Abstract
Parasitemia with a large Babesia species was identified in two domestic cats from Israel. One cat, also coinfected with feline immunodeficiency virus and “Candidatus Mycoplasma haemominutum,” had profound icterus and anemia which resolved after therapy, whereas a second cat was an asymptomatic carrier. Amplification and sequencing of the 18S rRNA gene, followed by phylogenetic analyses, indicated that infection was caused by Babesia canis. However, the sequences of the internal transcribed and 5.8S rRNA regions of the ribosomal operon used for subspeciation of B. canis were markedly different from the recognized subspecies of B. canis, which include B. canis vogeli, B. canis canis, and B. canis rossi. Based on phylogenetic comparisons of the 18S rRNA gene, 5.8S, and internal transcribed spacer sequences of the isolates from the cats and on the smaller sizes of the merozoite and trophozoite stages of this parasite, which distinguish it from the subspecies of B. canis present in dogs, we propose to identify the novel feline genotype of B. canis described in the present study as a new subspecies, B. canis subsp. presentii.
Babesia species are tick-borne intraerythrocytic apicomplexan parasites found in a variety of domestic and wild animals and in humans. Babesiosis in domestic cats has primarily been reported in South Africa, where infection is mainly due to Babesia felis, a small Babesia that causes anemia and icterus (24, 28). In addition, Babesia cati was reported from a cat (Felis catus) in India (23) and sporadic cases of infection in domestic cats by unidentified Babesia parasites have been reported in France, Germany, Thailand, and Zimbabwe (3, 16, 22, 29). Reports of Babesia in wild felids include Babesia herpailuri from a jaguarundi (Herpailurus yaguarundi) (7), Babesia pantherae from a leopard (Panthera pardus) (8), Babesia leo from lions (Panthera leo) (24), and a unidentified piroplasm in cheetahs (Acinonyx jubatus) (1). Cytauxzoon felis is a piroplasm that is phylogenetically related to Theileria and Babesia and infects the bobcat (Lynx rufus) (11) and domestic cats (34). In a recent study from Spain and Portugal, a partial DNA sequence from the small subunit RNA gene identified as Babesia canis canis was amplified from the blood of three cats in which parasites were not visualized by microscopy (6). This has provided initial molecular evidence for infection by Babesia canis in cats.
Babesia infections in domestic dogs are caused by large piroplasms described as B. canis and by smaller parasites that are mostly grouped under the species Babesia gibsoni. Three subspecies of B. canis have been recognized based on differences in the pathological and clinical syndromes caused by each subspecies, antigenic properties, transmission by different vector ticks, and genetic characterization (5, 13, 27, 36). B. canis rossi described in South Africa is transmitted by the tick Haemaphysalis leachi and causes a severe and often fatal hemolytic disease in dogs (25). B. canis vogeli is found in the Middle East, North Africa, Europe, Asia, and Australia (4, 36) and is transmitted by Rhipicephalus sanguineus. It commonly induces mild to moderate clinical signs in dogs. B. canis canis, described in Europe, is transmitted by Dermacentor reticulatus and causes hemolytic anemia with variable degrees of severity (33) and coagulation abnormalities (27). These subspecies were genotypically grouped by sequencing the first and second internal transcribed spacers (ITS1 and ITS2) and the intervening 5.8S coding region of the rRNA gene (36). The subspecies can also be differentiated by PCR-restriction fragment length polymorphism assays of the ITS regions (36) or of a partial 18S rRNA sequence (5). In addition to B. canis and B. gibsoni, other species and distinct strains of small Babesia parasites infect dogs. These include a Babesia microti-like parasite proposed as Theileria annae that was described and genotypically characterized from Spain (37) and a small piroplasm from California (17).
The present study describes a clinical infection and the morphological and genetic characterization of a Babesia from two domestic cats in Israel.
CASE REPORTS
Case 1.
