Abstract
An enzyme-linked immunosorbent assay to diagnose Chagas' disease by a serological test was performed with Trypanosoma cruzi recombinant antigens (JL8, MAP, and TcPo). High sensitivity (99.4%) and specificity (99.3%) were obtained when JL8 was combined with MAP (JM) and tested with 150 serum samples from chagasic and 142 nonchagasic individuals. Moreover, JM also diagnosed 84.2% of patients in the acute phase of T. cruzi infection.
A serological test is the most reliable and practical method for the diagnosis of Chagas' disease, an illness that is caused by the protozoan Trypanosoma cruzi and that affects millions of people in Latin America (22). The risk of T. cruzi transmission by transfusion in areas where Chagas' disease is endemic is assessed by performing at least two different tests to detect specific antibodies (23, 25, 26). In countries where Chagas' disease is not endemic, it is advisable to use serological tests on persons born in or given blood transfusions in countries where Chagas' disease is endemic (8, 9, 12, 14, 27). The acute phase of Chagas' disease is rarely diagnosed, because it is often without symptoms (22). Moreover, natural transmission by triatomine bugs is under control in some Latin American countries. Furthermore, there is still a need for continuing epidemiological surveillance in countries where transmission has not yet been controlled (5, 22). Conventional serological tests for Chagas (CSC tests) (e.g., indirect immunofluorescence [IIF], indirect hemagglutination [IHA], and enzyme-linked immunosorbent assay [ELISA]) usually employ semipurified antigens from the epimastigote form of T. cruzi. Consequently, CSC tests yield relatively large numbers of inconclusive and false-positive results (4, 19, 23, 25), mainly when a concomitant infection, such as leishmaniasis, is present (4, 31), and the sensitivities of CSC tests are far from ideal in the diagnosis of the early acute phase of disease (3, 28, 30) or in patients with low titers of anti-T. cruzi antibodies (31). This nonideal performance may have social, legal, and economic implications. To overcome these problems, several laboratories developed new serodiagnostic tests using antigens from infective trypomastigote forms (1, 28, 30) or a combination of T. cruzi recombinant proteins and/or synthetic peptides (4, 6, 7, 13, 20, 21, 24, 31).
The International Atomic Energy Agency organized a collaborative study to develop an ELISA with a mixture of T. cruzi recombinant antigens for immunodiagnosis of the acute and chronic phases of Chagas' disease. In this study, we evaluated the performance of three T. cruzi recombinant antigens (JL8, MAP, and TcPo) with serum samples from patients living in six Latin American countries (Table 1). Previous studies showed that JL8 and TcPo react with immunoglobulin G (IgG) antibodies of patients with chronic Chagas' disease (15-18), and assays with JL8 showed high sensitivity and specificity (4, 7, 13, 15-18). MAP is recognized by IgG antibodies from chronic and acute chagasic patients (11; unpublished data).
TABLE 1.
Geographical origin and distribution of serum samples of T. cruzi-infected individuals and nonchagasic individuals
| Country | No. of serum samples | No. of individuals
|
|||
|---|---|---|---|---|---|
|
T. cruzi infected
|
Nonchagasic
|
||||
| Acute phase | Chronic phase | Other diseases | Healthy | ||
| Bolivia | 51 | 0 | 15 | 0 | 36 |
| Brazil | 172 | 10 | 81 | 53 | 28 |
| Honduras | 40 | 0 | 35 | 0 | 5 |
| Mexico | 5 | 0 | 3 | 0 | 2 |
| Panama | 38 | 9 | 20 | 9 | 0 |
| Venezuela | 16 | 0 | 7 | 0 | 9 |
| Total | 322 | 19 | 161 | 62 | 80 |
Mixtures of recombinant antigens perform better than single proteins.
