Abstract
Published assays that use TaqMan PCR are consistently sensitive, rapid, and readily transferable. Here we describe a TaqMan PCR-based method for the detection of rabies virus (RV) RNA in tissue samples. We show that the method has an acceptable linear range, is both sensitive and specific, and, importantly, correlates with the concentration of infectious virus. In addition, the levels of RV-specific amplification are adjustable according to the levels of an endogenous control (β-actin mRNA), allowing the calculation of comparable quantities. We tested the capacity of this assay to cope with target sequence variations. The number of sequence mismatches between gene-specific oligonucleotides and the target sequence significantly affects amplification (P < 0.001), and point mutations at the center of the probe can result in false-negative results through the prevention of probe binding and subsequent fluorescence. This study demonstrates that the genetic heterogeneity of RVs may prove a serious obstacle in the development of a diagnostic assay based on TaqMan PCR; however, the quantification of RV levels may prove to be a valuable application of this assay.
TaqMan PCR is widely implemented as a detection and quantitation method for viruses isolated from a variety of sample types. Assays have been developed for a number of negative-strand RNA viruses, e.g., respiratory syncytial virus (8), Hendra virus (17) and Rift Valley fever virus (6), and have been found to be sensitive and specific, coupled with the advantages of a test that is rapid, requires no post-PCR manipulation, and is amenable to high-throughput testing.
The rabies virus (RV) is the type member of the Lyssavirus genus that includes Lagos bat virus, Mokola virus, Duvenhage virus, European bat lyssavirus 1, European bat lyssavirus 2, and Australian bat lyssavirus. Recently, a new lyssavirus discovered in central Asia has been proposed as a new genotype (2). Isolates of RV in the United States exhibit considerable sequence heterogeneity in nature (18), making problematic the designing of oligonucleotide primers for RNA detection. RNA virus heterogeneity may affect the performance of TaqMan PCR assays (16), but there are few data available on the tolerance of TaqMan technology to sequence variation. A TaqMan PCR assay to differentiate lyssaviruses by genotype has been developed (3), but there has been no formal assessment of the impact of RNA sequence diversity on this assay.
The direct fluorescent antibody test (25) is the current laboratory test of choice for detecting RV antigen in tissue samples and has adequate levels of sensitivity and specificity when it is performed correctly (22, 23). The advantages of RNA detection methods are in the transferability of the technology to a wide variety of other sample types that may be unsuitable for the direct fluorescent antibody test, such as saliva and cerebrospinal fluid (5). PCR has been used for the confirmatory diagnosis of human rabies when other tests could not be readily applied (4). For experimental studies, PCR-based techniques have been utilized for the detection of RV RNA from oral swabs (15). RNA can be buffered adequately at room temperature for considerable periods by using commercially available products such as RNAlater (Ambion) and TRIzol (Life Technologies).
In addition to its application to qualitative detection-based assays, TaqMan technology has been used to quantify viral loads from tissue samples (1). At present, methods for estimating the levels of RV in clinical samples are limited to titration in animals or tissue culture. The classic mouse inoculation test (10) can lead to a considerable delay in the estimation of an end point, requires facilities for the use of experimental animals, and is labor intensive. Cell culture isolation methods (26) are problematical due to the inability of certain RV variants to propagate easily in specific cell lines. The TaqMan PCR provides a potential means to accurately quantify levels of RV RNA in a wide variety of sample types. Such information is vital for investigating RV pathogenesis.
In this study, we assessed a TaqMan PCR for the detection of RV RNA in clinical samples. We also assessed the kinetics of the TaqMan reaction for application to quantitative virology and analyzed the effects of sequence divergence on the efficiency of this reaction.
MATERIALS AND METHODS
Experimental samples.
Experimental samples were used to investigate assay dynamics (linearity, sensitivity, and amenability to quantification). Tissue samples were obtained at necropsy from rabid, striped skunks (Mephitis mephitis) experimentally infected with either an RV variant epidemiologically associated with big brown bats (Eptesicus fuscus; herein referred to as AZ-EF) or an RV variant associated historically with skunks in the southwestern United States (herein referred to as AZ-SK). AZ-EF has recently been identified in naturally infected skunks (J. Smith, R. Rohde, B. Mayes, C. Parmely, and M. Leslie, Abstr. 12th Annu. Rabies Am. Conf., abstr., 2001). All animal care and experimental procedures were performed in compliance with the Centers for Disease Control and Prevention (CDC) Institutional Animal Care and Use Guidelines.
Following homogenization in phosphate-buffered saline, salivary gland (SG) samples were titrated in mice (10), and 250 μl of homogenate was added directly to TRIzol LS reagent (Invitrogen Life Technologies, Carlsbad, Calif.) for RNA extraction. A 3-mm3 sample of frozen brain tissue (BR) was homogenized directly in 750 μl of TRIzol reagent according to the manufacturer's instructions. All samples were stored at −80°C prior to processing.
Historical samples.
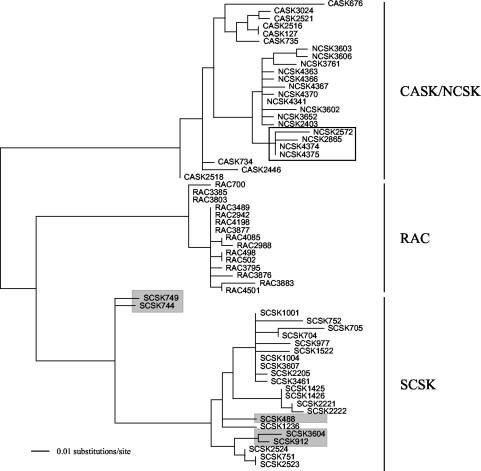
A selection of historical samples was used to test the effects of sequence variation on the kinetics of the TaqMan reaction. Four RV variants associated with the infection of terrestrial mammals (11) were selected for this study: California skunk (CASK), north central skunk (NCSK), raccoon (RAC), and south central skunk (SCSK). A total of 63 isolates, which were previously typed by molecular techniques, were selected from those available at the CDC where sequence data for the nucleoprotein (N) gene were available (Table 1 and Fig. 1). These samples are not representative of all circulating lineages of RV in the United States, but they provide a means to study the effect of sequence diversity on the TaqMan PCR. RNA extracted prior to November 1994 had been extracted by a method described elsewhere (19). After November 1994, RNA was extracted by using TRIzol according to the manufacturer's instructions.
