Abstract
Disseminated infection with the coelomycetous fungus Nattrassia mangiferae is a very rare disease affecting only the immunocompromised host. We report the first case of a disseminated infection with spondylodiscitis and granular skin lesions due to N. mangiferae in a renal transplant patient.
CASE REPORT
A 62-year-old male of Turkish origin, who had been living in Austria for more than 15 years, underwent cadaveric kidney transplantation because of vascular nephropathy in September 2001. Initial immunosuppressive therapy consisted of cyclosporin A, mycophenolate mofetil, and steroids.
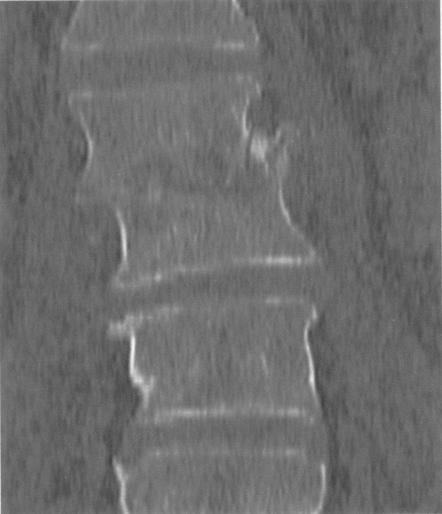
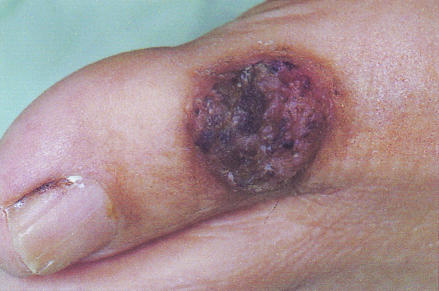
In August 2002, the patient stayed in Izmir, Turkey, for 4 weeks. During his stay, he developed fever. Moreover, coin-shaped lesions on the back of both hands and feet were described. The patient returned to Austria and was admitted with suspected pneumonia. In addition, the patient complained about lumbosacral pain, and black granulomatous lesions were now seen all over the body. Subsequent magnetic resonance imaging of the lumbosacral spine detected an L2/L3 spondylodiscitis (Fig. 1). Massive compression of the vertebral bodies together with the osteolytic process between the vertebral bodies and moderate swelling of the paraspinal soft tissues were described. Computed tomography (CT) showed pulmonary infiltrates and inflammatory destruction of ribs 8 and 10. An elevated C-reactive protein level of 13.1 mg/dl (usually <1 mg/dl) and leukocyte count of 15.8 per liter with 89% neutrophils and 5% lymphocytes were indicative of an inflammatory process. A CT-guided aspiration obtained from L2/L3, cerebrospinal fluid (CSF), and a skin biopsy of the coin-shaped lesions were cultured and histologically investigated. A black granulomatous lesion localized to the big toe was especially prominent and was recognized as one of the first skin lesions (Fig. 2). Histologically, the L2/L3 aspirate showed a chronic inflammatory process; the presence of tuberculosis could be excluded. Also, PCR performed repeatedly to detect Mycobacterium tuberculosis and Mycobacterium avium from CSF and urine showed only negative results. In the CSF, pleocytosis was observed (667 cells/μl with 95% neutrophils), but neither bacteria nor fungal elements could be detected. The aspirate and the skin biopsy showed growth of a fungus. The cultures from CSF and blood remained negative.
FIG. 1.
Coronal reconstructions in a bone display window. The figure shows massive compression of the vertebral bodies. Moreover, the cranial vertebral body deviates to the right.
FIG. 2.
Black granulomatous lesion localized to the big toe. This was recognized as one of the first skin lesions.
The aspirate and the skin biopsy were inoculated on blood agar (bioMérieux, L'Etoile, France), Sabouraud dextrose agar (Oxoid, Basingstoke, United Kingdom), and brain heart infusion agar (Oxoid) and incubated at both 30 and 35°C. After 24 h, cultures were positive with a rapidly expanding filamentous hyaline fungus. After 3 to 4 days, a hairy black growth that consisted of large brown arthroconidia identical to the Scytalidium synanamorph of Nattrassia mangiferae could be observed. Growth was observed on all media inoculated.
