Abstract
Gamma-interferon-inducible lysosomal thiol reductase (GILT) facilitates MHC class II-restricted processing though endocytic reduction of protein disulfide bonds and is necessary for efficient class II-restricted processing of melanocyte differentiation antigen, tyrosinase-related protein 1 (TRP1). Using class II-restricted, TRP1-specific T cell repector transgenic mice, we identify a novel role for GILT in the maintenance of tolerance to TRP1. TRP1-specific thymocytes are centrally deleted in the presence of GILT and TRP1. In contrast, CD4 single positive thymocytes and peripheral T cells develop in the absence of GILT or TRP1, demonstrating that GILT is required for negative selection of TRP1-specific thymocytes. Although TRP1-specific T cells escape thymic deletion in the absence of GILT, they are tolerant to TRP1 and do not induce vilitigo. TRP1-specific T cells that develop in the absence of GILT have diminished IL-2 and IFN-γ production. Furthermore, GILT-deficient mice have a four-fold increase in the percentage of TRP1-specific regulatory T cells compared to TRP1-deficient mice, and depletion of regulatory T cells partially restores the ability of GILT-deficient TRP1-specific CD4+ T cells to induce vitiligo. Thus, GILT plays a critical role in regulating CD4+ T cell tolerance to an endogenous skin-restricted antigen relevant to controlling autoimmunity and generating effective immunotherapy for melanoma.
Introduction
MHC class II-restricted antigen (Ag) presentation plays an essential role in the development the CD4+ T cell repertoire ((Klein et al., 2009)). Cortical thymic epithelial cells present self peptide:MHC complexes to CD4+CD8+ double positive (DP) thymocytes (Hogquist et al., 1994; Lo et al., 2009; Nikolic-Zugic and Bevan, 1990). Ligation of specific T cell receptors (TCRs) with their cognate self peptide:MHC complexes provides DP thymocytes with survival signals termed positive selection and lead to downregulation of the unused coreceptor. CD4+ thymocytes interact with dendritic cells (DCs) and medullary thymic epithelial cells (mTECs) presenting self peptide:MHC complexes. Thymocytes that bind self peptide:MHC complexes with high avidity die by apoptosis (negative selection) (Kisielow et al., 1988; McCaughtry et al., 2008; Swat et al., 1991). Autoreactive T cells that escape negative selection may differentiate into regulatory T (Treg) cells or be controlled by peripheral tolerance mechanisms (Apostolou et al., 2002; Fontenot et al., 2003; Jordan et al., 2001). Thus, factors that influence the generation of MHC class II-restricted peptides have the potential to shape CD4+ T cell tolerance to self Ags.
MHC class II-restricted epitopes are generated in the endocytic pathway by disulfide bond reduction and proteolytic degradation of endogenous and exogenous proteins in this compartment ((Bryant and Ploegh, 2004)). Gamma-interferon-inducible lysosomal thiol reductase (GILT) is the only reductase known to be localized to the class II loading compartment (Arunachalam et al., 2000; Luster et al., 1988; Maric et al., 2001). GILT facilitates the processing and presentation of certain class II epitopes through reduction of protein disulfide bonds (Hastings et al., 2006; Maric et al., 2001; Sealy et al., 2008). Melanocyte differentiation Ags, including tyrosinase, tyrosinase-related protein (TRP) 1 (gp75), TRP2 and gp100, are melanosomal integral membrane proteins involved in melanin synthesis. These Ags are clinically relevant for both the autoimmune destruction of melanocytes, which results in vitiligo, and anti-melanoma immune responses. Antibodies (Naughton et al., 1983) and CD8+ T cells (Lang et al., 2001; Mandelcorn-Monson et al., 2003; Ogg et al., 1998; Palermo et al., 2001) specific for melanocyte differentiation Ags have been identified in vitiligo patients, and CD4+ and CD8+ T cells from melanoma patients recognize multiple epitopes from melanocyte differentiation Ags (http://www.cancerimmunity.org/peptidedatabase/differentiation.htm). GILT is required for efficient MHC class II-restricted Ag processing of tyrosinase and TRP1 (Haque et al., 2002; Rausch et al., 2010; van Geel et al., 2010). Since this group of Ags is presented by MHC class II (Kobayashi et al., 1998; Muranski et al., 2008; Overwijk et al., 1999; Parkhurst et al., 2004; Robbins et al., 2002; Topalian et al., 1996; Touloukian et al., 2002; Touloukian et al., 2000; Wang et al., 1999) and contains disulfide bonds (Berson et al., 2001; Garcia-Borron and Solano, 2002; Negroiu et al., 2000), GILT is likely to be important in enhancing class II-restricted processing of multiple epitopes from this clinically-relevant group of skin-restricted Ags.
