Abstract
The diagnosis of canine leishmaniosis (CanL) is currently predominantly achieved by cytological or histological identification of amastigotes in biopsy samples, demonstration of specific anti-Leishmania antibodies and PCR-based approaches. All these methods have the advantage of being sensitive and more or less specific; nevertheless, most of them also have disadvantages. A chromogenic in situ hybridisation (ISH) procedure with a digoxigenin-labelled probe, targeting a fragment of the 5.8S rRNA was developed for the detection of all species of Leishmania parasites in routinely paraffin wax-embedded canine tissues. This method was validated in comparison with traditional techniques (histology, PCR), on various tissues from three dogs with histological changes consistent with a florid leishmaniosis. Amastigote forms of Leishmania gave clear signals and were easily identified using ISH. Various tissues from 10 additional dogs with clinical suspicion or/and a positive serological test but without histological presence of amastigotes did not show any ISH signals. Potential cross-reactivity of the probe was ruled out by negative outcome of the ISH against selected protozoa (including the related Trypanosoma cruzi) and fungi. Thus, ISH proved to be a powerful tool for unambiguous detection of Leishmania parasites in paraffin wax-embedded tissues.
OF the approximately 20 virulent species of Leishmania, a considerable number has been described in dogs (L infantum (syn L chagasi), L donovani, L tropica, L major, L arabica, L braziliensis, L peruviana, L panamensis) (Dantas-Torres 2007). Clinical disease in dogs, referred to as canine leishmaniosis (CanL) seems to be caused by only one species, L infantum (syn L chagasi) in many regions worldwide. These protozoal parasites of great medical and veterinary significance are transmitted by the bite of female blood-sucking phlebotomine sand fly vectors of the genera Phlebotomus in the Old World (Desjeux 1996) and Lutzomyia in the New World (Grimaldi and Tesh 1993).
Based on serological surveys, it has been estimated that at least 2.5 million dogs are infected in the south-western European countries (Moreno and Alvar 2002). Although most of these dogs are clinically asymptomatic, they constitute a very significant part of the reservoir of L infantum, the aetiological agent of zoonotic visceral leishmaniosis (Dantas-Torres 2007).
CanL is a complex disease and shares many clinical and pathological features with other canine diseases (Gomes and others 2008). However, the most common clinical signs are cutaneous alterations including exfoliative, ulcerative, nodular and pustular dermatitis, alopecia and hyperkeratosis, local or generalised lymphadenomegaly, hepatosplenomegaly, weight loss, ocular lesions (such as anterior uveitis, conjunctivitis, dry keratoconjunctivitis or blepharitis) and glomerulopathy (Blavier and others 2001, Tafuri and others 2001, Moreira and others 2007). The typical histopathological findings in tissues from infected dogs are chronic granulomatous inflammation in various tissues, especially skin, liver, spleen, hypertrophy and hyperplasia of cells of the mononuclear phagocyte system (Tafuri and others 2001). However, not all infected animals develop clinical manifestations, therefore the precise diagnosis of canine Leishmania infection may prove complex. Endemic CanL is spreading northwards into the foothills of the Alps and the Pyrenees (Ferroglio and others 2005, Dereure and others 2009). Furthermore, due to tourism and the importation of dogs from areas where the disease is endemic, the incidence of infected dogs in Central Europe is increasing (Baneth and others 2008).
Diagnosis of CanL is usually based on the use of direct diagnostic methods like microscopic examination, histopathology, immunohistochemistry (IHC), PCR, quantitative real-time PCR (qPCR) or indirect diagnostic methods such as ELISA and indirect immunofluorescent antibody test (IFAT) (Maia and Campino 2008, Miró and others 2008). Serological assays are widely and frequently used but their value for detecting asymptomatic infections may be questionable, as false-negative results occur. Thus, the infection rate of Leishmania in dog populations from endemic areas is often underestimated (Alvar and others 2004). Conversely, recovery from clinical disease may lead to persistence of specific antibodies despite clearance of the microorganisms and cross-reactions with antibodies against other pathogens such as T cruzi and Ehrlichia canis are possible (Barbosa-de-Deus and others 2002).
Frequently, skin biopsies and samples from lymphatic tissue are used for diagnosing CanL. Apart from the microscopic identification of amastigotes, IHC procedures are available. However, commercially available monoclonal antibodies failed to produce specific staining in paraffin wax-embedded tissue and alternatively used canine hyperimmune sera are not readily available for all laboratories and may be cross-reactive with related protozoa (Bourdoiseau and others 1997, Tafuri and others 2004). Thus the major aim of the present study was to fill this diagnostic gap and to establish an in situ hybridisation (ISH) procedure for Leishmania species which facilitates detection of parasites directly within the tissue and which should be specific and sensitive.