TABLE 1.
List of historical samples used for variant-specific TaqMan PCRs
| Variant | Sample no. | Datea | Stateb | Hostc | Mismatchesd
|
TaqMan resulte
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | R | P | T | CASK | NCSK | RAC | SCSK | |||||
| CASK | 127 | 08/15/91 | CA | SK | 0 | 0 | 0 | 0 | 21.47 | 40.00 | 40.00 | 40.00 |
| CASK | 676 | 02/02/94 | CA | SK | 1 | 0 | 1 | 2 | 32.66 | 40.00 | 40.00 | 40.00 |
| CASK | 734 | 08/15/91 | CA | SK | 1 | 1 | 0 | 2 | 22.34 | 40.00 | 40.00 | 40.00 |
| CASK | 735 | 08/15/91 | CA | SK | 0 | 0 | 0 | 0 | 18.93 | 40.00 | 40.00 | 40.00 |
| CASK | 2446 | 08/12/94 | CA | WD | 0 | 1 | 0 | 1 | 26.31 | 40.00 | 40.00 | 40.00 |
| CASK | 2516 | 09/01/94 | CA | SK | 0 | 0 | 0 | 0 | 17.65 | 40.00 | 40.00 | 40.00 |
| CASK | 2518 | 09/02/94 | CA | FX | 0 | 1 | 0 | 1 | 22.46 | 40.00 | 40.00 | 40.00 |
| CASK | 2521 | 09/06/94 | CA | SK | 1 | 0 | 0 | 1 | 34.56 | 40.00 | 40.00 | 40.00 |
| CASK | 3024 | 01/29/98 | CA | DG | 0 | 0 | 0 | 0 | 22.95 | 40.00 | 40.00 | 40.00 |
| NCSK | 2403 | 02/14/95 | TN | DG | 0 | 0 | 0 | 0 | 22.31 | 19.33 | 40.00 | 40.00 |
| NCSK | 2572 | 02/15/95 | KY | DG | 0 | 1 | 0 | 1 | 27.09 | 40.00 | 40.00 | 40.00 |
| NCSK | 2865 | 06/12/95 | TN | FX | 1 | 1 | 0 | 2 | 32.98 | 40.00 | 40.00 | 40.00 |
| NCSK | 3602 | 08/18/97 | OH | DG | 2 | 0 | 0 | 2 | 25.44 | 24.03 | 40.00 | 40.00 |
| NCSK | 3603 | 01/29/98 | AR | DG | 0 | 1 | 1 | 2 | 32.28 | 29.40 | 40.00 | 40.00 |
| NCSK | 3606 | 01/21/97 | AR | DG | 1 | 1 | 0 | 2 | 29.18 | 26.74 | 40.00 | 40.00 |
| NCSK | 3761 | 02/12/98 | KY | DG | 0 | 0 | 1 | 1 | 35.59 | 28.70 | 40.00 | 40.00 |
| NCSK | 4341 | 04/15/99 | MI | SK | 0 | 0 | 0 | 0 | 18.14 | 17.41 | 40.00 | 40.00 |
| NCSK | 4363 | 05/17/99 | KY | SK | 0 | 0 | 1 | 1 | 26.91 | 16.23 | 39.15 | 40.00 |
| NCSK | 4366 | 05/17/99 | KY | SK | 0 | 0 | 0 | 0 | 19.73 | 18.04 | 40.00 | 40.00 |
| NCSK | 4367 | 05/17/99 | KY | SK | 0 | 0 | 1 | 1 | 24.22 | 18.47 | 40.00 | 40.00 |
| NCSK | 4370 | 05/17/99 | KY | SK | 0 | 0 | 0 | 0 | 22.64 | 18.45 | 40.00 | 40.00 |
| NCSK | 4374 | 06/25/99 | VA | SK | 0 | 1 | 0 | 1 | 17.11 | 40.00 | 40.00 | 40.00 |
| NCSK | 4375 | 06/25/99 | KY | SK | 0 | 1 | 0 | 1 | 19.64 | 40.00 | 40.00 | 40.00 |
| NCSK | 3652 | 11/22/97 | TX | HR | 0 | 0 | 0 | 0 | 26.00 | 24.19 | 40.00 | 40.00 |
| RAC | 498 | 01/12/96 | FL | FX | 0 | 0 | 1 | 1 | 40.00 | 40.00 | 19.21 | 40.00 |
| RAC | 502 | 01/12/96 | FL | FX | 0 | 0 | 1 | 1 | 40.00 | 40.00 | 19.94 | 40.00 |
| RAC | 700 | 10/12/94 | FL | RC | 1 | 1 | 0 | 2 | 40.00 | 40.00 | 19.52 | 40.00 |
| RAC | 2942 | 02/11/98 | DE | DG | 0 | 0 | 0 | 0 | 40.00 | 40.00 | 21.38 | 40.00 |
| RAC | 2988 | 01/28/98 | AL | DG | 0 | 0 | 2 | 2 | 40.00 | 40.00 | 25.72 | 40.00 |
| RAC | 3385 | 04/08/97 | FL | DG | 0 | 1 | 0 | 1 | 40.00 | 40.00 | 23.15 | 40.00 |
| RAC | 3489 | 01/28/98 | WV | FX | 0 | 0 | 0 | 0 | 40.00 | 40.00 | 16.26 | 40.00 |
| RAC | 3795 | 06/28/98 | FL | FX | 0 | 0 | 0 | 0 | 40.00 | 40.00 | 20.88 | 40.00 |
