Abstract
Omega-3 fatty acids, which are found abundantly in fish oil, are increasingly being used in the management of cardiovascular disease. It is clear that fish oil, in clinically used doses (typically 4 g/d of eicosapentaenoic acid and docosahexaenoic acid) reduce high triglycerides. However, the role of omega-3 fatty acids in reducing mortality, sudden death, arrhythmias, myocardial infarction, and heart failure has not yet been established. This review will focus on the current clinical uses of fish oil and provide an update on their effects on triglycerides, coronary artery disease, heart failure, and arrhythmia. We will explore the dietary sources of fish oil as compared with drug therapy, and discuss the use of fish oil products in combination with other commonly used lipid-lowering agents. We will examine the underlying mechanism of fish oil’s action on triglyceride reduction, plaque stability, and effect in diabetes, and review the newly discovered anti-inflammatory effects of fish oil. Finally, we will examine the limitations of current data and suggest recommendations for fish oil use.
Keywords: omega-3, fatty acids, fish oil
Humans are unable to place double bonds beyond position 9 on long chain polyunsaturated fatty acids (FA), making the omega-3 FA synthesized in plants and in marine microalgae essential elements to the human diet.1 Fish contain high levels of 2 omega-3 FA, eicosapentaenoic acid (EPA; C20:5 n-3), and docosahexaenoic acid [DHA]; C22:6 n-3)2,3 (Fig. 1). Many claims about the role of these omega-3 FA have been made in the prevention and treatment of cardiovascular disease. For instance, fish oil is seen as having a therapeutic role in coronary artery disease (CAD), heart failure, fatal and nonfatal arrhythmias, as well as offering an alternative or adjunct to the standard therapy for hypertriglyceridemia and diabetes. This review will highlight the potential mechanisms of fish oil on cardiovascular disease and provide an update of clinical trial results. The established uses in the treatment of hypertriglyceridemia and sources of omega-3 FA—both dietary and drug therapy—will be iterated, along with its potential application in combination with standard hypolipidemic agents. Finally, the limitations of current data will be addressed, as well as suggested recommendations for clinical use.
FIGURE 1.
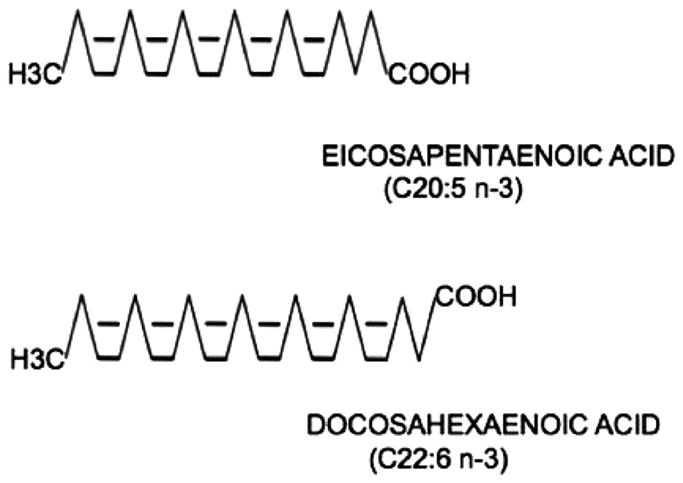
The chemical structure of eicosapentaenoic acid and docosahexaenoic acid. Eicosapentaenoic acid consists of 20 carbons (C20) with 5 double bonds, and the last unsaturated carbon is located third from the methyl end (n-3). Do-cosahexaenoic acid consists of 22 carbons (C22) with 6 double bonds, and also with the3 last unsaturated carbon located third from the methyl end (n-3). Adapted with permission from Frishman et al, eds. Cardiovascular Pharmacotherapeutics. New York, NY: McGraw Hill; 2003.3
MECHANISM OF ACTION
Inflammation Modulation
Fish oil’s most potent effect on atherosclerosis may be related to its potential to alter plaque inflammation, thereby stabilizing vulnerable plaques. In recent years there has been a growing body of evidence that is shifting the paradigm of how inflammation is contained and dissipated.4 In this new model, inflammation resolution is an active process mediated by lipid-derived compounds. Newly discovered families of chemical mediators, resolvins, and protectins5,6 are directly involved in blocking neutrophil migration, infiltration, and recruitment, as well as in blocking T-cell migration and promoting T-cell apoptosis.7–12 In addition, protectins can reduce tumor necrosis factor and interferon secretion.13 Interestingly, both protectins and resolvins are strictly derived from omega-3 FA. EPA is the substrate of the resolvins family and DHA can be converted to both resolvins and protectins.7 It may be that the effects of fish oil on inflammatory mediators underlie the positive findings demonstrated in several trials assessing fish oil and plaque stability.14–16
Triglyceride Reduction
Omega-3 FA most likely reduce serum triglyceride levels by modulating very-low-density lipoprotein (VLDL) and chylomicron metabolism. There is a consistent finding in the literature that the end effect of fish oil is decreased hepatic secretion of VLDL17—the major endogenous source of triglycerides. This effect occurs most likely through multiple mechanisms, including: (1) decreased synthesis of triglycerides because these omega-3 FA may not be the preferred substrates of the enzyme diacylglycerol O-acyltransferase,18 or they may interact with nuclear transcription factors that control lipogenesis19; cellular metabolism consequently shifts toward a decrease in triglyceride synthesis and an increase in FA oxidation; and (2) the promotion of apolipoprotein B degradation in the liver through the stimulation of an autophagic process.20 This means that fewer VLDL particles can be assembled and secreted. Fish oil may also accelerate VLDL and chylomicron clearance21 by inducing lipoprotein lipase activity.22
CLINICAL TRIALS
Arrhythmia and Sudden Death
Fish oil has been shown to have a direct electrophysiological effect on the myocardium. Initial experience with animal ischemia models demonstrated that the ventricular fibrillation threshold was increased in both animals fed or infused with omega-3 FA.23,24 This progressed to a demonstration, on a cellular and ion channel level, that omega-3 FA reduce both sodium currents and L-type calcium currents.25–29 It is hypothesized that during ischemia, a reduction in the sodium ion current protects hyperexcitable tissue, and a reduction in the calcium ion current reduces arrhythmogenic depolarizing currents.30
Heart rate variability, a possible surrogate outcome for the risk of sudden death, was assessed in a randomized trial of myocardial infarction (MI) survivors with an ejection fraction of 40%. In the 49 patients that were randomized to either fish oil or olive oil, Holter monitor recordings showed an increase in heart rate variability in the fish oil group.31 In a larger cohort assessed in the Japan EPA Lipid Intervention Study (JELIS),32 however, no difference in heart rate variability could be attributed to fish oil.
