Abstract
Introduction
There is a pressing need for effective measures to prevent the spread of cholera. Our systematic review assesses the effects of chemoprophylaxis in preventing cholera among exposed contacts.
Methods and Findings
We considered published and unpublished reports of studies up to July 2011. For this we searched: PubMed (1966 to July, 2011), Embase (1980 to July 2011), Cochrane Central Register of Controlled Trials (6; 2011), LILACS (1982 to July, 2011), the International Clinical Trials Registry Platform (July 2011) and references of identified publications. We included controlled clinical trials (randomized and non-randomized) in which chemoprophylaxis was used to prevent cholera among patient contacts. The main outcome measures were hospitalization and laboratory diagnosis of cholera in contacts for cholera patients. We assessed the risk of bias. We identified 2638 references and these included 2 randomized trials and 5 controlled trials that added up to a total of 4,154 participants. The risk of bias scored high for most trials. The combined results from two trials found that chemoprophylaxis reduced hospitalization of contacts during the follow-up period by 8–12 days (2826 participants; RR 0.54 95% CI 0.40–0.74;I2 0%). A meta-analysis of five trials found a significant reduction in disease among contacts with at least one positive sample who received chemoprophylaxis during the overall follow-up (range 4–15 days) (1,414 participants; RR 0.35 95% CI 0.18–0.66;I2 74%). A significant reduction in the number of positive samples was also found with chemoprophylaxis (3 CCT; 6,918 samples; RR 0.39 95% CI 0.29–0.51;I2 0%).
Conclusion
Our findings suggest that chemoprophylaxis has a protective effect among household contacts of people with cholera but the results are based on studies with a high risk of bias. Hence, there is a need for adequate reliable research that allows balancing benefits and harms by evaluating the effects of chemoprophylaxis.
Introduction
As we advance into the 21st century, an estimated one billion people remain without adequate access to safe water and sanitation and vulnerable to cholera epidemics [1]. It is estimated that there are 3–5 million cholera cases every year, leading to 100,000–130,000 deaths, mostly in Africa and Asia, and affecting both children and adults [2], [3]. The growing number of people affected involving major cholera outbreaks are cause for concern at the World Health Organization (WHO); WHO reported a 24% an increase in reported cases for the 2004 to 2008 period compared to the 2000 to 2004 period [4]. Most affected countries report an overall cholera case-fatality rate (CFR) under 5%, but in some locations the CFR approaches 50% during outbreaks, affecting highly vulnerable groups [3].
Currently (2011) the world is facing the so called “seventh cholera pandemic” that began in Indonesia in 1961 and is caused by an El Tor biotype of Vibrio cholera serogroup O1. An outbreak has happened in Haiti and it is of particular concern because of the devastation associated to the earthquake on 12 January 2010 that dramatically increased the vulnerability to the spread of cholera [5]. The strains of Vibrio cholera found in Haiti belong to a category known as hybrids which produce the classical type of cholera toxin and are a variant of the El Tor biotype [6]. According to the Ministère de la Santé Publique et de la Population (MSPP) of Haiti, from mid October 2010 to the third week of June 2011, a total of 363,117 cholera cases were reported in the country, of which 55% (191,508) were hospitalized and 5,506 died; the overall case fatality rate was 1.5% [7].
There is a pressing need for effective measures to prevent the spread of cholera. Although there are effective and efficient preventive measures, consisting of providing adequate access to safe water and sanitation, health education and proper food hygiene, in many settings such basic measures are difficult to implement.
The Strategic Advisory Group of Experts (SAGE) on immunization recommended the use of immunization with cholera vaccines in conjunction with other prevention and control strategies, in areas where the disease is endemic [8]. Chemoprophylaxis refers to the administration of medication to prevent disease or infection. In the case of cholera, healthy individuals are given antibiotics with the aim of protecting them against the disease, limiting the spread of the disease and curtailing an epidemic. Multiple infections in the same household are common due to shared sources of contaminated water and food. WHO does not recommend chemopophylaxis arguing that “routine treatment of a community with antibiotics, or mass chemoprophylaxis, has no effect on the spread of cholera, can have adverse effects by increasing antimicrobial resistance and provides a false sense of security” [9]. In addition, chemoprophylaxis with antibiotics is also limited by access, costs, and contraindications [9], [10].