| RAC | 3803 | 05/22/98 | FL | DG | 0 | 1 | 0 | 1 | 40.00 | 40.00 | 17.23 | 40.00 |
| RAC | 3876 | 06/28/98 | FL | FX | 0 | 0 | 0 | 0 | 40.00 | 40.00 | 15.52 | 40.00 |
| RAC | 3877 | 06/28/98 | FL | FX | 0 | 0 | 0 | 0 | 40.00 | 39.44 | 16.78 | 40.00 |
| RAC | 3883 | 06/12/98 | MD | BV | 2 | 0 | 0 | 2 | 40.00 | 40.00 | 14.76 | 40.00 |
| RAC | 4085 | 12/17/98 | AL | HR | 0 | 0 | 1 | 1 | 40.00 | 40.00 | 15.88 | 40.00 |
| RAC | 4198 | 02/09/99 | AL | RC | 0 | 0 | 0 | 0 | 40.00 | 40.00 | 15.27 | 40.00 |
| RAC | 4501 | 09/09/99 | VA | SK | 0 | 0 | 0 | 0 | 40.00 | 40.00 | 15.33 | 40.00 |
| SCSK | 488 | 05/01/92 | AZ | SK | 3 | 1 | 0 | 4 | 40.00 | 40.00 | 40.00 | 40.00 |
| SCSK | 704 | 03/15/93 | NM | FX | 0 | 0 | 0 | 0 | 40.00 | 40.00 | 40.00 | 18.12 |
| SCSK | 705 | 03/11/93 | NM | CT | 1 | 0 | 0 | 1 | 40.00 | 40.00 | 40.00 | 18.29 |
| SCSK | 744 | 09/08/94 | TX | SK | 3 | 2 | 0 | 5 | 40.00 | 40.00 | 40.00 | 37.55 |
| SCSK | 749 | 09/08/94 | TX | SK | 3 | 2 | 0 | 5 | 40.00 | 40.00 | 40.00 | 40.00 |
| SCSK | 751 | 12/30/91 | TX | SK | 2 | 0 | 2 | 4 | 40.00 | 40.00 | 40.00 | 19.58 |
| SCSK | 752 | 12/30/91 | TX | SK | 1 | 0 | 1 | 2 | 40.00 | 40.00 | 40.00 | 17.84 |
| SCSK | 912 | 11/25/92 | OK | BV | 3 | 2 | 1 | 6 | 40.00 | 40.00 | 40.00 | 40.00 |
| SCSK | 977 | 09/16/92 | MO | SK | 0 | 0 | 0 | 0 | 40.00 | 40.00 | 40.00 | 17.82 |
| SCSK | 1001 | 09/16/02 | KS | SK | 0 | 0 | 0 | 0 | 40.00 | 39.27 | 40.00 | 21.89 |
| SCSK | 1004 | 02/01/94 | KS | SK | 0 | 0 | 0 | 0 | 40.00 | 40.00 | 40.00 | 26.38 |
| SCSK | 1236 | 12/29/91 | TX | SK | 1 | 1 | 0 | 2 | 40.00 | 40.00 | 40.00 | 24.21 |
| SCSK | 1425 | 01/03/91 | CO | SK | 1 | 1 | 1 | 3 | 40.00 | 40.00 | 40.00 | 26.93 |
| SCSK | 1426 | 01/03/91 | CO | SK | 1 | 1 | 1 | 3 | 40.00 | 40.00 | 40.00 | 30.11 |
| SCSK | 1522 | 11/28/92 | KS | DG | 0 | 0 | 1 | 1 | 40.00 | 40.00 | 40.00 | 20.24 |
| SCSK | 2205 | 01/08/94 | LA | SK | 0 | 0 | 1 | 1 | 40.00 | 40.00 | 40.00 | 16.81 |
| SCSK | 2221 | 02/03/94 | NE | SK | 1 | 1 | 2 | 4 | 40.00 | 40.00 | 40.00 | 28.09 |
| SCSK | 2222 | 02/13/94 | NE | SK | 1 | 1 | 2 | 4 | 40.00 | 40.00 | 40.00 | 23.89 |
| SCSK | 2523 | 09/08/94 | TX | SK | 2 | 0 | 2 | 4 | 40.00 | 40.00 | 40.00 | 23.05 |
| SCSK | 2524 | 09/08/94 | TX | SK | 2 | 1 | 2 | 5 | 40.00 | 40.00 | 40.00 | 28.94 |
| SCSK | 3461 | 01/29/98 | AR | CT | 0 | 0 | 0 | 0 | 40.00 | 40.00 | 40.00 | 18.25 |
| SCSK | 3604 | 01/29/98 | AR | CT | 3 | 1 | 1 | 5 | 40.00 | 40.00 | 40.00 | 37.73 |
| SCSK | 3607 | 01/29/98 | AR | CT | 0 | 0 | 0 | 0 | 40.00 | 40.00 | 40.00 | 16.24 |
Date of RNA extraction.
State from which the sample was submitted to CDC. AL, Alabama; AR, Arkansas; AZ, Arizona; CA, California; CO, Colorado; DE, Delaware; FL, Florida; KS, Kansas; KY, Kentucky; LA, Louisiana; MD, Maryland; MI, Michigan; MO, Missouri; NE, Nebraska; NM, New Mexico; OH, Ohio; OK, Oklahoma; TN, Tennessee; TX, Texas; VA, Virginia; WV, West Virginia.