Although there are no randomized data on fish oil consumption and protection from sudden death, observational studies have linked omega-3 FA with the prevention of sudden death. In a population-based, case-control study of sudden cardiac death victims, the mean red blood cell membrane omega-3 FA level of the lowest quartile, when compared with the mean level of the third quartile, was associated with a relative risk reduction of 70%.33 A similar finding was appreciated in a nested, prospective, case-control study of the Physician Health Study cohort of 22,000 healthy males. In the 119 patients that succumbed to sudden death, baseline omega-3 FA blood levels were significantly lower than in matched controls.34 Finally, in an analysis of data from the Nurses Health Study, a cohort study of 84,688 women, an inverse association was shown between fish consumption and CAD-related death. The investigators concluded that the reduction in CAD deaths was likely due to a reduction in sudden deaths, as there was no difference in the rate of MI when comparing high and low fish consumption.35
The randomized trials assessing the efficacy of fish oil supplementation on secondary prevention of CAD lend further evidence to the findings that fish oil may protect from sudden cardiac death.36 The Diet and Reinfarction Trial (DART),37 one of the first randomized trials of fish oil in CAD, has been interpreted as potential support for fish oil’s role in sudden death reduction because the primary outcome of all-cause mortality occurred within 2 months of the trial’s onset.38 After such a short time span, it was believed that atherosclerosis would not be altered and therefore another mechanism was reducing mortality. This was further supported by the fact that nonfatal MIs were not reduced. Although the actual modes of death other than CAD-related deaths were not documented, it has been postulated to be secondary to a reduction in sudden death.39 The Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico-Prevenzione40 (GISSI-Prevenzione) trial, a larger randomized trial of fish oil in CAD, has also been interpreted as evidence for fish oil’s protection against sudden death. Sudden death, however, was not a primary end point. Rather, the reduction in fatal events was driven by a reduction in cardiovascular death, which included coronary death, cardiac death, and sudden death.
Three randomized trials assessing more than 600 patients with known malignant ventricular arrhythmia were carried out under the protection of implanted cardioverter defibrillator (ICD) therapy.41–43 In all 3 of the trials, 75% of the patients had ischemic heart disease, survived ventricular tachycardia or ventricular fibrillation and were randomized to 1 to 3 g/d of fish oil. In the first trial of its kind, 402 patients with ICDs were randomized to either a fish oil or an olive oil supplement.41 Although statistical significance was not reached, after approximately 1 year the primary end-point of time to first ICD cardioversion for ventricular tachycardia or fibrillation or death from any cause was longer in the fish oil group. This finding was not replicated in a trial of 200 patients who were randomized to either fish oil or a placebo and followed for a median of approximately 2 years.42 In fact, time to first ICD cardioversion was not changed and the incidence of recurrent ventricular tachycardia and fibrillation was more common in the group assigned to fish oil. In the largest trial, 546 patients were randomized to supplemental fish oil or a placebo and were followed for a mean period of 1 year.43 The primary outcome of the rate of ICD cardioversion or all-cause mortality was not reduced. It was concluded in a recent meta-analysis of these trials that fish oil did not have a protective effect.44
The effect of fish oil on incident atrial fibrillation has not been studied in large randomized trials, and observational population-based trials show mixed results. The Danish Diet, Cancer and Health Study, and the Rotterdam Study followed 47,000 and 5100 middle-aged adults, respectively.45,46 Neither study found that the consumption of fish oil affected the incidence of atrial fibrillation. Similar findings were seen in the Women’s Health Initiative where there was no association between fish and omega-3 FA intake regarding incident atrial fibrillation.47 However, in a 12-year prospective, observational study of 4815 adults over the age of 65, daily fish consumption was associated with a 31% risk reduction in incident atrial fibrillation.48
Coronary Artery Disease
The DART study, published in 1989, was the first randomized trial to show the efficacy of fish oil on CAD.37 In the trial, 2033 post-MI patients were randomized to receive 3 types of diets: a diet that was either high in cereal fiber, polyunsaturated fat, or fish oil. The fish oil group consumed 200 to 400 g/wk of fatty fish (2 portions of fish per week) or 0.5 g/d of Maxepa fish oil supplement. At 2 years, the primary end point of all-cause mortality was reduced by 29% in the fish oil group, whereas no improvement was seen in the other dietary advice groups.