Material and methods
Tissue samples and histology
Formalin-fixed, paraffin wax-embedded tissues from three dogs (case #1: skin (location not further specified); case #2: spleen; case #3: skin, liver, kidney, lung, heart muscle, intestine, bone marrow, brain, pancreas) with a histological diagnosis of cutaneous or/and visceral CanL were used for validation of the ISH assay in routinely prepared samples. Two of these dogs had a history of importation from Greece (case #2) and Italy (case #3), respectively, and from the third positive dog no information on its origin or travel history was available. Various tissues (spleen, liver, kidney, lung, heart muscle, intestine) from 10 additional dogs (numbers #4 to #13) with clinical suspicion but without histological presence of amastigotes were subjected to ISH. Three of these 10 dogs had a history of a stay abroad in an endemic area, and three other dogs were imported from countries of the Mediterranean basin. Serological test results were available in several cases (Table 1).
TABLE 1.
Information on breed, origin, travel history, serological data, results of histological examination, ISH and PCR of the 13 dogs included in the study
| Case number |
Breed | Origin/travel history | Serology | HE | Results of ISH |
PCR |
|---|---|---|---|---|---|---|
| 1 | Dobermann | No importation or stay abroad reported | Not available | Pos | Pos | Pos |
| 2 | Crossbreed | Imported from Greece | Not available | Pos | Pos | Pos |
| 3 | English setter | Imported from Italy | Pos | Pos | Pos | Pos |
| 4 | Great dane | No importation or stay abroad reported | Pos | Neg | Neg | Pos |
| 5 | German shepherd dog | No importation or stay abroad reported | Pos | Neg | Neg | Neg |
| 6 | German shepherd dog | Stay abroad in Spain, Morocco | Pos | Neg | Neg | Neg |
| 7 | English setter | Stay abroad in Greece | Pos | Neg | Neg | Pos |
| 8 | German shepherd dog | Stay abroad in Greece | Not available | Neg | Neg | Neg |
| 9 | Spaniel | Imported from Spain | Neg | Neg | Neg | Neg |
| 10 | Crossbreed | No importation or stay abroad reported | Pos | Neg | Neg | Neg |
| 11 | Setter-type | Imported from Italy | Not available | Neg | Neg | Pos |
| 12 | Dobermann | No importation or stay abroad reported | Not available | Neg | Neg | Neg |
| 13 | Crossbreed | Imported from Spain | Pos | Neg | Neg | Neg |
HE Histological examination, ISH In situ hybridisation
Supplementary paraffin wax-embedded tissues from mice experimentally infected with L amazonensis (courtesy of L. Q. Vieira; Brazil) and T cruzi (courtesy of Sonia G. Andrade; Brazil) were examined using the Leishmania species probe. To evaluate potential cross-hybridisation with other microorganisms, archival paraffin wax-embedded tissues from different animal species naturally infected with protozoal parasites of the genera Cryptosporidium, Sarcocystis, Eimeria, Toxoplasma, Giardia, Entamoeba or fungi (Aspergillus, Candida) and viruses (canine adenovirus type 2, canine parvovirus type 2) were tested with the Leishmania species probe (Table 2). Application of an irrelevant oligonucleotide probe (Giardia species probe) to Leishmania-positive control slides (case #1) was carried out as additional negative control. All sections used for ISH were also stained by Giemsa and with haematoxylin and eosin to demonstrate parasites.
TABLE 2.
Archived paraffin wax-embedded tissues used to exclude cross-hybridisation of the Leishmania species probe
| Infectious agent | Host | Tissue | Diagnostic method used for verification |
|---|---|---|---|
| Cryptosporidium species | Leopard gecko (Eublepharis macularius) | Small intestine | In situ hybridisation |
| Sarcocystis species | Sheep | Heart muscle | Histological examination |
| Eimeria species | Chicken | Intestine | Histological examination |
| Toxoplasma species | Manul (Felis manul) | Heart muscle | Immunohistochemistry |
| Giardia species | Rabbit | Intestine | Parasitological examination, in situ hybridisation |
| Entamoeba species | Boa constrictor (Boa constrictor) | Intestine | In situ hybridisation |
| Aspergillus | Humboldt penguin (Spheniscus humboldti) | Lung | Grocott’s methenamine silver stain |
| Candida | Gouldian finch (Erythrura gouldiae) | Gizzard | Grocott’s methenamine silver stain |
| CAV-2 | Dog | Lung | In situ hybridisation, PCR |
| CPV-2 | Dog | Intestine | Immunohistochemistry |
CAV-2 Canine adenovirus type 2, CPV-2 Canine parvovirus type 2
Probe design
An oligonucleotide probe labelled with digoxigenin at the 3′ end (Eurofins; MWG Operon; Ebersberg) was designed based on previously published sequences, obtained from GenBank, to detect 5.8S ribosomal RNA sequence specific to Leishmania species.
