002 | e | Oral isolate from a healthy 9-year-old boy | This study |
| NN2003 | f | Oral isolate from a healthy 7-year-old girl | This study |
| MT8148GD | k | GD mutant strain of MT8148 | This study |
| FT1 (NN2011) | k | Oral isolate from a healthy 3-year-old girl | This study |
| SU1 (NN2029) | k | Oral isolate from a healthy 10-year-old girl | This study |
| YK1 | k | Oral isolate from a 6-year-old girl with Down's syndrome | This study |
Generation of antisera against untypeable S. mutans cells.
Antisera to serotypes c, e, and f of S. mutans were taken from our laboratory stock (12), while antisera to the untypeable strains TW295 and TW871 were generated as follows. Whole cells of each strain were suspended in phosphate-buffered saline (PBS) and repeatedly injected intravenously into rabbits for five consecutive days (dry weight, 5 mg per day). One week after the final injection, immunization was repeated for another 2 weeks, five times each week. Blood was then taken from the auricular vein, and the antibody titer was checked using an immunodiffusion method with the Rantz-Randall (RR) polysaccharide antigen (5) to TW295 or TW871.
Construction of GD mutant strain.
A glucose side chain-defective (GD) mutant strain of MT8148 was constructed by insertional inactivation of the gluA gene. The gluA gene and its flanking region were amplified by PCR using AmpliTaq Gold polymerase (Applied Biosystems, Foster City, Calif.) with primers constructed on the basis of the gluA sequence from S. mutans strain Xc (21) (GenBank accession no. AB001562) and the S. mutans genomic database published by Oklahoma University (1) (GenBank accession no. AE014133). The amplified fragment was then cloned into a pST Blue-1 vector (Promega, Madison, Wis.) to generate pRN101. The middle of the open reading frame of gluA in pRN101 was cleaved by StuI and then ligated with the erythromycin resistance gene (erm) from the recombinant plasmid pKN100 carrying an 830-bp fragment of erm from pVA838 (10) to yield pRN102. After linearization by digestion at the unique restriction enzyme site of NotI, the plasmid was introduced into S. mutans MT8148 by the method of Tobian and Macrina (18). The transformants were screened on mitis salivarius (MS) agar (Difco Laboratories, Detroit, Mich.) plates containing erythromycin (10 μg/ml). The appropriate insertional inactivation of the mutant MT8148GD was confirmed by PCR amplification of the gluA gene and the immunodiffusion method.
Clinical specimens.
One thousand three hundred twenty-six S. mutans strains isolated from 571 children between 1982 and 1990 (the MT4000 and MT10000 series of isolates) were selected from our laboratory stock. In addition, strains from 100 subjects (3 to 17 years of age; average, 8.9 years) who visited the Pedodontics Clinic of Osaka University Dental Hospital, Suita, Osaka, Japan, from June to August 2002 were randomly selected (the NN2000 series of isolates). These subjects included 88 healthy children, along with 5 patients with cleft lip and palate, 3 with ventricular septal defect, 2 with amelogenesis imperfecta, 1 with spondyloschisis, and 1 autistic patient. Collection of clinical specimens was carried out in accordance with the Osaka University Health Guideline for Studies Involving Human Subjects. Plaque samples were collected in sterile PBS, diluted, and streaked onto MS agar plates containing bacitracin (0.2 U/ml; Sigma Chemical Co., St. Louis, Mo.) and 15% (wt/vol) sucrose. One colony from each subject was chosen on the basis of colony morphology and defined as S. mutans as described previously (4). Serotyping was done by the immunodiffusion method as described above, using rabbit antisera specific for serotypes c, e, and f (12). In addition, 50 colonies were isolated from each subject with a serologically untypeable strain.
Two thousand five hundred clinical isolates of S. mutans were obtained from another group of 50 subjects (3 to 19 years of age; average, 7.8 years) who came to the Pedodontics Clinic of Osaka University Dental Hospital in early 2003. These included 45 healthy children and 5 patients with general or oral health problems, i.e., Down's syndrome, congenital heart disorder, cleft lip and palate, amelogenesis imperfecta, and oligodontia.
