Abstract
Background and Purpose
Chronic pain is increasingly recognized as a consequence of stroke. This study aimed to describe the prevalence and pain types of new onset chronic pain (“novel pain”) in patients with stroke compared with a randomly selected reference group from the general population and to identify factors associated with pain development in stroke patients.
Methods
In a population-based follow-up design, development of chronic pain after stroke was assessed by a questionnaire sent to consecutive stroke patients, registered in a Danish national stroke database, two years after their stroke. A randomly selected sex- and age-matched reference group from the same catchment area received a similar questionnaire about development of new types of chronic pain in the same time period. A total of 608 stroke patients and 519 reference subjects were included in the study.
Results
Development of novel pain was reported by 39.0% of stroke patients and 28.9% of reference subjects (OR 1.57, CI 1.21-2.04), and was associated with low age and depression in a multivariate model. Daily intake of pain medication for novel pain was reported by 15.3% and 9.4% of the stroke and reference population, respectively. Novel headache, shoulder pain, pain from increased muscle stiffness, and other types of novel pain were more common in stroke patients, whereas joint pain was equally common in the two groups.
Conclusions
Development of chronic pain is more common in stroke patients compared with sex- and age-matched reference subjects. Evaluation of post-stroke pain should be part of stroke follow-up.
Introduction
Stroke is the third leading cause of mortality and the major cause of long-term disabilities, such as hemiparesis, language problems and cognitive deficits, in the developed world [1] [2]. The reported prevalence of chronic pain in stroke survivors varies considerably with figures ranging from 11 to 53% [3]–[13]. This variability may be related to differences in criteria and methodologies used. Only few of these studies are population based and none of them have included a reference group.
Post stroke pain (PSP) is often considered to be identical to central post stroke pain (CPSP) also known as thalamic pain (e.g. [14]). However, CPSP is a specific neuropathic pain condition in which pain is due to a lesion of the somatosensory pathways within the central nervous system, i.e., those pathways that conduct information of noxious and non-noxious stimuli from the periphery to the brain. The stroke lesion causing CPSP may be located either within or outside the thalamus [15]. It is now clear that patients with stroke may suffer from a range of other pain types such as hemiplegic shoulder pain [16], [17], musculoskeletal pain [4], [7], [18], and headache [7], [11], [12], besides central post-stroke pain (CPSP) [19]–[21]. Patients may have several types of PSP concomitantly [3], [11], and often have a history of chronic pain prior to their stroke [7], [22]. A detailed and a priori delineation of these types of pain and how they each influence the quality of life in this group of patients has not been systematically done before.
We combined clinical data from a national database of consecutive patients admitted with a stroke with data from a postal questionnaire mailed 2 years after stroke. The aim of the study was 1) to describe the prevalence and pain types of new onset chronic pain (“novel pain”) in stroke patients compared with a randomly selected sex- and age-matched reference group from the general population and 2) to identify factors associated with pain development in stroke patients. Part of this study has been reported elsewhere [23] and identified CPSP in this stroke sample has been published recently [13].
Materials and Methods
The NIP database
All hospitalized acute stroke patients in Denmark are diagnosed according to the WHO criteria (ICD-10) and registered in a National Indicator Project database (NIP) [24]. Patients with intracerebral hemorrhage (I 61), cerebral infarction (I 63), and unspecified stroke (I 64) were included in the study, while patients with transient ischemic attacks (G 45) or subarachnoid hemorrhages (I 60) were excluded. The NIP stroke database contains information on stroke risk factors, severity, evaluation, and treatment, and is subject to regular systematic audits. Stroke severity is measured on admission using the Scandinavian Stroke Scale (SSS), a validated 9-item scale with scores between 0 and 58, where a high score indicates preserved function [25]. Disability and activities of daily living (ADL) are measured on day 7 (±2 days) after stroke using the Barthel Index (BI) [26]. Hospital files were not available for this study.
Study Design
All patients hospitalized with a stroke diagnosis in the County of Aarhus (population 657,671 inhabitants, January 2005) between March 1, 2004 and February 28, 2005 [27] were included in the study. A questionnaire about the development of novel pain after stroke onset was sent out in October 2006 to all 964 surviving Danish patients (median days from stroke 794.5 (range 588–1099)). A similar questionnaire about the development of novel chronic pain within the last two years was sent to 957 (F = 456, M = 501) sex– and age-matched reference subjects. The reference group was randomly selected from the Danish general population in the same catchment area and was identified through the Central Office of Civil Registration. A reminder was sent out to non-responders after one month. If needed, participants were contacted by telephone for clarifying responses (152 stroke patients and 49 reference subjects). Proxy responders were allowed if the subjects could clearly communicate their pain experience.
Questionnaire: General
The questionnaire included sections on demographics, medical and stroke history, increased muscle tone and spasms, sensory symptoms, pain, and concomitant diseases (see the English translation of the questionnaires in the supplementary material, Figure S1, S2). In addition, all subjects were asked to draw areas of abnormal sensitivity on a body chart and to rate their quality of life and health on a rating scale from 0 to 10 (0 = “bad” and 10 = “excellent”).
Questionnaire: Pain
New onset chronic pain (in the following called “novel pain”) was defined as constant or remitting pain lasting more than 3 months and with onset at or after the stroke in patients and within the last 2 years in reference subjects. Subjects reporting development of novel pain were asked specifically about pain due to increased muscle stiffness, headache, shoulder pain, other joint pain, or “other pain” and to fill out a section on pain interference, including questions on how the pain affected their sleep, quality of life, mood, social life, and activities of daily living. Each pain interference item was rated on a 5 point scale: not at all, a little, some, quite a lot, and very much.