Host species from which virus was isolated. BV, bovine; CT, cat; DG, dog; FX, fox; HR, horse; ML, mountain lion; RC, raccoon; SK, skunk; WD, wolf hybrid.
The number of sequence mismatches between the target sequence and that of the TaqMan primers and probe designated as the phylogenetic variant. Mismatches are shown as those in the sequences of the forward primers (F), reverse primers (R), and TaqMan probes (P). The total number (T) of mismatches is also shown.
Samples were tested with all four sets of TaqMan primers and probe. Numbers are Ct values.
FIG. 1.
Maximum likelihood phylogenetic tree depicting the relationships of the 62 historical rabies virus isolates. The tree is rooted at the midpoint for purposes of clarity. The tree is based on 164 nucleotides of the N gene, corresponding to positions 1123 to 1287 of the Pasteur RV N gene (GenBank accession no. A492968) and generated with the PAUP* program (20). Samples within the clear box are those NCSK samples typed by the TaqMan PCR assay as CASK. Samples within the gray boxes are those that were weakly positive or negative by TaqMan PCR.
RNA extraction and cDNA generation.
RNA was extracted from experimental samples with TRIzol and TRIzol LS reagents according to the manufacturer's instructions and with 1 μl of Glycoblue (Ambion, Austin, Tex.) used as a coprecipitant. Dried RNA pellets were resuspended in 100 μl of nuclease-free water (Promega, Madison, Wis.). RNA samples were stored at −80°C until used.
Reverse transcription (RT) of all RNA samples (experimental and historical) was undertaken by using a reverse transcription system (Promega) according to the manufacturer's instructions with 0.5 μg of the supplied random primers. RT was performed with 1 μl of RNA from experimental samples (to avoid saturation) and 5 μl of RNA from historical samples.
Design of TaqMan primer and probe sets.
All TaqMan primers and probes were designed by the Primer Express computer program (Applied Biosystems, Foster City, Calif.). For the two isolates used for the experimental infection of the skunks, nucleotide sequences from the N gene of each variant (AZ-SK and AZ-EF with GenBank accession numbers AF483524 and AY170413, respectively) were inputted into the program, and the optimal primer and probe sequences were obtained by using the default settings of the program.
For historical samples, a consensus sequence was generated for the sets of sequences comprising each RV variant (Fig. 1) from a sequence alignment generated with the BioEdit computer program (7). From this alignment, areas of relative conservation were selected as target regions for placement of the TaqMan primers and probes. These regions were used as input for Primer Express to generate the optimal primer and probe sequences according to the default settings.
TaqMan primer and probe details are shown in Table 2. All TaqMan probes were labeled at the 5′ end with a fluorescent reporter dye (6-carboxy-fluorescein) and at the 3′ end with a quencher. The AZ-EF and AZ-SK probes were quenched with a minor groove binder-nonfluorescent quencher (Applied Biosystems), while the CASK, NCSK, RAC and SCSK probes were quenched with Black Hole Quencher (Biosearch Technologies, Novato, Calif.). Primer and probe concentrations were optimized according to the manufacturer's recommendations.
TABLE 2.
Oligonucleotide sequences for TaqMan primers and probes
| Variant | Rolea | Length (no. of oligonucleotides) | Tmaxb | Conc.c (nM) | Sequence | Positiond |
|---|---|---|---|---|---|---|
| AZ-EF | F | 21 | 60 | 900 | GAATCCTGATAGCACGGAGGG | 278-298 |
| R | 20 | 60 | 900 | CTTCCACATCGGTGCGTTTT | 333-352 | |
| P | 29 | 70 | 250 | CAAGATCACCCCAAATTCTCTTGTGGACA | 303-331 | |
| AZ-SK | F | 20 | 60 | 900 | GTCGGCTGCTATATGGGTCAG | 943-963 |
| R | 19 | 60 | 900 | ATCTCATGCGGAGCACAGG | 995-1013 | |
| P | 29 | 70 | 250 | TGAGGTCCTTGAATGCAACGGTAATAGCC | 965-993 | |
| CASK | F | 23 | 60 | 300 | TCATGATGAATGGAGGTCGACTC | 1226-1247 |
| R | 25 | 60 | 300 | TTGATGATTGGAACTGACTGAGACA | 1296-1272 | |
| P | 21 | 70 | 200 | AGAGATCGCATATACGGAGAT | 1249-1270 | |
| NCSK | F | 21 | 60 | 300 | GGTGAAACCAGAAGTCCGGAA | 1189-1209 |
| R | 25 | 60 | 300 | CCGTATATGCGATCTCTTTAGTCGA | 1266-1242 | |
| P | 21 | 69 | 250 | CTGTCTATACTCGAATCATGA | 1211-1227 | |
| RAC | F | 21 | 59 | 300 | TGGTGAAACCAGGAGTCCAGA | 1188-1208 |
| R | 19 | 60 | 300 | ATCTTTTGAGTCGGCCCCC | 1255-1235 | |
| P | 19 | 70 | 250 | CGGTCTATACTCGGATCAT | 1211-1227 | |
| SCSK | F | 26 | 60 | 300 | ATGATGAAGACTATTTCTCCGGTGAG | 1169-1191 |
| R | 20 | 60 | 300 | GTCGGCCTCCATTCATCATG | 1246-1226 | |
| P | 16 | 70 | 250 | CGGAGGCAGTCTATAC | 1202-1219 |
F, forward PCR primer; R, reverse PCR primer; P, TaqMan probe.
Tm calculated by using Primer Express.
Optimal final concentration.
Nucleotide position relative to the Pasteur virus N gene (GenBank accession number A492968).
TaqMan PCR.