Characterization of untypeable S. mutans.
The sucrose-dependent adhesion and cellular hydrophobicity of the untypeable clinical isolates were evaluated as described by Hamada et al. (6) and Rosenberg et al. (17), respectively. The expression of cell-associated (CA) or cell-free (CF) glucosyltransferases (GTFs) and the surface protein antigen (PA) were analyzed using Western blot analysis with antibodies specific to GTF and PA (4). Genomic DNA was extracted from the test organisms, and 16S rRNA was sequenced and compared with that of the reference strain NCTC10449 (2) (GenBank accession no. X58303 and S70358).
Phagocytosis assay.
The organisms were cultured in brain heart infusion broth (Difco) for 18 h at 37°C. After the bacterial cells were washed, cell concentrations were adjusted with PBS to 1.0 × 108 CFU/ml. Human peripheral blood (500 μl) was collected from a healthy volunteer and incubated with 500 μl (5.0 × 107 CFU) of the tested bacteria for 10 min at 37°C. Interactions between polymorphonuclear leukocytes (PMNs) and bacteria were observed with a light microscope (magnification, ×100; Olympus Optical, Tokyo, Japan) following Giemsa staining (Wako Pure Chemical Industries, Osaka, Japan). The rate of phagocytosis was expressed as the mean ratio of phagocytosed PMNs per 100 PMNs, with 500 PMNs examined. In addition, changes in the rate of phagocytosis in strains MT8148, MT8148GD, FT1, and TW295 were observed at 15, 30, 60, 90, and 120 min.
Statistical analysis.
Intergroup differences in various factors were estimated by a statistical analysis of variance for factorial models. Fisher's protected least-significant-difference test was used to compare individual groups.
RESULTS
Serotype distribution of past and recent strains.
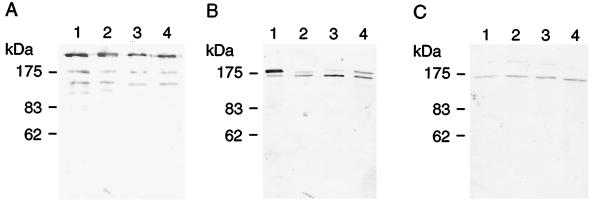
It was found that the antisera to TW295 and TW871 reacted specifically with RR extracts of TW295 and TW871, respectively, but not with those from serotype c, e, or f organisms (Fig. 1A and B). Furthermore, the precipitation bands were fused to each other. Our laboratory records indicate that all 1,326 of the strains from 571 children (the MT series) isolated between 1982 and 1990 were serologically classified as c, e, or f with no untypeable isolates found. On the other hand, 78, 17, and 3 of the recent 100 isolates (the NN2000 series) from 100 subjects were classified as serotypes c, e, and f, respectively, whereas the remaining 2 strains, FT1 (NN2011) and SU1 (NN2029), were found not to react with c-, e-, or f-specific antiserum (Table 2), though both showed rough colonies on MS agar plates; positive bacitracin resistance; γ hemolysis on blood agar; a positive fermentation profile for mannitol, sorbitol, raffinose, and melibiose; and a negative dextran agglutination, which are characteristics of S. mutans. Moreover, they also expressed PA and three types of GTFs (Fig. 2). Strains FT1 and SU1 showed high levels of sucrose-dependent adhesion and cellular hydrophobicity (data not shown), and their 16S rRNA gene sequences were identical to that of strain NCTC10449. In addition, the RR extracts of strain FT1 produced precipitation bands with antisera to strain TW871 (Fig. 1C) and TW295 (data not shown), which also fused to a precipitation band of the RR extract of TW871 with antiserum to TW871 (Fig. 1E). Furthermore, 50 strains from each subject with strain FT1 or SU1 were not reactive with c-, e-, or f-specific antiserum but were reactive with the antiserum to TW871. Based on these findings, we propose that these S. mutans strains be designated a new serotype, k.