To evaluate the role of GILT in the development of CD4+ T cell responses to melanocyte differentiation Ags, we use a class II-restricted TRP1-specific TCR transgenic (Tg) mouse strain (Muranski et al., 2008). RAG-expressing Tg mice spontaneously develop vitiligo (Rausch et al., 2010; Xie et al., 2010). In the absence of GILT, RAG-expressing Tg mice have a significant delay in vitiligo onset due to impaired class II-restricted processing of TRP1 and diminished T cell activation (Rausch et al., 2010). Paradoxically, we found an increased percentage of TRP1-specific T cells in the thymus and peripheral lymphoid organs of GILT-deficient, RAG-expressing Tg mice (Rausch et al., 2010), suggesting that GILT may play a role in T cell development and tolerance. Here, we demonstrate that GILT functions to regulate tolerance of CD4+ TRP1-specific T cells. GILT is required for negative selection of TRP1-specific thymocytes. Peripheral TRP1-specific CD4+ T cells that develop in the absence of GILT are tolerant to TRP1 in that they are unable to induce vitiligo and do not produce IL-2 and IFN-γ after Ag exposure. Tolerance of CD4+ TRP1-specific T cells from GILT-deficient mice is partially mediated by increased Treg cells.
Results
RAG-deficient TRP1-specific TCR Tg mice do not develop spontaneous vitiligo
To restrict the analysis to T cells specific for TRP1, Tg and GILT-/- Tg mice were backcrossed onto the RAG-/- background. All mouse strains in this study are on the RAG-/- background unless otherwise stated. Surprisingly, neither GILT-expressing Tg nor GILT-/- Tg mice developed vitiligo when followed for over one year (data not shown). To better understand the phenotype of these mice, the lymphoid organs were evaluated. The white-based brown (TRP1Bw) mouse strain contains a radiation-induced inversion interrupting the gene encoding TRP1 (Smyth et al., 2006) and serves as an Ag-negative control. No significant differences in total thymocyte number were observed among Ag-GILT+/+Tg, Ag+GILT-/-Tg and Ag+GILT+/+Tg mice (Fig.1a). In RAG-expressing Tg mice which develop vitiligo, CD4 single positive (CD4SP) thymocytes develop (Rausch et al., 2010). In contrast, in RAG-deficient Tg mice, CD4SP thymocytes did not readily develop indicating that TRP1-specific thymocytes are negatively selected in the presence of Ag and GILT (Fig.1b, upper right). Endogenous TCRs, which are present in RAG-expressing TRP1-specific Tg mice (Rausch et al., 2010), likely rescue autoreactive T cells from thymic deletion, as in other TCR Tg models (Zal et al., 1996).
Figure 1. GILT is required for negative selection of TRP1-specific thymocytes.