Subjects indicating development of “other pain” were asked to indicate the area of pain on a body chart; to score the intensity of their worst pain within the last week on a numeric rating scale (NRS) from 0–10, where 0 equals “no pain” and 10 “worst possible pain”; and to answer questions about the pain quality (from S-LANSS) [28]. A subset of patients who had indicated the presence of “other pain” and were suspected of central post-stroke pain (n = 51), were invited for a clinical examination (data reported elsewhere) [13].
Ethics statement
The study was approved by the local ethical committee (the Central Denmark Region Committees on Biomedical Research Ethics; ID 20060083), the steering group of the NIP database, and the Danish Data Protection Agency (ID 2006-41-6779) and was performed according to the Declaration of Helsinki. An accompanying letter was sent out with the questionnaire. Only patients who gave written consent to further contact were solicited by telephone. This procedure was approved by the ethical committee.
Statistics
Only subjects who had completed the screening question on novel pain (“Have you developed chronic pain following or in connection with the stroke, e.g., headache, joint pain or other pain in the body or the face?”) were included. Pain frequencies are based on the total number of included responders unless otherwise stated; responders with missing information to an item were excluded from the specific analysis.
Age was divided into 3 strata (<65, 65–74, and ≥75 years). Statistical analysis was performed using Intercooled Stata version 9.1 software (StataCorp LP, College Station, Texas, USA). Data are presented as mean and standard deviation (SD), with 95% confidence intervals (CI) or as median with 10% and 90% percentiles (p10-90) or range. P-values less than 0.05 were considered statistically significant. Parametric data were analyzed using Student's t-test. Non-parametric data were analyzed using Mann-Whitney and Kruskal-Wallis (rank sum). Dichotomous data were analyzed using Pearson's chi-square test and Fisher's exact test. Odds ratios are presented with 10% and 90% CI. A logistic regression analysis was performed to clarify the impact of variables identified to be associated with development of novel pain in the whole cohort and in stroke patients separately.
Results
Demographics
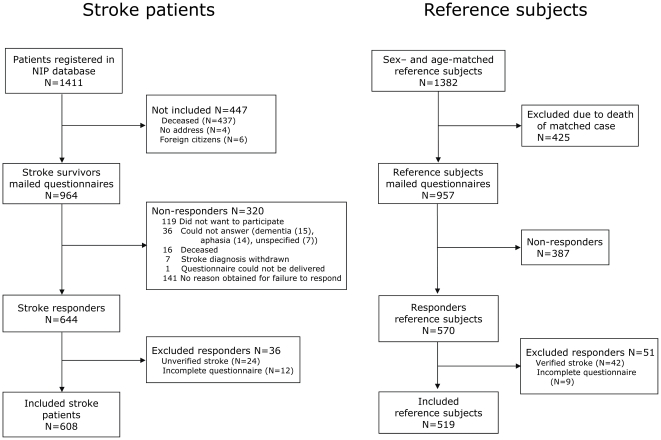
A total of 1411 patients were registered with stroke in the database. Of the 964 surviving stroke patients, 644 returned the questionnaires (response rate of 66.8%); 550 returned the primary questionnaire and 94 the reminder (figure 1). A total of 36 stroke responders were excluded: 24 denied having had the stroke episode and 12 had not completed the pain section of the questionnaire, leaving 608 (F = 268, M = 340) included stroke subjects (63.1%). Patient characteristics are seen in Table 1 and Table S1. Included stroke patients were younger and less severely affected by the stroke than the non-included.
Figure 1. Study flowchart.
Study flowchart of stroke patients (left side) and reference subjects (right side).
Table 1. Comparison between included stroke patients and reference subjects based on data from the questionnaires.
| Included subjects | ||
| Stroke patientsN = 608 | Reference subjectsN = 519 | |
| Included responders | ||
| Primary questionnaire | 520 | 471 |
| Reminder | 88 | 48 |
| Median age (years) | 72.6 | 71.0 |
| Median age females (years) | 74.9 | 74.0 |
| Median age males (years) | 70.9 | 69.0 |
| Male percentage (%) | 55.9 | 58.4 |
| Days stroke to questionnaire, median (range) | 794.5 (588–1099) | - |
| SSS score, median (p10-90) | 50 (26–58) | - |
| Overall QoL (NRS), median (p10-90)¤ | 7 (3–9) | 8 (5–10) |
| Overall QoH (NRS), median (p10-90)# | 6 (3–9) | 8 (5–10) |
| Self-reported diabetes (%) | 14.1 | 7.3 |
| Self-reported depression (%) | 20.9 | 5.6 |
| Self-reported CVD (%) | 33.4 | 16.6 |
| Cohabiting/married (%) | 58.6 | 64.7 |
| Living in nursing home (%) | 12.7 | 1.9 |
| Novel pain (%) | 39.0 | 28.9 |
| Headache (%) | 10.5 | 2.3 |
| Shoulder pain (%) | 15.1 | 9.8 |
| Other joint pain (%) | 22.0 | 18.5 |
| Pain due to muscle stiffness and spasms (%) | 17.4 | 5.2 |
| Other pain (%) | 22.9 | 13.5 |
A total of 570 out of 957 reference subjects returned the questionnaire (59.6%): 517 returned the primary questionnaire and 53 the reminder. Of the responders, 51 were excluded due to either stroke (n = 42) or an incomplete pain section (n = 9), leaving 519 (F = 216, M = 303) included reference subjects (54.2%) (figure 1).
The median response proportion to each question was 94.7% (range 82.2–100%). The lowest response proportion was observed in the questions describing the impact of pain on social life.