FIG. 1.
Immunodiffusion between RR extracts and rabbit serotype-specific antisera. (A) Well 1 contained RR extract from strain TW295. The outer wells contained antisera to strain TW295 (well 2) and TW871 (well 3), along with antisera specific to serotypes c (well 4), e (well 5), and f (well 6). (B) Well 7 contained antiserum to strain TW871. The outer wells contained RR extracts from strains TW295 (well 8), TW871 (well 9), MT4251 (f; well 10), MT4245 (e; well 11), and MT8148 (c; well 12). (C) Well 13 contained RR extract from strain FT1. The outer wells contained antisera specific to strain TW871 (well 14), serotype c (well 15), serotype e (well 16), and serotype f (well 17). (D) Well 18 contained antiserum specific to serotype c. The outer wells contained RR extracts from strains MT8148 (well 19) and MT8148GD (well 20). (E) Well 21 contained antiserum to strain TW871. The outer wells contained RR extracts from strains TW295 (well 22), TW871 (well 23), FT1 (well 24), SU1 (well 25), YK1 (well 26), and MT8148GD (well 27).
TABLE 2.
Serotype distribution in two series of clinical oral isolates of S. mutans
| Serotype | No. of isolates
|
|
|---|---|---|
| MT series taken between 1982 and 1990 (n = 1,326) | NN2000 series taken in 2002 (n = 100) | |
| c | 1,131 | 78 |
| e | 163 | 17 |
| f | 32 | 3 |
| k | 0 | 2 |
FIG. 2.
Western immunoblot detection of PA, CA-GTF, and CF-GTF expression in S. mutans. Whole cells (A and B) or culture supernatant concentrated by ammonium sulfate precipitation (C) were separated by SDS-7% polyacrylamide gel electrophoresis. The transferred protein bands on a polyvinylidene difluoride membrane were reacted with rabbit antibodies against PA (A), CA-GTF (B), or CF-GTF (C) of S. mutans. Lanes 1, strain MT8148; lanes 2, strain FT1; lanes 3, strain SU1; lanes 4, strain YK1.
Serotype distribution of 2,500 strains from 50 subjects.
Table 3 shows the serotype distribution of 2,500 recent isolates of S. mutans, of which 2,450 were classified as c, e, or f type. On the other hand, the RR extracts of the other 50 strains (YK1 through YK50) from a single subject (a girl with Down's syndrome) produced a precipitation band with antiserum to TW871, which also fused to the precipitation bands of RR extracts of TW295, TW871, FT1, and SU1 (Fig. 1E). All of these strains were considered to be serotype k. Table 4 shows the serotype distribution patterns in individual subjects, most of whom were found to possess serotype c of S. mutans alone, followed by serotype e alone, while 5 of the 50 subjects possessed multiple serotypes.
TABLE 3.
Serotype distribution of 2,500 S. mutans isolates obtained from 50 subjects
| Serotype | No. of isolates | No. of subjects |
|---|---|---|
| c | 1,769 | 38 |
| e | 551 | 13 |
| f | 130 | 4 |
| k | 50 | 1 |
TABLE 4.
Individual serotype distribution among 50 subjects
| Serotype | No. of subjects |
|---|---|
| Single | |
| c | 34 |
| e | 8 |
| f | 2 |
| k | 1 |
| Multiple | |
| c and e | 3 |
| e and f | 1 |
| c, e, and f | 1 |
Biological and serological characterization of a gluA-inactivated mutant.
A gluA-inactivated mutant strain, MT8148GD, showed typical biological features of S. mutans, including rough colonies on MS agar, positive profiles of sugar fermentation, expression of GTFs and PA, and high levels of sucrose-dependent adhesion and cellular hydrophobicity. The RR extract of MT8148GD produced a precipitation band with the antiserum to TW871 but not with serotype c-specific antiserum (Fig. 1D) and fused to precipitation bands of TW295, TW871, FT1, SU1, and YK1 (Fig. 1E).
Phagocytosis assay.