Homology studies were performed with the Sci Ed Central software package (Scientific & Educational software; Cary, North Carolina) and the relevant sequences were compared with sequences from closely related protozoa. Regions of homology were selected as probe sequence and submitted to Basic Local Alignment Search Tool (www.ncbi.nlm.nih.gov/blast.cgi) to eliminate unexpected cross-reactivity (Fig 1).
FIG 1.
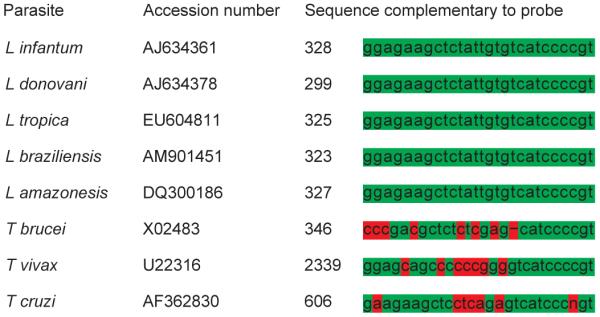
Alignment of the 5.8S rRNA gene sequences complementary to the Leishmania probe of different Leishmania species and other related protozoa of the order Kinetoplastida. The probe shows 100 per cent identity with representatives of the genus Leishmania but a sufficient number of mismatches with other related protozoa to prevent cross-hybridisation (homologous nucleotides: green; mismatches: red)
The probe sequence was: 5′ACGGGGATGACACAATAGAGCTTCTCC to 3′.
This sequence is complementary to a segment of 5.8S ribosomal RNA of all Leishmania species and offers adequate nucleotide differences from other related protozoa of the class Kinetoplastida to prevent cross-detection (Fig 1). Hence, according to in silico analysis this probe is highly specific for Leishmania (all relevant species) and cross-hybridisation with related protozoa is unlikely to occur.
ISH
The paraffin wax-embedded tissue samples were sectioned (~3 μm) and placed on Super frost Plus slides (Menzel Gläser; Braunschweig). They were dewaxed in Neoclear and rehydrated in a series of graded alcohols (100 per cent, 96 per cent, 70 per cent) and distilled water. Proteolytic treatment was done with proteinase K (Roche; Basel) 6 μg/ml in Tris-buffered saline at 37°C for 40 minutes and slides were rinsed with distilled water and dehydrated in 96 per cent ethanol and 100 per cent isopropanol followed by air-drying.
Afterwards, the slides were covered with hybridisation mixture, 100 μl of which were composed of 12 μl distilled water, 20 μl 20x standard sodium citrate (SSC), 50 μl formamide (50 per cent), 2 μl Denhardt’s solution, 10 μl dextran sulfate, 5 μl herring sperm DNA and 1 μl Leishmania probe at a concentration of 100 ng/ml.
Then the slides were incubated at 95°C for 6 minutes and immediately afterwards placed on crushed ice. After cooling, the slides were hybridised overnight in a humid chamber at 40°C. On the second day, the slides were washed in 2x SSC, 1x SSC and 0.1x SSC at room temperature (RT).
Then the slides were incubated with antidigoxigenin-AP Fab fragments (Roche; Basel) (Dilution 1:200) for one hour at RT. After washing, the signal was visualised with 5-bromo-4-chloro-3-indolyl phosphate (Roche; Basel) and 4-nitro blue tetrazolium chloride (Roche; Basel) for one hour at RT in the dark. The staining reaction was terminated by placing the slides in TE buffer (pH 8.0) for 10 minutes. Finally, the slides were counterstained with haematoxylin and mounted under coverslips with Aquatex (VWR International; Vienna).
PCR
For confirmation of all ISH results, a PCR assay (Gomes and others 2007) targeting part of L donovani species complex kinetoplastid DNA (kDNA) was applied.