Nevertheless, large-scale selective antibiotic prophylaxis has been provided to the contacts of people with cholera during outbreaks, as part of comprehensive community interventions [10]. Some experts argue that a well-targeted antibiotic prophylaxis can reduce direct human transmission of cholera [11], but it is difficult to evaluate the role of chemoprophylaxis in limiting cholera epidemics.
We conducted this systematic review of the findings of randomized clinical trials (RCT) and controlled clinical trials (CCT) to assess the effects of chemoprophylaxis and its effectiveness in preventing cholera in patient contacts.
Materials and Methods
We conducted structured searches in PubMed (1966 to July 31, 2011), Cochrane Central Register of Controlled Trials (Central) (6; 2011), LILACS (1982 to July 31, 2011), Embase (1980 to July 31, 2011), and Scirus (July 31, 2011) (Annex 1). We searched the International Clinical Trials Registry Platform search portal of WHO (ICTRP) to identify past and ongoing trials using the key words “cholera” or “cholerae”. References of identified publications were screened and relevant websites were searched (document S1). We reached out to authors and relevant key stakeholders to identify unpublished studies and related additional data from manuscripts.
Inclusion criteria and outcomes
We included randomized clinical trials (RCT) and controlled clinical trials (CCT) which assessed the effectiveness of antibiotics in preventing cholera among patients' contacts. We only considered RCTs/CCTs using chemoprophylaxis for contacts (children and/or adults) compared with no intervention, placebo, or other chemoprophylaxis regimens. Studies in which the intervention group included vaccines were excluded.
Clinical and bacteriological diagnoses of cholera (secondary cases) were the main outcomes considered in the review regardless of the microbiological method used to diagnose cholera. We included those adverse events reported in CCT/RCTs and did not search for additional adverse event studies or records. Due to variations in outcome reporting, we collected data which provided the number of subjects having at least one positive sample, the number of total subjects as well as the total number of positive samples, and the total number of samples obtained during follow-up. In addition, we collected data from trials that included or excluded contacts with positive samples on the first day of treatment. Findings are presented according to categories that were pre-specified by the trial.
Study selection
Two independent reviewers assessed separately the titles, abstracts, and studies identified in the literature search (LR, EC). All trials matching the inclusion criteria were screened by two reviewers. Disagreements between reviewers were to be resolved by consensus or eventually majority by involving a third author; eventually the third author option was not required.
Data extraction
At least two reviewers (LR, EC, TPK, PRP) independently extracted the relevant data using a predesigned data extraction form; disagreements between reviewers were resolved by referring to a third author. Key variables that were collected included identification data of the paper, treatment characteristics (type of intervention, dose, and duration), demographic variables (age, gender), number of participants, patients lost to follow up, proportion of patients with laboratory diagnoses of cholera (secondary cases), the methodology of the diagnosis, and all reported adverse events. Included studies were used to analyze the type and frequency of adverse effects, we only used included studies.
We performed an evaluation on the risk of bias for each trial following the Cochrane Collaboration tool for the assessment of these variables [12]. We also assessed inclusion and exclusion criteria; sample size calculation; and baseline comparability of age, gender, relevant clinical characteristics, and diagnoses. We registered data in the studies table (Table S1).
We obtained outcomes data from published articles and compared it using Review Manager Version 5.0 (RevMan 5). We contacted authors to obtain additional information about their studies.
Statistical analysis
We used the RevMan 5 software from the Cochrane Collaboration to assist the statistical analysis. For dichotomous primary outcomes the results, expressed as relative risk (RR) and 95% confidence intervals (CI), were calculated using the Mantel–Haenszel random effects model. For the pooled analysis we calculated the I square (I 2) statistic that describes the percentage of total variation across studies attributed to heterogeneity [12]; low, moderate, and high levels of heterogeneity are roughly estimated as I 2 values of 25%, 50%, and 75%, respectively. Where necessary, multiple arms of a study were combined grouping the relevant interventions into a single group, and all relevant control interventions as a single control group [12]. The PRISMA check list is provided as suppoting information (Document S2).