Stroke patients had a higher response proportion than reference subjects. There was no statistically significant difference in gender distribution and age between stroke patients and reference subjects (table 1). Female subjects in both groups were significantly older than male subjects. Stroke patients had a higher reported frequency of diabetes and depression than reference subjects, whereas joint disease, gastrointestinal problems, and other pain-causing diseases were equally common.
Incidence of novel pain in stroke patients compared with reference subjects
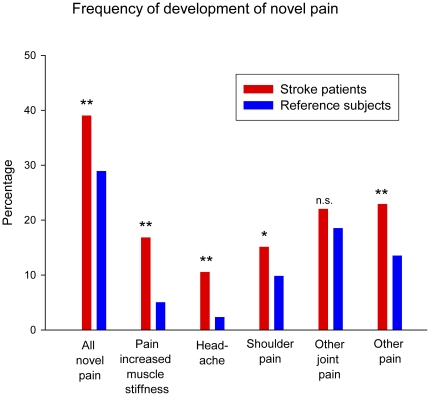
Development of novel pain after stroke or within the last 2 years was reported by 39.0% (35.1–43.0%) of stroke patients compared with 28.9% (25.0–33.0%) of reference subjects, OR 1.57 (1.21–2.04) (Figure 2). The highest frequency of novel pain was in the youngest age group of stroke patients in contrast to the reference group, where the pain frequency increased with age (Table S2).
Figure 2. Frequency of development of novel pain in stroke patients and reference subjects.
The reported prevalence of novel pain development in stroke patients (red columns) and reference subjects (blue columns). Stroke patients had a significantly higher prevalence of pain due to muscle stiffness or spasms, headache, shoulder pain, and other pain. * p<0.05, **p<0.001.n.s. not significant.
In a univariate model, stroke was associated with a higher odds ratio (1.57) for development of novel pain as compared to the reference group (Table 2, model 1). This association was only slightly lower (OR 1.53) when correcting for age, gender, and diabetes in a multiple regression analysis (Table 2, model 2). When depression was included in the analysis (Table 2, model 3), there was no longer a significant difference in odds between stroke patients and reference subjects (OR 1.28, CI: 0.98–1.66). We tested for possible effect modification of gender, age, diabetes, and depression and found that a likelihood ratio test after estimation was significant for age (p = 0.0082), but not for gender (p = 0.25), diabetes (p = 0.49), and depression (p = 0.076). Self-reported cardiovascular disease (CVD) was not included in the analysis because the self-reported data to this item also covered ischemic pain in lower limbs.
Table 2. Logistic regression.
| Model | Odds ratio for pain in stroke patients vs reference subjects | Comment |
| Univariable logistic regression | ||
| Model 1 | 1.57 (CI 1.22–2.02) | Odds ratio for pain development in stroke patients as compared to reference subjects |
| Multiple variable logistic regression | ||
| Model 2 | 1.53 (CI 1.19–1.97) | Confounder analysis including self-reported diabetes, gender, agegroup |
| Model 3 | 1.28 (CI: 0.98–1.66) | Confounder analysis including self-reported diabetes, gender, agegroup and depression |
| Stratified analysis (Model 2) | ||
| Gender | 1.76 (1.24–2.49) | Males |
| 1.29 (0.89–1.86) | Females | |
| Age | 2.67 (CI: 1.69–4.22) | Age<65 years |
| 1.33 (CI: 0.81–2.18) | Age 65–74 years | |
| 1.09 (CI: 0.74–1.61) | Age>75 years | |
| Diabetes | 1.20 (CI: 0.54–2.67) | With diabetes |
| 1.57 (CI: 1.20–2.06) | Without diabetes | |
| Depression | 2.82 (CI: 1.22–6.52) | With depression |
| 1.17 (CI: 0.88–1.54) | Without depression | |
Odds for pain development in stroke patients vs. reference subjects.
Daily intake of pain medication was required by two-thirds of the subjects with development of novel pain, corresponding to the use of daily pain medication for novel pain in 15.3% of the stroke population and 9.4% of the reference population (p = 0.003). Pain interference was higher in stroke patients compared with reference subjects with respect to quality of life (p = 0.006), mood (p = 0.003), social life (p<0.001), and activities of daily living (p<0.001), but not with respect to interference of sleep (p = 0.69) (mean response rate 83.5% (81.9–85.3%)). In the subjects reporting development of novel pain, there was a significant association between high pain interference and depression in stroke subjects (p<0.001), but not in control subjects (p = 0.15).
Subtypes of novel pain
Headache
Development of headache was more common in stroke patients than in reference subjects (10.5% vs. 2.3%, p<0.001), OR 4.97 (2.62–10.23) (Figure 2, Table 2).The frequency and severity of the headache were the same in the two groups. In stroke patients with novel headache, 63.5% reported headache more than 7 days per month and 46.9% had severe or unbearable headache. A history of headache prior to the development of the novel headache was common. The proportion of novel headache was highest in the youngest stroke patients (p = 0.03), but equally common in men and women.
Shoulder and other joint pain
Development of shoulder pain was more common in stroke patients than in reference subjects (15.1% vs. 9.8%, p<0.001), OR 1.64 (1.12–2.40) (Table S2). In stroke subjects, the proportion of novel shoulder pain decreased with age (p = 0.02).
Development of pain from other joints was equally common in stroke patients and reference subjects (22.0% vs. 18.5%, p = 0.14), OR 1.25 (0.92–1.69), but more common in women compared with men (p = 0.002). Novel pain from multiple other joints was common in stroke patients and included pain in hips, ankles, feet, knees, neck, back, wrist, fingers and elbows.