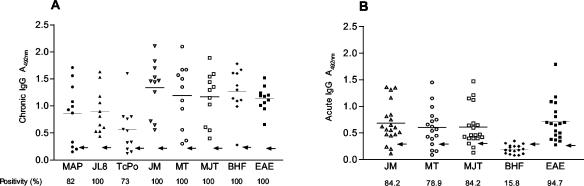
Several studies have shown that the use of a single antigen in an assay does not confer the required sensitivity (4, 7, 16, 18, 21, 29, 31). In this study, the reactivities of recombinant antigens, used singly or in different combinations, were compared with the reactivities of the following tests: (i) CSC tests (IIF, IHA, epi-ELISA [ELISA that uses semipurified antigens from the epimastigote form of T. cruzi], and EAE-ELISA [in-house ELISA that uses the epimastigote form of T. cruzi]) (31); (ii) a test using a mixture of recombinant proteins (BHF) developed for diagnosis of chronic cases (31); and (iii) a Western blot assay that uses antigens from T. cruzi trypomastigotes (TESA blot assay) (28, 31). The diagnostic performance of ELISA with JL8, MAP, and TcPo antigens used singly or in various combinations of two or three antigens was evaluated first, using a panel of serum samples from 11 Brazilian patients with the chronic phase of Chagas' disease that were positive by CSC tests. The optimal concentration of each component was determined by cross-titration: the optimal serum and conjugate dilutions were determined to be 1:50 and 1:6,000, respectively. Microtiter plates (high binding; Costar) were coated with 50 μl of antigen/well. The antigens used follow: antigens JL8 (1,000 ng ml−1), MAP (200 ng ml−1), and TcPo (200 ng ml−1) alone; mixtures of two antigens, such as JL8 and MAP (JM) (250 ng ml−1), MAP and TcPo (MT), and JL8 and TcPo (JT) (300 ng ml−1); or all three antigens together, namely, MAP, JL8, and TcPo (MJT) (350 ng ml−1). Titration of antigen binding to microtiter plates was performed by recombinant proteins labeled with iodine (125I), as previously described (29). Higher average absorbance (A492) (mean ± standard deviation [SD]) and sensitivity values were obtained with JL8 (0.89 ± 0.41 and 100%, respectively), followed by MAP (0.85 ± 0.56 and 82%, respectively) and TcPo (0.56 ± 0.42 and 73%, respectively) (Fig. 1A). Using different combinations of antigens in ELISAs resulted in a sensitivity of 100%, with higher reactivities than those of the single recombinant antigens. The reactivities of the antigen combinations were 1.34 ± 0.50 for JM, 1.19 ± 0.59 for MT, and 1.17 ± 0.48 for MJT. BHF and EAE-ELISA had a sensitivity of 100%, with averages of 1.26 ± 0.42 and 1.13 ± 0.22, respectively (Fig. 1A). The JT combination had a low sensitivity (not shown), so its use was discontinued.
FIG. 1.
(A) Reactivity data of T. cruzi recombinant antigens MAP, JL8, and TcPo individually or in various combinations of two or three proteins (JM, MT, and MJT) with sera from 11 Brazilian patients with well-defined chronic-phase Chagas' disease. (B) Reactivity data of recombinant mixtures JM, MT, MJT, and BHF with 19 acute-phase sera. The sensitivities of the different antigens or tests are shown at the bottom of the figure. EAE-ELISA data are shown in panels A and B. For each antigen, the average is indicated by the short horizontal line, and the arrow indicates the cutoff value.
Some mixtures of recombinant proteins also detect anti-T. cruzi IgG antibodies from acute-phase patients.
The capacities of mixtures of recombinant antigens (JM, MT, and MJT) to detect acute-phase antibodies were tested. JM and MJT were able to detect 84.2% and MT was able to detect 78.9% of acute cases (9 samples from Panama and 10 from Brazil) (Table 1 and Fig. 1B). JL8 and MAP antigens are made up of 14- and 38-amino-acid repeats, respectively, that are strongly conserved in strains and isolates of T. cruzi (11, 15), which improved the sensitivity of diagnosis of acutely infected individuals. These results were quite similar to those described for recombinant SAPA (shed acute-phase antigen) that has been employed in the diagnosis of the acute phase of Chagas' disease (2, 7, 16, 20); its sensitivity varied from 80.8 to 90% (2, 16). The specificities for acute-phase sera were 15.8% by BHF, 94.7% by EAE-ELISA (Table 2 and Fig. 1B), and 100% by TESA blotting (IgG and IgM [not shown]).