A 6-year-old male castrated domestic longhair cat was presented for veterinary care in central Israel with a history of acute lethargy and anorexia of 2 days' duration. Physical examination revealed pyrexia (40.5°C) and icterus. The cat was reported to have been infested with ticks in the past; however, no ticks were found during the physical examination. Hematological abnormalities included a severe normocytic hypochromic anemia (hematocrit, 16.7% [normal range, 32 to 48]; red blood cells, 3.23 × 106/μl [normal range, 5.3 to 8.9 × 106/μl]; mean cell volume, 51.8 fl [normal range, 39 to 55 fl]; mean corpuscular hemoglobin concentration, 28.8 g/dl [normal range, 30 to 36 g/dl]), leukopenia, lymphopenia, and thrombocytopenia. Pear-shaped pairs of merozoites and single trophozoite forms of Babesia piroplasms were noted in 2% of the erythrocytes on examination of a May-Grunwald-Giemsa-stained blood smear. Biochemical abnormalities in serum included marked hyperbilirubinemia (bilirubin, 11.6 mg/dl; normal range, 0.1 to 0.5 mg/dl) and increased activities of alanine aminotransferase, creatine kinase, and lactate dehydrogenase. EDTA anticoagulated blood was frozen at −70°C for future molecular analysis. The cat was treated with a single dose of 2.5 mg of imidocarb dipropionate (Imizol; Schering-Plough)/kg intramuscularly (12), 10 mg of doxycycline (Vibramycin; Pfizer)/kg/day orally for 21 days, and intravenous fluids. By the following day, the cat had clinically improved and was more alert and active. After 12 days of treatment the cat was clinically normal. Repeat hematological and serum biochemical testing at this stage revealed improvements in all parameters. The hematocrit was 25.2%, the leukocytes and thrombocytes returned to near-normal numbers, the lymphocytes were within the normal range, the bilirubin level decreased to 0.62 mg/dl, the alanine aminotransferase and creatine kinase activities were normal, and lactate dehydrogenase activity decreased to near normal. No Babesia parasites were evident in blood smears. The cat tested positive for feline immunodeficiency virus (FIV) antibodies and negative for feline leukemia virus (FeLV) p27 antigen by using a commercial test kit (Idexx, Inc., Westbrook, Maine). Since cat 1 had a clinical syndrome that was also compatible with hemoplasmosis, PCR analysis of a pretreatment EDTA anticoagluated blood sample that was stored frozen at −70°C was performed for feline hemoplasma species. It was found to be positive for “Candidatus Mycoplasma haemominutum” and negative for Mycoplasma haemofelis.
Case 2.
A 2-year-old noncastrated male Persian cat with a history of hematuria from the same household was presented for veterinary care. The cat was normal on clinical examination. Hematological parameters and a serum biochemistry panel were normal, and testing for FIV, FeLV, and PCR for the two feline hemoplasma species were negative. A low parasitemia (<1% of the red blood cells) with Babesia piroplasms was evident on examination of a May-Grunwald-Giemsa-stained blood smear. EDTA anticoagulated blood was frozen at −70°C for future molecular analysis. The cat was diagnosed with cystitis and treated orally with 20 mg of the antibiotic cephalexin (Ceforal; Teva)/kg three times daily for 14 days. After 10 days, the owners reported that the cat had normal-appearing urine, and they did not return for a follow-up examination.
MATERIALS AND METHODS
Parasites.
Babesia parasites were viewed for morphological description by light microscopy. The sizes of parasites were determined by using a micrometer at ×1,000 magnification.
Blood samples were taken from two additional in-contact cats (one living in the same household and another living in a neighboring house) for microscopic examination of blood smears and for Babesia PCR analysis. In addition, blood from two dogs with confirmed babesiosis (one from Beer Sheva in south-central Israel and the other from the Nahriya in northern Israel) was collected prior to babesiacidal treatment. All blood samples were frozen until use and then thawed, and DNA was extracted from 100 μl of blood by using the phenol-chloroform method. The lyophilized DNA was sent to the Department of Clinical Veterinary Science at the University of Bristol (United Kingdom) for PCR analysis and characterization of Babesia DNA.
Amplification of Babesia DNA.
The extracted DNA was reconstituted in 100 μl of TE buffer. All PCRs were performed in 20-μl total volumes by using Extensor Hi-Fidelity PCR Master Mix (Abgene, Ltd., Epsom Surrey, United Kingdom). Primers were synthesized by Sigma-Genosys (Cambridge, United Kingdom). Portions (2 μl) of template DNA solution were used in each PCR, and samples were analyzed in duplicate. The primers were used at a final concentration of 0.5 μM. All PCRs were performed on a Tetrad DNA Engine (MJ Research, Waltham, Mass.). The initial screens for apicomplexan protozoa were performed with primers BabgenF (GAA ACT GCG AAT GGC TCA TTA) and BabgenR (CGG TAG GCC AAT ACC CTA CCG TC), which flank a region of ca. 270 bp in all Babesia spp. and some other related apicomplexans. The thermal cycler program used was 94°C for 15 min, followed by 40 cycles of 94°C for 30 s, 65°C for 30 s, and 72°C for 30 s. Samples that were positive were then tested with the primer pairs RIB-19 and RIB-20 (37), which amplify the majority of the Babesia 18S rRNA gene, and RIB-3 and RIB-13 for the intergenic spacer as previously described (36).