Amplification of parasite DNA was attempted using the primer pair RV1/RV2 (Gomes and others 2007) that amplifies a 145 bp sequence from the LT1 fragment of L donovani species complex kDNA minicircles. For PCR amplification, paraffin wax sections (10 μm) from skin, liver and spleen tissue samples of all 13 dogs were dewaxed in xylene and afterwards washed in ethanol and dried. DNA was extracted using Nexttec Clean Columns (Nexttec; Leverkusen) according to the manufacturer’s instructions. The 25 μl reaction mixture was composed of 10 μl HotMasterMix (5Prime; Eppendorf; Hamburg), 1 μl of each forward and reverse primer, 11 μl of distilled water and 2 μl of template DNA. The PCR amplification started with denaturation for two minutes at 94°C, was followed by 40 cycles of heat denaturation at 94°C for 30 seconds, primer annealing at 60°C for 30 seconds and DNA extension at 72°C for one minute, and ended with a final extension at 72°C for 10 minutes. A 10 μl aliquot of each PCR product was analysed by gel electrophoresis on a 2 per cent Tris acetate-EDTA-agarose gel. The agarose gel was stained with ethidium bromide and the bands obtained were visualised with a BioSens SC-Series 710 gel documentation system using the BioSens gel imaging system software (GenXpress; Wiener Neudorf). Positive PCR controls contained 2 μl of DNA extracted from cultured L infantum (Courtesy of Jaroslav Kulda; Prague) and L donovani (Courtesy of Roman Peschke; Vienna) parasites. Negative PCR controls contained paraffin wax sections of mice experimentally infected with L amazonensis and 2 μl of laboratory grade water.
Results
Histology
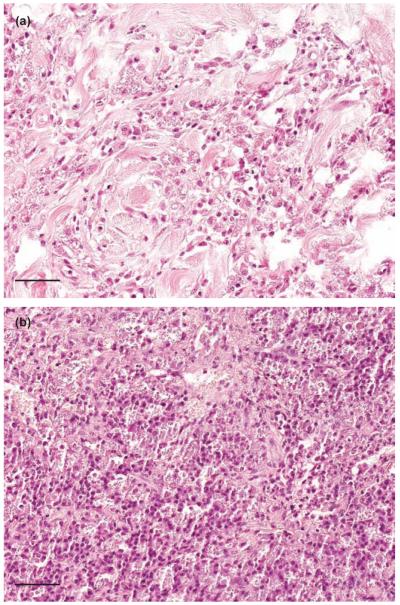
The three positive dogs (dogs #1 to 3) used to validate the ISH procedure had chronic granulomatous inflammation in various tissues. Case #1 was represented by a skin sample with an extreme degree of hyperkeratosis and a severe granulomatous inflammation of corium and subcutis dominated by numerous macrophages with large parasitophorous vacuoles containing many amastigotes presenting as ovoid organisms of 2 to 4 μm in diameter. Case #2 had undergone a splenectomy due to not further specified reasons. The macroscopically severely enlarged spleen was submitted for histological examination. The red pulp was infiltrated with large numbers of macrophages the majority of which contained numerous amastigotes in huge parasitophorous vacuoles. Macrophages containing amastigotes were also present in the capsule and the trabeculae. The splenic pulp also contained many plasma cells but only few lymphocytes. Dog #3 was a postmortem examination case; the animal had a two years clinical history of chronic dermatitis. Histologically, moderate multifocal granulomatous inflammation was observed in skin (corium and subcutis), kidney, lung, liver, heart and intestine (Lamina propria; tela submucosa, tunica muscularis). All inflammatory infiltrates were dominated by macrophages, containing many amastigotes in parasitophorous vacuoles. (Fig 2a, b). In other organs, (bone marrow, brain, pancreas) no Leishmania parasites were discovered.
FIG 2.
Histological sections of skin (a, dog #1) and spleen (b, dog #2) of dogs naturally infected with Leishmania donovani species complex. In both tissues, several vacuolised macrophages containing numerous amastigotes are present. Haematoxylin and eosin. Bar 80 μm
The additional ISH-negative 10 (seven postmortem examination cases, three skin biopsies) cases showed an array of different histological lesions many of which were compatible with chronic leishmaniosis (granulomatous dermatitis [five]; glomerulonephritis and interstitial nephritis [four]; interstitial hepatitis [three]; hyperplasia of lymph nodes and/or spleen [three]; bronchopneumonia [three]). However, no amastigotes were noticed in any of the tissues investigated (Table 1).
ISH
Leishmania parasites were readily identifiable by a distinct purple to black signal decoring amastigotes within the parasitophorous vacuoles of macrophages.
