Abstract
OBJECTIVE
Articular chondrocytes respond to osmotic stress with transient changes in cell volume and the intracellular concentration of calcium ion ([Ca2+]i). The goal of this study was to examine the hypothesis that interleukin-1 (IL-1), a pro-inflammatory cytokine associated with osteoarthritis, influences osmotically-induced Ca2+ signaling.
METHODS
Fluorescence ratio imaging was used to measure [Ca2+]i and cell volume in response to hypo- or hyper-osmotic stress in isolated porcine chondrocytes, with or without pre-exposure to 10 ng/ml IL-1α. Inhibitors of IL-1 (IL-1 receptor antagonist, IL-Ra), Ca2+ mobilization (thapsigargin, an inhibitor of Ca-ATPases), and cytoskeletal remodeling (Toxin B, an inhibitor of the Rho family of small GTPases) were used to determine the mechanisms involved in increased [Ca2+]i, F-actin remodeling, volume adaptation and active volume recovery.
RESULTS
In response to osmotic stress, chondrocytes exhibited transient increases in [Ca2+]i, generally followed by decaying oscillations. Pre-exposure to IL-1 significantly inhibited regulatory volume decrease following hypo-osmotic swelling and reduced the change in cell volume and the time to peak [Ca2+]i in response to hyper-osmotic stress, but did not affect the peak magnitudes of [Ca2+]i in those cells that did response. Co-treatment with IL-1Ra, thapsigargin, or Toxin B restored these responses to control levels. The effects were associated with alterations in F-actin organization.
CONCLUSIONS
IL-1 alters the normal volumetric and Ca2+ signaling response of chondrocytes to osmotic stress through mechanisms involving F-actin remodeling via small Rho GTPases. These findings provide further insights into the mechanisms by which IL-1 may interfere with normal physiologic processes in the chondrocyte, such as the adaptation or regulatory responses to mechanical and osmotic loading.
Keywords: cartilage, chondrocyte, osteoarthritis, cytoskeleton, calcium channel, stretch-activated ion channel, volume regulation, cytokine
Introduction
Under normal physiologic conditions, articular cartilage is subjected to both static and dynamic compressive loads, which are believed to play an important role in the health and homeostasis of the synovial joint [1]. The precise mechanisms by which the chondrocytes respond to their mechanical environment, either physiologically or pathologically, remain to be elucidated. There is significant evidence that changes in the physicochemical properties of the pericellular environment, secondary to mechanical loading, may be an important factor in biophysical transduction of signals [2–6]. Joint loading results in deformation of the cartilage layer and associated loss of interstitial water, followed by recovery of this fluid as the load is released [7–9]. As the proteoglycans in cartilage possess large numbers of negatively charged sulfate and carboxyl groups, the loss and gain of water changes the effective “fixed” charge density of the tissue, and thus alters the effective concentration of positively charged counterions (e.g., Na+, K+, Ca2+) in a manner that is directly related to magnitude of tissue dilatation [10, 11]. Previous radiographic studies have reported strains of up to 20% in cartilage in an intact joint [12], while other magnetic resonance imaging studies in vivo showed mean loss of cartilage volume of 5.9% a following dynamic loading [9]. These physicochemical changes expose the chondrocytes to diurnal hyper- and hypo-osmotic stresses that vary with time and location in the tissue [5, 13, 14]. Importantly, one of the early characteristics of osteoarthritis is a loss of proteoglycan content secondary to disruption of the collagen fibers and/or degradation of aggrecan and smaller proteoglycans. These factors lead to a concomitant increase in tissue hydration, resulting in a decrease of the fixed charge density and interstitial osmolarity [15]. The magnitude of the osmotic change within cartilage subjected to dynamic loading has not been measured directly, but has been estimated to be on the order of 100 mOsm, using multiphasic models of cartilage that account for the fixed and mobile charges within the tissue [4, 10, 11, 14, 16].
Due to the high permeability of the cell membrane to water [17–19], either mechanical loading or changes in the extracellular osmolarity result in rapid changes in cell volume [20–28]. In many cell types, alterations in cell volume are responsible for triggering recovery mechanisms that involve the activation of pumps and channels on the cell membrane and the initiation of intracellular signaling cascades in an effort to restore steady state cell volume [26, 29–34]. In particular, the concentration of intracellular calcium ion ([Ca2+]i) is believed to play an important role in regulatory volume control [35] and has been shown to increase in certain cells in response to osmotic stress [36]. The F-actin cytoskeleton, which can serve as a store of Ca2+, is also modulated by osmotic stress in a number of cell types. Dynamic reorganization of the F-actin network after exposure to hypo-osmotic stress has been described in chondrocytes [27, 37, 38] as well as other cell types [39–41], and appears to be necessary for chondrocytes volume regulation [21, 22]. Isolated articular chondrocytes exposed to hypo-osmotic stress undergo a rapid depolymerization of the cortical F-actin network, mediated by the IP3/PIP2/gelsolin pathway, followed by a gradual recovery [27]. This breakdown and reconstitution of F-actin is thought to play an important role in cell volume recovery by facilitating solute transport and enabling intracellular transport of proteins involved in volume regulation to the cell membrane [31]. Indeed, there is increasing evidence that F-actin serves as an important Ca2+ store within the cytoplasm [42]. In contrast to hypo-osmotic stress, exposure to hyper-osmotic conditions can result in F-actin stabilization, which can modulate both Ca2+ signaling and cell volume decrease in response to osmotic stress [40, 43–45].
Interleukin-1 (IL-1) is a pro-inflammatory cytokine that is upregulated in the joints of patients with osteoarthritis and is implicated in the destruction of cartilage extracellular matrix in various joint diseases. IL-1 increases the production of proteases responsible for matrix degeneration, suppresses matrix biosynthesis, and induces pro-inflammatory mediators, such as nitric oxide and prostaglandins [46–48]. While the effect of IL-1 on matrix synthesis and turnover is well established in articular cartilage, the early signaling events in chondrocytes remain unclear. Recent studies suggest that one of the earliest events in the response to IL-1 in chondrocytes is a transient increase in [Ca2+]i by a mechanism which may involve Ca2+ influx from the extracellular space, release from intracellular stores, or mobilization via activation of G-protein coupled receptors [49]. Exposure to IL-1 also stabilizes F-actin in chondrocytic cells by a pathway involving activation of members of the Rho family of small GTPases [49]. Of particular interest are findings showing that the response of chondrocytic cells to mechanical loading is altered by IL-1 [50, 51]. An improved understanding of the mechanisms of interaction between physical factors (i.e., mechanical or osmotic stresses) and biochemical factors (e.g., IL-1) may provide new insight into the pathology of diseases such as osteoarthritis.
The objective of this study was to determine the effect of IL-1 on the response of isolated articular chondrocytes to physicochemical changes that are associated with mechanical loading and unloading of the cartilage, i.e., hypo- and hyper-osmotic stress. The effects of osmotic stress were measured on the initiation of [Ca2+]i transients in single chondrocytes, as well as the adaptation and recovery of cell volume. Furthermore, the effect of IL-1 exposure on the reorganization of F-actin after hypo-osmotic stress was determined. Finally, the role of IL-1-induced [Ca2+]i transients and Rho GTPase activation in the response to osmotic stress was examined.