Results
Characteristics of studies
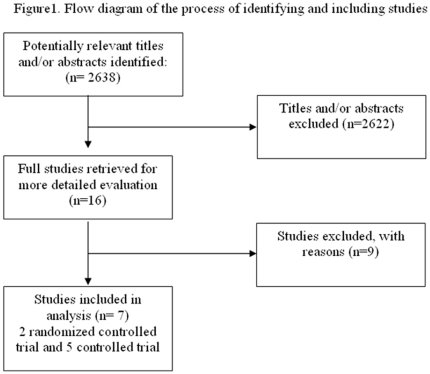
We identified 2638 references of interest (Figure 1) through the literature search and deemed relevant 16 studies of chemoprophylaxis amongst contacts of individuals diagnosed with cholera. We included and analyzed 7 trials: two randomized controlled trials (RCTs) and five controlled trials (CCTs), with 4.154 participants altogether (2606 of them received chemoprophylaxis) (Table S1) [13]–[19]. Nine references did not comply with eligibility criteria and were excluded (Table S2) [10], [20]–[27].
Figure 1. Flow diagram of study selection process.
Three CCTs were conducted in India [13], [15], [19]; the remaining four CCTs were in Bangladesh [16], Pakistan [18], Ivory Coast [17] and Peru [14]. One CCT allocated families, rather than individuals, to prophylaxis [18]. One of the seven trials was published in the 1960s [18], four in the 1970s [13], [15], [17], [19], one in the 1980s [16], and one in the 1990s [14]. The follow-up time (range 8 to 15 days), as well as the number of stool samples collected (range 0 to 15), varied among studies. A number of studies included contacts having positive bacteriological cholera on the first day, while others excluded such contacts. Outcomes were also reported in various formats across the studies; few trials reported clinical outcomes (Table S1).
Assessment of risk of bias
Overall the quality of the reporting and design of the RCTs was poor (Table S3). Two studies were randomized controlled trials [14], [17] and five were controlled clinical trials [13], [15], [16], [18], [19]. We found one RCT with unclear risk of bias [14] and the remaining six trials were scored as having a high risk of bias mainly because of inadequate methods to generate the sequence of randomization (alternation), lack of allocation concealment, and/or incomplete outcome data. One trial [16] included a “no treatment” arm with most patients receiving vitamins instead of an inert placebo [13], [15], [17]–[19]. One study [17] reported data only for those contacts that developed cholera. The majority of trials did not provide a sample size framework and a scientific rationale for the sample size determination.
Effects of Interventions
Overall chemoprophylaxis
The seven trials assessed the effects of different medications (tetracycline, doxycycline, ciprofloxacine, sulfadoxine) vs. vitamins (used as placebo) or no treatment. They also determined effectiveness based on different outcomes; data for each individual outcome were not available in all trials (Table S4). Only one RCT used an adequate placebo [14].
Clinical outcomes
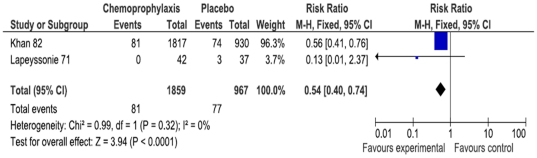
Few trials reported data on clinical outcomes, including adverse effects (Table S4). No reports of mortality were found in any trial. When we pooled two trials [16], [17], chemoprophylaxis (which included tetracycline and sulfadoxine) was significantly more effective in preventing hospitalization of contacts during the follow-up (2826 participants; RR 0.55 95% CI 0.41 to 0.75; I2 0%; Figure 2). However, both RCTs did not provide sufficient information to determine if the authors reported all cause hospitalizations or only cholera related hospitalizations.
Figure 2. Prevention of hospitalization of contacts of cholera patients during the follow-up.