The TaqMan reactions were performed in a 25-μl reaction volume comprised of 12.5 μl of TaqMan master mix (Applied Biosystems), 2.5 μl of each TaqMan primer (at the concentrations shown in Table 2), 2.5 μl of probe (at the concentrations shown in Table 2), 4 μl of nuclease-free water, and 1 μl of cDNA. All TaqMan PCRs were performed as uniplex reactions with one set of TaqMan primers and probe per well. All reactions were carried out in MicroAmp optical 96-well reaction plates (Applied Biosystems) sealed with MicroAmp optical caps (Applied Biosystems). Plates were transferred to an ABI Prism 7700 sequence detection system (Applied Biosystems), and DNA was amplified according to the following program: 1 cycle each of 50°C for 2 min and 95°C for 10 min, followed by 40 cycles each of 95°C for 15 s and 60°C for 1 min.
TaqMan runs of experimental samples contained at least four replicates each of a known positive control (from RV-positive BR), an RV-negative control cDNA, and nuclease-free water. Each historical sample was run in triplicate with each of the four variant-specific TaqMan primer and probe sets alongside a positive control cDNA for each variant (from RV-positive BR), negative control cDNA (from BR infected with a big brown bat RV variant), and nuclease-free water (3 wells). Previous testing showed that all four terrestrial TaqMan primer and probe sets did not amplify the big brown bat RV variant used for the control reactions with no amplification. All sample replicates were run on the same plate, thus measuring variability only within the run. In addition, a number of samples were run on separate plates to assess variability between runs.
For each PCR, a threshold cycle number (Ct) was obtained corresponding to the PCR cycle number during which the fluorescence of the reaction rose above a threshold value statistically determined by the computer software. The Ct values are inversely proportional to the log10 of the amount of template in the PCR. A difference of 1 Ct corresponds to a twofold difference in template amounts. A Ct value less than the mean plus two standard deviations of the negative control wells was considered positive. A Ct value of 40 corresponds to no amplification.
Levels of β-actin, determined by TaqMan PCR, were used to normalize levels of all samples (both experimental and historic). For amplification of β-actin mRNA, the PCR assay was performed with a β-actin detection kit (Applied Biosystems) according to the manufacturer's instructions. The adjustment according to the levels of β-actin was performed by subtracting the highest mean β-actin Ct value (i.e., the lowest levels of β-actin) from the mean β-actin Ct of each sample. This difference was subtracted from the mean Ct value obtained from the RV-specific TaqMan PCR. This provided a method to account for differences in the levels of viral RNA due to sample heterogeneity. Data were adjusted in sets according to tissue type because the suitability of endogenous controls can be tissue specific (14). Standard curves were not generated for quantification experiments as all total RNA levels were within the linear and equal amplification range of the assay and thus applicable to quantification through normalization with β-actin mRNA.
Conventional PCR and sensitivity assay.
A conventional, heminested PCR was used to compare the sensitivity of the TaqMan assay and was performed as described elsewhere (15). Briefly, fivefold serial dilutions of a known RV-positive RNA sample were made in nuclease-free water, and RT was carried out by using a reverse transcription system with random primers. The same cDNA sample was used for heminested (10 μl) and TaqMan (1 μl/replicate) PCRs. Products of conventional PCRs (15 μl) were visualized on a 2% agarose gel stained with ethidium bromide (0.5 μg/ml).
Statistical analysis.
Differences in the RNA yields were assessed by means of the Mann-Whitney U test. Associations between variables were determined according to Spearman's rank correlation coefficient (rs) or by linear regression analysis. For parametric tests, data were checked for normality by using the Kolmogorov-Smirnov test. All analyses were performed with SPSS version 11.0 (SPSS Inc., Chicago, Ill.).
RESULTS
Linearity of the TaqMan PCR.
Total RNA yields from tissue, determined by spectrophotometry, were typically in the range of 5 to 10 μg although in several cases the concentration was below the level accurately determinable (∼0.1 μg/μl). For experimental samples for which accurate measurement was possible, there were no significant differences in RNA yields from BR and SG samples (P = 0.805).
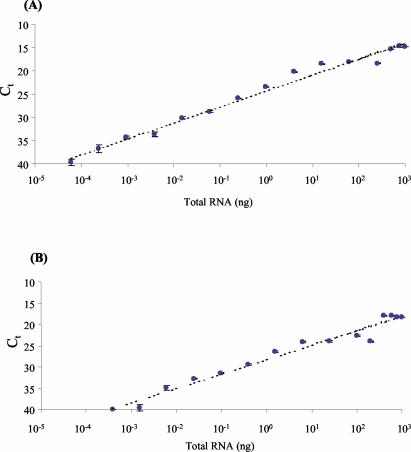
To test for potential saturation of the RT reaction, fourfold serial dilutions of total RNA from SG and BR samples were made in nuclease-free water and used for RT. The RNA quantities ranged from 1 μg to ∼17 pg. The linear ranges of the assay were comparable for RNA from both SG and BR (Fig. 2). Over this range, and for both tissue types, there was no evidence that amplification of β-actin mRNA was nonlinear (results not shown). There were no differences in the linear ranges of the assay between TaqMan primer and probe sets or between samples of the same tissue but from different animals (results not shown). For both sample types, a departure from linearity was observed with the addition of between 15 and 63 ng of total RNA. In all cases this increase required the addition of more than 1 μl of stock RNA. Within the linear range (<63 ng for SG; <15 ng for BR), there was a strongly significant inverse correlation between the amount of total RNA and corresponding Ct (for both, rs = 1.000, P < 0.0001). The amplification efficiencies of the RV-specific target sequence and β-actin mRNA were assessed by subtracting the β-actin Ct from the RV Ct over this dilution series and assessing the stability of this new value with various amounts of RNA. Stable amplification efficiencies were evident for both SG and BR samples when <15 ng of total RNA was added (results not shown).