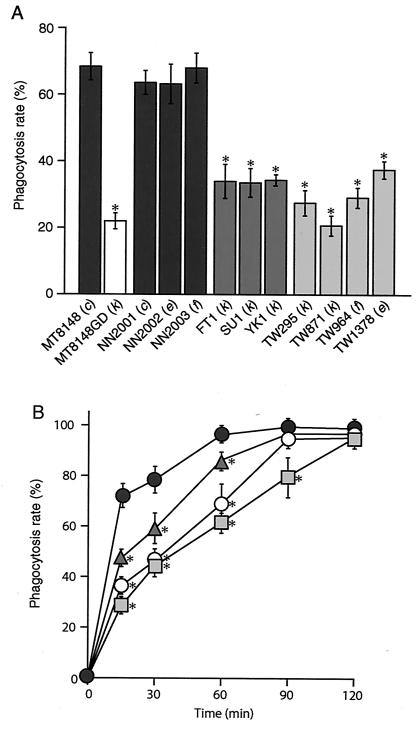
The phagocytic capabilities of these strains following 10 min of incubation are presented in Fig. 3A. The phagocytosis rate of strain MT8148GD was 22.0% ± 2.4%, which was significantly lower than that of the parent strain, MT8148 (68.4% ± 4.1%) (P < 0.001). Furthermore, oral isolates of NN2001 (c), NN2002 (e), and NN2003 (f) showed phagocytosis rates similar to that of MT8148, while the serotype k oral strains FT1, SU1, and YK1 also showed lower phagocytosis rates than MT8148 (P < 0.001). In addition, the phagocytosis rates of all four blood isolates were significantly lower than those of the oral isolates (P < 0.001), while those of MT8148GD, FT1, and TW295 were significantly lower than that of MT8148 until 60 min of incubation (P < 0.001), and the rate of TW295 was also significantly lower than that of MT8148 after 90 min of incubation (P < 0.001) (Fig. 3B).
FIG. 3.
Phagocytosis rates of oral and blood isolates of S. mutans. The results show the mean ± standard deviation from five experiments. (A) The phagocytosis rates of the 12 strains were examined following 10 min of incubation. (B) Changes in the phagocytosis rates of strains MT8148 (•), MT8148GD (○), FT1 (▴), and TW295(▪) at 15, 30, 60, 90, and 120 min of incubation. There were statistically significant differences between MT8148 (serotype c) and the other strains, as determined by Fisher's protected least-significant-difference analysis (*, P < 0.001).
DISCUSSION
The RR extracts of two blood isolates (TW295 and TW871), 152 oral isolates (FT1 through FT51, SU1 through SU51, and YK1 through YK50), and a gluA-inactivated strain (MT8148GD) produced precipitation bands with antiserum to TW295 or TW871 (Fig. 1E). These isolates were shown to possess biological properties typical of S. mutans, including high levels of sucrose-dependent adhesion and hydrophobicity and GTFs, while the culture supernatants contained a 190-kDa PA that produced precipitation bands with antiserum to PA of MT8148 (data not shown). On the other hand, the serological properties of these strains were different from those of the known S. mutans serotypes (c, e, and f), and based on our findings, we propose a new S. mutans serotype, k.
The isolation frequencies of S. mutans serotypes c, e, and f in the present study were closely similar to those reported in a Danish study (16). In that report, 1 of 76 mutans streptococci from human dental-plaque samples and 7 of 70 from human carious lesions could not be serologically classified as serotype a through g. A biochemical analysis revealed that these untypeable strains belonged to S. mutans, while there was no description of the structures of the serotype-specific polysaccharide. In another study, all 1,047 S. mutans or S. sobrinus isolates taken from human dental plaque, carious dentine, or feces samples could be classified as serotype c, e, f, d, or g (5). Furthermore, in a recent study conducted in another area of Japan, 144 strains of S. mutans or S. sobrinus from dental plaque were classified as serotype c, e, d, or g, and there were no serotype f strains or untypeable strains detected (8). On the other hand, 9 out of 103 subjects were reported to harbor serologically untypeable S. mutans in Japan recently, although a precise description of the properties of the serotype-specific polysaccharide was not provided (7). Taking these data together, we speculated that serotype k organisms have appeared in Japan only recently.