The Lyon Diet Heart Study, performed shortly after the DART study, was a prospective trial of 607 survivors of MI who were randomized to either a Mediterranean diet or a regular Western diet.49 At a mean follow-up of 27 months, the primary end point of death from cardiovascular causes and nonfatal deaths had a 73% relative risk reduction—a positive effect that continued at follow up assessment at a mean of 46 months.50 FA analysis of plasma lipids showed that in the patients randomized to a Mediterranean diet, there was a higher concentration of alpha-linolenic acid as well as EPA. Fish, however, was consumed in similar amounts by both the Western and Mediterranean diet groups. The higher blood level of EPA in the Mediterranean diet arm was attributed to its synthesis from alpha-linolenic acid, which was 60-times higher than the plasma concentration of EPA. In addition, the risk reduction that occurred in this trial could not be attributed to one particular diet intervention because as the consumption of fruits and vegetables increased, the consumption of monounsaturated fat increased, while saturated fat and cholesterol were decreased.
The GISSI-Prevenzione trial40 showed similar findings. In this open-label trial, 11,324 post-MI patients were followed for 3.5 years after randomization to either 1 g/d of omega-3 FA, vitamin E, both, or none. In the 2836 patients assigned to only omega-3 FA, the primary end point of death, nonfatal MI or stroke, was reduced by 10%. This decreased risk occurred despite a minimal triglyceride-lowering effect because of the relatively low dose of omega-3 FA. Of note, the GISSI-Prevenzione trial was done prior to the pervasive use of lipid-lowering agents. Only about 40% of patients were on any form of lipid-lowering therapy.
Heart Failure
The GISSI-Heart Failure trial was the first blinded, randomized trial to assess the efficacy of fish oil supplements in patients with heart failure.51 The trial enrolled 7046 subjects with heart failure; 60% with New York Heart Association class II symptoms and 40% with a history of MI. The majority of patients were on a standard heart failure regimen, including angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, beta-blockers, and spironolactone, but only 22% were on a statin. At an average of 3.9 years, the coprimary end points of death and death or hospital admission for cardiovascular reasons were reduced by approximately 9% with fish oil supplementation. Sudden cardiac death, a secondary end-point, showed a statistically nonsignificant relative risk reduction of 7% with fish oil. There was also a reduction in 2 other arrhythmia-related secondary end-points: first hospitalization for ventricular arrhythmia and presumed arrhythmic death.
Diabetes
To date, no studies have assessed mortality or nonfatal MI in diabetic patients treated with fish oil.52–54 A recent comprehensive meta-analysis analyzed the effect of fish oil supplements on metabolic parameters when added to usual care in patients with type 2 diabetes mellitus or impaired glucose tolerance.54 The meta-analysis included a total of 23 small, randomized trials with over 1000 patients that were assessed for lipid and insulin resistance parameters. At a mean follow-up of approximately 9 weeks, triglyceride reduction was accomplished but no significant changes were seen in total cholesterol, high-density lipoprotein-cholesterol, HgA1c levels, fasting glucose levels, fasting insulin, or in body weight. The largest randomized trial to date assessed approximately 400 patients with impaired glucose tolerance or insulin-dependent diabetes mel-litus, and as reflected in the larger meta-analysis, found no effect of moderate to high doses of fish oil on diabetic parameters.55 There are insufficient randomized data to comment on the combination of fish oil and specific diabetes medications and related mortality and/or morbidity.
COMBINATION THERAPY
HMG CoA-Reductase Inhibitors
Several small studies have shown that combination therapy with fish oil and HMG CoA reductase inhibitors is safe.56–61 The largest trial to date, the JELIS trial,32 was an open label trial of 18,645 Japanese adults with hypercholesterolemia who were randomized to a standard statin regimen or a fish oil formulation containing 1.8 g of EPA added to a statin medication. The cohort was made up mostly of postmenopausal, nonobese women with a 15% to 20% incidence of diabetes, tobacco use, or CAD. The primary outcome of any major cardiovascular event, at a mean of 4.6 years, was moderately reduced by a relative risk reduction of 26%. Both unstable angina and nonfatal MI were reduced, but no change was seen in sudden death. Overall, the findings were remarkable because at baseline approximately 90% of Japanese consumed at least 900 mg of EPA and DHA per day.62 The rates of cancer, joint pain, lumbar pain, or muscle pain were similar in the 2 groups. There was a similar rate of increase in measures of creatine phosphokinase, but more patients had an increase in aspartate aminotransferase levels (0.6% vs. 0.4%) in the fish oil group. The rate of bleeding was 1.1% in the fish oil combination group versus 0.6% in the HMG–CoA reductase inhibitor group.
Fenofibrate
Fish oil combined with fenofibrate has not been studied extensively in randomized controlled trials. Data to date, however, suggest that the combination is safe and effective.63,64 A recent randomized controlled trial of 100 patients with severe hypertriglyceridemia and HIV on highly active antiretroviral therapy showed that a regimen of fenofibrate and 3 g/d of fish oil for 8 weeks was well tolerated. The median baseline triglyceride level of 650 mg/dL was reduced by 65%.63 Another recent randomized, 2 month, double-blind, placebo-controlled trial, which was set up to assess the safety and efficacy of fenofibrate with 4 g of fish oil, showed that in the 81 patients assigned to combination therapy, triglyceride levels were reduced by 61%. Therapy was well-tolerated without significant adverse reactions at 8 weeks or at the end of a 2-year open label extension.64 The combination of fish oil and niacin requires further study.