FIG. 2.
Linearity of the TaqMan reaction for RNA extracted from SG homogenates (A) and BR homogenates (B) from an experimentally infected rabid skunk. Error bars show 95% CL of the mean of three replicate TaqMan PCRs.
Repeatability.
Due to the limited quantity of the samples, repeat RNA extraction was not possible. Therefore, the repeatability of the assay could only be assessed by repeat RT reactions and TaqMan PCRs. For experimental tissue samples, results were consistent, showing little variability within runs. The ranges of Ct values for the three replicates of each sample were low for both BR (mean range, 0.2; 95% confidence limits [CL], 0.09 to 0.31) and SG (mean range, 0.7; 95% CL, 0.12 to 1.28). Interrun variability was found to be insignificant (rs = 0.900, n = 10, P < 0.001).
Correlation with concentration of infectious virus.
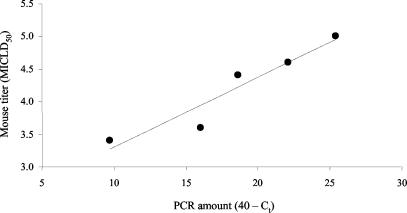
The SG homogenates from experimentally infected skunks were inoculated intracerebrally into mice, and a titer was calculated according to the Karber method (9). The MICLD50 correlated significantly (P < 0.01) with the corresponding TaqMan PCR quantity for samples from experimental animals (Fig. 3).
FIG. 3.
Correlation between infectious concentration of RV (mouse inoculation test) and TaqMan PCR quantity for SG tissues obtained from experimentally infected rabid skunks (rs = −1.00, n = 5, P < 0.01). For ease of interpretation, TaqMan quantities are given as 40 minus the mean Ct.
Sensitivity.
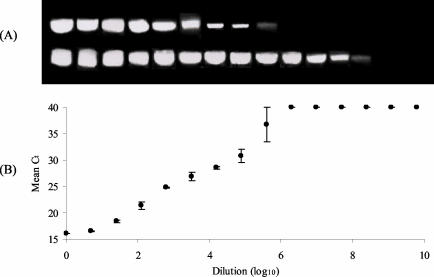
The sensitivity of the TaqMan PCR was assessed against the results of a heminested PCR (Fig. 4). The TaqMan PCR was as sensitive as the primary PCR in that both could detect viral RNA from a 105.6 dilution of the stock RNA solution. The heminested PCR, however, was able to detect viral RNA from a 107.7 dilution of the stock RNA.
FIG. 4.
Comparative sensitivity of a conventional heminested PCR and TaqMan PCR for detection of RV RNA. (A) Products of conventional PCR. The upper band shows the 397-bp product from the first-round reaction; the lower band shows the 376-bp product from the heminested reaction. (B) Results of TaqMan PCR. A Ct value of 40 indicates a negative reaction. Error bars show 95% CL of the mean of three replicate TaqMan PCRs.
To test for an increase in sensitivity, the TaqMan PCR was run for a greater number of cycles (60 rather than 40) and with increased volumes of cDNA in the PCR (3 to 5 μl). Neither of these methods resulted in an increased detection limit (results not shown).
Detection of rabies virus RNA from historical samples.
Of the 62 historical isolates tested, 44 (71%) were typed correctly by this assay. That is, cDNA was successfully amplified by the TaqMan primers and probe designed to detect the variant to which the isolate was placed (Fig. 1). Only three samples (5%) were negative against all TaqMan primer and probe sets (Table 1).
There was considerable cross-reaction between the NCSK and CASK TaqMan assays: 11 samples were positive in the assays using both NCSK and CASK primer and probe sets, but in each case the lowest Ct (i.e., the highest PCR quantity) corresponded to the phylogenetic placement of the isolate (Fig. 1). Additionally, four samples designated NCSK by phylogeny were negative with the NCSK primer and probe set but positive with the CASK TaqMan primer and probe set (sample numbers 2865, 2572, 4374, and 4375).
Three samples were negative in assays that used all four sets of TaqMan primers and probes. Additionally, two samples had a mean Ct of >35. These five samples were all SCSK variants with a high number of mismatches between the viral sequence and that of the TaqMan primer and probe set (4, 5, 6, 5, and 5 mismatches for sample numbers 488, 744, 749, 912, and 3604, respectively). Furthermore, all of these samples contained a mismatch close to the center of the probe.
To assess the effect on Ct values of time since RNA extraction, samples with no differences in sequences from the sequences of the TaqMan primer and probe sets were selected. A correlation analysis showed that the age of the sample significantly reduced the unadjusted Ct value (rs = 0.549, n = 22, P = 0.008) but not the β-actin-adjusted values (rs = 0.339, n = 22, P = 0.123). The method of RNA extraction did not significantly alter the results of the TaqMan PCRs (results not shown).
Influence of sequence mismatches on amplification.
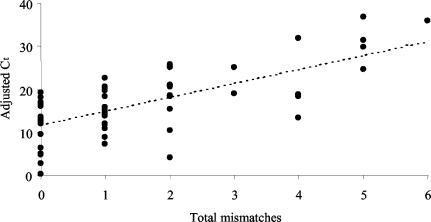
The results of TaqMan PCR assays using historical samples were normalized according to the levels of β-actin mRNA. For each sample, adjusted values correlated significantly with raw values (rs = 0.851, n = 62, P < 0.001). Mean and adjusted data showed no significant departure from normality (P < 0.05). The total number of mismatches between the target sequences and those of the TaqMan primer and probe sets showed a significant positive association with Ct values (Fig. 5).
FIG. 5.
Association of TaqMan PCR values and sequence mismatches between the TaqMan primer and probe sets and the viral target sequences. The two variables are significantly associated in terms of β-actin-adjusted data (F1,60 = 57.86, P < 0.001) and raw data (values not shown; F1,60 = 61.27, P < 0.001).