Materials and Methods
Cell Culture
Unless otherwise noted, reagents and chemicals were obtained from GibcoBRL (Grand Island, NY). Articular cartilage was harvested from the femoral condyles of skeletally mature pigs (N=8) obtained from a local abattoir. The tissue was minced and stored at room temperature in wash medium containing DMEM-high glucose supplemented with 100 μg/ml kanamycin (Sigma Chemical, St. Louis, MO), 150 μg/ml gentamicin, and 1 μg/ml fungizone. Cells were isolated from the tissue by a sequential pronase/collagenase digestion similar to a previously published protocol [52]. The tissue was digested for 60 minutes in 1320 PUK/ml pronase (Calbiochem, San Diego, CA), followed by 2 hour digestion in 0.4% type 2 collagenase. Isolated cell suspensions were filtered through a 70 μm nylon mesh filter (FALCON Cell Strainer; Becton Dickinson, Franklin Lakes, NJ) to remove debris. The cell pellet was then washed by sequential rinsing and centrifugation at 400× g for 10 minutes. Cell viability was determined using a trypan blue exclusion assay, and viability was found to be greater than 95% in all cases. The cells were plated at a density of 1.5 million cells/ml on 31 mm glass coverslips (#1.5, VWR Scientific, West Chester, PA) in 6-well culture plates. The cells were allowed to adhere for 45 minutes, and then each well was filled with 2 ml feed medium (DMEM/F12, 10% fetal bovine serum, no antibiotics). The cells were cultured overnight at 37°C, and all tests were performed between 12 and 24 hours after the cells were plated on glass to ensure that the cells maintained a rounded morphology, similar to that in situ. Cells from a minimum of 3 joints were used for each experimental condition, and the total number of chondrocytes per experiment is shown in the figure captions.
Interleukin-1 Exposure
Isolated chondrocytes were exposed to 10 ng/ml recombinant porcine IL-1α (R&D Systems, Minneapolis, MN) for 1 hour prior to osmotic stimulation. IL-1 was reconstituted in phosphate buffered saline (PBS) and diluted in culture medium to the desired concentration.
Osmotic Stress
Coverslips with adherent chondrocytes were mounted in a custom-built heated perfusion chamber containing iso-osmotic medium (DMEM-HG, 10mM HEPES, 340 mOsm). All experiments were performed at 37ºC. In control experiments, cells were perfused with iso-osmotic medium to ensure that the cells were not actively responding to the perfusion. Isolated cells were perfused with either hypo-osmotic medium (medium adjusted by the addition of distilled water to 240 mOsm), or hyper-osmotic medium (medium adjusted by the addition of sterile sucrose to 440 mOsm) in the presence and absence of IL-1. The osmolality of the solutions was measured using a freezing point osmometer (Osmette 2007, Precision System Inc., Natick, MA). Perfusion was performed by drawing the iso-osmotic media out of the chamber through a syringe, and perfusing with hypo- or hyper-osmotic media through a second syringe mounted on the opposite side of the chamber. The exchange of medium was performed in approximately 5 seconds.
Calcium Imaging
Transient changes in [Ca2+]i induced by osmotic stress were measured using fluorescence ratio imaging of single cells using laser scanning microscopy (LSM 510, Zeiss). Control and IL-1-treated cells were loaded with Fura-Red AM (20 μM) and Fluo-3 AM (15 μM) (Molecular Probes), and [Ca2+]i was measured in single cells using fluorescence ratio imaging as previously described [39, 49, 53]. Images were recorded at a scan rate of 0.33 Hz for 11 minutes to track relative [Ca2+]i and an increase of 10% above baseline of the intensity ratio was considered to represent a positive [Ca2+]i response. The percentage of cells responding to osmotic stress and the percentage of cells displaying oscillations in [Ca2+]i were reported. The peak fluorescence ratio and the time to peak following osmotic stress were also analyzed.
Cell Volume Measurement
Cell volume was measured using a custom-written image analysis program in the PV-WAVE programming language (Visual Numerics, San Ramon, California, USA). Differential interference contrast (DIC) images were collected using laser scanning microscopy (LSM 510, Zeiss) at a scan rate of 0.10 Hz for 20 minutes. The projected surface area of the cells was measured using an edge-detection algorithm and cell volume was calculated assuming a spherical morphology [54]. Cell volume was normalized to the iso-osmotic cell volume and minimum, maximum, and final (recovered) volumes were recorded.
The Rate of Change of Cell Volume
The time constant (τ) describing the rate of change in cell volume was determined using a nonlinear regression of the volume data to the following exponential models (Kaleidagraph, Synergy Software, Reading, Pennsylvania, USA):
where V is the volume, Vmax and Vmin are the maximum and minimum volumes, t is the time and τswell and τshrink are the time constants for swelling and shrinking. The value of τ was reported for each cell.
Inhibitors of Calcium Signaling and F-actin Stabilization
To examine the mechanisms involved in mediating the effect of IL-1 on the response of chondrocytes to osmotic stress, inhibitors of IL-1-induced Ca2+ signaling were used, based on our previous findings [49]. Thapsigargin (Calbiochem) has previously been shown to inhibit the mobilization of intracellular Ca2+ by IL-1 in chondrocytes [49]. A 30 minute pre-treatment with thapsigargin (3 μM) was used to determine if the effect of IL-1 on osmotically induced volume change and recovery was dependent on IL-1 induced Ca2+ mobilization. Toxin B (100 pg/mL, 3 hours, Sigma) was used to determine if activation of Rho GTPases mediated the effect of IL-1 on the response of chondrocytes to osmotic stress [49].
F-Actin Reorganization
Isolated chondrocytes in suspension were cultured for 1 hour either in control medium, or in medium containing 10 ng/mL IL-1. After the 1 hour pre-treatment, cells were exposed to hypo-osmotic stress for 0, 2, and 10 minutes and then fixed, permeabilized and labeled for F-actin with fluorescent phalloidin. The fluorescent label was solubilized with methanol and cellular F-actin was assayed using a plate reader. Specimens were normalized to the control levels at time-zero.
F-actin distribution following exposure to hypo-osmotic stress was determined using confocal microscopy. Isolated chondrocytes plated on glass coverslips were exposed to IL-1 and hypo-osmotic medium as described and then fixed, permeabilized, and their F-actin labeled with Alexa Fluor 488-phalloidin (Molecular Probes). Images of F-actin distribution were obtained using a 63×, 1.2 NA water immersion objective lens (Zeiss). The distribution of F-actin across the cell was visually compared between control and IL-1-treated groups, and between the 0, 2, and 10 minute time points.
Statistical Analysis
For all test conditions, the percentage of chondrocytes showing an increase in [Ca2+]i was reported. In addition, where appropriate, the percentage of cells displaying oscillations in [Ca2+]i, time to peak [Ca2+]i, and magnitude of [Ca2+]i increase was also reported. The percentage of cells responding and oscillating in each group was compared by chi-squared analysis of proportions (StatView). The peak [Ca2+]i and the time to peak in each group were compared by ANOVA with Fishers PLSD post hoc test (StatView). To characterize the volume adaptation after osmotic stress, the maximum or minimum and final volumes, as well as the time constant for cell shrinking or swelling were recorded. Each of these parameters was compared between groups using ANOVA with Fishers PLSD.