The presence of amplicons of the appropriate size was determined by agarose gel electrophoresis of 5-μl aliquots of the reaction mixtures. Where a product was present, the contents of the duplicate tubes were pooled, and the amplified DNA was purified from unreacted primers and nucleotides by using NucleoSpin Extract (Machery-Nagel, Duren, Germany) according the manufacturer's instructions. DNA nucleotide sequence determination was carried out by the Sequencing Service, University of Dundee, Dundee, Scotland, with the same primers as were used for amplification. Sequences were edited and aligned by using the Chromas and Omiga suites of software (Accelrys, Ltd., Cambridge, United Kingdom), and global sequence comparisons were performed by using basic local alignment search tool (BLAST) hosted by the National Center for Biotechnology Information (NCBI; http://www.ncbi.nlm.nih.gov:80/BLAST/). New sequences were deposited in the GenBank database.
PCR for feline hemoplasma species.
PCR analysis for feline hemoplasma species was performed by using a real-time PCR assay as previously described (30).
Phylogenetic analysis.
The feline babesial DNA sequences were subjected to phylogenetic analysis with other 18S or intergenic sequences chosen from NCBI BLAST searches or from known related organisms. For both the 18S gene and the intergenic spacer region, sequences were aligned by using CLUSTAL X (version 1.81; EMBL) by using a DNA weight matrix that scores residue matches as 1.9 and mismatches as 0 (32). The alignments were further adjusted manually by eye. Phylogenetic trees were constructed by using the algorithms implemented in the PAUP v.4 program (PAUP Phylogenetic Analysis, version 4; Sinauer Associates, Sunderland, Mass.) by using parsimony and other methods. Trees were computed by using the neighbor-joining method (26) from a distance matrix corrected for nucleotide substitutions by the Kimura two-parameter model with a transition/tranversion ratio set at 2. The data was resampled 100 times to give bootstrap percentage values.
RESULTS
Morphological description and size measurements. (i) Trophozoites.
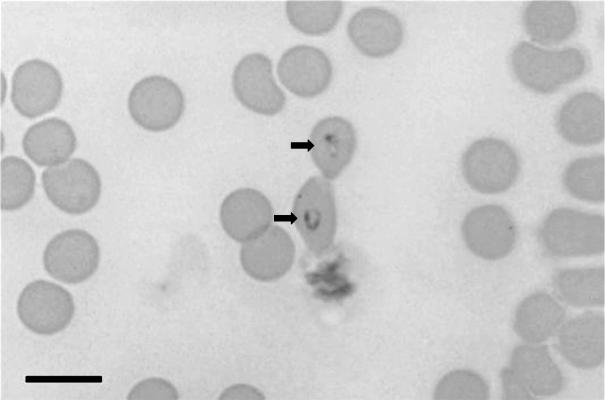
Trophozoites were round to oval (or ring-shaped) (Fig. 1) and, in some cases, elongated with a pointed end. These single forms were usually present close to the center of erythrocytes. The nuclei appeared as rounded forms along the trophozoite outer limits. The mean size of trophozoites was 2.7 ± 0.42 μm by 1.7 ± 0.38 μm (range, 3.2 to 1.8 μm by 2.5 to 1.2 μm [n = 16]).
FIG. 1.
Trophozoites of B. canis in feline blood (arrows). Bar, 10 μm.
(ii) Merozoites and merogony.
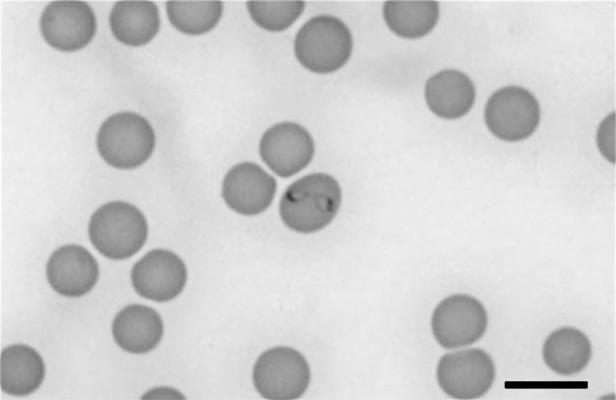
Pear-shaped elongated merozoites were observed to divide by binary fission. Most merozoites were in pairs (Fig. 2) or, rarely, up to four merozites were present in a single erythrocyte. The merozoites did not form tetrads (Maltese cross form), as is found in some species of Babesia, and were large enough to extend across the erythrocyte diameter when attached in pairs. The merozoites had a crescent-shaped nucleus found in the pole distal to the attachment connecting between the pair of merozoites. The mean size of merozoites was 2.5 ± 0.36 μm by 1.4 ± 0.3 μm (range, 2.9 to 1.3 μm by 2.3 to 0.7 μm [n = 28]).