Bacteriological outcomes
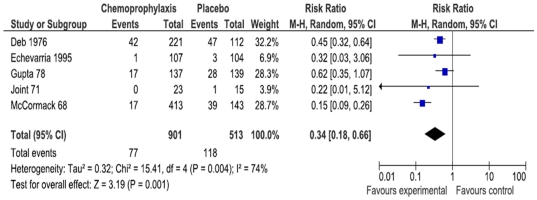
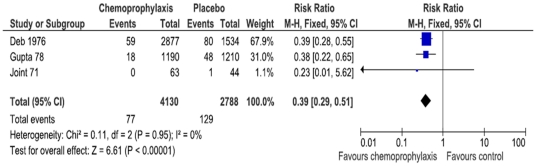
Meta-analysis of five trials [13]–[15], [18], [19] found a significant reduction in the rate of at least one positive sample during the overall follow-up amongst contacts receiving chemoprophylaxis compared with the control group (1414 participants; RR 0.34 95% CI 0.18 to 0.66; Figure 3); there was significant heterogeneity among the 5 studies (I2 = 74%, P<0.01). Chemoprophylaxis was also found to reduce the total number of positive samples (one patient could have multiple positive samples on different days) (3 trials; 6918 samples; RR 0.39 95% CI 0.29 to 0.51;I2 0%; Figure 4) [13], [15], [19]. When positive samples of contacts from day one during the reported follow-up period were included, we found that chemoprophylaxis reduced the rate of contacts presenting at least one positive sample (2 trials, 832 participants; RR 0.40 95% CI 0.14 to 1.11;I2 82%) [18], [19] and the overall rate of positive samples (4 trials, 8.885 samples; RR 0.34 (0.20 to 0.59;I2 64%) [13]–[15], [19]. Meta-analysis of trials that reported data on 4, 7, and 15 days of follow-up are shown in Table S4. When considering only trials assessing the effects of tetracycline and doxycycline, the combination of tetracycline and doxycycline prevented contacts from having positive samples, as compared with placebo, and reduced the proportion of at least one positive sample amongst contacts (Table S4).
Figure 3. Proportion of patients with at least one positive cholera sample during the overall follow-up.
Figure 4. Proportion of total positive cholera samples/total number of samples during the overall follow-up.
Vibrio cholera susceptibility
One RCT [14] reported that V. cholera 01 strains isolated from index cases and household contacts had in vitro susceptibility to ciprofloxacin (MIC, 0.007 µg/mL). One CCT [13] concluded that all the strains of V. cholera isolated during the study were biotype El Tor, with no indications of emergence of resistant strains when sulfadoxine was used as a single dose.
Adverse events
Few trials reported adverse events [13], [14], [19] and none assessed systematically the specific adverse effects observed. Reports noted that adverse events were mild, but criteria and/or judgment with regards to categorization of the severity were not explicit. In combining the data of two trials [13], [14] that assessed utcomes after two weeks of treatment, we found no significant difference in the incidence of diarrhea; one CCT [19] reported that 7.3% of contacts that received doxycycline complained of nausea, and 2.9% suffered from mild vomiting.
Discussion
Main Findings
This systematic review suggests that hospitalization of contacts of cholera patients can be reduced by 26% to 60% with the prophylactic administration of antimicrobial agents (the hospitalization rate was 4.4% versus 8.0% in the control group). The studies available for analysis are a highly heterogeneous group that assessed different interventions and different outcomes with different methods and at different follow-up times, with a variety of sampling methods and outcome reporting. Most of the studies focused on microbiological outcomes, documenting the excretion of V. cholera after chemoprophylaxis [13]–[15], [18], [19], but there is limited information about impact. Household contacts were followed for short periods of time. It is uncertain whether those who received chemoprophylaxis ultimately had the same rates of cholera and hospitalization compared to those in the control group. Other limitations of this review mainly concern the methodological quality and poor reporting of most of the included studies. Because most studies were conducted during the 1960's and 1970's, it is likely that the epidemiological conditions (e.g.resistance, dissemination patterns) have changed. The external and internal validity of included studies is likely compromised by factors that increase bias and imprecision, with a failure to address clinically important outcomes and adequate assessment of the balance between benefits and harms for participating individuals or populations. Most clinical trials included in the review were performed at a time when antibiotic resistance to cholera was rare, although a number of studies worldwide suggest an increase in resistance of V. cholera to several antibiotics worldwide [28]–[41]. Some of those studies strongly suggest that mass chemoprophylaxis, and nonselective chemoprophylaxis of adult family members, can contribute to the emergence of resistance [38]–[40]. In addition, attempts to provide chemoprophylaxis to household contacts require resources and time, and may have associated risks and result in unexpected harms. It is difficult with the available data to draw conclusions that could be adequately used to inform policy and practice.