Results
Control experiments confirmed that perfusion of the chondrocytes with an iso-osmotic solution (340 mOsm) did not elicit changes in [Ca2+]i. Perfusion with either hypo- (240 mOsm) or hyper-osmotic (440 mOsm) medium led to an increase in [Ca2+]i accompanied by oscillations that decreased in magnitude over time (Figure 1). In untreated chondrocytes, virtually all cells tested exhibited transient increases in [Ca2+]i after exposure to hypo-osmotic (92.3%) or hyper-osmotic stress (93.3%) (Table 1). Nearly all of the responding cells displayed oscillations in [Ca2+]i (87.5% and 100% for hypo- and hyper-osmotic stress, respectively) (Table 1).
Figure 1. Representative trace of [Ca2+]i response of chondrocytes to hypo-osmotic stress.
Articular chondrocytes exposed to hypo- or hyper-osmotic stress medium responded by mobilizing [Ca2+]i, followed by decaying oscillations. The y-axis on this plot represents the fluorescence ratio (Fluo-3 AM excitation: Fura Red AM excitation) normalized to the ratio before exposure to osmotic stress.
Table 1. The percentage of cells showing increased [Ca2+]i and displaying oscillations in [Ca2+]i after acute exposure to osmotic stress.
Isolated chondrocytes responded to hypo and hyper-osmotic stress by initiating [Ca2+]i with oscillations in virtually all cells. Pre-treatment with IL-1 decreased the percentage of cells responding after both hypo and hyper-osmotic stress, and decreased the percentage displaying oscillations in the hypo-osmotic case only. Inhibition of the IL-1 effect with IL-1Ra restored the percentage of cells responding and oscillating to levels that were not significantly different from control. Pre-treatment with toxin B increased the percentage of cells responding and oscillating relative to the IL-1 treated group, with only the percentage of cells responding to hypo-osmotic stress significantly different from control.
| 10 ng/mL IL-1 | |||||
|---|---|---|---|---|---|
| Control | No inhibitor | IL-1Ra | Toxin B | ||
| Hyper-osmotic stress | % responding | 93.3% | 53.3% * | 90.5 % | 83.3% |
| % oscillating | 100% | 87.5% | 100% | 86.7% | |
| Hypo-osmotic stress | % responding | 92.3% | 27.3% * | 88.9% | 63.3% * |
| % oscillating | 87.5% | 0% * | 79.2% | 76.7% | |
p<0.01 vs. untreated control group, n=22–26 cells, Chi-squared analysis of proportions.
Pre-treatment with IL-1 for one hour significantly decreased the percentage of cells responding to osmotic stress. Only 27.3% of IL-1-treated cells displayed [Ca2+]i transients after exposure to hypo-osmotic stress, and none of these cells displayed oscillations (Table 1). In response to hyper-osmotic stress, only 53.3% of IL-1-treated cells displayed [Ca2+]i transients, and 87.5% of these responding cells displayed oscillations (Table 1). To confirm the specificity of the IL-1 response, IL-1 receptor antagonist (IL-1Ra, R & D Systems) was added simultaneously with the IL-1. In these experiments, the percentage of cells mobilizing Ca2+ and displaying oscillations was indistinguishable from that of control experiments.
In chondrocytes exposed to toxin B for 3 hours prior to IL-1 exposure and then perfused with hypo-osmotic medium, a significant decrease was observed in the [Ca2+]i response, with 63.3% of cells showing increased [Ca2+]i and 76.7% of these displaying oscillations, Table 1. In contrast, toxin B had no effect on the percentage of cells responding or oscillating after hyper-osmotic stress in the presence of IL-1 (Table 1).Hypo-osmotic stress increased the peak fluorescence ratio by a factor of 2.57±0.66 in control cells (Figure 2a), while hyper-osmotic stress increased this ratio by a factor of 3.19±0.60 (Figure 2b) over baseline. The time to peak [Ca2+]i in the untreated cells was 83±28.9 seconds for hypo-osmotic stress and 234±74.4 seconds for hyper-osmotic stress (Figure 2a and 2b).
Figure 2.
(a) Magnitude of peak [Ca2+]i and time to peak in chondrocytes after exposure to hypo-osmotic stress Control chondrocytes responded to hypo-osmotic stress with a 2.5-fold increase in [Ca2+]i within 83 seconds of exposure. Pre-treatment of cells with IL-1 had no effect on the fold increase of [Ca2+]i or the time to peak. Data is expressed as mean ± standard deviation. * p<0.05 vs. control, n=14–20 cells, ANOVA with Fisher’s PLSD post hoc test. (b) Magnitude of peak [Ca2+]i and time to peak in chondrocytes after exposure to hyper-osmotic stress. Control chondrocytes responded to hyper-osmotic stress with a 3-fold increase in [Ca2+]i within 234 seconds of exposure. Pre-treatment of cells with IL-1 had no effect on the fold increase of [Ca2+]i but significantly increased the time to peak to 412 seconds. Data is expressed as mean ± standard deviation. * p<0.05 vs. control, n=14–20 cells, ANOVA with Fisher’s PLSD post hoc test.
The presence of IL-1 had no effect on the peak increase in fluorescence magnitude in response to hypo- or hyper-osmotic stress (2.12±0.82 and 3.08±0.62, Figure 2). IL-1 had no effect on the time to peak [Ca2+]i after hypo-osmotic stress (74.8±36.4 seconds, Figure 2a), but significantly increased the time to peak to 412.6±86.4 seconds after hyper-osmotic stress (Figure 2b).
The magnitude of the [Ca2+]i peak and the time to peak in cells exposed to IL-1Ra and toxin B before IL-1 treatment was not significantly different from control for either hypo- or hyper-osmotic stress.
Control experiments confirmed that chondrocytes perfused with iso-osmotic solution did not demonstrate any volume change. Cells exposed to hypo-osmotic medium rapidly swelled and exhibited a significant recovery of their volume (Figure 3a). In contrast, cells exposed to a hyper-osmotic solution displayed rapid volume decrease to 0.79±0.03 of their original volume (Figure 3b) with no measurable volume recovery over the course of the experiment.
Figure 3.
(a) Characteristic volume response of chondrocytes to hypo-osmotic stress Cell volume change after acute osmotic stress was measured using a custom image analysis algorithm. This trace shows cell volume over time for an untreated chondrocyte and an IL-1 treated chondrocyte exposed to 240 mOsm medium at time t=0. Cells respond to hypo-osmotic stress by rapidly swelling and attempting to regulate their volume. IL-1 treatment inhibited volume regulation in chondrocytes. (b) Characteristic volume response of chondrocytes to hyper-osmotic stress. Cell volume change after acute osmotic stress was measured using a custom image analysis algorithm. This trace shows cell volume over time for a single untreated chondrocyte exposed to 440 mOsm medium at time t=0. Cells respond to hyper-osmotic stress by rapidly shrinking with no appreciable volume regulation.