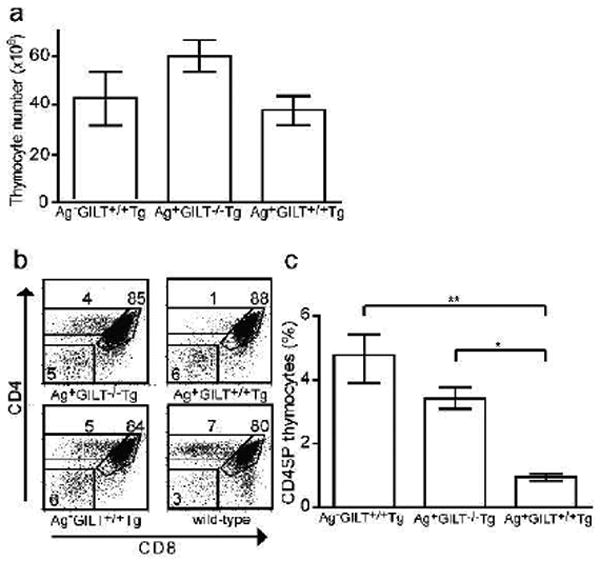
(a) Total thymocyte numbers from Ag-GILT+/+Tg (n = 11), Ag+GILT-/-Tg (n = 10) and Ag+GILT+/+Tg (n = 9) mice. Columns in (a) and (c) represent the mean ± standard error. Data is from three pooled experiments. (b) CD4 and CD8 staining of thymocytes with dead cell exclusion using 7-AAD from representative Ag-GILT+/+Tg, Ag+GILT-/-Tg, Ag+GILT+/+Tg and wild-type mice. Numbers represent percentage in gate. (c) Percentage of TRP1-specific CD4SP thymocytes in Ag- GILT+/+Tg, Ag+GILT-/-Tg and Ag+GILT+/+Tg mice compared by ANOVA with the Bonferroni correction for multiple comparisons (**, p < 0.01; *, p < 0.05). Data are representative of three experiments with 3-4 mice per group.
GILT is required for negative selection of TRP1-specific thymocytes
In comparison to Ag+GILT+/+Tg mice, an increased percentage of CD4SP thymocytes developed in Ag+GILT-/-Tg mice (Fig. 1b, upper left), demonstrating that GILT is required for central deletion of TRP1-specific thymocytes. In the absence of TRP1, TRP1-specific thymocytes are positively selected and CD4SP cells develop (Fig. 1b, lower left). Both Ag+GILT-/- Tg and Ag-GILT+/+Tg mice had a significant increase in the percentage of CD4SP thymocytes compared to Ag+GILT+/+Tg mice (p < 0.05 and p < 0.01, respectively) (Fig. 1c). No difference in the percentage of CD4SP thymocytes was observed between Ag+GILT-/-Tg and Ag-GILT+/+Tg mice (Fig. 1c), indicating the absence of GILT and absence of TRP1 have similar effects on thymic selection. T cells can escape thymic deletion by downregulation of coreceptors (Mamalaki et al., 1996). However, there was no evidence of CD4 downregulation, as no differences in the percentage of CD4-CD8- double negative thymocytes were seen (Fig. 1b). These data indicate that GILT is required for negative selection of TRP1-specific thymocytes.
Peripheral CD4+ TRP1-specific T cells develop in the absence of GILT
Next, we examined the skin-draining lymph nodes for the presence of CD4+ TRP1-specific T cells. No significant differences in total lymph node cell numbers were observed; the mean number of lymph node cells in Ag-GILT+/+Tg, Ag+GILT-/-Tg and Ag+GILT+/+Tg mice were 1.2×106 ± 5.9×105, 2.5×106 ± 8.5×105 cells, and 1.3×106 ± 2.9×105 cells, respectively. TRP1-specific T cells were identified by expression of CD4 and TCR Vβ14. Large percentages of CD4+Vβ14+ T cells were present in the periphery of Ag-GILT+/+Tg and Ag+GILT-/-Tg, but not Ag+GILT+/+Tg, mice (Fig. 2a), consistent with the thymic analyses (Fig. 1). Both Ag-GILT+/+Tg and Ag+GILT-/-Tg mice had a significant increase in the percentage of TRP1-specific T cells in lymph nodes compared to Ag+GILT+/+Tg mice (p < 0.001) (Fig 2b). In addition, Ag+GILT-/-Tg mice had a decreased percentage of CD4+Vβ14+ cells (p < 0.001) (Fig. 2b) and an increased percentage of CD4-Vβ14+ cells (Fig. 2a) compared to Ag-GILT+/+Tg mice. Analysis of the CD4-Vβ14+ cells from Ag+GILT-/-Tg mice showed that 87% were CD3+ and the remaining CD3- cells expressed the natural killer cell marker CD49b (Fig. 2c). Approximately 1/3 of the CD4-Vβ14+ cells were CD8+ and 1/2 were CD4-CD8- (Fig. 2c), suggesting that the TRP1-specific TCR may allow selection of CD8+ and double negative T cells. Next, since Ag+GILT-/-Tg mice express TRP1 and can potentially present low levels of TRP1 peptide:MHC class II complexes, we evaluated markers of naïve and activated T cells. As expected, TRP1-specific T cells from Ag-deficient mice were naïve (CD62L+CD44-) (Fig 2d, left). In Ag+GILT-/-Tg mice the majority of TRP1-specific cells were naïve, and a small percentage of T cells had an effector memory phenotype (CD62L-CD44+) (Fig 2d, right), suggesting the level of TRP1 presentation in GILT-deficient mice supports T cell activation.