FIG. 2.
Pair of merozoites of B. canis in a feline erythrocyte. Bar, 10 μm.
Of 60 parasitized erythrocytes examined from case 1, 29 (48.3%) contained merozoites in pairs and 31 (51.7%) had single forms; of these 31, 20 were elongated trophozoites with a pointed end, and 11 were oval shaped.
Amplification and sequencing of DNA segments (18S segment, ITS).
An amplicon of 1,693 bp comprising the majority of the 18S rRNA gene was sequenced from the blood of cat 1 (GenBank accession no. AY272047). A second amplicon of 640 bp covering the ITS1, 5.8S, and ITS2 region from cat 1 was also sequenced (GenBank accession no. AY272048). A 270-bp fragment of the Babesia 18S gene from cat 2 was amplified, sequenced, and found to be identical with that of cat 1. The two additional blood samples from cats living in the same household or at a neighboring house were negative for Babesia by microscopy and PCR, seronegative for FIV and FeLV, and also PCR negative for the two feline hemoplasma species. The segments of the 18S rRNA gene (270 bp) that were amplified and sequenced from the two canine Babesia isolates from northern and central Israel were found to be identical with reported sequences of B. canis vogeli.
Phylogenetic analysis.
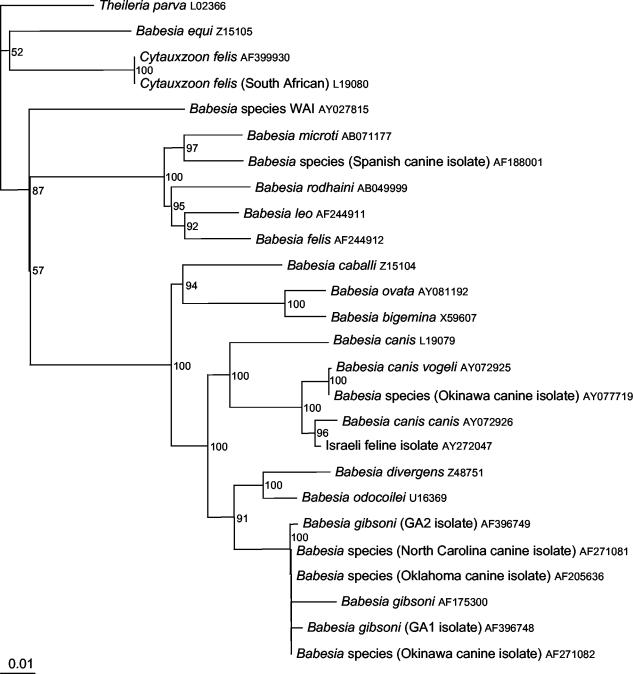
Pairwise sequence comparisons for the 18S and ITS sequences are shown in Table 1 and 2, respectively. The cat sequence clustered within the B. canis species having 97% sequence identity with the 18S rRNA gene of B. canis canis and 96% with B. canis vogeli. Since a full B. canis rossi 18S rRNA gene sequence is not available in GenBank, a comparison with this subspecies was not possible. Pairwise comparison of the ITS region sequences of known B. canis subspecies and the feline Babesia described here showed nucleotide similarities ranging from 62% with B. canis vogeli to 79% with B. canis rossi. Phylogenetic analyses were performed for both the 18S rRNA gene (Fig. 3) and the ITS (Fig. 4) by using the sequences generated from cat 1 and related GenBank sequences. Figure 3 shows that, based on 18S rRNA phylogeny, the Israeli cat sequence groups with the canine large Babesia species, notably B. canis canis. B. canis vogeli is also closely related. Figure 4, based on ITS phylogeny, also shows grouping of the Israeli cat sequence with canine large Babesia species, although only a limited number of sequences are available for inclusion in the ITS phylogenetic tree.
TABLE 1.