Control cells exposed to a hypo-osmotic solution displayed rapid volume increase to 1.48±0.09 times their original volume and then recovered partially to 1.27±0.08 (Figure 4). Exposure to 10 ng/mL IL-1 for 1 hour prior to osmotic stress had no effect on the swelling phase in isolated chondrocytes (1.48±0.11 times original volume, Figure 4), though the extent of volume regulation was significantly inhibited (1.41±0.06, Figure 4). Co-treatment with IL-1Ra, thapsigargin or toxin B had no effect on the volume increase though they antagonized the IL-1 effect by allowing comparable volume recovery to control (1.50±0.10 and 1.25±0.06; 1.46±0.12 and 1.22±0.11; 1.50±0.13 and 1.30±0.07 for maximum and recovered volume, respectively, for IL-1Ra, thapsigargin and toxin B, Figure 4).
Figure 4. Cell swelling and regulatory volume decrease in chondrocytes exposed to hypo-osmotic stress.
Control chondrocytes responded to osmotic stress with a rapid increase in cell volume to approximately 150% of initial volume followed by RVD to approximately 125% of initial volume. Pre-treatment of cells with IL-1 had no effect on cell swelling, but reduced the capacity for RVD. Inhibition of the IL-1 effect by IL-1Ra and thapsigargin successfully restored normal RVD. Cells pre-treated with toxin B before IL-1 exposure swelled normally, and showed significant volume regulation although final volume was slightly higher than in the control cells. * p<0.05 vs. untreated control, n=31–43 cells, ANOVA with Fisher’s PLSD post hoc test.
Exposure to 10 ng/mL IL-1 for 1 hour prior to hyper-osmotic stress significantly diminished but did not eliminate the extent of volume decrease in isolated chondrocytes (0.87±0.05 final volume, Figure 5). As expected, IL-1Ra antagonized the effect of IL-1. Furthermore, treatment with thapsigargin or toxin B restored the extent of volume decrease back to levels indistinguishable from the control (0.77±0.03 to 0.80±0.04, Figure 5).
Figure 5. Extent of cell shrinking in chondrocytes exposed to hyper-osmotic stress.
Control chondrocytes responded with a rapid decrease in cell volume to 80% of initial volume. Pre-treatment of cells with IL-1 reduced the extent of cell shrinking to 86% of initial volume. Inhibition of the IL-1 effect by IL-1Ra, thapsigargin, or toxin B restored volume adaptation to levels similar to control. * p<0.05 vs. untreated control, n=32–40 cells, ANOVA with Fisher’s PLSD post hoc test.
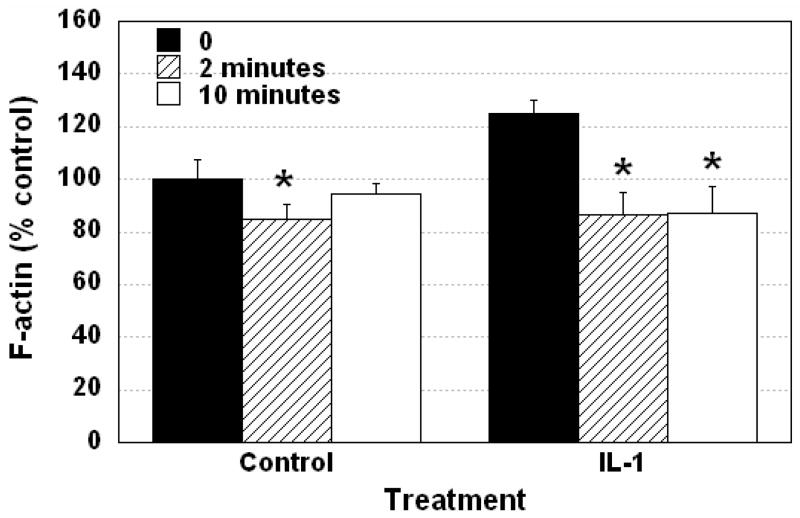
The transient volume data for both shrinking and swelling was fit using non-linear regression to an exponential model in order to determine the rate of volume change (τ). The curve fits were generally excellent with correlation coefficients (R) of greater than 0.9 in all cases, with R2=0.89±0.06 and R2=0.93±0.04 for shrinking and swelling, respectively. Control cells exposed to hypo-osmotic stress swelled rapidly (τ=23.7±6.2 seconds). Pre-treatment of cells with IL-1 with and without IL-1Ra, thapsigargin or toxin B had no effect on the rate of cell swelling (data not shown). Control experiments confirmed that chondrocytes perfused with an iso-osmotic solution (340 mOsm) did not demonstrate F-actin reorganization. In untreated chondrocytes exposed to hypo-osmotic stress, F-actin was transiently disrupted at 2 minutes, and had begun to recover back towards baseline levels by 10 minutes (Figure 6). In IL-1-treated chondrocytes exposed to hypo-osmotic stress, F-actin was transiently disrupted at 2 minutes, with little recovery apparent at 10 minutes (Figure 6).
Figure 6. Cellular F-actin content in isolated chondrocytes after exposure to hypo-osmotic stress with and without IL-1 treatment.
Hypo-osmotic stress led to a decrease in cellular F-actin within 2 minutes in both control and IL-1 treated chondrocytes. This disruption was followed by a gradual recovery to levels indistinguishable from the initial condition within 10 minutes. Conversely, after IL-1 treatment, chondrocytes did not significantly recover their cellular F-actin content by 10 minutes. Data is expressed as mean ± standard deviation. * p<0.05 vs. control group at 1 hour, n= 14–20, ANOVA with Fisher’s PLSD post hoc test.
Control cells exposed to hyper-osmotic stress shrank rapidly with τ=17.9±3.1 seconds, while cells pre-treated with IL-1 shrank significantly more slowly, with a τ=32.4±4.4 seconds (Figure 7). Inhibition of the IL-1 response by IL-1Ra (τ=19.8±3.7 seconds), thapsigargin (τ=16.6±5.2 seconds), or toxin B (τ=20.2±2.4 seconds) all reduced τ to levels similar to control (Figure 7).
Figure 7. Time constant of cell shrinking in chondrocytes exposed to hyper-osmotic stress.
Control chondrocytes responded to hyper-osmotic stress with a rapid decrease in cell volume (τ=17.9 seconds). Pre-treatment of cells with IL-1 reduced the rate of cell shrinking as reflected by the greater time constant (τ=32.4 seconds). Inhibition of the IL-1 effect by IL-1Ra ((τ=19.8 seconds), thapsigargin (τ=16.6 seconds), and toxin B (τ=20.2 seconds) restored the rate of cell volume change to levels similar to control. * p<0.05 vs. untreated control, n=32–40 cells, ANOVA with Fisher’s PLSD post hoc test.
Discussion
The findings of this study indicate that chondrocytes respond to physiologically relevant changes in extracellular osmolarity with rapid alterations in cell volume, followed by a transient increase in [Ca2+]i that is characterized by decaying oscillations. These responses were significantly altered in the presence of IL-1, a pro-inflammatory cytokine that is associated with cartilage catabolism in rheumatoid arthritis and osteoarthritis. The effects of IL-1 on intracellular Ca2+ mobilization and cell volume recovery appear to be due to altered structure and remodeling of the F-actin cytoskeleton that is mediated by Rho family GTPases [49]. Taken together, these findings provide further insights into the mechanisms by which IL-1 may interfere with normal physiologic processes in the chondrocyte, such as the adaptation or regulatory responses to mechanical and osmotic loading.