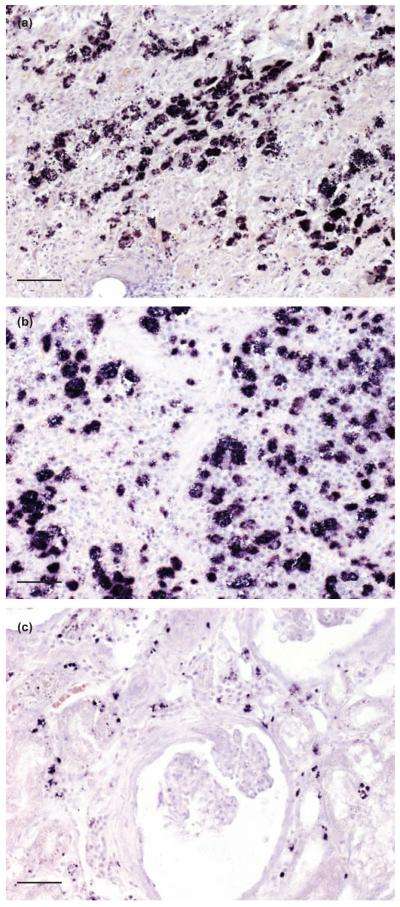
Clearly labelled parasites were found in skin and spleen, respectively, of dogs #1 and #2, and in skin, kidney, liver, lung, heart and intestine of dog #3 (Fig 3a to c). In other organs, (bone marrow, brain, pancreas) no Leishmania parasites were observed. No Leishmania parasites were detected in any of the tissue samples of the remaining 10 dogs (dog #4 to #13).
FIG 3.
In situ hybridisation of skin (a, dog #1), spleen (b, dog #2) and kidney (c, dog #3) show numerous Leishmania amastigotes readily discernible by their distinct purple to black signal, bar 80 μm
In mice, experimentally infected with L amazonensis, large numbers of labelled parasites were present in tissue sections of skin (Fig 4). There was no cross-reactivity either with T cruzi (Fig 5), or with other protozoal parasites, fungi and viruses as listed in Table 2. Leishmania-positive slides hybridised with the irrelevant Giardia probe showed total absence of signals (Fig 6).
FIG 4.
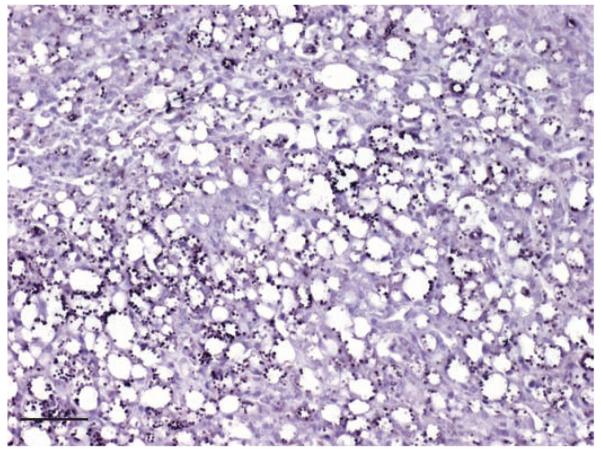
In situ hybridisation of skin of a mouse experimentally infected with L amazonensis. Many amastigotes within macrophages are clearly labelled, bar 80 μm
FIG 5.
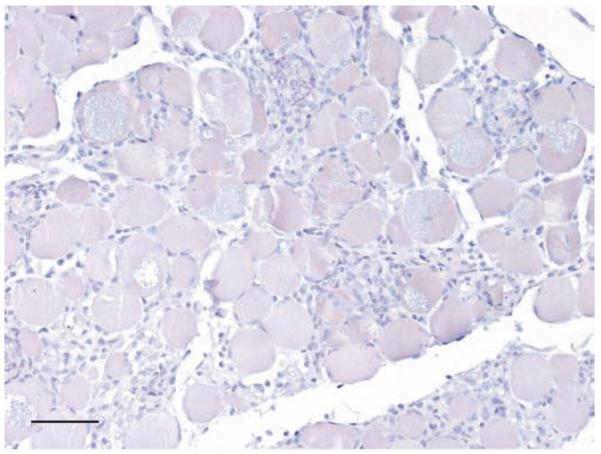
In situ hybridisation of heart muscle of a mouse experimentally infected with T cruzi shows complete absence of signals, bar 80 μm
FIG 6.
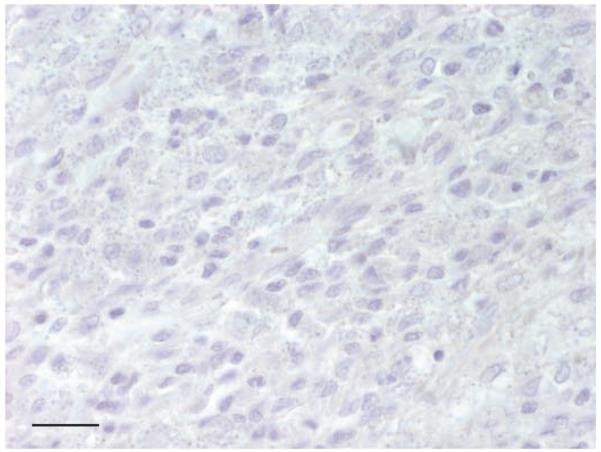
In situ hybridisation of skin of a dog (dog #1) naturally infected with Leishmania donovani species complex, using the irrelevant Giardia probe shows complete absence of signals, bar 40 μm
PCR
The PCR yielded distinct amplification products of the expected size (145 bp) from various DNA extracts of spleen, liver, skin, kidneys, lung, heart muscle and small intestine of the three ISH-positive dogs and confirmed the tentative diagnosis of an infection with L donovani species complex, presumably L infantum. As expected, L amazonensis DNA was not amplified with this procedure. Although histological examination and ISH had shown absence of Leishmania parasites in tissue samples of the remaining 10 dogs, PCR yielded amplification products of the expected size in three ISH-negative cases (Table 1).