Pairwise differences between pairs of B. canis subspecies or Babesia species that infect felines for the 18S rRNA gene (1,693 bases)
| Organism | Pairwise differences (%)
|
|||
|---|---|---|---|---|
| B. canis canis | B. felis | B. leo | Cat isolate Israel 1 | |
| B. canis vogeli | 97 | 83 | 83 | 96 |
| B. canis canis | 82 | 82 | 97 | |
| B. felis | 97 | 83 | ||
| B. leo | 83 | |||
TABLE 2.
Pairwise differences for pairs of B. canis subspecies and cat isolate Israel 1 for the ITS region (640 bases)a
| Organism | Pairwise differences (%)
|
||
|---|---|---|---|
| B. canis vogeli | B. canis rossi | Cat isolate Israel 1 | |
| B. canis canis | 81 | 63 | 62 |
| B. canis vogeli | 79 | 63 | |
| B. canis rossi | 79 | ||
The sequences were edited to cover the portion available for the Israeli cat Babesia isolate.
FIG. 3.
Phylogeny of 18S gene of the feline Babesia isolate and related species. The phylogenetic tree was constructed by using the neighbor-joining program from a distance matrix corrected for nucleotide substitutions by the Kimura two-parameter model. Theileria parva was included as an outgroup. Evolutionary distances are to the scale shown. Bootstrap percentage values are given to the right of the tree nodes. GenBank accession numbers are shown.
FIG. 4.
Phylogeny of the ITS region of the feline Babesia isolate and related species. The phylogenetic tree was constructed by using the neighbor-joining program from a distance matrix corrected for nucleotide substitutions by the Kimura two-parameter model. T. parva was included as an outgroup. Evolutionary distances are to the scale shown. Bootstrap percentage values are given to the right of the tree nodes. GenBank accession numbers are shown.
DISCUSSION
In the past, Babesia species have been described on the basis of their morphology and animal hosts. More recently, genetic and antigenic analyses have enhanced taxonomic studies. The subspeciation of B. canis was originally proposed on the basis of geographical variation in tick vectors, antigenic properties, and the clinical manifestations of infection. This division was subsequently supported by DNA analyses indicating genetic dissimilarity between the subspecies (5, 36). The 18S rRNA gene relatedness of the Israeli feline Babesia indicated that it clustered with the B. canis species. However, the marked sequence difference in the ITS, the region used previously for subspeciating of B. canis (36), and the distinctly smaller size of the merozoite and trophozoite stages have led us to propose that this feline genotype be recognized as a new B. canis subspecies. We propose to name this subspecies B. canis subsp. presentii after the late Ben-Zion Presenti, the founder of the Hematology Department at the Hebrew University School of Veterinary Medicine.
The 18S rRNA and phylogenetic and pairwise analyses showed that the Israeli cat Babesia was most closely related to B. canis canis. B. canis vogeli and the Okinawa canine isolate (GenBank accession number AY077719) were also closely related. The relatedness of the Israeli cat Babesia to the canine large Babesia species was further assessed with the ITS sequence because a full 18S sequence for B. canis rossi was not available, and since this part of the rRNA operon has been previously used for the comparison of B. canis subspecies (36). The homogeneity of the ITS-1 can be used to infer phylogeny between organisms that have diverged within the last 50 million years (14). It has been suggested for use in species level analysis (2) and used for the description of new apicomplexan parasites (21). The analyses of the Israeli feline ITS sequence supported again the clustering of this cat Babesia within the B. canis species. However, the cat ITS sequence was different from B. canis canis and B. canis vogeli and closer yet dissimilar with B. canis rossi (79% pairwise base similarity).
B. canis infection in cats is supported by a recent report in which a 395-bp fragment of the 18S rRNA gene was amplified from the blood of two cats from Portugal and one from Spain by using a seminested PCR (6). Although identified as B. canis canis, only a relatively short fragment of the 18S gene was sequenced, and Babesia parasites were not evident on microscopic examination of stained blood smears. However, a sequence comparison of the 18S gene fragment amplified by Criado-Forenelio (6) (accession number AY15057) and the corresponding part of the 18S gene sequence from the cats described in this report (accession number AY272047), showed 99.5% identity with variation in only two bases. Further sequence and morphological information for the Iberian feline B. canis parasite may indicate identity or a close relatedness with the Israeli feline B. canis. Interestingly, two of these cats from the Iberian Peninsula were coinfected with FeLV or FIV.