These results are consistent with previous studies showing that hyper-osmotic stress induces a rapid decrease in volume and mobilizes Ca2+ in articular chondrocytes, with little apparent regulatory volume increase, consistent with previous studies of isolated or in situ chondrocytes [22, 26]. Exposure of chondrocytes to IL-1 decreased the percentage of cells exhibiting [Ca2+]i transients in response to hyper-osmotic stress, and delayed the time to peak [Ca2+]i in those cells that did respond. The inhibition of IL-1-induced Ca2+ signaling or F-actin stabilization, via IL-1Ra, thapsigargin, or toxin B rescued the IL-1 effect. However, the presence of IL-1 did not alter the peak increase in fluorescence magnitude in response to hypo- or hyper-osmotic stress (Figure 2), suggesting that cells that did respond to osmotic stress were likely responding by similar mechanisms. These results are consistent with previous studies suggesting that stabilization of F-actin using phalloidin can modulate osmotically-induced Ca2+ signaling in cells of the intervertebral disc [45]. These findings support the hypotheses of Lange and coworkers, who have shown that the existence of a dense, organized cortical F-actin shell can act as an effective barrier to the movement of small molecules such as glucose, as well as signaling molecules such as Ca2+, into and out of the cell [31, 42, 55, 56]. In this case, signaling must be initiated first by breakdown of the F-actin barrier followed by diffusion of the molecule of interest [57]. In general, previous studies in a number of cell types have shown that F-actin is stabilized by hyper-osmotic stress [40, 43, 44, 58]. Furthermore, extracellular influx of Ca2+ is necessary for Ca2+ signaling after hyper-osmotic stress [26]. Taken together, these findings suggest that one mechanism for inhibition of [Ca2+]i signaling by IL-1 may involve inhibition of Ca2+ transport through a stabilized F-actin in the cell cortex or microvilli, which may then be responsible for inhibiting other Ca2+ mechanisms such as those required for osmotic signaling.
IL-1 also significantly affected the magnitude and rate of volume adaptation in response to hyper-osmotic stress. These effects may be due to changes in the viscoelastic mechanical properties of the chondrocyte, caused by IL-1 induced stabilization of intracellular F-actin [49]. Numerous studies have shown that F-actin is a primary determinant of cellular mechanical properties, and that both the stiffness and apparent viscosity of isolated cells is modulated by altering the F-actin cytoskeleton. For example, cytochalasin D, a disruptor of F-actin microfilaments, significantly decreases chondrocyte stiffness and viscosity [59] and alters the relationship between the deformation of the cell, nucleus, and extracellular matrix [60]. Cells of the intervertebral disc show similar trends in response to cytochalasin D, while stabilization of F-actin with phalloidin increases cell stiffness and viscosity [45]. In many chondrocytic cells, cell stiffness and viscosity are correlated with F-actin organization [59, 61–63].
In response to hypo-osmotic stress, chondrocytes exhibited cell swelling, transient increases in [Ca2+]i, and regulatory volume decrease that was associated with F-actin reorganization. Under control conditions, chondrocytes swelled rapidly, with a time constant of An important finding of this study was that exposure to IL-1 significantly inhibited the ability of chondrocytes to mobilize intracellular Ca2+ in response to hypo-osmotic stress. The ability of thapsigargin and toxin B to reverse the IL-1 effect suggests that both IL-1 induced Ca2+ signaling and activation of Rho GTPases play an important role in this response. Hypo-osmotic Ca2+ signaling in articular chondrocytes has been shown to require influx from the extracellular space as well as a contribution by Ca2+ release from IP3 sensitive intracellular stores [27, 38]. The mechanism of the effect of IL-1 on the response of chondrocytes to hypo-osmotic stress is not fully understood, and appears to differ from the mechanisms involved in the hyper-osmotic stress response. Rather than F-actin acting as a transport barrier, hypo-osmotically induced Ca2+ signaling appears to involve Ca2+ release from the F-actin network as it is dissociated [27, 42]. Furthermore, the stiffness of the F-actin cortex and membrane are significantly lower than the osmotic stresses applied, and thus the cell is likely unable to withstand the physical forces generated during cell swelling [37, 64]. In support of these mechanisms, recent studies have shown that disruption of F-actin in isolated chondrocytes stimulates regulatory volume decrease (RVD) but does not affect the initial swelling response [21]. However, the overall rate of RVD in our study was somewhat slower than that reported previously for bovine chondrocytes in monolayer
IL-1 had no effect on either the rate or the extent of cell swelling, but significantly altered RVD in response to hypo-osmotic stress (Figure 7). The ability of IL-1 to inhibit RVD was reversed by preventing IL-1 induced Ca2+ signaling, using IL-1Ra, a natural antagonist of IL-1. More interestingly, both thapsigargin and toxin B also counteracted the IL-1-induced inhibition of RVD implicating intracellular Ca2+ stores and Rho activation, respectively. These findings are consistent with previous studies on other cells types showing that disruption or stabilization of F-actin alters RVD [44, 57, 65, 66]. Specific to chondrocytes, Kerrigan and Bush showed that disruption of F-actin with latrunculin B increased the overall rate RVD [21]. One common hypothesis to explain these results involves the ability of F-actin to bind and in some cases physically regulate a number of diverse ion channels at the cell membrane [44, 65]. It is thought that impairing the ability of cells to modulate activity of these channels through dynamic reorganization of the cytoskeleton may directly or indirectly influence the RVD process. This finding is consistent with previous studies showing that IL-1 stabilizes F-actin in clusters at the cell membrane and inhibits the ability of cells to reorganize F-actin after swelling induced disruption [49].
Changes in the extracellular osmolarity represent one component of the diverse biophysical environment to which the chondrocyte is exposed due to mechanical loading of the extracellular matrix, and direct deformation of chondrocytes may also initiate Ca2+ signaling [67–70]. Hyper-osmotic stress has been implicated as a potential surrogate for static compressive loading in cartilage [71], and the osmotic environment plays an important role in controlling synthesis and breakdown of components of the extracellular matrix [72]. Recent studies suggest that the effects of static compression on cartilage may be due to upregulation of IL-1. [73]. Hyper-osmotic stress acts synergistically with IL-1 to stimulate COX-2 expression and PGE2 production in articular cartilage [74]. In addition, transfection of chondrocytes with a constitutively active form of Rho has been shown to upregulate expression of MMP-13, a protease which is activated by IL-1 exposure in cartilage [75]. Conversely, the ability of chondrocytes to mobilize [Ca2+]i and adapt and regulate their volume after hypo-osmotic stress may represent the “recovery” response to tissue compression, and are thought to represent an important step in the process of mechanotransduction in chondrocytes [21, 27, 76, 77]. The downstream consequences of this response are not well characterized, but are thought to include changes in matrix synthesis and breakdown and altered gene expression among others [78]. An improved understanding of the sequence of biophysical and biochemical events involved in the process of mechanical signal transduction by chondrocytes will hopefully provide new insight into normal physiology of articular cartilage, as well as the pathology of diseases such as osteoarthritis.