Figure 2. Peripheral CD4+ TRP1-specific T cells develop in the absence of GILT.
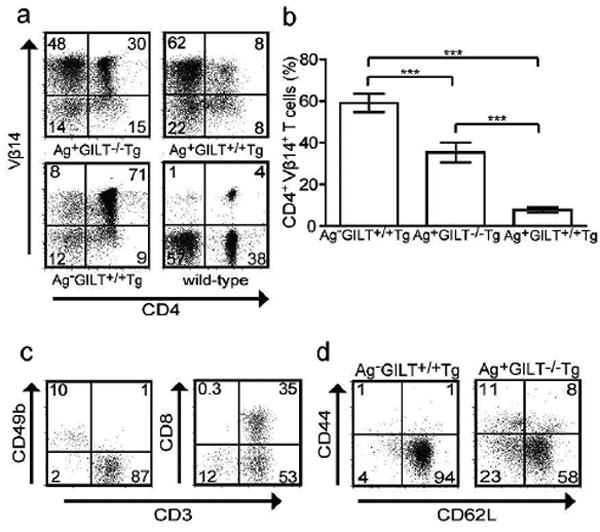
(a) Vβ14 (β chain in the TCR Tg) and CD4 staining of lymph node cells with dead cell exclusion using 7-AAD from representative Ag-GILT+/+Tg, Ag+GILT-/-Tg, Ag+GILT+/+Tg, and wild-type mice. Numbers indicate percentage in quadrants in (a), (c), and (d). (b) Percentage of CD4+Vβ14+ lymph node cells in Ag-GILT+/+Tg, Ag+GILT-/-Tg, and Ag+GILT+/+Tg mice compared by ANOVA with the Bonferroni correction for multiple comparisons (***, p < 0.001). Columns represent the mean ± standard error. Data represent three pooled experiments with at least 3 mice per group in each. (c) CD3, CD8, and CD49b staining of CD4-Vβ14+ gated cells from the lymph nodes of Ag+GILT-/-Tg mice. (d) CD62L and CD44 staining of CD4+Vβ14+ T cells from representative Ag- GILT+/+Tg and Ag+GILT-/-Tg mice.
CD4+ TRP1-specific T cells from GILT-/- mice do not induce vitiligo
Previous studies have demonstrated that CD4+ T cells from Ag- GILT+/+Tg mice induce severe vitiligo and have anti-melanoma activity (Muranski et al., 2008; Quezada et al., 2010; Rausch et al., 2010; Xie et al., 2010). To evaluate the function of T cells that develop in the absence of GILT, we performed adoptive transfer of CD4+ T cells from Ag-GILT+/+Tg and Ag+GILT-/-Tg mice into RAG-/- recipients. Adoptive transfer of CD4+ T cells from Ag-GILT+/+Tg mice into RAG-/- recipients produced large, confluent patches of depigmented fur and ocular damage with a median onset of four weeks (Fig. 3a and 3b), consistent with prior studies and expression of TRP1 in melanocytes located in the hair follicles and eye. In contrast, adoptive transfer of CD4+ T cells from Ag+GILT-/-Tg mice did not produce vitiligo after 15-22 weeks (Fig. 3a and 3c). These data demonstrate that although CD4+ TRP1-specific T cells escape negative selection in the absence of GILT, they maintain tolerance to TRP1 and are functionally distinct from those that develop in the absence of Ag.