The feline B. canis described here is markedly smaller in size than B. canis measured in canine erythrocytes. The mean sizes of the trophozite and merozoites are 2.5 by 1.7 μm and 2.7 by 1.4 μm, respectively, which is nearly half of the size reported for B. canis in dogs (5 by 2.5 to 3.0 μm) (18). Some size variation might be explained by species differences between erythrocytes of cats and dogs, the former being smaller and more compact, whereas the latter are large and biconcave. However, the marked differences in size most probably reflect true variation in parasite morphology. The shape of the merozoites and trophozoites described in this report is similar to B. canis in dogs and different to that of other feline species. No tetrad forms (Maltese cross), which are typical of some small feline Babesia species such as B. felis and B. leo (24), were found, and the maximum number of parasites within an erythrocyte was four, whereas up to eight parasites were reported for B. cati (23) and for the uncharacterized domestic feline Babesia from Zimbabwe (29).
Although smaller than B. canis subspecies in dogs, the Israeli feline B. canis is larger than most of the wild and domestic feline piroplasm species previously recorded. Only B. herpailuri, which is 2.7 by 2.2 μm, is larger (7). B. felis is 0.9 by 0.7 μm (7), B. leo is 1.05 by 1.0 μm (24), Cytauxzoon felis is 1 to 1.2 μm in length (34), B. cati is 0.5 to 2.0 μm in length (23), and B. panthera is 2.0 by 1.8 μm (7). According to the size classification of feline Babesia species suggested by Denning (8), the Babesia species described here is considered a large feline Babesia.
Molecular confirmation of B. canis vogeli DNA in the two dogs with clinical babesiosis in the present study indicated that this subspecies of B. canis is present in dogs in Israel. This was expected because R. sanguineus, the vector of B. canis vogeli, is prevalent in Israel and the tick vectors of other Babesia canis subspecies have not been reported there. Future research may show whether the feline B. canis described here is also present in dogs or other animals, or if it is found exclusively in domestic cats.
Babesiosis can range from an asymptomatic or mild infection to a severe illness depending on the virulence of the infecting Babesia species and the susceptibility of the individual host. The cat with clinical babesiosis had a higher parasitemia and was FIV antibody positive and coinfected with “Candidatus M. haemominutum.” In contrast, the cat with a low parasitemia was apparently asymptomatic and negative for FIV antibody and hemoplasma species. It is possible that either immunosuppression induced by FIV or coinfection with hemoplasma species played a role in exacerbating Babesia infection and triggering clinical signs of icterus and anemia. “Candidatus M. haemominutum” infection in cats is usually regarded as being relatively apathogenic since experimental infection does not usually result in significant clinical signs (9, 35), and no association between anemia and infection was found in two studies evaluating naturally infected cats (15, 31). However, others have reported the development of mild to moderate anemia in some cats inoculated with “Candidatus M. haemominutum,” and the degree of anemia may be more severe in cats coinfected with retroviruses (10). In the case of cat 1, the rapid response to therapy with the elevation in red blood cell counts just several days after treatment probably indicated that FIV was not a major contributor to the cat's anemia. Both B. canis infection in dogs (19) and feline hemoplasma infections (20) respond to imidocarb diproprionate therapy. Consequently, a therapeutic response in cat 1 does not indicate whether either or both infections played a role in the development of clinical disease. Given the fact that cat 1, which did develop a severe clinical disease, was coinfected with FIV and “Candidatus M. haemominutum,” it is likely that the interaction between the Babesia and one or two of these pathogens was responsible for the severe disease found in that cat.
Further research is indicated to investigate the tick species responsible for transmission of the feline B. canis proposed subspecies, the spectrum of host species it infects, the prevalence of symptomatic and asymptomatic infections in cats, and the role of coinfecting agents such as FIV, FeLV, or hemoplasmas in the pathogenesis of infection.