Acknowledgments
Supported by NIH grants AG15768, AR50245, AR48182, and a grant from GlaxoSmithKline, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Griffin TM, Guilak F. The role of mechanical loading in the onset and progression of osteoarthritis. Exerc Sport Sci Rev. 2005;33:195–200. doi: 10.1097/00003677-200510000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Grodzinsky AJ, Levenston ME, Jin M, Frank EH. Cartilage tissue remodeling in response to mechanical forces. Annu Rev Biomed Eng. 2000;2:691–713. doi: 10.1146/annurev.bioeng.2.1.691. [DOI] [PubMed] [Google Scholar]
- 3.Guilak F, Sah R, Setton L. Physical regulation of cartilage metabolism. In: Hayes W, Mow V, editors. Basic Orthopaedic Biomechanics. Philadelphia: Lippincott-Raven; 1997. pp. 179–207. [Google Scholar]
- 4.Mow VC, Wang CC, Hung CT. The extracellular matrix, interstitial fluid and ions as a mechanical signal transducer in articular cartilage. Osteoarthritis Cartilage. 1999;7:41–58. doi: 10.1053/joca.1998.0161. [DOI] [PubMed] [Google Scholar]
- 5.Guilak F, Mow VC. The mechanical environment of the chondrocyte: a biphasic finite element model of cell-matrix interactions in articular cartilage. J Biomech. 2000;33:1663–1673. [PubMed] [Google Scholar]
- 6.Guilak F, Alexopoulos LG, Upton ML, Youn I, Choi JB, Cao L, et al. The pericellular matrix as a transducer of biomechanical and biochemical signals in articular cartilage. Ann N Y Acad Sci. 2006;1068:498–512. doi: 10.1196/annals.1346.011. [DOI] [PubMed] [Google Scholar]
- 7.Mow VC, Holmes MH, Lai WM. Fluid transport and mechanical properties of articular cartilage: a review. Journal of Biomechanics. 1984;17:377–394. doi: 10.1016/0021-9290(84)90031-9. [DOI] [PubMed] [Google Scholar]
- 8.Soltz MA, Ateshian GA. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J Biomech. 1998;31:927–934. doi: 10.1016/s0021-9290(98)00105-5. [DOI] [PubMed] [Google Scholar]
- 9.Eckstein F, Lemberger B, Stammberger T, Englmeier KH, Reiser M. Patellar cartilage deformation in vivo after static versus dynamic loading. J Biomech. 2000;33:819–825. doi: 10.1016/s0021-9290(00)00034-8. [DOI] [PubMed] [Google Scholar]
- 10.Maroudas A, Muir H, Wingham J. The correlation of fixed negative charge with glycosaminoglycan content of human articular cartilage. Biochim Biophys Acta. 1969;177:492–500. doi: 10.1016/0304-4165(69)90311-0. [DOI] [PubMed] [Google Scholar]
- 11.Lai WM, Hou JS, Mow VC. A triphasic theory for the swelling and deformation behaviors of articular cartilage. J Biomech Eng. 1991;113:245–258. doi: 10.1115/1.2894880. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong CG, Bahrani AS, Gardner DL. In vitro measurement of articular cartilage deformations in the intact human hip joint under load. J Bone Joint Surg Am. 1979;61:744–755. [PubMed] [Google Scholar]
- 13.Likhitpanichkul M, Guo XE, Mow VC. The effect of matrix tension-compression nonlinearity and fixed negative charges on chondrocyte responses in cartilage. Mol Cell Biomech. 2005;2:191–204. [PubMed] [Google Scholar]
- 14.Haider MA, Schugart RC, Setton LA, Guilak F. A mechano-chemical model for the passive swelling response of an isolated chondron under osmotic loading. Biomech Model Mechanobiol. 2006;5:160–171. doi: 10.1007/s10237-006-0026-1. [DOI] [PubMed] [Google Scholar]
- 15.Maroudas A, Ziv I, Weisman N, Venn M. Studies of hydration and swelling pressure in normal and osteoarthritic cartilage. Biorheology. 1985;22:159–169. doi: 10.3233/bir-1985-22206. [DOI] [PubMed] [Google Scholar]
- 16.Gray ML, Pizzanelli AM, Grodzinsky AJ, Lee RC. Mechanical and physiochemical determinants of the chondrocyte biosynthetic response. J Orthop Res. 1988;6:777–792. doi: 10.1002/jor.1100060602. [DOI] [PubMed] [Google Scholar]
- 17.Verkman AS, van Hoek AN, Ma T, Frigeri A, Skach WR, Mitra A, et al. Water transport across mammalian cell membranes. American Journal of Physiology. 1996;270:C12–C30. doi: 10.1152/ajpcell.1996.270.1.C12. [DOI] [PubMed] [Google Scholar]
- 18.Mobasheri A, Marples D. Expression of the AQP-1 water channel in normal human tissues: a semiquantitative study using tissue microarray technology. Am J Physiol Cell Physiol. 2004;286:C529–537. doi: 10.1152/ajpcell.00408.2003. [DOI] [PubMed] [Google Scholar]
- 19.Mobasheri A, Trujillo E, Bell S, Carter SD, Clegg PD, Martin-Vasallo P, et al. Aquaporin water channels AQP1 and AQP3, are expressed in equine articular chondrocytes. Vet J. 2004;168:143–150. doi: 10.1016/j.tvjl.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Bush PG, Hall AC. Passive osmotic properties of in situ human articular chondrocytes within non-degenerate and degenerate cartilage. J Cell Physiol. 2005;204:309–319. doi: 10.1002/jcp.20294. [DOI] [PubMed] [Google Scholar]
- 21.Kerrigan MJ, Hall AC. Stimulation of regulatory volume decrease (RVD) by isolated bovine articular chondrocytes following F-actin disruption using latrunculin B. Biorheology. 2005;42:283–293. [PubMed] [Google Scholar]
- 22.Kerrigan MJ, Hook CS, Qusous A, Hall AC. Regulatory volume increase (RVI) by in situ and isolated bovine articular chondrocytes. J Cell Physiol. 2006;209:481–492. doi: 10.1002/jcp.20758. [DOI] [PubMed] [Google Scholar]
- 23.Chao PG, Tang Z, Angelini E, West AC, Costa KD, Hung CT. Dynamic osmotic loading of chondrocytes using a novel microfluidic device. Journal of Biomechanics. 2005;38:1273–1281. doi: 10.1016/j.jbiomech.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 24.Ateshian GA, Likhitpanichkul M, Hung CT. A mixture theory analysis for passive transport in osmotic loading of cells. J Biomech. 2006;39:464–475. doi: 10.1016/j.jbiomech.2004.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ateshian GA, Costa KD, Hung CT. A theoretical analysis of water transport through chondrocytes. Biomech Model Mechanobiol. 2007;6:91–101. doi: 10.1007/s10237-006-0039-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erickson GR, Alexopoulos LG, Guilak F. Hyper-osmotic stress induces volume change and calcium transients in chondrocytes by transmembrane, phospholipid, and G-protein pathways. J Biomech. 2001;34:1527–1535. doi: 10.1016/s0021-9290(01)00156-7. [DOI] [PubMed] [Google Scholar]