SOURCES OF FISH OIL
For patients without documented CAD, the American Heart Association 2006 Diet and Lifestyle Recommendations advise the consumption of at least 2 servings of fish per week, preferably fatty fish high in DHA and EPA.65 The guidelines also recommend a daily fish intake equivalent to 1 g/d of EPA and DHA for secondary prevention of CAD. Fish oil supplements containing EPA and DHA are suggested as an alternative to fatty fish consumption for secondary prevention.
Today the only Food and Drug Administration (FDA)-approved form of dietary omega-3 FA supplement is Lovaza (omega-3-acid ethyl esters; GlaxoSmithKline), which contains 375 mg of DHA and 465 mg of EPA per 1 g capsule. The myriad of dietary supplements of fish oil, including Kosher capsules, vary from comparable content to insignificant amounts, and for the most part can include other fats and cholesterols. In comparison, to achieve approximately 1 g of EPA and DHA in a meal, 12 ounces of canned light tuna, 2 to 3 ounces of sardines, 1.5 to 2.5 ounces of farmed Atlantic salmon, or 20 ounces of farmed catfish must be consumed (Table 1).65 Unfortunately, potentially high levels of harmful pollutants offset this source of omega-3 FA. The FDA action level for unacceptably high mercury content in fish is 1.0 μg/g. The mercury level in most fish is at or below 0.1 μg/g, but tilefish, swordfish, and king mackerel have high levels of mercury. The majority of fish species also contain <100 ng/g of polychlorinated biphenyls, which is below the FDA action level of 2000 ng/g. Dioxins, which do not have FDA action levels, are present in the majority of marine life.66
TABLE 1.
Common Dietary Fish and Corresponding Ounces Required to Obtain 1 g/d of Omega 3-FA’s (EPA + DHA)65
| Oz. Required for 1 g/d EPA + DHA | |
|---|---|
| Herring, Pacific | 1.5 |
| Herring, Atlantic | 2 |
| Sardines | 2–3 |
| Tuna, light canned | 12 |
| Tuna, white canned | 4 |
| Salmon, Atlantic wild | 2–3.5 |
| Salmon, Atlantic farmed | 1.5–2.5 |
CLINICAL USE FOR TRIGLYCERIDE REDUCTION
The hypotriglyceridemic effect of fish oil is well established and is related to both dose and baseline triglyceride level. Patients with triglycerides <90 mg/dL will be negligibly affected unless very high doses of omega-3 FA are used.67,68 However, in patients with triglycerides >200 mg/dL, who are treated with 4 g/d of fish oil, a 30% reduction in triglycerides is expected.17,69 For patients with triglycerides >500 mg/dL who are at risk for pancreatitis, the National Cholesterol Education Program Adult Treatment Panel III guidelines recommend using fish oil supplements as an adjunctive therapy to fibrates and nicotinic acid.70 Lovaza capsules have been shown to be effective, safe, and comparable to gemfibrozil in treating triglycerides at this range.71,72 The official label recommendation for Lovaza is for patients with triglycerides >500 mg/dL.73
EFFECT ON LIPOPROTEIN SUBFRACTIONS
The most extensive data of the effect of fish oil on lipoprotein subfractions are based on trials performed before the widespread use of statins. This data were aggregated over a decade ago in a meta-analysis of 16 randomized trials including over 1500 patients.17 In this analysis, low-density lipoprotein (LDL) was increased by an average of 5% and high-density lipoprotein was marginally changed. Although a shift toward less atherogenic, larger and more buoyant LDL particle composition has been shown,74 this has been offset by the observation that the number of apolipoprotein B 100 particles increases and may be more susceptible to oxidation.75 Increased conversion of remnant particles (intermediate density lipoprotein) to LDL has also been observed.76
ADVERSE EFFECTS
The FDA product label on Lovaza warns of potential bleeding complications with the coadministration of anticoagulants. This warning is based on observational studies that suggested a prolonged bleeding time in populations ingesting high levels of fish oil77 and on in vitro studies that demonstrated an effect on pro-thrombotic mediators such as a reduction in thromboxane A2 production78 and platelet activation factor.79 The same trend, however, has not been clearly demonstrated in measurements of clotting times or in factors of fibrinolysis.80 In addition, in randomized clinical trials of patients undergoing coronary artery bypass graft surgery, percutaneous transluminal coronary angioplasty, endarterectomy and diagnostic angiography, no adverse bleeding related events have been demonstrated.81 For example, in a trial of 500 patients randomized to pretreatment with 6.9 g of DHA and EPA preparation 2 weeks before balloon percutaneous transluminal coronary angioplasty (where all the patients received 325 mg/d of aspirin and heparin bolus periprocedure), no difference was seen in bleeding complications.82 Similar results were seen in a trial of 610 patients undergoing coronary artery bypass graft surgery, randomized to either placebo or 4 g/d of fish oil and then further randomized to aspirin or warfarin (dosed to an international normalized ratio [INR] goal of 2.5–4.2). At 1 year, the number of bleeding complications was not increased.15 The effect of fish oil on INR values has not been studied extensively, but a small, randomized trial showed that fish oil did not alter the Coumadin dosing regimen.83 There is very little evidence that a lower target INR is necessary in patients receiving chronic warfarin therapy and fish oil.