DISCUSSION
The TaqMan PCR assay to detect RV RNA was as sensitive as the primary PCR assay but had a considerably reduced detection limit compared to the detection limit of a heminested PCR. Methods adopted to increase sensitivity, such as increasing the template concentration and the number of thermocycles, failed to increase the detection limit of the assay. For this reason, it is unlikely that this assay, in its present form, could improve upon the existing nested-PCR methods used for laboratory detection of RV RNA (15). However, the sensitivity of the assay can be increased to the level of the heminested PCR assay by using a first-round PCR product as the template for a TaqMan PCR (V. Shankar, personal communication). The sensitivity of our TaqMan assay compared to those of conventional PCR methods from other laboratories (13, 21) has not been tested.
The use of a large number of historical samples allowed an assessment of the effect of sequence mismatches between the TaqMan primers and probes and the viral target sequences. The primers and probes designed for this study were not intended to be optimal for the detection of the variants of interest but were chosen to allow a thorough, quantitative study of the effect of sequence mismatches on sensitivity and specificity. The genomic region from which the oligonucleotides were designed was limited to that where sufficient sequence data were available.
Mismatches between TaqMan primer and probe sequences and the target sequence are clearly detrimental to amplification. The number of mismatches reduces the efficiency of the reaction such that more than four differences can result in a weakly positive or negative result. A point mutation at the center of the probe (the site used for single nucleotide polymorphism detection) can prevent generation of a fluorescent signal although the PCR itself proceeds normally (results not shown).
For these reasons, which are based on existing sequence data, it seems unlikely that sufficient homology exists to generate TaqMan primer and probe sets capable of detecting a wide range of RV variants. For over 250 bat RV variants from the United States (where sequence data were available over a 300-nucleotide region of the N gene), based on the results of this study, ∼20 sets of TaqMan primers and probes would be required to detect every isolate (results not shown). An alignment of bat RV sequence data for the region covered by the TaqMan primer and probe set recently described to detect genotype 1 lyssaviruses (3) suggests that a number of these samples would not be detectable by existing TaqMan methodology (results not shown). The level of microdiversity within clades of RV may prove highly problematic in applying TaqMan technology to RV surveillance. Current PCR methods that utilize degenerate primers with a reduced annealing temperature allow amplification despite reduced sequence homology (15), although such methods do generate nonspecific products, requiring confirmation of the PCR results by nucleotide sequencing.
The possibility remains, however, of using TaqMan technology as an adjunct to existing detection methods. If the epidemiology of rabies is well defined within a geographical region, then screening with RV variant-specific TaqMan primer and probe sets could serve as a substitute for nucleotide sequencing. Multiplex assays have been developed that can simultaneously detect different variants and/or viruses (12, 24). Our TaqMan assay used random primers for cDNA generation and identical thermocycling conditions for each TaqMan primer and probe set, ensuring that the same cDNA sample can be used for multiple reactions on the same TaqMan plate, irrespective of the TaqMan primers and probes.
We propose that this TaqMan assay can be readily applied to quantitative studies of RV infection. Over a range of RNA levels there was little distortion of relative values when RNA concentrations that occur with clinical samples were used. In fact, these results suggest that samples for which high levels of total RNA are expected (e.g., tissues) should be suspended in >100 μl of water. The repeatability of the assay (as determined on the basis of different cDNA samples) was excellent in all cases. However, the variability due to repeat RNA extraction was not tested. Importantly, the quantity of RV determined by the TaqMan PCR assay correlated significantly with that estimated through virus isolation by the mouse inoculation test. Such an association is crucial when this technology is applied to pathogenesis studies, such as those used for foot-and-mouth disease virus infection of pigs (1). Although we have only assessed relative quantities of RV RNA, there is no evidence to suggest that absolute quantitation (according to quantitative standards) could not be developed if desired.
Crucial to the success of quantitative PCR is the ability to adjust the levels of RV determined by the TaqMan PCR according to levels of an endogenous control to account for variation in tissue samples and RNA extraction efficiency. Here we have shown that the levels of RV RNA determined by TaqMan PCR can be adjusted according to those of β-actin mRNA. The amplification of both β-actin mRNA and RV RNA had acceptable linear ranges and amplification efficiencies over the levels of total RNA used in this study and those likely to be used from biological samples. Normalization was successfully applied to correct for the effects of RNA degradation over time.
Acknowledgments
We thank Ivan Kuzmin, Staci Murphy, Mike Niezgoda, and Lillian Orciari for their contributions to this work. We also thank the staff in the Viral and Rickettsial Zoonoses Branch and the Animal Resources Branch at the CDC for their outstanding technical expertise and contributions.
G.J.H. was funded by an American Society for Microbiology and National Centers for Infectious Disease postdoctoral fellowship.