REFERENCES
- 1.Averbeck, G. A., K. E. Bjork, C. Packer, and L. Herbst. 1990. Prevalence of haematozoans in lions (Panthera leo) and cheetah (Acinonyx jubatus) in Serengeti National Park and Ngorongoro Crater, Tanzania. J. Wildl. Dis. 26:392-394. [DOI] [PubMed] [Google Scholar]
- 2.Barta, J. R. 1997. Investigating phylogenetic relationships within the Apicomplexa using sequence data: the search for homology. Methods 13:81-88. [DOI] [PubMed] [Google Scholar]
- 3.Bourdeau, P. 1996. Les babesioses felines. Le Point Vet. 27:947-953. (In French.) [Google Scholar]
- 4.Caccio, S. M., B. Antunovic, C. Moretti, V. Mangli, A. Marinculic, R. R. Baric, S. Slemenda, and N. J. Pieniazek. 2002. Molecular characterization of Babesia canis canis and Babesia canis vogeli from naturally infected European dogs. Vet. Parasitol. 106:285-292. [DOI] [PubMed] [Google Scholar]
- 5.Carret, C., F. Walas, B. Carcy, N. Grande, E. Precigout, K. Moubri, T. P. Schetters, and A. Gorenflot. 1999. Babesia canis canis, Babesia canis vogeli, Babesia canis rossi: differentiation of the three subspecies by a restriction fragment length polymorphism analysis on amplified small subunit rRNA genes. J. Eukaryot. Microbiol. 46:298-303. [DOI] [PubMed] [Google Scholar]
- 6.Criado-Fornelio, A., A. Martinex-Marcos, A. Buling-Sarana, and J. C. Barba-Carretero. 2003. Presence of Mycoplasma haemofelis, Mycoplasma haemominutum and piroplasmids in cats from southern Europe: a molecular study. Vet. Microbiol. 93:307-317. [DOI] [PubMed] [Google Scholar]
- 7.Denning, H. K. 1967. Eine unbekannte Babesienart beim Jaguarundi (Herpailurus yaguarundi). Kleintierpraxis 12:146-152. (In German.) [Google Scholar]
- 8.Denning, H. K., and D. W. Brockelsby. 1972. Babesia pantherae sp. nov., a piroplasm of the leopard (Pantera pardus). Parasitology 64:525-532. [DOI] [PubMed] [Google Scholar]
- 9.Foley, J. E., S. Harrus, A. Poland, B. Chomel, and N. C. Pedersen. 1998. Molecular, clinical, and pathologic comparison of two distinct strains of Haemobartonella felis in domestic cats. Am. J. Vet. Res. 59:1581-1588. [PubMed] [Google Scholar]
- 10.George, J. W., B. A. Rideout, S. M. Griffey, and N. C. Pedersen. 2002. Effect of preexisting FeLV infection or FeLV and feline immunodeficiency virus coinfection on pathogenicity of the small variant of Haemobartonella felis in cats. Am. J. Vet. Res. 63:1172-1178. [DOI] [PubMed] [Google Scholar]
- 11.Glenn, B. L., A. A. Kocan, and E. F. Blouin. 1983. Cytauxzoonosis in bobcats. J. Am. Vet. Med. Assoc. 183:1155-1158. [PubMed] [Google Scholar]
- 12.Greene, C. E., K. Latimer, E. Hopper, G. Shoeffler, K. Lower, and F. Cullens. 1999. Administration of diminazene aceturate or imidocarb dipropionate for treatment of cytauxzoonosis in cats. J. Am. Vet. Med. Assoc. 215:497-500. [PubMed] [Google Scholar]
- 13.Hauschild, S., and E. Schein. 1996. The subspecies specificity of Babesia canis. Berl. Munch. Tierarztl. Wochenschr. 109:216-219. [PubMed] [Google Scholar]
- 14.Hillis, D. M., and T. M. Dixon. 1991. Ribosomal DNA: molecular evolution and phylogenetic inference. Q. Rev. Biol. 66:411-453. [DOI] [PubMed] [Google Scholar]
- 15.Jensen, W. A., M. R. Lappin, S. Kamkar, and W. J. Reagen. 2001. Use of a polymerase chain reaction assay to detect and differentiate two strains of Haemobartonella felis infection in naturally infected cats. Am. J. Vet. Res. 62:604-608. [DOI] [PubMed] [Google Scholar]
- 16.Jittapalapong, S., and W. Jansawan. 1993. Preliminary survey on blood parasites of cats in Bangkhen District area. Kasetsart J. (Nat. Sci.) 27:330-335. [Google Scholar]
- 17.Kjemtrup, A. M., A. A. Kocan, L. Whitworth, J. Meinkoth, A. J. Birkenheuer, J. Cumming, M. K. Boudreaux, S. L. Stockham, A. Irizarry-Rovira, and P. A. Conrad. 2000. There are at least three genetically distinct small piroplasms from dogs. Int. J. Parasitol. 120:487-493. [DOI] [PubMed] [Google Scholar]
- 18.Kuttler, K. L. 1988. World-wide impact of babesiosis, p. 1-22. In M. Ristic (ed.), Babesiosis of domestic animals and man. CRC Press, Inc., Boca Raton, Fla.
- 19.Kuttler, K. L. 1988. Chemotherapy of canine babesiosis, p. 227-242. In M. Ristic (ed.), Babesiosis of domestic animals and man. CRC Press, Inc., Boca Raton, Fla.