RECOMMENDATIONS FOR USE
The evidence that fish oil consumption should be used for primary prevention of CAD is based on observational studies. The only randomized trial for primary prevention, the JELIS trial, showed a moderate relative risk reduction and was conducted in a very specific group. Nevertheless, to date, there has been no strong signal suggesting any serious adverse effects of having high DHA and EPA oils in the diet. We agree with the national guidelines that one should consume moderate amounts of fish oil— either in supplement or through the dietary intake of fatty fish with low mercury levels.
Secondary prevention fish oil studies demonstrate a significant reduction in MI. But unfortunately, both the observational and randomized trials were conducted in an era before the widespread use of HMG-CoA reductase inhibitors, and therefore, the incremental benefit is still unknown. Nevertheless, in patients receiving antiplatelet and anticoagulant therapy in addition to fish oil supplementation (even at doses as high as 4 g per day), no serious adverse complications have been reported.
Fish oil therapy is efficacious and safe for patients with severe to moderate hypertriglyceridemia. Combination therapy with HMG-CoA reductase inhibitors is also efficacious and has not been associated with any serious adverse reactions. Fish oil therapy added to fenofibrate in patients with severe hypertriglyceridemia is also effective and safe. Accordingly, it may be a safe and effective adjunct in the pharmacotherapy of the mixed lipid disorder that is frequently encountered in patients with the metabolic syndrome and/or type II diabetes mellitus.
CONCLUSIONS
The addition of omega-3 FA to a healthy diet appears to be safe when used for the primary and secondary prevention of CAD. The potential benefits are not limited to a reduction in triglycerides. However, the incremental benefits to modern therapy and a prudent diet are yet to be fully evaluated.
Acknowledgments
Dr. Fisher’s research on fish oil is supported by grant RO1 HL58541.
References
- 1.Guschina IA, Harwood JL. Lipids and lipid metabolism in eukaryotic algae. Prog Lipid Res. 2006;45:160–186. doi: 10.1016/j.plipres.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Kris-Etherton PM, Harris WS, Appel LJ, et al. American Heart Association, Nutrition Committee. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 3.Frishman WH, Sonnenblick EH, Sica DA, editors. Cardiovascular Pharmacotherapeutics. 2. New York, NY: McGraw Hill; 2003. p. 382. [Google Scholar]
- 4.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serhan CN, Hong S, Gronert K, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong S, Gronert K, Devchand P, et al. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood and glial cells: autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 7.Tjonahen E, Oh S, Siegelman S, et al. Resolvin E2: identification and anti-inflammatory actions: pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chem Biol. 2006;13:1193–1202. doi: 10.1016/j.chembiol.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Arita M, Bianchini F, Aliberti J, et al. Stereochemical assignment, anti-inflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arita M, Oh S, Chonan T, et al. Metabolic inactivation of resolvin E1 and stabilization of its anti-inflammatory actions. J Biol Chem. 2006;281:22847–22854. doi: 10.1074/jbc.M603766200. [DOI] [PubMed] [Google Scholar]
- 10.Hasturk H, Kantarci A, Goguet-Surmenian E, et al. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 2007;179:7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- 11.Vassiliou EK, Kesler OM, Tadros JH, et al. Bone marrow-derived dendritic cells generated in the presence of resolvin E1 induce apoptosis of activated CD4+ T cells. J Immunol. 2008;181:4534–4544. doi: 10.4049/jimmunol.181.7.4534. [DOI] [PubMed] [Google Scholar]
- 12.Arita M, Ohira T, Sun YP, et al. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 13.Ariel A, Li PL, Want W, et al. The docosatriene protectin D1 is produced by TH2 skewing and promotes human T cell apoptosis via lipid raft clustering. J Biol Chem. 2005;280:43079–43086. doi: 10.1074/jbc.M509796200. [DOI] [PubMed] [Google Scholar]
- 14.Von Schacky C, Angerer P, Kothny W, et al. The effect of dietary omega-3 fatty acids on coronary atherosclerosis: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1999;130:554–562. doi: 10.7326/0003-4819-130-7-199904060-00003. [DOI] [PubMed] [Google Scholar]
- 15.Eritsland J, Arnesen H, Gronseth K, et al. Effect of dietary supplementation with n-3 fatty acids on coronary artery bypass graft patency. Am J Cardiol. 1996;77:31–36. doi: 10.1016/s0002-9149(97)89130-8. [DOI] [PubMed] [Google Scholar]
- 16.Thies F, Garry JM, Yaqoob P, et al. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomized controlled trial. Lancet. 2003;361:477–485. doi: 10.1016/S0140-6736(03)12468-3. [DOI] [PubMed] [Google Scholar]
- 17.Harris WS. N-3 fatty acids and serum lipoproteins: human studies. Am J Clin Nutr. 1997;65(suppl 5):1645S–1654S. doi: 10.1093/ajcn/65.5.1645S. [DOI] [PubMed] [Google Scholar]