REFERENCES
- 1.Alexandersen, S., M. B. Oleksiewicz, and A. I. Donaldson. 2001. The early pathogenesis of foot-and-mouth disease virus in pigs infected by contact: a quantitative time-course study using TaqMan RT-PCR. J. Gen. Virol. 82:747-755. [DOI] [PubMed] [Google Scholar]
- 2.Arai, Y. T., I. V. Kuzmin, Y. Kameoka, and A. D. Botvinkin. 2003. New lyssavirus genotype from the lesser mouse-eared bat (Myotis blythi), Kyrghyzstan. Emerg. Infect. Dis. 9:333-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black, E. M., J. P. Lowings, J. Smith, P. R. Heaton, and L. M. McElhinney. 2002. A rapid RT-PCR method to differentiate six established genotypes of rabies and rabies-related viruses using TaqMan technology. J. Virol. Methods 105:25-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 1997. Human rabies-Kentucky and Montana, 1996. Morb. Mortal. Wkly. Rep. 46:397-400. [PubMed] [Google Scholar]
- 5.Crepin, P., L. Audry, Y. Rotivel, A. Gacoin, C. Caroff, and H. Bourhy. 1998. Intravitam diagnosis of human rabies by PCR using saliva and cerebrospinal fluid. J. Clin. Microbiol. 36:1117-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia, S., J.-M. Crance, A. Billecoco, A. Peinnequin, A. Jouan, M. Bouloy, and D. Garin. 2001. Quantitative real-time PCR detection of Rift Valley fever virus and its application to evaluation of antiviral compounds. J. Clin. Microbiol. 39:4456-4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall, T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95-98. [Google Scholar]
- 8.Hu, A., M. Colella, J. S. Tam, R. Rappaport, and S.-M. Cheng. 2003. Simultaneous detection, subgrouping, and quantitation of respiratory syncytial virus A and B by real-time PCR. J. Clin. Microbiol. 41:149-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karber, G. 1931. 50% end point calculation. Arch. Exp. Pathol. Pharmakol. 162:480-483. [Google Scholar]
- 10.Koprowski, H. 1996. The mouse inoculation test, p. 80-86. In F.-X. Meslin, M. M. Kaplan, and H. Koprowski (ed.), Laboratory techniques in rabies, 4th ed. World Health Organization, Geneva, Switzerland.
- 11.Krebs, J. W., H. R. Noll, C. E. Rupprecht, and J. E. Childs. 2001. Rabies surveillance in the United States during 2001. J. Am. Vet. Med. Assoc. 221:1690-1701. [DOI] [PubMed] [Google Scholar]
- 12.Meng, Q., C. Wong, A. Rangachari, S. Tamatsukuri, M. Sasaki, E. Fiss, L. Cheng, T. Ramankutty, D. Clarke, H. Yawata, Y. Sakakura, T. Hirose, and C. Impraim. 2001. Automated multiplex assay system for simultaneous detection of hepatitis B virus DNA, hepatitis C virus RNA, and human immunodeficiency type 1 RNA. J. Clin. Microbiol. 39:2937-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nadin-Davis, S. A. 1998. Polymerase chain reaction protocols for rabies virus discrimination. J. Virol. Methods 75:1-8. [DOI] [PubMed] [Google Scholar]
- 14.Oleksiewicz, M. B., A. I. Donaldson, and S. Alexandersen. 2001. Development of a novel real-time RT-PCR assay for quantitation of foot-and-mouth disease virus in diverse porcine tissues. J. Virol. Methods 92:23-35. [DOI] [PubMed] [Google Scholar]
- 15.Orciari, L. A., M. Niezgoda, C. A. Hanlon, J. H. Shaddock, D. W. Sanderlin, P. A. Yager, and C. E. Rupprecht. 2001. Rapid clearance of SAG-2 rabies virus from dogs after oral vaccination. Vaccine 19:4511-4518. [DOI] [PubMed] [Google Scholar]
- 16.Schutten, M., B. van den Hoogen, M. E. van der Edne, R. A. Gruters, A. M. D. E. Osterhaus, and H. G. M. Niesters. 2000. Development of a real-time quantitative RT-PCR for the detection of HIV-2 RNA in plasma. J. Virol. Methods 88:81-87. [DOI] [PubMed] [Google Scholar]
- 17.Smith, I. L., K. Halpin, D. Warrilow, and G. A. Smith. 2001. Development of a fluorogenic RT-PCR assay (TaqMan) for the detection of Hendra virus. J. Virol. Methods 98:33-40. [DOI] [PubMed] [Google Scholar]
- 18.Smith, J. S. 2002. Molecular epidemiology, p. 79-111. In A. C. Jackson and W. H. Wunner (ed.), Rabies. Academic Press, New York, N.Y.
- 19.Smith, J. S., D. B. Fishbein, C. E. Rupprecht, and K. Clark. 1991. Unexplained rabies in three immigrants in the United States: a virologic investigation. N. Engl. J. Med. 324:205-211. [DOI] [PubMed] [Google Scholar]
- 20.Swofford, D. L. 2000. PAUP* version 4: phylogenetic analysis using parsimony (*and other methods). Sinauer Associates, Sunderland, Mass.
- 21.Tordo, N., H. Bourhy, and D. Sacremento. 1995. Polymerase chain reaction technology for rabies virus, p. 125-145. In J. P. Clewley (ed.), The polymerase chain reaction (PCR) for human viral diagnosis. CRC Press, Boca Raton, Fla.
- 22.Trimarchi, C. V. 2000. Rabies, p. 335-338. In S. Specter, R. Hodinka, and S. Young (ed.), Clinical virology manual. ASM Press, Washington, D.C.
- 23.Trimarchi, C. V., and J. S. Smith. 2002. Diagnostic evaluation, p. 307-349. In A. C. Jackson and W. H. Wunner (ed.), Rabies. Academic Press, New York, N.Y.
- 24.Van Elden, L. J. R., M. Nijhuis, P. Schipper, R. Schuurman, and A. M. Van Loon. 2001. Simultaneous detection of influenza viruses A and B using real-time quantitative PCR. J. Clin. Microbiol. 39:196-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Velleca, W. M., and F. T. Forrester. 1981. Detection and identification, p. 69-107. In Laboratory methods for detecting rabies. Centers for Disease Control and Prevention, Atlanta, Ga.
- 26.Webster, W. A., and G. A. Casey. 1996. Virus isolation in neuroblastoma cell culture, p. 96-104. In F.-X. Meslin, M. M. Kaplan, and H. Koprowski (ed.), Laboratory techniques in rabies, 4th ed. World Health Organization, Geneva, Switzerland.