Figure 3. CD4+ TRP1-specific T cells from GILT-deficient mice do not induce vitiligo.
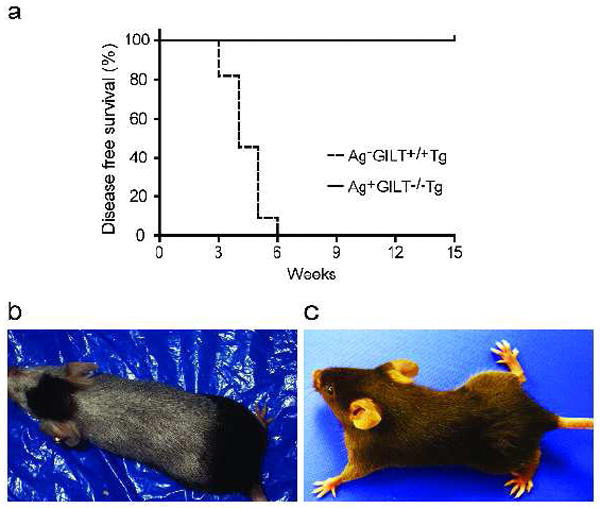
(a) CD4+ TRP1-specific T cells were isolated from Ag-GILT+/+Tg (n = 11) and Ag+GILT-/-Tg (n = 14) mice and adoptively transferred into RAG-/- mice by tail vein injection. Animals were monitored for vitiligo onset. Disease free survival curves were compared by the log rank test. One-half of the mice receiving Ag+GILT-/-Tg cells were followed for an additional seven weeks and did not develop vitiligo. Data represent three pooled experiments. The group that received Ag-GILT+/+Tg T cells was previously reported and is reproduced with permission (Rausch et al., 2010) (Copyright 2010. The American Association of Immunologists, Inc.). (b) Adoptive transfer of Ag-GILT+/+Tg T cells induced large patches of depigmented fur and ocular damage. (c) Representative, unaffected mouse.
GILT-deficient TRP1-specific T cells have diminished cytokine production following Ag exposure
To explore the functional differences between CD4+ TRP1-specific T cells from Ag-GILT+/+Tg and Ag+GILT-/-Tg mice, IL-2 production was assessed in response to TRP1 stimulation in vitro. CD4+ T cells from Ag-GILT+/+Tg and Ag+GILT-/-Tg mice were cocultured with bone marrow-derived DCs and TRP1 peptide (which does not require intracellular processing), melanoma cell lysate (as a source of TRP1 protein) or squamous cell carcinoma lysate (negative control). CD4+ T cells from Ag+GILT-/-Tg mice produced dramatically less IL-2 than those from Ag-GILT+/+Tg mice in response to TRP1 peptide or TRP1 protein (Fig. 4a). In addition, anti-CD3 and anti-CD28 stimulation resulted in less IL-2 production by CD4+ T cells from GILT-deficient mice (Fig. 4a).
Figure 4. TRP1-specific T cells from GILT-deficient mice have diminished cytokine production after Ag exposure.