- 18.Madsen L, Rustan AC, Vaagenes H, et al. Eicosapentaenoic and docosahexaenoic acid affect mitochondrial and peroxisomal fatty acid oxidation in relation to substrate preference. Lipids. 1999;34:951–963. doi: 10.1007/s11745-999-0445-x. [DOI] [PubMed] [Google Scholar]
- 19.Davidson MH. Mechanisms for the hypotriglyceridemic effect of marine omega-3 fatty acids. Am J Cardiol. 2006;98:27i–33i. doi: 10.1016/j.amjcard.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 20.Pan M, Maitin V, Parathath S, et al. Presecretory oxidation, aggregation, and autophagic destruction of apoprotein-B: a pathway for late-stage quality control. Proc Natl Acad Sci U S A. 2008;105:5862–5867. doi: 10.1073/pnas.0707460104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park Y, Harris WS. Omega-3 fatty acid supplementation accelerates chylo-micron triglyceride clearance. J Lipid Res. 2003;44:455–463. doi: 10.1194/jlr.M200282-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Weintraub MS, Zechner R, Brown A, et al. Dietary polyunsaturated fats of the W-6 and W-3 series reduce postprandial lipoprotein levels. Chronic and acute effects of fat saturation on postprandial lipoprotein metabolism. J Clin Invest. 1988;82:1884–1893. doi: 10.1172/JCI113806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Billman GE, Hallaq H, Leaf A. Prevention of ischemia-induced arrhythmias by n-3 fatty acids. Proc Natl Acad Sci U S A. 1994;91:4427–4430. doi: 10.1073/pnas.91.10.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobsen AN, Du XJ, Dart AM, et al. Ins-(1,4.5)-P and arrhythmogenic responses during myocardial reperfusion. Am J Physiol. 1997;273:H1119–H1125. doi: 10.1152/ajpheart.1997.273.3.H1119. [DOI] [PubMed] [Google Scholar]
- 25.Kang JX, Leaf A. Effects of long-chain polyunsaturated fatty acids on the contraction of neonatal rat cardiac myocytes. Proc Natl Acad Sci U S A. 1994;91:9886–9890. doi: 10.1073/pnas.91.21.9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang JX, Xiao YF, Leaf A. Free long-chain polyunsaturated fatty acids reduce membrane electrical excitability in neonatal rat cardiac myocytes. Proc Natl Acad Sci U S A. 1995;92:3997–4001. doi: 10.1073/pnas.92.9.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao YF, Kang JX, Morgan JP, et al. Blocking effects of polyunsaturated fatty acids on Na+ channels of neonatal rat ventricular myocytes. Proc Natl Acad Sci U S A. 1995;92:11000–11004. doi: 10.1073/pnas.92.24.11000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao YF, Wright SN, Wang GK, et al. Coexpression with the [beta]1-subunit modifies the kinetics and fatty-acid block of hH1[alpha] Na+ channels. Am J Physiol. 2000;279:H35–H46. doi: 10.1152/ajpheart.2000.279.1.H35. [DOI] [PubMed] [Google Scholar]
- 29.Xiao YF, Gomez AM, Morgan JP, et al. Suppression of voltage-gated L-type Ca2+ currents by polyunsaturated fatty acids in adult and neonatal rat cardiac myocytes. Proc Natl Acad Sci U S A. 1997;94:4182–4187. doi: 10.1073/pnas.94.8.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leaf A, Kang J, Xiao YF, et al. Clinical prevention of sudden cardiac death by n-3 polyunsaturated fatty acids and mechanism of prevention of arrhythmias by n-3 fish oils. Circulation. 2003;107:2646–2652. doi: 10.1161/01.CIR.0000069566.78305.33. [DOI] [PubMed] [Google Scholar]
- 31.Christensen JH, Gustenhoff P, Korup E, et al. Effect of fish oil on heart rate variability in survivors of myocardial infarction: a double blind randomized controlled trial. BMJ. 1996;312:677–678. doi: 10.1136/bmj.312.7032.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid (EPA) on major coronary events in hypercholesterolemic patients (JELIS): a randomized open-label blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 33.Siscovick DS, Raghunathan TE, King I, et al. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA. 1995;274:1363–1367. doi: 10.1001/jama.1995.03530170043030. [DOI] [PubMed] [Google Scholar]
- 34.Albert CM, Campos H, Stampfer MJ, et al. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med. 2002;346:1113–1118. doi: 10.1056/NEJMoa012918. [DOI] [PubMed] [Google Scholar]
- 35.Hu F, Bronner L, Willet WC, et al. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA. 2002;287:1815–1821. doi: 10.1001/jama.287.14.1815. [DOI] [PubMed] [Google Scholar]
- 36.Majmudar MD, Tompkins C, Bachmann JM, et al. Effects of lipid-altering therapies on ventricular arrhythmias and sudden cardiac death. Cardiol Rev. 2009;17:60–69. doi: 10.1097/CRD.0b013e3181861be8. [DOI] [PubMed] [Google Scholar]
- 37.Burr ML, Fehily AM, Gilbert JF, et al. Effects of changes in fat, fish, and fiber intakes on death and myocardial reinfarction: Diet and Reinfarction Trial (DART) Lancet. 1989;334:757–761. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- 38.Burr M. Secondary prevention of CHD in UK men: the Diet and Reinfarction Trial and its sequel. Proc Nutr Soc. 2007;66:9–15. doi: 10.1017/S0029665107005241. [DOI] [PubMed] [Google Scholar]
- 39.Burr M. Reflections on the Diet and Reinfarction Trial (DART) Eur Heart J. 2001;3(suppl D):D75–D78. [Google Scholar]