Muscle stiffness, spasms and pain
Muscle stiffness or spasms were reported by 32.8% of stroke subjects compared with 9.8% of reference subjects (p<0.001), OR 4.50 (3.16–6.46). Pain directly due to muscle stiffness or spasms was reported by more than half of the subjects with these symptoms, corresponding to 17.4% of stroke patients and 5.2% of reference subjects (p<0.001), OR 3.82 (2.41–6.28) (Table S2).
Other novel pain
In stroke patients, 22.9% had developed other pains (i.e., not pain from increased muscle stiffness or spasms, headache, shoulder, or other joints) compared with 13.5% of reference subjects (p<0.001), OR 1.90 (1.37–2.64) (Table S2). In stroke patients, the proportion of patients reporting other novel pain tended to decrease with age (p = 0.08). Diabetes was not associated with development of other novel pain.
The localization of other pain is summarized in Figure S3. The area of other novel pain (“worst pain”) was more often unilateral in stroke patients compared with reference subjects (Table S1). A sensation of “pins and needles” and unpleasantness or pain in response to light touch was more common in stroke patients than reference subjects reporting other novel pain, whereas burning pain was described equally common in the two groups (Table S1).
Concomitant types of novel pain
Stroke patients were more likely to report development of more than one concomitant pain type compared with controls (61.2% vs. 48.0%, p = 0.011). The likelihood of reporting several novel pain types concomitantly decreased significantly with age in stroke patients (p<0.001), but not in reference subjects (p = 0.40).
Factors related to pain in the stroke patients
The stroke diagnosis did not differ between stroke patients with or without development of novel pain (p = 0.32), but the median SSS score on admission was lower, i.e. indicating a more severe stroke, in stroke patients reporting novel pain than in patients without novel pain (p = 0.0018) (Table 3). A history of prior stroke was more common in stroke patients with novel pain compared with stroke patients without novel pain (27.9% vs. 20.1%, p = 0.027), whereas a diagnosis of atrial fibrillation was more common in stroke patients without novel pain (p = 0.031). There were no significant differences between the stroke patients with and without novel pain with respect to other risk factors at the time of stroke.
Table 3. Comparison between stroke patients and reference subjects with development of novel types of pain vs. no pain development at time of questionnaire.
| Stroke patients | |||
| Pain | No pain | P-value | |
| Included responders | 237 | 371 | - |
| Primary questionnaire | 193 | 327 | 0.022 |
| Reminder | 44 | 44 | |
| Percentage of included responders, % (95% CI) | 39.0 (35.1–43.0) | 61.0 | - |
| Median age (years) | 70.8 | 73.2 | 0.018 |
| Median age females (years) | 72.4 | 77.0 | 0.0075 |
| Median age males (years) | 68.9 | 71.2 | 0.28 |
| Male percentage (%) | 52.3 | 58.2 | 0.15 |
| Days stroke to questionnaire, median | 801 | 794 | 0.98 |
| SSS score, median (p10-90) | 48 (24–58) | 51 (30–58) | 0.0018 |
| Overall QoL (NRS), median (p10-90)¤ | 5 (2–8) | 7 (4–10) | <0.001 |
| Overall QoH (NRS), median (p10-90)# | 5 (2–8) | 7 (4–10) | <0.001 |
| Self-reported diabetes (%) | 15.6 | 13.2 | 0.41 |
| Self-reported depression (%) | 34.6 | 12.1 | <0.001 |
| Self-reported CVD (%) | 45.2 | 25.9 | <0.001 |
| Cohabiting/married (%) | 57.0 | 59.6 | 0.53 |
| Living in nursing home (%) | 13.9 | 11.9 | 0.46 |
In a multiple logistic regression of all included stroke patients (n = 608) (Table 4), low age, and depression were identified as significant risk factors for development of post-stroke pain. When the SSS score was included in the same analysis (n = 527), a low SSS score was also a significant risk factor for pain development.
Table 4. Logistic regression in stroke patients only.
| Variable | Odds ratio for development of pain after stroke (95% CI) (n = 608)# | P-value | Odds ratio for development of pain after stroke (95% CI) (n = 527)¤ | P-value |
| Diabetes (vs no diabetes) | 1.08 (0.65–1.78) | 0.77 | 1.08 (0.63–1.88) | 0.76 |
| Males (vs females) | 0.77 (0.53–1.11) | 0.16 | 0.90 (0.61–1.32) | 0.58 |
| Depression (vs no depression) | 3.43 (2.25–5.25) | <0.001 | 3.13 (1.99–4.91) | <0.001 |
| Diagnosis (vs hemorrhage) | NA | - | ||
| Infarction | 0.73 (0.43–1.26) | 0.27 | ||
| Unspecified | 1.09 (0.57–2.09) | 0.79 | ||
| Age (vs <65 years) | ||||
| Age 65–74 years | 0.57 (0.36–0.90) | 0.015 | 0.57 (0.35–0.93) | 0.026 |
| Age≥75 years | 0.65 (0.43–0.99) | 0.043 | 0.63 (0.40–0.98) | 0.041 |
| SSS (<45) | NA | - | 0.60 (0.40–0.89) | 0.011 |
Multiple regression model of all included stroke patients, including the same variables as in the multiple variable resgression analysis of all included subjects (table 2).
Multiple variable regression model including the SSS ( = Scandinavian Stroke Score).
Discussion
To our knowledge, this is the first published population-based study on PSP including a control group. Stroke patients compared with age and sex-matched reference subjects more often reported development of novel pain including pain due to increased muscle stiffness, headache, shoulder pain, and other types of novel pain. Two-thirds of the patients with development of novel pain were taking daily pain medication, corresponding to 15% of the total stroke population. Taken together these findings indicate that novel pain after stroke is a symptom with a major impact on the stroke patient, in addition to the other well-known motor and cognitive sequelae that often accompany stroke. Former studies have shown that stroke represents the disease condition associated with the highest degree of disability [2]. The present findings raise the possibility that pain is another contributing factor to the disability seen in stroke survivors.