The containment of cholera needs adequate scientific basis. This review highlights knowledge gaps and provides recommendations for future studies to address the impact of chemoprophylaxis in crowded or enclosed settings (e.g. prisons, camps, nurseries, orphanages, nursing homes, and ships). Future studies should evaluate the effectiveness of relevant existing and new single dose therapeutic options (e.g. azythromycin). The effectiveness of chemoprophylaxis in reducing hospitalization rates should be confirmed in well designed and conducted RCTs of adequate sample size. Longer follow-up periods than in the past studies are needed to determine the effectiveness of antibiotics. It seems likely that only large cluster randomized trials, using an appropriate placebo can provide the data required to make informed decisions regarding the use of prophylactic antibiotics in cholera outbreaks. RCTs should also conform to the guidelines from the Consolidated Standards of Reporting Trials (CONSORT) statement [42].
Supporting Information
Characteristics of studies included in the review.
(DOC)
Studies excluded from the review.
(DOC)
Assessment of bias risk of randomized & controlled clinical trials.
(DOC)
Main outcomes of clinical trials.
(DOC)
Search strategies.
(DOC)
Prisma check list.
(DOC)
Acknowledgments
The authors wish to thank Adam McCutcheon and the Department of Health Administration & Policy at George Mason University and the Joint Campbell & Cochrane Equity Methods Group for their help in obtaining complete papers; and Professor David Sack for his useful commentaries for the discussion section.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The authors have no funding or support to declare.
References
- 1.United Nations. The Millennium Development Goals Report. New York. 2008. Available: http://mdgs.un.org/unsd/mdg/Resources/Static/Products/Progress2008/MDG_Report_2008_En.pdf. Accessed 2010 December 9.
- 2.World Health Organization. 2010. Available: http://www.who.int/immunization/newsroom/news_revised_position_paper_cholera_vaccines/en/index.html Accessed: 2010 December 9.
- 3.Zuckerman JN, Rombo L, Fisch A. The true burden and risk of cholera: implications for prevention and control. Lancet Infectious Diseases. 2007;7(8):521–30. doi: 10.1016/S1473-3099(07)70138-X. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Weekly epidemiological record. 31July 2009, 84th year. No. 31, 2009, 84, 309–24. 2009. Available: http://www.who.int/wer/2009/wer8431.pdf Accessed 2010 November 29.
- 5.Pan American Health Organization. PAHO responds to cholera outbreak in Haiti. 2010. Available: http://new.paho.org/disasters/index.php?option=com_content&task=view&id=1423&Itemid=1 Accessed 2010 November 29.
- 6.CDC update cholera outbreak – Haiti. MMWR Morb Mortal Weekly Report. 2010;59:1473–79. [PubMed] [Google Scholar]
- 7.Préliminaire data Ministère de la Sante Publique et de la Population de Haïti. 2011. Available: http://www.mspp.gouv.ht/site/downloads/Rapport%20journalier%20MSPP%20du%2002%20fevrier%202011.pdf Accessed 2011 February 14.
- 8.World Health Organization. Cholera vaccines: WHO position paper. Wkly Epidemiol Rec. 2010;85:117–128. Available: http://www.who.int/wer/2010/wer8513.pdf Accessed 2010 December 9. [PubMed] [Google Scholar]
- 9.Global Task Force on Cholera Control. Prevention and control of cholera outbreaks: WHO policy and recommendations. Available: http://www.who.int/cholera/technical/prevention/control/en/index2.html. Accessed 2010 December 9.
- 10.Guévart E, Noeske J, Sollé J, Mouangue A, Bikoti JM. Large-scale selective antibiotic prophylaxis during the 2004 cholera outbreak in Douala (Cameroon). Sante. 2007;17(2):63–8. [PubMed] [Google Scholar]
- 11.Weil AA, Khan AI, Chowdhury F, Larocque RC, Faruque AS, et al. Clinical outcomes in household contacts of patients with cholera in Bangladesh. Clin Infect Dis. 2009;49(10):1473–9. doi: 10.1086/644779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. 2009. The Cochrane Collaboration. Accessed 2010 November 9.