- 40.GISSI-Prevenzione Investigators. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 41.Raitt MH, Connor WE, Morris C, et al. Fish oil supplementation and risk of ventricular tachycardia and ventricular fibrillation in patients with implantable defibrillators: a randomized controlled trial. JAMA. 2005;293:2884–2891. doi: 10.1001/jama.293.23.2884. [DOI] [PubMed] [Google Scholar]
- 42.Leaf A, Albert CM, Josephson M, et al. Prevention of fatal arrhythmias in high-risk subjects by fish oil n-3 fatty acid intake. Circulation. 2005;112:2762–2768. doi: 10.1161/CIRCULATIONAHA.105.549527. [DOI] [PubMed] [Google Scholar]
- 43.Brouwer IA, Zock PL, Camm AJ, et al. Effect of fish oil on ventricular tachyarrhythmia and death in patients with implantable cardioverter defibrillators: the Study on Omega-3 Fatty Acids and Ventricular Arrhythmia (SOFA) randomized trial. JAMA. 2006;295:2613–2619. doi: 10.1001/jama.295.22.2613. [DOI] [PubMed] [Google Scholar]
- 44.Brouwer IA, Raitt MH, Dullemeijer C, et al. Effect of fish oil on ventricular tachyarrhythmia in three studies in patients with implantable cardioverter defibrillators. Eur Heart J. 2009;30:820–826. doi: 10.1093/eurheartj/ehp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frost L, Vestergaard P. n-3 fatty acids consumed from fish and risk of atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Clin Nutr. 2005;81:50–54. doi: 10.1093/ajcn/81.1.50. [DOI] [PubMed] [Google Scholar]
- 46.Brouwer IA, Heeringa J, Geleijnse JM, et al. Intake of very long chain n-3 fatty acids from fish and incidence of atrial fibrillation: the Rotterdam Study. Am Heart J. 2006;151:857–862. doi: 10.1016/j.ahj.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 47.Berry JD, Prineas RJ, van Horn L, et al. Dietary fish intake and incident atrial fibrillation (from the Women’s Health Initiative) Am J Cardiol. 2010;105:844–848. doi: 10.1016/j.amjcard.2009.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mozaffarian D, Psaty B, Rimm EB, et al. Fish intake and risk of incident atrial fibrillation. Circulation. 2004;110:368–373. doi: 10.1161/01.CIR.0000138154.00779.A5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Lorgeril M, Renaud S, Mamelle N, et al. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet. 1994;343:1454–1459. doi: 10.1016/s0140-6736(94)92580-1. [DOI] [PubMed] [Google Scholar]
- 50.De Lorgeril M, Salen P, Martin JL, et al. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999;99:779–785. doi: 10.1161/01.cir.99.6.779. [DOI] [PubMed] [Google Scholar]
- 51.Tavazzi L, Maggioni AP, Marchioli R, et al. GISSI-HF Investigators. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomized, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 52.Montori VM, Farmer A, Wollan PC, et al. Fish oil supplementation in type 2 diabetes: a quantitative systematic review. Diabetes Care. 2000;23:1407–1415. doi: 10.2337/diacare.23.9.1407. [DOI] [PubMed] [Google Scholar]
- 53.Farmer A, Montori V, Dinneen S, et al. Fish oil in people with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2001:CD003205. doi: 10.1002/14651858.CD003205. [DOI] [PubMed] [Google Scholar]
- 54.Hartweg J, Perera R, Montori VM, et al. Omega-3 polyunsaturated fatty acids (PUFA) for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2008:CD003205. doi: 10.1002/14651858.CD003205.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sirtori CR, Paoletti R, Mancini M, et al. n-3 fatty acids do not lead to an increased diabetic risk in patients with hyperlipidemia and abnormal glucose tolerance. Italian Fish Oil Multicenter Study. Am J Clin Nutr. 1997;65:1874–1881. doi: 10.1093/ajcn/65.6.1874. [DOI] [PubMed] [Google Scholar]
- 56.Davidson MH, Stein EA, Bays HE, et al. COMBination of prescription Omega-3 with Simvastatin (COMBOS) Investigators. Efficacy and tolerability of adding prescription omega-3 fatty acids 4 g/d to simvastatin 40 mg/d in hypertriglyceridemic patients: an 8-week, randomized, double-blind, placebo-controlled study. Clin Ther. 2007;29:1354–1367. doi: 10.1016/j.clinthera.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 57.Durrington PN, Bhatnagar D, Mackness MI, et al. An omega-3-polyunsaturated fatty acid concentrate administered for one year decreased triglycerides in simvastatin treated patients with coronary heart disease and persisting hypertriglyceridemia. Heart. 2001;85:544–548. doi: 10.1136/heart.85.5.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gosai P, Liu J, Doyle RT, et al. Effect of omega-3-acid ethyl esters on the steady-state plasma pharmacokinetics of rosuvastatin in healthy adults. Expert Opin Pharmacother. 2008;9:2947–2953. doi: 10.1517/14656560802532640. [DOI] [PubMed] [Google Scholar]
- 59.Nordøy A, Bønaa KH, Nilsen H, et al. Effects of simvastatin and omega-3 fatty acids on plasma lipoproteins and lipid peroxidation in patients with combined hyperlipidaemia. J Intern Med. 1998;243:163–170. doi: 10.1046/j.1365-2796.1998.00297.x. [DOI] [PubMed] [Google Scholar]