In an univariate model in this study, stroke patients were more likely to report development of chronic pain following stroke (39.0%) compared with reference subjects (28.9%), developing pain within the last 2 years (OR: 1.57). The difference between the two groups (risk difference 10.1%, CI 4.6–15.6%) can be interpreted as the proportion of pain that is stroke related, and supports findings from other studies [5], [7], [8]. Consistent with previous observations pain was associated with depression.
When correcting for possible confounders in a multiple regression analysis that included depression, there was a significant reduction in the difference in odds between stroke patients and reference subjects. These findings suggest that the higher odds for pain development in stroke patients were partly due to an associated depression, rather than stroke per se. The difficulty in dissecting the relationship between depression and pain in general and pain due to stroke in particular is underscored by the fact that a) risk of depression is increased after stroke [29], b) patients with chronic pain are more likely to report depression [30], and c) depressed patients are more likely to have pain than non-depressed patients [31]. In the present study, we found a significant association between high pain interference on mood and depression in stroke patients reporting development of pain, but not in control subjects reporting pain. Clinical and experimental studies have shown a high concordance of depression and pain. It is still unclear if chronic pain and mood disorders share common pathophysiological mechanisms or whether they are both caused by separable and distinct mechanisms. It is of interest to speculate on the mechanisms behind this higher frequency of depression in stroke patients compared with reference subjects. The mechanisms underlying pain and depression have been linked to disturbances in the monoaminergic neurotransmission systems originating in the brainstem and projecting down both into the spinal cord and into the forebrain [30], [32], [33]. Disruption of serotonergic and noradrenergic systems is likely to occur in patients suffering a stroke affecting the brainstem and subcortical structures [34], [35]. In chronic pain there is pharmacological evidence that restoring serotonergic and noradrenergic neurotransmission with specific serotonergic and noradrenergic re-uptake inhibitors can reduce pain in patients with peripheral or central neuropathic pain conditions (for review see Finnerup et al. 2010) [36]. Thus a more profound reduction of central serotonin and noradrenaline tone in stroke patients with pain than in the reference group with pain may be one possibility for the association of depression in the stroke pain group and not in the reference pain group.
In this study, and also in a recent study [12], pain was more prevalent in the youngest age group of stroke patients. This is in contrast to what is seen in the reference group, where the prevalence of novel pain, increased with age as we expected [37]. The mechanism for this higher pain frequency in the youngest stroke patients is not clear. It has been shown that stroke in the brainstem and thalamus more frequently are associated with central pain than other locations (for review see Klit et al [38]) and that posterior territory infarcts, including brainstem and thalamic strokes, are relatively more frequent in the younger age groups [39]–[41]. Whether this age-dependent effect may account for the present observation requires further studies.
The presence of specific subtypes of pain was assessed by asking about pain due to increased muscle stiffness including spasms, headache, shoulder pain, other joint pain, and “other pain”. The reported proportion of muscle stiffness or spasms was higher in stroke patients compared with reference subjects (OR: 4.50), and more than half of these subjects reported pain directly due to these symptoms. Our findings are in accordance with previous studies reporting a prevalence of spasticity of 17–38% using clinical assessment scales [42]–[44]. Development of chronic headache was more common in stroke patients than in reference subjects (OR: 4.97). In other studies, post-stroke headache has been reported by 10% [7], [12]. The present study supports the notion of headache being a common consequence of stroke, and we have previously suggested that the development of headache might be pathophysiologically linked to the stroke [45]. However, the high prevalence of post-stroke headache in this study may be partly ascribed to the routine use of dipyridamole in ischemic stroke patients at the time of data collection [46]. Development of shoulder pain was reported by 15% of stroke patients and 10% of reference subjects (OR: 1.64). In previous studies, the range of reported prevalence of post-stroke shoulder pain is wide, ranging from 6–64% [8], [16], [47], [48]. Differences in study populations and criteria used may explain this variability.
The strength of this study is the inclusion of a sex- and age-matched reference group randomly chosen in the same area as the stroke population. The inclusion of a control group is important, as chronic pain is common in all population groups, and in particular among the elderly [49], [50]. Stroke and reference subjects were comparable with respect to age, sex, and concomitant diseases apart from diabetes, CVD, and depression. To exactly determine the influence of stroke for the subsequent development of pain, the ideal control group would have the same risk factors as the study group.
The present study population was selected from a stroke database including 95% of all stroke patients in the area [51]. The NIP stroke database undergoes regular audit and the quality and reliability of the data are high. As regards the reliability of the information from the questionnaires, the overall quality of the responses was good and response rates of the individual questions were generally high (94.7%). In cases of uncertainty responders were contacted by telephone in order to clarify responses.
The present study has a few shortcomings. The response rate was not high, but is consistent with response rates of other questionnaire studies in stroke survivors [52], [53]. It is a retrospective study with a risk of recall bias. However, the reference group is assumed to be exposed to a similar bias, so it is unlikely that the retrospective character of the study would change the relative frequency of pain between the two groups. The pain prevalence before the study is not known and may therefore differ between the groups. The pain frequency increased from the primary questionnaire to the reminder in the stroke patients (37.1% vs. 50.0%, p = 0.022), but not in the reference group (29.0% vs. 27.1%, p = 0.77), implying that pain frequencies for stroke patients were not overrated in this study. Stroke severity has, in this and previous studies [7], [17], been associated to pain prevalence. In this study, the included stroke patients were less severely affected than non-responders; however, the study group is likely to be representative for the stroke survivors. The pain intensity was not recorded for all subgroups of pain but only for headache and novel types of pain. In these two latter types of pain, there was no difference in pain intensity between stroke patients and reference subjects. What is of importance in a study like this may not be the pain intensity per se, but whether the pain has an intensity that needs daily medication. In this study 15% of stroke patients with novel pain after stroke took daily medication for their pains compared with 9% in the reference pain group.