- 27.Erickson GR, Northrup DL, Guilak F. Hypo-osmotic stress induces calcium-dependent actin reorganization in articular chondrocytes. Osteoarthritis Cartilage. 2003;11:187–197. doi: 10.1053/s1063-4584(02)00347-3. [DOI] [PubMed] [Google Scholar]
- 28.Trickey WR, Baaijens FP, Laursen TA, Alexopoulos LG, Guilak F. Determination of the Poisson's ratio of the cell: recovery properties of chondrocytes after release from complete micropipette aspiration. J Biomech. 2006;39:78–87. doi: 10.1016/j.jbiomech.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Guizouarn H, Motais R, Garcia Romeu F, Borgese F. Cell volume regulation: the role of taurine loss in maintaining membrane potential and cell pH. Journal of Physiology. 2000;523(Pt 1):147–154. doi: 10.1111/j.1469-7793.2000.t01-1-00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffmann EK, Dunham PB. Membrane mechanisms and intracellular signalling in cell volume regulation. International Review of Cytology. 1995;161:173–262. doi: 10.1016/s0074-7696(08)62498-5. [DOI] [PubMed] [Google Scholar]
- 31.Lange K. Regulation of cell volume via microvillar ion channels. Journal of Cellular Physiology. 2000;185:21–35. doi: 10.1002/1097-4652(200010)185:1<21::AID-JCP2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 32.O'Neill C. Physiological significance of volume-regulatory transporters. American Journal of Physiology. 1999;276:C995–C1011. doi: 10.1152/ajpcell.1999.276.5.C995. [DOI] [PubMed] [Google Scholar]
- 33.Perlman DF, Goldstein L. Organic osmolyte channels in cell volume regulation in vertebrates. Journal of Experimental Zoology. 1999;283:725–733. [PubMed] [Google Scholar]
- 34.Waldegger S, Steuer S, Risler T, Heidland A, Capasso G, Massry S, et al. Mechanisms and clinical significance of cell volume regulation. Nephrology, Dialysis, Transplantation. 1998;13:867–874. doi: 10.1093/ndt/13.4.867. [DOI] [PubMed] [Google Scholar]
- 35.McCarty NA, O'Neil RG. Calcium signaling in cell volume regulation. Physiological Reviews. 1992;72:1037–1061. doi: 10.1152/physrev.1992.72.4.1037. [DOI] [PubMed] [Google Scholar]
- 36.Lang F, Busch GL, Volkl H. The diversity of volume regulatory mechanisms. Cellular Physiology and Biochemistry. 1998;8:1–45. doi: 10.1159/000016269. [DOI] [PubMed] [Google Scholar]
- 37.Guilak F, Erickson GR, Ting_Beall HP. The effects of osmotic stress on the viscoelastic and physical properties of articular chondrocytes. Biophysical Journal. 2002;82:720–727. doi: 10.1016/S0006-3495(02)75434-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chao PH, West AC, Hung CT. Chondrocyte intracellular calcium, cytoskeletal organization, and gene expression responses to dynamic osmotic loading. Am J Physiol Cell Physiol. 2006;291:C718–725. doi: 10.1152/ajpcell.00127.2005. [DOI] [PubMed] [Google Scholar]
- 39.Pritchard S, Guilak F. The role of F-actin in hypo-osmotically induced cell volume change and calcium signaling in anulus fibrosus cells. Ann Biomed Eng. 2004;32:103–111. doi: 10.1023/b:abme.0000007795.69001.35. [DOI] [PubMed] [Google Scholar]
- 40.Cantiello HF. Role of actin filament organization in cell volume and ion channel regulation. Journal of Experimental Zoology. 1997;279:425–435. doi: 10.1002/(sici)1097-010x(19971201)279:5<425::aid-jez4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 41.Moran J, Sabanero M, Meza I, Pasantes_Morales H. Changes of actin cytoskeleton during swelling and regulatory volume decrease in cultured astrocytes. The American Journal of Physiology. 1996;271:C1901–1907. doi: 10.1152/ajpcell.1996.271.6.C1901. [DOI] [PubMed] [Google Scholar]
- 42.Lange K, Gartzke J. F-actin-based Ca signaling-a critical comparison with the current concept of Ca signaling. J Cell Physiol. 2006;209:270–287. doi: 10.1002/jcp.20717. [DOI] [PubMed] [Google Scholar]
- 43.Cornet M, Isobe Y, Lemanski LF. Effects of anisosmotic conditions on the cytoskeletal architecture of cultured PC12 cells. Journal of Morphology. 1994;222:269–286. doi: 10.1002/jmor.1052220305. [DOI] [PubMed] [Google Scholar]
- 44.Henson JH. Relationships between the actin cytoskeleton and cell volume regulation. Microscopy Research and Technique. 1999;47:155–162. doi: 10.1002/(SICI)1097-0029(19991015)47:2<155::AID-JEMT7>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 45.Pritchard S, Erickson GR, Guilak F. Hyperosmotically induced volume change and calcium signaling in intervertebral disk cells: the role of the actin cytoskeleton. Biophys J. 2002;83:2502–2510. doi: 10.1016/S0006-3495(02)75261-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cernanec J, Guilak F, Weinberg JB, Pisetsky DS, Fermor B. Influence of hypoxia and reoxygenation on cytokine-induced production of proinflammatory mediators in articular cartilage. Arthritis Rheum. 2002;46:968–975. doi: 10.1002/art.10213. [DOI] [PubMed] [Google Scholar]
- 47.Goldring MB. Osteoarthritis and cartilage: the role of cytokines. Curr Rheumatol Rep. 2000;2:459–465. doi: 10.1007/s11926-000-0021-y. [DOI] [PubMed] [Google Scholar]
- 48.van den Berg WB, van de Loo FA, Otterness I, Arntz O, Joosten LA. In vivo evidence for a key role of IL-1 in cartilage destruction in experimental arthritis. Agents and Actions Supplements. 1991;32:159–163. doi: 10.1007/978-3-0348-7405-2_21. [DOI] [PubMed] [Google Scholar]
- 49.Pritchard S, Guilak F. Effects of interleukin-1 on calcium signaling and the increase of filamentous actin in isolated and in situ articular chondrocytes. Arthritis Rheum. 2006;54:2164–2174. doi: 10.1002/art.21941. [DOI] [PubMed] [Google Scholar]
- 50.Shin SJ, Fermor B, Weinberg JB, Pisetsky DS, Guilak F. Regulation of matrix turnover in meniscal explants: role of mechanical stress, interleukin-1, and nitric oxide. J Appl Physiol. 2003;95:308–313. doi: 10.1152/japplphysiol.00131.2003. [DOI] [PubMed] [Google Scholar]
- 51.Patwari P, Cook MN, DiMicco MA, Blake SM, James IE, Kumar S, et al. Proteoglycan degradation after injurious compression of bovine and human articular cartilage in vitro: interaction with exogenous cytokines. Arthritis Rheum. 2003;48:1292–1301. doi: 10.1002/art.10892. [DOI] [PubMed] [Google Scholar]
- 52.Kuettner KE, Pauli BU, Gall G, Memoli VA, Schenk RK. Synthesis of cartilage matrix by mammalian chondrocytes in vitro. I. Isolation, culture characteristics, and morphology. J Cell Biol. 1982;93:743–750. doi: 10.1083/jcb.93.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lipp P, Niggli E. Ratiometric confocal Ca(2+)-measurements with visible wavelength indicators in isolated cardiac myocytes. Cell Calcium. 1993;14:359–372. doi: 10.1016/0143-4160(93)90040-d. [DOI] [PubMed] [Google Scholar]