TABLE 2.
Sensitivity of ELISA with mixtures of recombinant proteins JM, MT, and MJT compared with those of BHF and EAE-ELISA
| Individual | No. of individuals | Sensitivity (%)
|
||||
|---|---|---|---|---|---|---|
| ELISA with:
|
BHFa | EAEb | ||||
| JM | MT | MJT | ||||
| Chagasic | 180 | |||||
| Acute | 19 | 84.2 | 78.9 | 84.2 | 15.8 | 94.7 |
| Chronic | 11 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Chronic | 150c | 99.4 | NDd | ND | ND | 100.0 |
| Nonchagasic | 142 | |||||
| Healthy | 80 | 0 | 0 | 0 | 0 | 0 |
| Other diseasese | 43 | 2.3 | 7.0 | 2.3 | 2.3 | 0 |
| T. rangeli | 9 | 0 | 0 | 0 | 0 | 0 |
| Leishmaniasis | 10 | 0 | 20.0 | 10.0 | 0 | 60.0 |
| Specificityf | 142 | 99.3 | 96.5 | 98.6 | 99.3 | 95.8 |
Mixture of recombinant antigens (BHF) developed for diagnosis of chronic patients (31).
EAE-ELISA, an in-house test with the epimastigote form of T. cruzi (31).
Sera positive in three CSC tests (IIF, IHA, and epi-ELISA).
ND, not determined.
Five individuals had toxoplasmosis, four had malaria, five had paracoccidioidomycosis, five had schistosomiasis, 19 had connective tissue disorders positive for antinuclear antibodies, and 5 had rheumatic fever.
Specificity data obtained with 142 samples from nonchagasic individuals.
Mixtures of recombinant proteins are more specific than whole-epimastigote antigens.
The specificities of recombinant mixtures were determined with sera from 142 nonchagasic individuals (62 of these individuals had other diseases) (Table 2). The specificities of JM, MT, and MJT recombinant proteins were 99.3, 96.5, and 98.6%, respectively (Table 2). BHF and TESA blotting presented specificities of 99.3 (Table 2) and 100% (not shown), respectively. The sensitivity of EAE was 95.8%, since 6 of 10 sera from patients with leishmaniasis showed a cross-reaction (Table 2). The cross-reactivity of sera from individuals infected with Trypanosoma rangeli in CSC tests has been the subject of controversy (32), but our samples from T. rangeli-infected individuals were not reactive in assays that use T. cruzi recombinant proteins or in CSC tests.
Evaluation of the JM mixture to diagnose T. cruzi-infected individuals from different geographical areas.
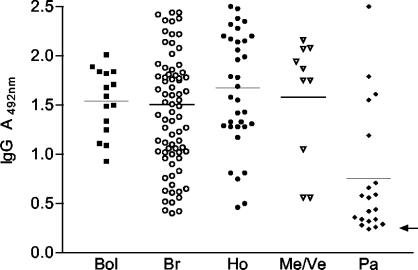
The JM mixture gave a sensitivity of 99.3% (Table 2 and Fig. 2) with sera from chagasic individuals (n = 150) (Table 1) that showed a sensitivity of 100% in three CSC tests (not shown) and with EAE-ELISA (Table 2). The average absorbance (± SD) or reactivity obtained with JM was high for chagasic patients from Bolivia (1.54 ± 0.33), Brazil (1.50 ± 0.63), Honduras (1.67 ± 0.59), and Mexico/Venezuela (1.58 ± 0.62) but low for Panama (0.75 ± 0.64) (Fig. 2). The sensitivity presented by JM (99.4%) was similar to those described with mixtures of recombinant antigens and/or synthetic peptides (4, 10, 20, 21, 31). Notwithstanding the fact that several reports have reported greater diagnostic ability of recombinant protein mixtures, there are always a few chagasic patients in whom anti-T. cruzi antibodies were not detected or the reactivity was near the cutoff value (10, 20, 21, 24, 31). This could be due to the heterogeneity of different parasite strains and/or variability in individual host immune responses. In conclusion, our results show that recombinant proteins in mixtures, such as JM, are very useful as antigens for immunodiagnosis of acute and chronic Chagas' disease and that the specificity was superior to that of CSC tests that employ whole or semipurified fractions from epimastigote forms of T. cruzi.