In conclusion, pain represents an important disability following stroke. In this population-based study, which included a sex and age-matched reference group, about 40% of the stroke patients had developed chronic pain within two years of their stroke and this pain was associated with depression and low age.
Supporting Information
English translation of the questionnaire to the stroke subjects.
(DOCX)
English translation of the questionnaire to the reference subjects.
(DOC)
Localization of other novel pain. The reported location of worst “other pain” in stroke patients (red bars) and reference subjects (blue bars). A hemibody distribution of pain, i.e., pain localized to one side of the body, with or without involvement of the face and trunk, and pain in parts of both upper and lower limbs, was more common in stroke patients than in reference subjects (hemibody: 21.3% vs. 4.5%, p = 0.002; parts of upper and lower limbs: 22.1% vs. 4.6%, p = 0.002), whereas pain with other localizations, including wide spread pain, pain in multiple sites, back pain and neck pain, was more common in reference subjects (15.0% vs. 34.9%, p = 0.003).
(TIF)
Characterization of the included stroke patients at time of stroke based on NIP data.
(DOC)
Characterization of stroke patients and reference subjects with development of all types of novel pain and subtypes of pain.
(DOC)
Acknowledgments
The authors would like to thank Helle O. Andersen for secretarial and language assistance and Christina Dahm for statistical and epidemiological advice.
Footnotes
Competing Interests: The authors have read the journal's policy and have the following conflicts. Dr. Klit has received funding for congress participation from Eli Lilly and Grünenthal and research support from the Ludvig & Sara Elsass Foundation and the Velux Foundation. Dr. Finnerup has received honoraria and research support in the past two years from Grunenthal. Dr. Andersen has been a member of the advisory board for Boehringer-Ingelheim and has received lecture honoraria from Boehringer-Ingelheim, Pfizer, and Lundbeck. Prof. Jensen has received honoraria or research support from Pfizer, Grünenthal, Eli-Lilly, GlaxoSmithKline, PharmEste, AstraZeneca, and Daiichi Sankyo. There are no patents, products in development or marketed products to declare. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials, as detailed online in the guide for authors.
Funding: The study was supported by the Ludvig & Sara Elsass Foundation and the Velux Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.National Stroke Association website. Available: http://www.stroke.org/site/PageServer?pagename=acute. Accessed 2011 Oct 21.
- 2.Bonita R. Epidemiology of stroke. Lancet. 1992;339:342–344. doi: 10.1016/0140-6736(92)91658-u. [DOI] [PubMed] [Google Scholar]
- 3.Kong KH, Woon VC, Yang SY. Prevalence of chronic pain and its impact on health-related quality of life in stroke survivors. Arch Phys Med Rehabil. 2004;85:35–40. doi: 10.1016/s0003-9993(03)00369-1. [DOI] [PubMed] [Google Scholar]
- 4.Kuptniratsaikul V, Kovindha A, Suethanapornkul S, Manimmanakorn N, Archongka Y. Complications during the rehabilitation period in Thai patients with stroke: a multicenter prospective study. Am J Phys Med Rehabil. 2009;88:92–99. doi: 10.1097/PHM.0b013e3181909d5f. [DOI] [PubMed] [Google Scholar]
- 5.Appelros P. Prevalence and predictors of pain and fatigue after stroke: a population-based study. Int J Rehabil Res. 2006;29:329–333. doi: 10.1097/MRR.0b013e328010c7b8. [DOI] [PubMed] [Google Scholar]
- 6.Indredavik B, Rohweder G, Naalsund E, Lydersen S. Medical complications in a comprehensive stroke unit and an early supported discharge service. Stroke. 2008;39:414–420. doi: 10.1161/STROKEAHA.107.489294. [DOI] [PubMed] [Google Scholar]
- 7.Jonsson AC, Lindgren I, Hallstrom B, Norrving B, Lindgren A. Prevalence and intensity of pain after stroke: a population based study focusing on patients' perspectives. J Neurol Neurosurg Psychiatry. 2006;77:590–595. doi: 10.1136/jnnp.2005.079145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lundstrom E, Smits A, Terent A, Borg J. Risk factors for stroke-related pain 1 year after first-ever stroke. Eur J Neurol. 2009;16:188–193. doi: 10.1111/j.1468-1331.2008.02378.x. [DOI] [PubMed] [Google Scholar]
- 9.Sackley C, Brittle N, Patel S, Ellins J, Scott M, et al. The prevalence of joint contractures, pressure sores, painful shoulder, other pain, falls, and depression in the year after a severely disabling stroke. Stroke. 2008;39:3329–3334. doi: 10.1161/STROKEAHA.108.518563. [DOI] [PubMed] [Google Scholar]
- 10.Langhorne P, Stott DJ, Robertson L, MacDonald J, Jones L, et al. Medical complications after stroke: a multicenter study. Stroke. 2000;31:1223–1229. doi: 10.1161/01.str.31.6.1223. [DOI] [PubMed] [Google Scholar]
- 11.Widar M, Samuelsson L, Karlsson-Tivenius S, Ahlstrom G. Long-term pain conditions after a stroke. J Rehabil Med. 2002;34:165–170. doi: 10.1080/16501970213237. [DOI] [PubMed] [Google Scholar]