Discussion
In this study, a chromogenic ISH procedure with a digoxigenin-labelled oligonucleotide probe for detection and identification of Leishmania parasites in various paraffin wax-embedded canine tissues was established.
Diagnosis of leishmaniosis is frequently based on cytological or histological identification of amastigotes in biopsy samples. However, detection of small numbers of parasites, free or within macrophages may be cumbersome (Xavier and others 2006) and long searches may be required.
In addition, other objects viewed by light microscopy (for example, Histoplasma species, Trypanosoma species) may be erroneously considered as amastigotes. In contrast, amastigotes were easily identified by ISH, even at low magnification and in different canine tissues. This method has the advantage of being highly specific due to nucleic acid detection and offers the possibility of correlating the presence of parasites with associated lesions.
Other commonly used diagnostic approaches are PCR, qPCR, ELISA and IFAT. All these methods have the advantage of being sensitive and more or less specific; nevertheless, most of them have also disadvantages: localisation of parasites within tissue lesions and simultaneous evaluation of morphology and distribution is not feasible with these methods. PCR detects parasite DNA, which does not necessarily imply presence of intact parasites, and does not allow inferences regarding the stage or the severity of disease (Moreira and others 2007). PCR yielded a positive result in 3 ISH-negative cases of this study. As also histological examination did not reveal any organisms suspicious of Leishmania amastigotes in the tissues of the PCR positive dogs, it is very likely that entire parasites were not present in the examined tissues of these cases. It is also necessary to note that the samples used for DNA extraction were taken from the same tissue blocks which had been subjected to ISH. Maybe the amastigotes were present in very small numbers and thus escaped morphological detection or PCR picked up only Leishmania DNA, which might have been released into the circulation from remote sites. Alternatively, although less likely, the unexpected PCR positivity of the ISH-negative samples could be explained by the cross-linking properties of formaldehyde which might have limited the access of the probe to the target nucleic acid sequences (Zsikla and others 2004, Babic and others 2010). However, with certain ISH applications fixation times of up to one year are tolerated without obvious reduction of signal (Mostegl and others 2011). Because of the retrospective character of this study, duration of fixation is not exactly known in these cases. Other diagnostic approaches like ELISA or IFAT have the disadvantage that cross-reactivity with other protozoal parasites, especially T cruzi, Ehrlichia species, Rickettsia species and Toxoplasma species may lead to false-positive results (Mohammed and others 1986, Harith and others 1987, Da Costa and others 1991, Barbosa-de-Deus and others 2002).
Despite their sensitivity, traditional diagnostic approaches and the ISH technique described here have been found inadequate for species discrimination. The most powerful of these methods is the multilocus enzyme electrophoresis technique, based on the variability of 15 isoenzymes in different Leishmania species. This technique requires isolation of parasites and growth in culture medium and definitive species identification takes up to two or three months. Thus, this method is unsuitable for routine diagnosis (Degrave and others 1994, Schallig and Oskam 2002).
In CanL, parasitism in the skin occurs consistently, because it is the primary site of phlebotomine bites and consecutive disease transmission. Clinical stage of infection does not necessarily correlate with parasitic load of tissues, especially in the skin. Nevertheless, direct parasite detection in skin biopsy samples is a good tool for definitive diagnosis and clinical follow up, although examination of routinely prepared histological sections is frequently inconclusive (Bourdoiseau and others 1997, Tafuri and others 2001).
The ISH procedure described here proved to be a powerful tool for unambiguous detection of Leishmania parasites in paraffin wax-embedded tissues, which will also be applicable for examination of skin biopsies.
Acknowledgments
Thanks are due to Dr. Sonia G. Andrade and Dr. Leda Q. Vieira for the generous gift of paraffin wax-embedded tissues from mice experimentally infected with T cruzi and L amazonensis. The authors are grateful to Klaus Bittermann for the professional digital artwork. This study was funded by the Austrian Science Fund (FWF) grant P20926.