FIG. 2.
Reactivity data of recombinant antigens JM with 150 serum samples from patients infected with T. cruzi from regions of Bolivia (Bol), Brazil (Br), Honduras (Ho), Mexico and Venezuela (Me/Ve), and Panama (Pa) where Chagas' disease is endemic. For each country, the average is indicated by the short horizontal line, and the arrow indicates the cutoff value.
Acknowledgments
This work was supported in part by grants from the International Atomic Energy Agency CRP E1.50.17 (Argentina, grant 10667 [M.J.L.]; Bolivia, grant 10668 [S.R.]; grants BRA-10670 [E.S.U.], 10669 [J.F.D.S.], and 11060 [A.O.L.]; Honduras, grant 10673 [E.P. and C.P.]; México, grant 10673 [B.E.]; Venezuela, grant 106775 [D.H.]; Panama, grant 10674 [O.S.]), CNPq, FAPESP, LIM49-FMUSP (Brazil), and CONICIT (Argentina). T. Bellido was supported by a scholarship from the International Atomic Energy Agency.
We thank T. Bellido, R. A. Oliveira, and S. B. N. Tavares (Goiás, Brazil) for technical cooperation.
REFERENCES
- 1.Almeida, I. C., D. T. Covas, L. M. T. Soussumi, and L. R. Travassos. 1997. A highly sensitive chemiluminescence enzyme-linked immunosorbent assay for diagnosis of active Trypanosoma cruzi infection. Transfusion 37:850-857. [DOI] [PubMed] [Google Scholar]
- 2.Breniere, S. F., N. Yaksic, N. Telleria, M. F. Bosseno, F. Noireau, P. Wincker, and D. Sanchez. 1997. Immune response to Trypanosoma cruzi shed acute phase antigen in children from an endemic area for Chagas' disease in Bolívia. Mem. Inst. Oswaldo Cruz 92:503-507. [DOI] [PubMed] [Google Scholar]
- 3.Camargo, M. E., and V. Amato-Neto. 1974. Anti-Trypanosoma cruzi IgM antibodies as serological evidence of recent infection. Rev. Inst. Med. Trop. São Paulo 16:200-202. [PubMed] [Google Scholar]
- 4.Carvalho, M. R., M. A. Krieger, W. Oeleman, M. A. Shikanai-Yasuda, A. W. Ferreira, J. B. Pereira, A. Saez-Alquezar, D. F. Dorlhiac-Llacer, D. F. Chamone, and S. Goldenberg. 1993. Chagas' disease diagnosis: evaluation of several tests in blood bank screening. Transfusion 33:830-834. [DOI] [PubMed] [Google Scholar]
- 5.Feliciangeli, M. D., D. Campbell-Lendrum, C. Martinez, D. Gonzalez, P. Coleman, and C. Davies. 2003. Chagas' disease control in Venezuela: lessons for the Andean region and beyond. Trends Parasitol. 19:44-49. [DOI] [PubMed] [Google Scholar]
- 6.Ferreira, A. W., Z. R. Belem, E. A. Lemos, S. G. Reed, and A. Campos-Neto. 2001. Enzyme-linked immunosorbent assay for serological diagnosis of Chagas' disease employing a Trypanosoma cruzi recombinant antigen that consists of four different peptides. J. Clin. Microbiol. 39:4390-4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franco da Silveira, J., E. S. Umezawa, and A. O. Luquetti. 2001. Chagas' disease: recombinant Trypanosoma cruzi antigens for serological diagnosis. Trends Parasitol. 17:286-291. [DOI] [PubMed] [Google Scholar]
- 8.Frank, M., B. Hegenscheid, K. Janitschke, and T. Weinke. 1997. Prevalence and epidemiological significance of Trypanosoma cruzi infection among Latin American immigrants in Berlin, Germany. Infection 25:355-358. [DOI] [PubMed] [Google Scholar]