- 12.Naess H, Lunde L, Brogger J, Waje-Andreassen U. Post-stroke pain on long-term follow-up: the Bergen stroke study. J Neurol. 2010;257:1446–1452. doi: 10.1007/s00415-010-5539-y. [DOI] [PubMed] [Google Scholar]
- 13.Klit H, Finnerup NB, Andersen G, Jensen TS. Central poststroke pain: a population-based study. Pain. 2011;152:818–824. doi: 10.1016/j.pain.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 14.Kim JS. Post-stroke pain. Expert Rev Neurother. 2009;9:711–721. doi: 10.1586/ern.09.19. [DOI] [PubMed] [Google Scholar]
- 15.Klit H, Finnerup NB, Jensen TS. Central post-stroke pain: clinical characteristics, pathophysiology, and management. Lancet Neurol. 2009;8:857–868. doi: 10.1016/S1474-4422(09)70176-0. [DOI] [PubMed] [Google Scholar]
- 16.Gamble GE, Barberan E, Laasch HU, Bowsher D, Tyrrell PJ, et al. Poststroke shoulder pain: a prospective study of the association and risk factors in 152 patients from a consecutive cohort of 205 patients presenting with stroke. Eur J Pain. 2002;6:467–474. doi: 10.1016/s1090-3801(02)00055-1. [DOI] [PubMed] [Google Scholar]
- 17.Lindgren I, Jonsson AC, Norrving B, Lindgren A. Shoulder pain after stroke: a prospective population-based study. Stroke. 2007;38:343–348. doi: 10.1161/01.STR.0000254598.16739.4e. [DOI] [PubMed] [Google Scholar]
- 18.Hettiarachchi C, Conaghan P, Tennant A, Bhakta B. Prevalence and impact of joint symptoms in people with stroke aged 55 years and over. J Rehabil Med. 2011;43:197–203. doi: 10.2340/16501977-0648. [DOI] [PubMed] [Google Scholar]
- 19.Weimar C, Kloke M, Schlott M, Katsarava Z, Diener HC. Central poststroke pain in a consecutive cohort of stroke patients. Cerebrovasc Dis. 2002;14:261–263. doi: 10.1159/000065663. [DOI] [PubMed] [Google Scholar]
- 20.Bowsher D. Stroke and central poststroke pain in an elderly population. J Pain. 2001;2:258–261. doi: 10.1054/jpai.2001.24549. [DOI] [PubMed] [Google Scholar]
- 21.Andersen G, Vestergaard K, Ingeman-Nielsen M, Jensen TS. Incidence of central post-stroke pain. Pain. 1995;61:187–193. doi: 10.1016/0304-3959(94)00144-4. [DOI] [PubMed] [Google Scholar]
- 22.Leijon G, Boivie J, Johansson I. Central post-stroke pain–neurological symptoms and pain characteristics. Pain. 1989;36:13–25. doi: 10.1016/0304-3959(89)90107-3. [DOI] [PubMed] [Google Scholar]
- 23.Klit H, Finnerup NB, Overvad K, Andersen G, Jensen TS. A postal survey on post-stroke pain. Cerebrovascular Dis. 2008;25:79. [Google Scholar]
- 24.National Indicator Project website. Available: http://www.nip.dk/aboutthedanishnationalindicatorproject. Accessed 2011 Oct 21.
- 25.Lindenstrom E, Boysen G, Waage Christiansen L, á Rogvi Hansen B, Würtzen Nielsen P. Reliability of Scandinavian Neurological Stroke Scale. Cerebrovascular Diseases. 1991;1:103–107. [Google Scholar]
- 26.Mahony FI, Barthel DW. Functional Evaluation: The Barthel Index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 27.Statistics Denmark website. Statistical yearbook 2005. Available: http://www.dst.dk/pukora/epub/upload/8958/komp2005.pdf. Accessed 2011 Oct 21.
- 28.Bennett MI, Smith BH, Torrance N, Potter J. The S-LANSS score for identifying pain of predominantly neuropathic origin: validation for use in clinical and postal research. J Pain. 2005;6:149–158. doi: 10.1016/j.jpain.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Kumar S, Selim MH, Caplan LR. Medical complications after stroke. Lancet Neurol. 2010;9:105–118. doi: 10.1016/S1474-4422(09)70266-2. [DOI] [PubMed] [Google Scholar]
- 30.Gormsen L, Rosenberg R, Bach FW, Jensen TS. Depression, anxiety, health-related quality of life and pain in patients with chronic fibromyalgia and neuropathic pain. Eur J Pain. 2010;14:127–128. doi: 10.1016/j.ejpain.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 32.Millan MJ. Descending control of pain. Progress in Neurobiology. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki R, Dickenson A. Spinal and Supraspinal Contributions to Central Sensitization in Peripheral Neuropathy. Neurosignals. 2005;14:175–181. doi: 10.1159/000087656. [DOI] [PubMed] [Google Scholar]
- 34.Andersen G, Ingeman-Nielsen M, Vestergaard K, Riis JO. Pathoanatomic correlation between poststroke pathological crying and damage to brain areas involved in serotonergic neurotransmission. Stroke. 1994;25:1050–1052. doi: 10.1161/01.str.25.5.1050. [DOI] [PubMed] [Google Scholar]
- 35.Moller M, Andersen G, Gjedde A. Serotonin 5HT1A receptor availability and pathological crying after stroke. Acta Neurol Scand. 2007;116:83–90. doi: 10.1111/j.1600-0404.2007.00869.x. [DOI] [PubMed] [Google Scholar]
- 36.Finnerup NB, Sindrup SrH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010;150:573–581. doi: 10.1016/j.pain.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 37.Helme RD, Gibson SJ. The epidemiology of pain in elderly people. Clin Geriatr Med. 2001;17:417–31. doi: 10.1016/s0749-0690(05)70078-1. [DOI] [PubMed] [Google Scholar]