In the present study, S. mutans strains with this new serotype were found in plaque samples from three children, with a total of 152 classified as serotype k. The origin of these clinical isolates has not yet been identified, but serotype k strains comprised the majority of the S. mutans strains found in dental-plaque samples from those three subjects, and all showed high levels of cellular hydrophobicity and sucrose-dependent adherence, as well as lower rates of phagocytosis. These findings suggest that serotype k strains of S. mutans are present in the oral cavity in humans because of their high hydrophobicity and sucrose-dependent adhesion, along with the expression of GTFs and PA, and may be able to survive in blood due to their lower phagocytotic capabilities. Therefore, special care must be taken with children with heart disorders. In particular, it may be more risky for Down's syndrome patients with S. mutans serotype k strains in the oral cavity, as they have been reported to have a possible congenital basis for disorders of PMN functions (13) and are also known to be susceptible to ventricular septal defect (11). As detection of the new serotype k in these patients may indicate an increased risk, a clinical approach, such as prescription of antibiotics prior to dental treatment, may be required and should be kept in mind.
Infective endocarditis is known to be initiated by an invasion of pathogenic bacteria into the bloodstream; however, the mechanisms of invasion and survival of S. mutans in blood have not been clarified. In the present study, we found that the phagocytosis rate of the GD isogenic mutant strain was lower than that of the parent strain (Fig. 3). In addition, the serotype k oral and blood isolates presented similar capabilities. Together, these findings suggest that serotype k strains, which are considered to lack a glucose side chain in the serotype-specific polysaccharide, may be possible pathogenic bacteremia strains. On the other hand, the blood isolates TW964 (e) and TW1378 (f) were less susceptible to phagocytosis than the oral isolates NN2002 (e) and NN2003 (f), though it has been shown that TW964 lacks a glucan-binding protein A (15) whereas no such alteration of TW1378 has been found. Thus, there may be an alteration of cell surface structures other than the serotype-specific polysaccharide that has an effect on phagocytic ability.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research (B) 14370693 from the Japan Society for Promotion of Science.
REFERENCES
- 1.Ajdic, D., W. M. McShan, R. E. McLaughlin, G. Savic, J. Chang, M. B. Carson, C. Primeaux, R. Tian, S. Kenton, H. Jia, S. Lin, Y. Qian, S. Li, H. Zhu, F. Najar, H. Lai, J. White, B. A. Roe, and J. J. Ferretti. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. USA 99:14434-14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bentley, R. W., J. A. Leigh, and M. D. Collins. 1991. Intrageneric structure of Streptococcus based on comparative analysis of small-subunit rRNA sequences. Int. J. Syst. Bacteriol. 41:487-494. [DOI] [PubMed] [Google Scholar]
- 3.Coykendall, A. L. 1989. Classification and identification of the viridans streptococci. Clin. Microbiol. Rev. 2:315-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujiwara, T., K. Nakano, M. Kawaguchi, T. Ooshima, S. Sobue, S. Kawabata, I. Nakagawa, and S. Hamada. 2001. Biochemical and genetic characterization of serologically untypable Streptococcus mutans strains isolated from patients with bacteremia. Eur. J. Oral Sci. 109:330-334. [DOI] [PubMed] [Google Scholar]
- 5.Hamada, S., N. Masuda, and S. Kotani. 1980. Isolation and serotyping of Streptococcus mutans from teeth and feces of children. J. Clin. Microbiol. 11:314-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamada, S., M. Torii, S. Kotani, and Y. Tsuchitani. 1981. Adherence of Streptococcus sanguis clinical isolates to smooth surfaces and interactions of the isolates with Streptococcus mutans glucosyltransferase. Infect. Immun. 32:364-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirakawa, M., and K. Takada. 2003. A new selective medium for Streptococcus mutans and the distribution of S. mutans and S. sobrinus and their serotypes in dental plaque. Caries Res. 37:212-217. [DOI] [PubMed] [Google Scholar]
- 8.Kozai, K., R. Nakayama, U. Tedjosasongko, S. Kuwahara, J. Suzuki, M. Okada, and N. Nagasaka. 1999. Intrafamilial distribution of mutans streptococci in Japanese families and possibility of father-to-child transmission. Microbiol. Immunol. 43:99-106. [DOI] [PubMed] [Google Scholar]
- 9.Linzer, R., M. S. Reddy, and M. J. Levine. 1986. Immunochemical aspects of serotype carbohydrate antigens of Streptococcus mutans, p. 29-38. In S. Hamada, S. M. Michalek, H. Kiyono, L. Manaker, and J. R. McGhee (ed.), Molecular microbiology and immunology of Streptococcus mutans. Elsevier Science Publishers, Amsterdam, The Netherlands.