- 9.Galel, S. A., and L. V. Kirchoff. 1996. Risk factors for Trypanosoma cruzi infection in California blood donors. Transfusion 36:227-231. [DOI] [PubMed] [Google Scholar]
- 10.Houghton, R. L., D. R. Benson, L. D. Reynolds, P. D. McNeill, P. R. Sleath, M. J. Lodes, D. A. Leiby, R. Badaro, and S. G. Reed. 1999. A multi-epitope synthetic peptide and recombinant protein for the detection of antibodies to Trypanosoma cruzi in radioimmunoprecipitation-confirmed and consensus-positive sera. J. Infect. Dis. 179:1226-1234. [DOI] [PubMed] [Google Scholar]
- 11.Kerner, N., P. Ligeard, M. J. Levin, and M. Hontebeyrie-Joskowicz. 1991. Trypanosoma cruzi: antibodies to a MAP-like protein in chronic Chagas' disease cross-react with mammalian cytoskeleton. Exp. Parasitol. 73:451-459. [DOI] [PubMed] [Google Scholar]
- 12.Kirchoff, L. V. 1993. American trypanosomiasis (Chagas' disease)—a tropical disease now in the United States. N. Engl. J. Med. 329:639-644. [DOI] [PubMed] [Google Scholar]
- 13.Krieger, M. A., E. Almeida, W. Oelemann, J. J. Lafaille, J. B. Pereira, H. Krieger, M. R. Carvalho, and S. Goldenberg. 1992. Use of recombinant antigens for the accurate immunodiagnosis of Chagas' disease. Am. J. Trop. Med. Hyg. 46:427-434. [DOI] [PubMed] [Google Scholar]
- 14.Leiby, D. A., R. M. Herron, E. J. Read, B. A. Lenes, and R. J. Stumpf. 2002. Trypanosoma cruzi in Los Angeles and Miami blood donors: impact of evolving donor demographics on seroprevalence and implications for transfusion transmission. Transfusion 42:549-555. [DOI] [PubMed] [Google Scholar]
- 15.Levin, M. J., E. A. Mesti, R. Benarous, G. Levitus, A. Schijman, P. Levy-Yeyati, A. Ruiz, A. Kahan, M. B. Rosenbaum, H. N. Torres, and E. L. Segura. 1989. Identification of major Trypanosoma cruzi antigenic determinants in chronic Chagas' heart disease. Am. J. Trop. Med. Hyg. 41:530-539. [DOI] [PubMed] [Google Scholar]
- 16.Levin, M. J., J. Franco da Silveira, A. C. C. Frasch, M. E. Camargo, S. Lafon, W. M. Degrave, and R. Rangel-Aldao. 1991. Recombinant antigens and Chagas' disease diagnosis: analysis of a workshop. FEMS Microbiol. Immunol. 89:11-20. [DOI] [PubMed] [Google Scholar]
- 17.Lopez Bergami, P., J. Scaglione, and M. J. Levin. 2001. Antibodies against the C-terminal end of Trypanosoma cruzi ribosomal P proteins are pathogenic. FASEB J. 15:2602-2612. [DOI] [PubMed] [Google Scholar]
- 18.Moncayo, A., and A. O. Luquetti. 1990. Multicentre double blind study for evaluation of Trypanosoma cruzi defined antigens as diagnostic reagents. Mem. Inst. Oswaldo Cruz 85:489-495. [DOI] [PubMed] [Google Scholar]
- 19.Oelemann, W. M. R., M. G. M. Teixeira, G. C. Verissimo-da-Costa, J. Borges-Pereira, J. A. De Castro, J. R. Coura, and J. M. Peralta. 1998. Evaluation of three commercial enzyme-linked immunosorbent assays for diagnosis of Chagas' disease. J. Clin. Microbiol. 36:2423-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pastini, A. C., S. R. Iglesias, V. C. Carricarte, M. E. Guerin, D. O. Sanchez, and A. C. Frasch. 1994. Immunoassay with recombinant Trypanosoma cruzi antigens potentially useful for screening donated blood and diagnosing Chagas' disease. Clin. Chem. 40:1893-1897. [PubMed] [Google Scholar]