Footnotes
Provenance: not commissioned; externally peer reviewed
References
- ALVAR J, CAÑAVATE C, MOLINA R, MORENO J, NIETO J. Canine leishmaniasis. Advances in Parasitology. 2004;57:1–88. doi: 10.1016/S0065-308X(04)57001-X. [DOI] [PubMed] [Google Scholar]
- BABIC A, LOFTIN IR, STANISLAW S, WANG M, MILLER R, WARREN SM. The impact of pre-analytical processing on staining quality for H&E, dual hapten, dual color in situ hybridization and fluorescent in situ hybridization assays. Methods. 2010;52:287–300. doi: 10.1016/j.ymeth.2010.08.012. OTHERS. [DOI] [PubMed] [Google Scholar]
- BANETH G, KOUTINAS AF, SOLANO-GALLEGO L, BOURDEAU P, FERRER L. Canine leishmaniosis-new concepts and insights on an expanding zoonosis: part one. Trends in Parasitology. 2008;24:324–330. doi: 10.1016/j.pt.2008.04.001. [DOI] [PubMed] [Google Scholar]
- BARBOSA-DE-DEUS R, MARES-GIUA ML, NUNES AZ, COSTA KM, JUNQUEIRA RG, MAYRINK W, GENARO O, TAVARES CA. Leishmania major-like antigen for specific and sensitive serodiagnosis of human and canine visceral leishmaniasis. Clinical and Diagnostic Laboratory Immunology. 2002;9:1356–1361. doi: 10.1128/CDLI.9.6.1361-1366.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLAVIER A, KEROACK S, DENEROLLE P, GOY-THOLLOT I, CHABANNE L, CADORÉ JL, BOURDOISEAU G. Atypical forms of canine leishmaniosis. Veterinary Journal. 2001;162:108–120. doi: 10.1053/tvjl.2000.0556. [DOI] [PubMed] [Google Scholar]
- BOURDOISEAU G, MARCHAL T, MAGNOL JP. Immunohistochemical detection of Leishmania infantum in formalin-fixed, paraffin-embedded sections of canine skin and lymph nodes. Journal of Veterinary Diagnostic Investigations. 1997;9:439–440. doi: 10.1177/104063879700900421. [DOI] [PubMed] [Google Scholar]
- DA COSTA CA, GENARO O, LANA M, MAGALHÃES PA, DIAS M, MICHALICK MS, MELO MN, DA COSTA RT, MAGALHÃES-ROCHA NM, MAYRINK W. Canine visceral leishmaniasis: evaluation of the serologic method used in epidemiologic studies. Revista da Sociedade Brasileira de Medicina Tropical. 1991;24:21–25. doi: 10.1590/s0037-86821991000100004. [DOI] [PubMed] [Google Scholar]
- DANTAS-TORRES F. The role of dogs as reservoirs of Leishmania parasites, with emphasis on Leishmania (Leishmania) infantum and Leishmania (Viannia) braziliensis. Veterinary Parasitology. 2007;149:139–146. doi: 10.1016/j.vetpar.2007.07.007. [DOI] [PubMed] [Google Scholar]
- DEGRAVE W, FERNANDES O, CAMPBELL D, BOZZA M, LOPES U. Use of molecular probes and PCR for detection and typing of Leishmania-a mini-review. Memórias do Instituto Oswaldo Cruz. 1994;89:463–469. doi: 10.1590/s0074-02761994000300032. [DOI] [PubMed] [Google Scholar]
- DEREURE J, VANWAMBEKE SO, MALÉ P, MARTINEZ S, PRATLONG F, BALARD J, DEDET JP. The potential effects of global warming on changes in canine leishmaniasis in a focus outside the classical area of the disease in Southern France. Vector Borne and Zoonotic Diseases. 2009;9:687–694. doi: 10.1089/vbz.2008.0126. [DOI] [PubMed] [Google Scholar]
- DESJEUX PH. Leishmaniasis. Public health aspects and control. Clinics in Dermatology. 1996;14:417–423. doi: 10.1016/0738-081x(96)00057-0. [DOI] [PubMed] [Google Scholar]
- FERROGLIO E, MAROLI M, GASTALDO S, MIGNONE W, ROSSI L. Canine leishmaniasis. Emerging Infectious Diseases. 2005;11:1618–1620. doi: 10.3201/eid1110.040966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOMES AHS, FERREIRA IMR, LIMA MLSR, CUNHA EA, GARCIA AS, ARAÚJO MF, PEREIRA-CHIOCCOLA VL. PCR identification of Leishmania in diagnosis and control of canine leishmaniasis. Veterinary Parasitology. 2007;144:234–241. doi: 10.1016/j.vetpar.2006.10.008. [DOI] [PubMed] [Google Scholar]
- GOMES YM, PAIVA CAVALCANTI M, LIRA RA, ABATH FG, ALVES LC. Diagnosis of canine visceral leishmaniasis: biotechnological advances. Veterinary Journal. 2008;175:45–52. doi: 10.1016/j.tvjl.2006.10.019. [DOI] [PubMed] [Google Scholar]