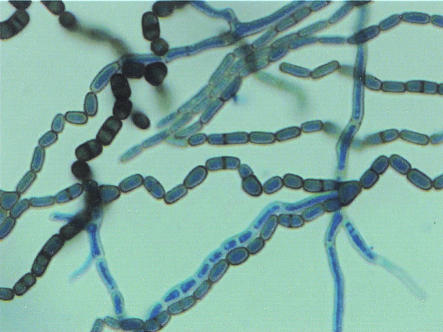
The fungus was identified as N. mangiferae by its colonial and microscopic features. Identification was confirmed by the national reference laboratory, the Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands. The colonies grew rapidly, filling the petri dish within 3 to 5 days and turning from olivaceous gray to black. The reverse darkened to a grayish black after 10 days of incubation. Microscopic examination showed septate, branched, subhyaline-to-dark brown hyphae fragmented to form arthroconidia, which were smooth walled and initially cylindrical, but soon became ellipsoidal (Fig. 3). Conidia were also smooth walled, one celled, and hyaline at first and septate and dark brown during later stages. Pycnidia were observed after 2 to 3 weeks.
FIG. 3.
Lactophenol blue stain of N. mangiferae, synanamorph of S. dimidiatum. Shown are broad hyphae and lateral branches fragmenting to form nonseptate or one-septate arthroconidia.
Susceptibility tests were performed by means of the E-test as described by the manufacturer (AB Biodisk, Solna, Sweden). The MICs of most antifungal agents were relatively high (itraconazole, 0.5 μg/ml; ketoconazole, 1.0 μg/ml; flucytosine, >32 μg/ml; fluconazole, 24 μg/ml), with the exceptions of amphotericin B (0.032 μg/ml) and voriconazole (0.012 μg/ml). No data exist defining breakpoints for N. mangiferae. Because the MICs of amphotericin B and voriconazole were very low, the isolate was assumed to be susceptible to these drugs.
The patient was treated for 9 weeks with continuously administered amphotericin B (1.5 mg/kg of body weight), which was well tolerated. Repeated skin biopsies showed growth of N. mangiferae for 7 weeks and then became negative. Because a moderate rise in the level of creatinine was observed, therapy with amphotericin B was terminated, and oral voriconazole was administered at 400 mg/day. Skin lesions became less prominent, the pulmonary infiltrates also resolved under this therapy, and the patient was gradually recovering. After 4 weeks, therapy was switched again to continuously administered amphotericin B (1 mg/kg of body weight), because his clinical condition deteriorated due to pneumonia. However, N. mangiferae could not be detected. Therefore, therapy with amphotericin B was stopped again, and voriconazole was continued. A month later, the patient, on therapy with voriconazole, died due to bacterial pneumonia. An autopsy was refused by the patient's family, but permission was given for limited sampling. Thus, lung tissue, CSF, and skin were obtained by puncture for culturing. All of the postmortem cultures were negative for N. mangiferae.
Nattrassia mangiferae, formerly Hendersonula toruloidea and the synanamorph of Scytalidium dimidiatum, is a dematiaceous coelomycete and a known plant pathogen in tropical and subtropical woods. Gentles and Evans (4) were the first to report on N. mangiferae isolates causing superficial dermatomycosis and onychomycosis clinically undistinguishable from typical dermatophyte infections. Since then, a number of reports have described patients who had immigrated to the United Kingdom (7, 11). Other papers documented the incidence in residents of countries where the fungus is known to be endemic (5, 12).
Reviews of the literature on N. mangiferae infections and infections due to Scytalidium species have been provided by Frankel and Rippon, Moore, and Sigler and Sutton (3, 4, 8, 11-13), but there have been very few reports of deep or disseminated infections. Reports have involved mycetoma (2) and subcutaneous abscesses of the foot (14), facial lesions (10), fungemia and abdominal abscesses (1), endophthalmitis following trauma (6), and abscess following trauma to the base of a finger (9).
It has been presumed that infection is the result of direct contact with soil or plants, especially in those individuals typically residing in or having visited areas of endemicity. Proven transmission from human to human or from animal to human is unknown. In this case, we were unable to trace the portal of entry of N. mangiferae. Whether the patient contracted the infection during his stay in Izmir or had contact with tropical woods remains unclear. Although we are not aware of any reports concerning N. mangiferae occurring in Turkey, the climate in Izmir and the surrounding area may be favorable enough to allow growth and colonization of this fungus. Thus, we speculate that the patient acquired the infection during his stay in Izmir, and the big toe showing the first skin lesion may have been the portal of entry.
Optimal therapy of disseminated Nattrassia infections seems to be difficult. Therapy with amphotericin B has been effective in a patient with facial lesions and underlying immunosuppression and in a French diabetic patient with dual infection of the maxillary sinus caused by a zygomycete and N. mangiferae (12). In another report, management of neutropenia combined with amphotericin B therapy was found to be effective in a child with fungemia and abdominal lesions caused by Scytalidium dimidiatum (1). In our case, only two agents exhibited low MICs. Because our isolate seemed to be susceptible in vitro to amphotericin B with a MIC of 0.032 μg/ml, systemic amphotericin B was chosen as the first-line therapy. Our patient improved gradually, the skin lesions became less prominent, and the cultures of skin biopsies became negative after 7 weeks of therapy. Voriconazole also showed a low MIC and was used when renal impairment was suspected after 9 weeks of therapy, and a change to oral therapy seemed to be indicated.