- 10.Macrina, F. L., R. P. Evans, J. A. Tobian, D. L. Hartley, D. B. Clewell, and K. R. Jones. 1983. Novel shuttle plasmid vehicles for Escherichia-Streptococcus transgeneric cloning. Gene 25:145-150. [DOI] [PubMed] [Google Scholar]
- 11.Marino, B. 1996. Patterns of congenital heart disease and associated cardiac anomalies in children with Down syndrome, p. 133-140. In B. Marino and S. M. Pueschel (ed.), Heart disease in persons with Down syndrome. Paul Brookes, Baltimore, Md.
- 12.Masuda, N., T. Shimamoto, K. Kitamura, S. Sobue, and S. Hamada. 1985. Transmission of Streptococcus mutans in some selected families. Microbios 44:223-232. [PubMed] [Google Scholar]
- 13.Mills, E. L., and P. G. Quie. 1980. Congenital disorders of the function of polymorphonuclear neutrophils. Rev. Infect. Dis. 2:505-517. [DOI] [PubMed] [Google Scholar]
- 14.Minami, T., T. Fujiwara, T. Ooshima, Y. Nakajima, and S. Hamada. 1990. Interaction of structural isomers of sucrose in the reaction between sucrose and glucosyltransferases from mutans streptococci. Oral Microbiol. Immunol. 5:189-194. [DOI] [PubMed] [Google Scholar]
- 15.Nakano, K., M. Matsumura, M. Kawaguchi, T. Fujiwara, S. Sobue, I. Nakagawa, S. Hamada, and T. Ooshima. 2002. Attenuation of glucan-binding protein C reduces the cariogenicity of Streptococcus mutans: analysis of strains isolated from human blood. J. Dent. Res. 81:376-379. [DOI] [PubMed] [Google Scholar]
- 16.Perch, B., E. Kjems, and T. Ravn. 1974. Biochemical and serological properties of Streptococcus mutans from various human and animal sources. Acta Pathol. Microbiol. Scand. B 82:357-370. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg, M., D. Gutnick, and E. Rosenberg. 1980. Adherence of bacteria to hydrocarbon: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol. Lett. 9:29-33. [Google Scholar]
- 18.Tobian, J. A., and F. L. Macrina. 1982. Helper plasmid cloning in Streptococcus sanguis: cloning of a tetracycline resistance determinant from the Streptococcus mutans chromosome. J. Bacteriol. 152:215-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ullman, R. F., S. J. Miller, M. J. Strampfer, and B. A. Cunha. 1988. Streptococcus mutans endocarditis: report of three cases and review of the literature. Heart Lung 17:209-212. [PubMed] [Google Scholar]
- 20.Vose, J. M., P. W. Smith, M. Henry, and D. Colan. 1987. Recurrent Streptococcus mutans endocarditis. Am. J. Med. 82:630-632. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita, Y., Y. Tsukioka, Y. Nakano, K. Tomihisa, T. Oho, and T. Koga. 1998. Biological functions of UDP-glucose synthesis in Streptococcus mutans. Microbiology 144:1235-1245. [DOI] [PubMed] [Google Scholar]