- 21.Peralta, J. M., M. G. M. Teixeira, W. G. Shreffler, J. B. Pereira, J. M. Burns, P. R. Sleath, and S. G. Reed. 1994. Serodiagnosis of Chagas' disease by enzyme-linked immunosorbent assay using two synthetic peptides as antigens. J. Clin. Microbiol. 32:971-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prata, A. 2001. Clinical and epidemiological aspects of Chagas' disease. Lancet Infect. Dis. 1:92-100. [DOI] [PubMed] [Google Scholar]
- 23.Saez-Alquézar, A., M. M. Otani, E. C. Sabino, G. Ribeiro-dos-Santos, N. Salles, and D. F. Chamone. 1998. Evaluation of the performance of Brazilian blood banks in testing for Chagas' disease. Vox Sang. 74:228-231. [PubMed] [Google Scholar]
- 24.Saez-Alquézar, A., E. C. Sabino, N. Salles, D. Chamone, F. Hulstaert, H. Pottel, E. Stoops, and M. Zrein. 2000. Serological confirmation of Chagas' disease by a recombinant and peptide antigen line immunoassay: INNO-LIA Chagas. J. Clin. Microbiol. 38:851-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salles, N. A., E. C. Sabino, M. G. Cliquet, J. Eluf-Neto, A. Mayer, C. Almeida-Neto, M. C. Mendonça, P. Dorliach-Llacer, D. F. Chamone, and A. Saez-Alquézar. 1996. Risk of exposure to Chagas' disease among seroreactive Brazilian blood donors. Transfusion 36:969-973. [DOI] [PubMed] [Google Scholar]
- 26.Schmunis, G. A. 1999. Prevention of transfusional T. cruzi infection in Latin America. Mem. Inst. Oswaldo Cruz 94:93-101. [DOI] [PubMed] [Google Scholar]
- 27.Shulman, I. A. 1999. Intervention strategies to reduce the risk of transfusion-transmitted Trypanosoma cruzi infection in the United States. Transfus. Med. Rev. 13:227-234. [DOI] [PubMed] [Google Scholar]
- 28.Umezawa, E. S., M. S. Nascimento, N. Kesper, Jr., J. R. Coura, J. Borges-Pereira, A. C. V. Junqueira, and M. E. Camargo. 1996. Immunoblot assay using excreted-secreted antigens of Trypanosoma cruzi in serodiagnosis of congenital, acute, and chronic Chagas' disease. J. Clin. Microbiol. 34:2143-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Umezawa, E. S., S. F. Bastos, M. E. Camargo, L. M. Yamauchi, M. R. Santos, A. Gonzalez, B. Zingales, M. J. Levin, O. Souza, R. Rangel-Aldao, and J. Franco da Silveira. 1999. Evaluation of recombinant antigens for Chagas' disease serodiagnosis in South and Central America. J. Clin. Microbiol. 37:1554-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Umezawa, E. S., M. S. Nascimento, and A. M. Stolf. 2001. Enzyme-linked immmunosorbent assay with T. cruzi excreted-secreted antigens (TESA-ELISA) for serodiagnosis of acute and chronic Chagas' disease. Diagn. Microbiol. Infect. Dis. 39:169-176. [DOI] [PubMed] [Google Scholar]
- 31.Umezawa, E. S., S. F. Bastos, J. R. Coura, M. J. Levin, A. Gonzalez, R. Rangel-Aldao, B. Zingales, A. O. Luquetti, and J. Franco da Silveira. 2003. An improved serodiagnostic test for Chagas' disease employing a mixture of Trypanosoma cruzi recombinant antigens. Transfusion 43:91-97. [DOI] [PubMed] [Google Scholar]
- 32.Vasquez, J. E., J. Krusnell, A. Orn, O. E. Souza, and R. A. Harris. 1997. Serological diagnosis of Trypanosoma rangeli infected patients. A comparison of different methods and its implications for the diagnosis of Chagas' disease. Scand. J. Immunol. 45:322-330. [DOI] [PubMed] [Google Scholar]