- GRIMALDI G, TESH RB. Leishmaniasis of the New World: current concepts and implications for future research. Clinical Microbiology Reviews. 1993;6:230–250. doi: 10.1128/cmr.6.3.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARITH AE, KOLK AHJ, KAGER PA, LEEUWENBURG J, FABER FJ. Evaluation of a new developed direct agglutination test (DAT) for serodiagnosis and seroepidemiological studies of visceral leishmaniasis: comparison with IFAT and ELISA. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1987;81:603–606. doi: 10.1016/0035-9203(87)90423-8. [DOI] [PubMed] [Google Scholar]
- MAIA C, CAMPINO L. Methods for diagnosis of canine leishmaniasis and immune response to infection. Veterinary Parasitology. 2008;158:274–287. doi: 10.1016/j.vetpar.2008.07.028. [DOI] [PubMed] [Google Scholar]
- MIRÓ G, CARDOSO L, PENNISI MG, OLIVA G, BANETH G. Canine leishmaniosis-new concepts and insights on an expanding zoonosis: part two. Trends in Parasitology. 2008;24:371–377. doi: 10.1016/j.pt.2008.05.003. [DOI] [PubMed] [Google Scholar]
- MOHAMMED EAER, WRIGHT EP, ABDEL RAHMAN AMA, KOLK A, LAARMAN JJ, PONDMAN KW. Serodiagnosis of Sudanese visceral and mucosal leishmaniasis: comparison of ELISA-immunofluorescence and indirect haemagglutination. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1986;80:271–274. doi: 10.1016/0035-9203(86)90033-7. [DOI] [PubMed] [Google Scholar]
- MOREIRA MAB, LUVIZOTTO MCR, GARCIA JF, CORBETT CEP, LAURENTI MD. Comparison of parasitological, immunological and molecular methods for the diagnosis of leishmaniasis in dogs with different clinical signs. Veterinary Parasitology. 2007;145:245–252. doi: 10.1016/j.vetpar.2006.12.012. [DOI] [PubMed] [Google Scholar]
- MORENO J, ALVAR J. Canine leishmaniasis: epidemiological risk and the experimental model. Trends in Parasitology. 2002;18:399–405. doi: 10.1016/s1471-4922(02)02347-4. [DOI] [PubMed] [Google Scholar]
- MOSTEGL MM, RICHTER B, DINHOPL N, WEISSENBÖCK H. Influence of prolonged formalin fixation of tissue samples on the sensitivity of chromogenic in situ hybridization. Journal of Veterinary Diagnostic Investigation. 2011 doi: 10.1177/1040638711425584. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHALLIG HDFH, OSKAM L. Molecular biological applications in the diagnosis and control of leishmaniasis and parasite identification. Tropical Medicine and International Health. 2002;7:641–651. doi: 10.1046/j.1365-3156.2002.00911.x. [DOI] [PubMed] [Google Scholar]
- TAFURI WL, DE OLIVEIRA MR, DE MELO MN, TAFURI WL. Canine visceral leishmaniosis: a remarkable histopathological picture of one case reported from Brazil. Veterinary Parasitology. 2001;96:203–212. doi: 10.1016/s0304-4017(00)00436-2. [DOI] [PubMed] [Google Scholar]
- TAFURI WL, DE LIMA SANTOS R, ESTEVES ARANTES RM, GONCALVES R, DE MELO MN, MARQUES MICHALICK MS, TAFURI WL. An alternative immunohistochemical method for detecting Leishmania amastigotes in paraffin-embedded canine tissues. Journal of Immunological Methods. 2004;292:17–23. doi: 10.1016/j.jim.2004.05.009. [DOI] [PubMed] [Google Scholar]
- XAVIER SC, DE ANDRADE HM, MONTE SJ, CHIARELLI IM, LIMA WG, MICHALICK MS, TAFURI WL, TAFURI WL. Comparison of paraffin-embedded skin biopsies from different anatomical regions as sampling methods for detection of Leishmania infection in dogs using histological, immunohistochemical and PCR methods. BMC Veterinary Research. 2006;8:2–17. doi: 10.1186/1746-6148-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZSIKLA V, BAUMANN M, CATHOMAS G. Effect of buffered formalin on amplification of DNA from paraffin wax embedded small biopsies using real-time PCR. Journal of Clinical Pathology. 2004;57:654–656. doi: 10.1136/jcp.2003.013961. [DOI] [PMC free article] [PubMed] [Google Scholar]