- 38.Klit H, Finnerup NB, Jensen TS. Clinical Characteristic of Central Poststroke Pain. In: Henry JL, Panju A, Yashpal K, editors. Central Neuropathic Pain: Focus on Poststroke Pain. Seattle: IASP Press; 2007. pp. 27–42. [Google Scholar]
- 39.Putaala J, Metso AJ, Metso TM, Konkola N, Kraemer Y, et al. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke: the Helsinki young stroke registry. Stroke. 2009;40:1195–1203. doi: 10.1161/STROKEAHA.108.529883. [DOI] [PubMed] [Google Scholar]
- 40.Kristensen B, Malm J, Carlberg B, Stegmayr B, Backman C, et al. Epidemiology and etiology of ischemic stroke in young adults aged 18 to 44 years in northern Sweden. Stroke. 1997;28:1702–1709. doi: 10.1161/01.str.28.9.1702. [DOI] [PubMed] [Google Scholar]
- 41.Cerrato P, Grasso M, Imperiale D, Priano L, Baima C, et al. Stroke in young patients: etiopathogenesis and risk factors in different age classes. Cerebrovasc Dis. 2004;18:154–159. doi: 10.1159/000079735. [DOI] [PubMed] [Google Scholar]
- 42.Watkins CL, Leathley MJ, Gregson JM, Moore AP, Smith TL, et al. Prevalence of spasticity post stroke. Clin Rehabil. 2002;16:515–522. doi: 10.1191/0269215502cr512oa. [DOI] [PubMed] [Google Scholar]
- 43.Lundstrom E, Terent A, Borg J. Prevalence of disabling spasticity 1 year after first-ever stroke. European Journal of Neurology. 2008;15:533–539. doi: 10.1111/j.1468-1331.2008.02114.x. [DOI] [PubMed] [Google Scholar]
- 44.Welmer AK, Widen HL, Sommerfeld DK. Location and severity of spasticity in the first 1–2 weeks and at 3 and 18 months after stroke. Eur J Neurol. 2010;17:720–725. doi: 10.1111/j.1468-1331.2009.02915.x. [DOI] [PubMed] [Google Scholar]
- 45.Vestergaard K, Andersen G, Nielsen MI, Jensen TS. Headache in stroke. Stroke. 1993;24:1621–1624. doi: 10.1161/01.str.24.11.1621. [DOI] [PubMed] [Google Scholar]
- 46.Sacco RL, Diener HC, Yusuf S, Cotton D, Ounpuu S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238–1251. doi: 10.1056/NEJMoa0805002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aras MD, Gokkaya NK, Comert D, Kaya A, Cakci A. Shoulder pain in hemiplegia: results from a national rehabilitation hospital in Turkey. Am J Phys Med Rehabil. 2004;83:713–719. doi: 10.1097/01.phm.0000138739.18844.88. [DOI] [PubMed] [Google Scholar]
- 48.Roosink M, Renzenbrink GJ, Buitenweg JR, Van Dongen RT, Geurts AC, et al. Persistent shoulder pain in the first 6 months after stroke: results of a prospective cohort study. Arch Phys Med Rehabil. 2011;92:1139–1145. doi: 10.1016/j.apmr.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 49.Moulin DE, Clark AJ, Speechley M, Morley-Forster PK. Chronic pain in Canada–prevalence, treatment, impact and the role of opioid analgesia. Pain Res Manag. 2002;7:179–184. doi: 10.1155/2002/323085. [DOI] [PubMed] [Google Scholar]
- 50.Crook J, Rideout E, Browne G. The prevalence of pain complaints in a general population. Pain. 1984;18:299–314. doi: 10.1016/0304-3959(84)90824-8. [DOI] [PubMed] [Google Scholar]
- 51.The National Indicator Project for Stroke. 2006. [Quality in treatment of Stroke in Aarhus Region March-December 2005]. Available: http://www.sundhed.dk/Artikel.aspx?id=30107.1. Accessed 2011 Oct 21.
- 52.Mead GE, Graham C, Dorman P, Bruins SK, Lewis SC, et al. Fatigue after stroke: baseline predictors and influence on survival. Analysis of data from UK patients recruited in the International Stroke Trial. PLoS One. 2011;6:e16988. doi: 10.1371/journal.pone.0016988. 10.1371/journal.pone.0016988 [doi]. Available: http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0016988. Accessed 2011 October 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naess H, Lunde L, Brogger J, Waje-Andreassen U. Post-stroke pain on long-term follow-up: the Bergen stroke study. J Neurol. 2010;257:1446–1452. doi: 10.1007/s00415-010-5539-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
English translation of the questionnaire to the stroke subjects.
(DOCX)
English translation of the questionnaire to the reference subjects.
(DOC)
Localization of other novel pain. The reported location of worst “other pain” in stroke patients (red bars) and reference subjects (blue bars). A hemibody distribution of pain, i.e., pain localized to one side of the body, with or without involvement of the face and trunk, and pain in parts of both upper and lower limbs, was more common in stroke patients than in reference subjects (hemibody: 21.3% vs. 4.5%, p = 0.002; parts of upper and lower limbs: 22.1% vs. 4.6%, p = 0.002), whereas pain with other localizations, including wide spread pain, pain in multiple sites, back pain and neck pain, was more common in reference subjects (15.0% vs. 34.9%, p = 0.003).
(TIF)
Characterization of the included stroke patients at time of stroke based on NIP data.
(DOC)
Characterization of stroke patients and reference subjects with development of all types of novel pain and subtypes of pain.
(DOC)