- 20.Lappin, M. R., M. Brewer, and S. Radecki. 2002. Effects of imidocarb dipropionate in cats with chronic hemobartonellosis. Vet. Ther. 2:144-149. [PubMed] [Google Scholar]
- 21.Marsh, A. E., B. C. Barr, A. E. Packham, and P. A. Conrad. 1998. Description of a new Neospora species (Protozoa: Apicomplexa: Sarcocystidae). J. Parasitol. 84:983-991. [PubMed] [Google Scholar]
- 22.Moik, K., and R. Gothe. 1997. Babesin-infektionen der Feliden und Fallbeschreibung bei einer Katze in Deutschland. Tierarztl. Prax. 25:532-535. (In German.) [Google Scholar]
- 23.Mudaliar, S. V., G. R. Achary, and V. S. Alwar. 1950. On a species of Babesia in an Indian wild cat (Felis catus). Indian Vet. J. 26:391-395. [PubMed] [Google Scholar]
- 24.Penzhorn, B. L., A. M. Kjemtrup, L. M. Lopez-Rebollar, and P. A. Conrad. 2001. Babesia leo n. sp. from lions in the Kruger National Park, South Africa, and its relation to other small piroplasms. J. Parasitol. 87:681-685. [DOI] [PubMed] [Google Scholar]
- 25.Reyers, F., A. L. Leisewitz, R. G. Lobetti, R. J. Milner, L. S. Jacobson, and M. van Zyl. 1998. Canine babesiosis in South Africa: more than one disease. Does this serve as a model for falciparum malaria? Ann. Trop. Med. Parasitol. 92:503-511. [PubMed] [Google Scholar]
- 26.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 27.Schetters, P. M., K. Moubri, E. Precigout, J.Kleuskens, N. C. Scholtes, and A. Gorenflot. 1997. Different Babesia isolates, different diseases. Parasitology 115:485-493. [DOI] [PubMed] [Google Scholar]
- 28.Schoeman, T., R. G. Lobetti, L. S. Jacobson, and B. L. Penzhorn. 2001. Feline babesiosis: signalment, clinical pathology and concurrent infections. J. S. Afr. Vet. Assoc. 72:4-11. [DOI] [PubMed] [Google Scholar]
- 29.Stewart, C. G., Hackett, K. J. W., and M. G. Collett. 1980. An unidentified Babesia of the domestic cat (Felis domesticus). J. S. Afr. Vet. Assoc. 51:219-221. [PubMed] [Google Scholar]
- 30.Tasker, S., C. R. Helps, M. J. Day, T. J. Gruffydd-Jones, and D. A. Harbour. 2003. Use of real-time PCR to detect and quantify Mycoplasma haemofelis and “Candidatus Mycoplasma haemominutum” DNA. J. Clin. Microbiol. 41:439-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tasker, S., S. H. Binns, M. J. Day, T. J. Gruffydd-Jones, D. A. Harbour, C. R. Helps, W. A. Jensen, C. S. Olver, and M. R. Lappin. 2003. Use of a PCR assay to assess prevalence and risk factors for Mycoplasma haemofelis and “Candidatus Mycoplasma haemominutum” in cats in the United Kingdom. Vet. Rec. 152:193-198. [DOI] [PubMed] [Google Scholar]
- 32.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uilenberg, G., Franssen, F. F. J., N. M. Perie, and A. M. Spanjer. 1989. Three groups of Babesia canis distinguished and a proposal for nomenclature. Vet. Q. 11:33-40. [DOI] [PubMed] [Google Scholar]
- 34.Wagner, J. E. 1975. Cytauxzoonosis in domestic cats (Felis domesticus) in Missouri. J. Am. Vet. Med. Assoc. 167:874. [Google Scholar]
- 35.Westfall, D. S., W. A. Jensen, W. J. Reagan, S. V. Radecki, and M. R. Lappin. 2001. Inoculation of two genotypes of Haemobartonella felis (California and Ohio variants) to induce infection in cats and the response to treatment with azithromycin. Am. J. Vet. Res. 62:687-691. [DOI] [PubMed] [Google Scholar]
- 36.Zahler, M., E. Schein, H. Rinder, and R. Gothe. 1998. Characteristic genotypes discriminate between Babesia canis isolates of differing vector specificity and pathogenicity to dogs. Parasitol. Res. 84:544-548. [DOI] [PubMed] [Google Scholar]
- 37.Zahler, M., H. Rinder, E. Schein, and R. Gothe. 2000. Detection of a new pathogenic Babesia microti-like species in dogs. Vet. Parasitol. 89:241-248. [DOI] [PubMed] [Google Scholar]