- 13.Deb BC, Sen Gupta PG, De SP, Sil J, Sikdar SN, et al. Effect of sulfadoxine on transmission of Vibrio cholera infection among family contacts of cholera patients in Calcutta. Bull World Health Organ. 1976;54:171–175. [PMC free article] [PubMed] [Google Scholar]
- 14.Echevarría J, Seas C, Carrillo C, Mostorino R, Ruiz R, et al. Efficacy and tolerability of ciprofloxacin prophylaxis in adult household contacts of patients with cholera. Clin Infect Dis. 1995;20(6):1480–4. doi: 10.1093/clinids/20.6.1480. [DOI] [PubMed] [Google Scholar]
- 15.Joint ICMR-GWB-WHO Cholera Study Group. Serological studies on cholera patients and their household contacts in Calcutta in 1968. Joint ICMR-GWB-WHO Cholera Study Group, Calcutta, India. Bull World Health Organ. 1970;43(3):389–99. [PMC free article] [PubMed] [Google Scholar]
- 16.Khan MU. Efficacy of short course antibiotic prophylaxis in controlling cholera in contacts during epidemic. J Trop Med Hyg. 1982;85(1):27–9. [PubMed] [Google Scholar]
- 17.Lapeyssonnie L. Chemoprophylaxis of cholera: value, expectations, and limitations. Med Trop (Mars); 1971;31(1):127–32. [PubMed] [Google Scholar]
- 18.McCormack WM, Chowdhury AM, Jahangir N, Ahmed AB, Mosley WH. Tetracycline prophylaxis in families of cholera patients. Bull World Health Organ. 1968;38(5):787–92. [PMC free article] [PubMed] [Google Scholar]
- 19.Sen Gupta PG, Sircar BK, Mondal S, De SP, Sen D, et al. Effect of doxycycline on transmission of Vibrio cholerae infection among family contacts of cholera patients in Calcutta. Bull World Health Organ; 1978;56:323–326. [PMC free article] [PubMed] [Google Scholar]
- 20.Guévart E, Solle J, Noeske J, Amougou G, Mouangue A, et al. Mass antibiotic prophylaxis against cholera in the New Bell central prison in Douala during the 2004 epidemic. Sante. 2005;15(4):225–7. [PubMed] [Google Scholar]
- 21.Guévart E, Solle J, Mouangue A, Noeske J, Bita A, et al. Antibiotic susceptibility of Vibrio cholerae 01: evolution after prolonged curative and preventive use during the 2004 cholera epidemics in Douala (Cameroon). Med Mal Infect. 2006;36(6):329–34. doi: 10.1016/j.medmal.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 22.Gharagozloo RA, Naficy K. Comparative Trial of Tetracycline, Chloramphenicol, and Trimethoprim/Sulphamethoxazole in Eradication of Vibrio cholerae El Tor. British Medical Journal. 1970;4:281–282. doi: 10.1136/bmj.4.5730.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pierce NF, Banwell JG, Mitra RC, Caranasos GJ, Keimowitz RI, et al. Controlled Comparison of Tetracycline and Furazolidone in Cholera. British Medical Journal. 1968;3:277–280. doi: 10.1136/bmj.3.5613.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Usubütün S, Ağalar C, Diri C, Türkyilmaz R. Single dose ciprofloxacin in cholera. Eur J Emerg Med. 1997;4(3):145–9. [PubMed] [Google Scholar]
- 25.Islam MR. Single dose tetracycline in cholera. Gut. 1987;28:1029–1032. doi: 10.1136/gut.28.8.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sack D, Islam S, Rabbani H, Islam A. Single-Dose Doxycycline for Cholera. Antimicrobial Agents And Chemotherapy; 1978;14(3):462–464. doi: 10.1128/aac.14.3.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gangarosa EJ, Saghari H, Emile J, Sanati A, Siadat H, et al. Search for a mass chemotherapeutic drug for cholera control. Bull World Health Organization. 1966;35:669–674. [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention (CDC) Cholera outbreak — Haiti, October 2010. MMWR Morb Mortal Wkly Rep. 2010;59(43):1411. [PubMed] [Google Scholar]