- 54.Alexopoulos LG, Erickson GR, Guilak F. A method for quantifying cell size from differential interference contrast images: validation and application to osmotically stressed chondrocytes. J Microsc. 2002;205:125–135. doi: 10.1046/j.0022-2720.2001.00976.x. [DOI] [PubMed] [Google Scholar]
- 55.Lange J, Schlieps K, Lange K, Knoll-Kohler E. Activation of calcium signaling in isolated rat hepatocytes is accompanied by shape changes of microvilli. Exp Cell Res. 1997;234:486–497. doi: 10.1006/excr.1997.3652. [DOI] [PubMed] [Google Scholar]
- 56.Lange K. Microvillar Ca++ signaling: a new view of an old problem. J Cell Physiol. 1999;180:19–34. doi: 10.1002/(SICI)1097-4652(199907)180:1<19::AID-JCP3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 57.Lange K. Microvillar ion channels: cytoskeletal modulation of ion fluxes. J Theor Biol. 2000;206:561–584. doi: 10.1006/jtbi.2000.2146. [DOI] [PubMed] [Google Scholar]
- 58.Hallows KR, Law FY, Packman CH, Knauf PA. Changes in cytoskeletal actin content, F-actin distribution, and surface morphology during HL-60 cell volume regulation. Journal of Cellular Physiology. 1996;167:60–71. doi: 10.1002/(SICI)1097-4652(199604)167:1<60::AID-JCP7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 59.Trickey WR, Lee GM, Guilak F. Viscoelastic properties of chondrocytes from normal and osteoarthritic human cartilage. J Orthop Res. 2000;18:891–898. doi: 10.1002/jor.1100180607. [DOI] [PubMed] [Google Scholar]
- 60.Guilak F. Compression-induced changes in the shape and volume of the chondrocyte nucleus. J Biomech. 1995;28:1529–1541. doi: 10.1016/0021-9290(95)00100-x. [DOI] [PubMed] [Google Scholar]
- 61.Darling EM, Hu JCY, Athanasiou KA. Zonal and topographical differences in articular chondrocyte gene expression. J Orthop Res. 2004;22:1182–1187. doi: 10.1016/j.orthres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 62.Darling EM, Zauscher S, Block JA, Guilak F. A thin-layer model for viscoelastic, stress-relaxation testing of cells using atomic force microscopy: do cell properties reflect metastatic potential? Biophys J. 2007;92:1784–1791. doi: 10.1529/biophysj.106.083097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guilak F, Ting_Beall HP, Baer AE, Trickey WR, Erickson GR, Setton LA. Viscoelastic properties of intervertebral disc cells. Identification of two biomechanically distinct cell populations. Spine. 1999;24:2475–2483. doi: 10.1097/00007632-199912010-00009. [DOI] [PubMed] [Google Scholar]
- 64.Trickey WR, Vail TP, Guilak F. The role of the cytoskeleton in the viscoelastic properties of human articular chondrocytes. J Orthop Res. 2004;22:131–139. doi: 10.1016/S0736-0266(03)00150-5. [DOI] [PubMed] [Google Scholar]
- 65.Moustakas A, Theodoropoulos PA, Gravanis A, Haussinger D, Stournaras C. The cytoskeleton in cell volume regulation. Contributions to Nephrology. 1998;123:121–134. doi: 10.1159/000059925. [DOI] [PubMed] [Google Scholar]
- 66.Papakonstanti EA, Vardaki EA, Stournaras C. Actin cytoskeleton: a signaling sensor in cell volume regulation. 2000;10:257–264. doi: 10.1159/000016366. [DOI] [PubMed] [Google Scholar]
- 67.Guilak F, Zell RA, Erickson GR, Grande DA, Rubin CT, McLeod KJ, et al. Mechanically induced calcium waves in articular chondrocytes are inhibited by gadolinium and amiloride. J Orthop Res. 1999;17:421–429. doi: 10.1002/jor.1100170319. [DOI] [PubMed] [Google Scholar]
- 68.Donahue HJ, Guilak F, Vander Molen MA, McLeod KJ, Rubin CT, Grande DA, et al. Chondrocytes isolated from mature articular cartilage retain the capacity to form functional gap junctions. J Bone Miner Res. 1995;10:1359–1364. doi: 10.1002/jbmr.5650100913. [DOI] [PubMed] [Google Scholar]
- 69.Pingguan-Murphy B, Lee DA, Bader DL, Knight MM. Activation of chondrocytes calcium signalling by dynamic compression is independent of number of cycles. Arch Biochem Biophys. 2005;444:45–51. doi: 10.1016/j.abb.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 70.Roberts SR, Knight MM, Lee DA, Bader DL. Mechanical compression influences intracellular Ca2+ signaling in chondrocytes seeded in agarose constructs. J Appl Physiol. 2001;90:1385–1391. doi: 10.1152/jappl.2001.90.4.1385. [DOI] [PubMed] [Google Scholar]
- 71.Schneiderman R, Keret D, Maroudas A. Effects of mechanical and osmotic pressure on the rate of glycosaminoglycan synthesis in the human adult femoral head cartilage: an in vitro study. J Orthop Res. 1986;4:393–408. doi: 10.1002/jor.1100040402. [DOI] [PubMed] [Google Scholar]
- 72.Urban JP, Hall AC, Gehl KA. Regulation of matrix synthesis rates by the ionic and osmotic environment of articular chondrocytes. Journal of Cellular Physiology. 1993;154:262–270. doi: 10.1002/jcp.1041540208. [DOI] [PubMed] [Google Scholar]
- 73.Murata M, Bonassar LJ, Wright M, Mankin HJ, Towle CA. A role for the interleukin-1 receptor in the pathway linking static mechanical compression to decreased proteoglycan synthesis in surface articular cartilage. Archives of Biochemistry and Biophysics. 2003;413:229–235. doi: 10.1016/s0003-9861(03)00129-2. [DOI] [PubMed] [Google Scholar]
- 74.Le D, Mankin H, Towle C. Effect of medium osmolality on cox-2 expression and PGE2 production in IL-1 activated articular cartilage. Trans ORS. 2005;30:1474. [Google Scholar]
- 75.Weemhoff J, Boehm A, Fortier L. The small G-protein RhoA increases catabolic signaling in chondrocytes. Trans ORS. 2005;30:1447. [Google Scholar]
- 76.Bush PG, Hall AC. Regulatory volume decrease (RVD) by isolated and in situ bovine articular chondrocytes. Journal of Cellular Physiology. 2001;187:304–314. doi: 10.1002/jcp.1077. [DOI] [PubMed] [Google Scholar]
- 77.Kerrigan MJ, Hall AC. Control of chondrocyte regulatory volume decrease (RVD) by [Ca(2+)](i) and cell shape. Osteoarthritis Cartilage. 2008;16:312–322. doi: 10.1016/j.joca.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 78.Hung CT, LeRoux MA, Palmer GD, Chao PH, Lo S, Valhmu WB. Disparate aggrecan gene expression in chondrocytes subjected to hypotonic and hypertonic loading in 2D and 3D culture. Biorheology. 2003;40:61–72. [PubMed] [Google Scholar]