- 60.Chan DC, Watts GF, Mori TA, et al. Factorial study of the effects of atorvastatin and fish oil on dyslipidaemia in visceral obesity. Eur J Clin Invest. 2002;32:429–436. doi: 10.1046/j.1365-2362.2002.01001.x. [DOI] [PubMed] [Google Scholar]
- 61.Contacos C. Effect of pravastatin and omega-3 fatty acids on plasma lipids and lipoproteins in patients with combined hyperlipidemia. Arterioscler Thromb. 1993;12:1755–1762. doi: 10.1161/01.atv.13.12.1755. [DOI] [PubMed] [Google Scholar]
- 62.Mozaffarian D. JELIS, fish oil, and cardiac events. Lancet. 2007;369:1062–1063. doi: 10.1016/S0140-6736(07)60504-2. [DOI] [PubMed] [Google Scholar]
- 63.Gerber JG, Kitch DW, Fichtenbaum CJ, et al. Fish oil and fenofibrate for the treatment of hypertriglyceridemia in HIV-infected subjects on antiretroviral therapy: results of ACTG A5186. J Acquir Immune Defic Syndr. 2008;47:459–466. doi: 10.1097/QAI.0b013e31815bace2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roth EM, Bays HE, Forer AD, et al. Prescription omega-3 fatty acid as an adjunct to fenofibrate therapy in hypertriglyceridemic subjects. J Cardiovasc Pharmacol. 2009;54:196–203. doi: 10.1097/FJC.0b013e3181b0cf71. [DOI] [PubMed] [Google Scholar]
- 65.Lichtenstein AH, Appel LJ, Brands M, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 66.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296:1885–1899. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- 67.Harris WS, Connor WE, McMurry MP. The comparative reductions of the plasma lipids and lipoproteins by dietary polyunsaturated fats: salmon oil versus vegetable oils. Metabolism. 1983;32:179–184. doi: 10.1016/0026-0495(83)90226-3. [DOI] [PubMed] [Google Scholar]
- 68.Harris WS, Rothrock DW, Fanning A, et al. Fish oils in hypertriglyceridemia: a dose-response study. Am J Clin Nutr. 1990;51:399–406. doi: 10.1093/ajcn/51.3.399. [DOI] [PubMed] [Google Scholar]
- 69.Balk EM, Lichtenstein AH, Chung M, et al. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis. 2006;189:19–30. doi: 10.1016/j.atherosclerosis.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 70.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 71.Harris WS, Ginsberg HN, Arunakul N, et al. Safety and efficacy of Lovaza in severe hypertriglyceridemia. J Cardiovasc Risk. 1997;4:385–391. [PubMed] [Google Scholar]
- 72.Stalenhoef AF, de Graaf J, Wittekoek ME, et al. The effect of concentrated n-3 fatty acids versus gemfibrozil on plasma lipoproteins, low density lipoprotein heterogeneity and oxidizability in patients with hypertriglyceridemia. Atherosclerosis. 2000;153:129–138. doi: 10.1016/s0021-9150(00)00381-6. [DOI] [PubMed] [Google Scholar]
- 73.Bays H. Clinical overview of omacor: a concentrated formulation of omega-3 polyunsaturated fatty acids. Am J Cardiol. 2006;98:71–76. doi: 10.1016/j.amjcard.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 74.Calabresi L, Donati D, Pazzucconi F, et al. Lovaza in familial combined hyperlipidemia: effects on lipids and low density lipoprotein subclasses. Atherosclerosis. 2000;148:387–396. doi: 10.1016/s0021-9150(99)00267-1. [DOI] [PubMed] [Google Scholar]
- 75.Suzukawa M, Abbey M, Howe PR, et al. Effects of fish oil fatty acids on low density lipoprotein size, oxidizability and uptake by macrophages. J Lipid Res. 1995;36:473–484. [PubMed] [Google Scholar]
- 76.Fisher WR, Zech LA, Stacpoole PW. Apolipoprotein B metabolism in hypertriglyceridemic diabetic patients administered either a fish oil- or vegetable oil-enriched diet. J Lipid Res. 1998;39:388–401. [PubMed] [Google Scholar]
- 77.Lichtenstein AH. Remarks on clinical data concerning dietary supplements that affect antithrombotic therapy. Thromb Res. 2005;117:71–73. doi: 10.1016/j.thromres.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 78.Knapp HR, Reilly IA, Alessandrini P, et al. In vivo indexes of platelet and vascular function during fish-oil administration in patients with atherosclerosis. N Engl J Med. 1986;314:937–942. doi: 10.1056/NEJM198604103141501. [DOI] [PubMed] [Google Scholar]
- 79.Mayer K, Merfels M, Muhly-Reinholz S, et al. Omega-3 fatty acids suppress monocyte adhesion to human endothelial cells: role of endothelial PAF generation. Am J Physiol Heart Circ Physiol. 2002;283:H811–H818. doi: 10.1152/ajpheart.00235.2002. [DOI] [PubMed] [Google Scholar]
- 80.Knapp HR. Dietary fatty acids in human thrombosis and hemostasis. Am J Clin Nutr. 1997;65(suppl 5):1687S–1698S. doi: 10.1093/ajcn/65.5.1687S. [DOI] [PubMed] [Google Scholar]
- 81.Harris WS. Expert opinion: omega-3 Fatty acids and bleeding-cause for concern? Am J Cardiol. 2007;99:S44–S46. doi: 10.1016/j.amjcard.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 82.Leaf A, Jorgensen MB, Jacobs AK, et al. Do fish oils prevent restenosis after coronary angioplasty? Circulation. 1994;90:2248–2257. doi: 10.1161/01.cir.90.5.2248. [DOI] [PubMed] [Google Scholar]
- 83.Bender NK, Kraynak MA, Chiquette E, et al. Effects of marine fish oils on the anticoagulation status of patients receiving chronic warfarin therapy. J Thromb Thrombolysis. 1998;5:257–261. doi: 10.1023/A:1008852127668. [DOI] [PubMed] [Google Scholar]