- 29.Ranjbar M, Rahmani E, Nooriamiri A, Gholami H, Golmohamadi A, et al. High prevalence of multidrug-resistant strains of Vibrio cholerae, in a cholera outbreak in Tehran-Iran, during June–September 2008. Trop Doct. 2010;40(4):214–6. doi: 10.1258/td.2010.100015. [DOI] [PubMed] [Google Scholar]
- 30.Manga NM, Ndour CT, Diop SA, Dia NM, Ka-Sall R, et al. Cholera in Senegal from 2004 to 2006: lessons learned from successive outbreaks. Med Trop (Mars) 2008;68(6):589–92. [PubMed] [Google Scholar]
- 31.Karki R, Bhatta DR, Malla S, Dumre SP. Cholera incidence among patients with diarrhea visiting National Public Health Laboratory, Nepal. Jpn J Infect Dis. 2010;63(3):185–7. [PubMed] [Google Scholar]
- 32.Kingston JJ, Zachariah K, Tuteja U, Kumar S, Batra HV. Molecular characterization of Vibrio cholerae isolates from cholera outbreaks in North India. J Microbiol. 2009;47(1):110–5. doi: 10.1007/s12275-008-0162-7. [DOI] [PubMed] [Google Scholar]
- 33.Keramat F, Hashemi SH, Mamani M, Ranjbar M, Erfan H. Survey of antibiogram tests in cholera patients in the 2005 epidemic in Hamadan, Islamic Republic of Iran. East Mediterr Health J. 2008;14(4):768–75. [PubMed] [Google Scholar]
- 34.Ngandjio A, Tejiokem M, Wouafo M, Ndome I, Yonga M, et al. Antimicrobial resistance and molecular characterization of Vibrio cholerae O1 during the 2004 and 2005 outbreak of cholera in Cameroon. Foodborne Pathog Dis. 2009;6(1):49–56. doi: 10.1089/fpd.2008.0127. [DOI] [PubMed] [Google Scholar]
- 35.Saha D, Khan WA, Karim MM, Chowdhury HR, Salam MA, et al. Single-dose ciprofloxacin versus 12-dose erythromycin for childhood cholera: a randomised controlled trial. Lancet. 2005;366:1085–1093. doi: 10.1016/S0140-6736(05)67290-X. [DOI] [PubMed] [Google Scholar]
- 36.Mhalu FS, Mmari PW, Ijumba J. Rapid emergence of El Tor Vibrio cholerae resistant to antimicrobial agents during first six months of fourth cholera epidemic in Tanzania. Lancet. 1979;1:345–347. doi: 10.1016/s0140-6736(79)92889-7. [DOI] [PubMed] [Google Scholar]
- 37.Dromigny JA, Rakoto-Alson O, Rajaonatahina D, Migliani R, Ranjalahy J, et al. Emergence and rapid spread of tetracycline-resistant Vibrio cholerae strains, Madagascar. Emerg Infect Dis. 2002;8:336–338. doi: 10.3201/eid0803.010258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Towner KJ, Pearson NJ, Mhalu FS, O'Grady F. Resistance to antimicrobial agents of Vibrio cholerae E1 Tor strains isolated during the fourth cholera epidemic in the United Republic of Tanzania. Bull World Health Organ; 1980;58(5):747–51. [PMC free article] [PubMed] [Google Scholar]
- 39.Weber JT, Mintz ED, Cañizares R, Semiglia A, Gomez I, et al. Epidemic cholera in Ecuador: multidrug-resistance and transmission by water and seafood. Epidemiol Infect; 1994;112(1):1–11. doi: 10.1017/s0950268800057368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okuda J, Hayashi N, Wakahara Y, Gotoh N. Reduced Expression of the vca0421 Gene of Vibrio cholerae O1 Results in Innate Resistance to Ciprofloxacin. Antimicrob Agents Chemother; 2010;54(11):4917–4919. doi: 10.1128/AAC.01652-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghosh A, Ramamurthy T. Antimicrobials & cholera: are we stranded? Indian J Med Res. 2011;133(2):225–31. [PMC free article] [PubMed] [Google Scholar]
- 42.Schulz KF, Altman DG, Moher D for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Lancet. 2010;375(9721):1144–6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of studies included in the review.
(DOC)
Studies excluded from the review.
(DOC)
Assessment of bias risk of randomized & controlled clinical trials.
(DOC)
Main outcomes of clinical trials.
(DOC)
Search strategies.
(DOC)
Prisma check list.
(DOC)