The fact that the cultures remained negative and the patient improved steadily indicates that voriconazole may be an alternative for therapy of disseminated Nattrassia infections.
Because autopsy was refused, the exact cause of death is unknown. However, because all postmortem cultures were negative for N. mangiferae, we are convinced that the patient did not die of the fungal infection.
To our knowledge, this is the first report of a disseminated infection including spondylodiscitis due to N. mangiferae in an immunosuppressed patient. This case illustrates the potential for disseminated infection and pathogenicity of this fungus when underlying conditions are present. From our experience, amphotericin B and voriconazole seem to be of value in the treatment of disseminated infections caused by N. mangiferae.
REFERENCES
- 1.Benne, C. A., C. Neeleman, M. Bruin, G. S. de Hoog, and A. Fleer. 1993. Disseminating infection with Scytalidium dimidiatum in a granulocytopenic child. Eur. J. Clin. Microbiol. Infect. Dis. 12:118-121. [DOI] [PubMed] [Google Scholar]
- 2.Drouhet, E., and B. Dupont. 1983. Laboratory and clinical assessment of ketoconazole in deep-seated mycoses. Am. J. Med. 74(Suppl. 1B):30-47. [DOI] [PubMed] [Google Scholar]
- 3.Frankel, D. H., and J. W. Rippon. 1989. Hendersonula toruloidea infection in man. Mycopathologia 105:175-186. [DOI] [PubMed] [Google Scholar]
- 4.Gentles, J. C., and E. G. V. Evans. 1970. Infection of the feet and nails with Hendersonula toruloidea. Sabouraudia 8:72-75. [PubMed] [Google Scholar]
- 5.Goon, A. T.-J., and C.-S. Seow. 2002. Three cases of Nattrassia mangiferae (Scytalidium dimidiatum) infection in Singapore. Int. J. Dermatol. 41:53-55. [DOI] [PubMed] [Google Scholar]
- 6.Gumbo, T., N. Mkanganwi, V. J. Robertson, and P. Masvaire. 2002. Case report. Nattrassia mangiferae endophthalmitis. Mycoses 45:118-119. [DOI] [PubMed] [Google Scholar]
- 7.Hay, R. J., and M. K. Moore. 1984. Clinical features of superficial fungal infections caused by Hendersonula toruloidea and Scytalidium hyalinum. Br. J. Dermatol. 110:677-683. [DOI] [PubMed] [Google Scholar]
- 8.Lacaz, C. S., A. D. Pereira, E. M. Heins-Vaccari, L. C. Cucé, C. Benatti, R. S. Nunes, N. T. de Melo, R. S. de Freitas-Leite, and G. L. Hernández-Arriagada. 1999. Onychomycosis caused by Scytalidium dimidiatum. Report of two cases. Review of the taxonomy of the synanamorph and anamorph forms of this coelomycete. Rev. Inst. Med. Trop. Sao Paulo 41:319-323. [PubMed] [Google Scholar]
- 9.Levi, M. E., and J. W. Smith. 1994. Posttraumatic infection due to Scytalidium dimidiatum. Clin. Infect. Dis. 18:128-129. [DOI] [PubMed] [Google Scholar]
- 10.Mariat, F., B. Liautaud, M. Liautaud, and F.-G. Marill. 1978. Hendersonula toruloidea, agent d'une dermatite verruqueuse mycosique observee en Algerie. Sabouraudia 16:133-140. [PubMed] [Google Scholar]
- 11.Moore, M. K. 1988. Morphological and physiological studies of isolates of Hendersonula toruloidea Nattrass cultures from human skin and nail samples. J. Med. Vet. Mycol. 26:25-39. [DOI] [PubMed] [Google Scholar]
- 12.Sigler, L., R. C. Summerbell, L. Poole, M. Wieden, D. A. Sutton, M. G. Rinaldi, M. Aguirre, G. W. Estes, and J. N. Galgiani. 1997. Invasive Nattrassia mangiferae infections: case report, literature review, and therapeutic and taxonomic appraisal. J. Clin. Microbiol. 35:433-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutton, D. A. 1999. Coelomycetes fungi in human disease. A review: clinical entities, pathogenesis, identification and therapy. Rev. Iberoam. Micol. 16:171-179. [PubMed] [Google Scholar]
- 14.Zaatari, G. S., G. Reed, and R. Morewessel. 1984. Subcutaneous hyphomycosis caused by Scytalidium hyalinum. Am. J. Clin. Pathol. 82:252-256. [DOI] [PubMed] [Google Scholar]