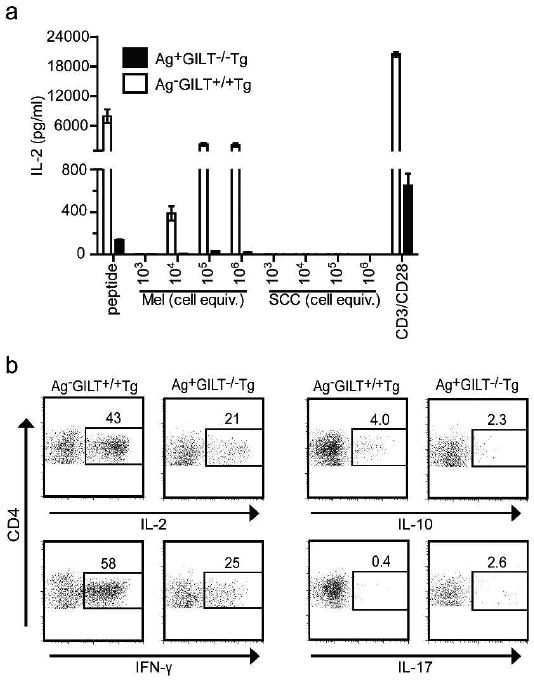
(a) CD4+ lymph node cells from Ag-GILT+/+Tg and Ag+GILT-/-Tg mice were cocultured with bone marrow-derived DCs and TRP1-expressing B16 melanoma (Mel) lysate, TRP1-deficient squamous cell carcinoma (SCC) lysate, or TRP1 peptide. Some cells were cultured with anti-CD3 and anti-CD28 antibodies. IL-2 production was measured by ELISA. Columns and bars represent means ± standard error of triplicate samples from one experiment. Data are representative of two experiments. (b) CD4+ T cells from Ag- GILT+/+Tg and Ag+GILT-/-Tg mice were adoptively transferred into RAG-/- recipients. One week after transfer, lymph node cells were collected from recipients and re-stimulated in vitro with PMA and ionomycin for 3 hours. IL-2, IFN-γ, IL-10 and IL-17 expression is shown in CD4+ cells. Data are representative of two experiments.
Next, we measured cytokine production following in vivo Ag exposure. CD4+ TRP1-specific T cells from Ag+GILT-/-Tg and Ag-GILT+/+Tg mice were adoptively transferred into TRP1-expressing RAG-/- hosts. Consistent with our in vitro data, a smaller percentage of CD4+ TRP1-specific T cells from Ag+GILT-/-Tg mice produced IL-2 compared to those from Ag-GILT+/+Tg mice (Fig 4b). Given that T cell-derived IFN-γ is essential for the anti-melanoma activity of CD4+ T cells from Ag-GILT+/+Tg mice (Quezada et al., 2010; Xie et al., 2010), we investigated IFN-γ production. Similarly, a decreased percentage of CD4+ TRP1-specific T cells from Ag+GILT-/-Tg mice produced IFN-γ following in vivo Ag exposure (Fig 4b). Since IL-10 production by Treg cells can contribute to tolerance and the absence of Treg cells accelerates vilitigo onset in RAG-expressing TRP1-specific Tg mice (Xie et al., 2010), we evaluated IL-10 production. While a greater percentage of CD4+ T cells from Ag-GILT+/+Tg mice produced IL-10 compared to those from Ag+GILT-/-Tg mice in response to in vivo TRP1 exposure, the percentage of CD4+IL-10+ cells in both strains was lower than the percentage of cells expressing IL-2 or IFN-γ (Fig. 4b). Since previous studies have demonstrated that CD4+ TRP1-specific T cells from Ag-GILT+/+Tg mice differentiated in vitro under Th17 polarizing conditions had superior anti-tumor activity (Muranski et al., 2008), we assessed IL-17 production. Very few TRP1-specific T cells from either strain produced IL-17 after in vivo TRP1 exposure (Fig 4b), suggesting that these cells do not readily differentiate into Th17 cells in vivo. Reduced IL-2 and IFN-γ production by TRP1-specific T cells from Ag+GILT-/-Tg mice following in vitro and in vivo Ag exposure is consistent with the inability of these cells to induce vitiligo (Fig. 3) and further demonstrates that TRP1-specific T cells that develop in the absence of GILT are tolerant.
Increased percentage of Treg cells in GILT-deficient mice contributes to TRP1-specific CD4+ T cell tolerance
Since Treg cells have been shown to delay spontaneous vitiligo in RAG-expressing TRP1-specific Tg mice and Treg cells develop in RAG-deficient Ag-GILT+/+Tg mice (Xie et al., 2010), we investigated whether GILT expression impacted the development of TRP1-specific Treg cells. Skin-draining lymph nodes from Ag+GILT-/-Tg mice had an approximately four-fold increase in the percentage of CD4+Vβ14+CD25+Foxp3+ Treg cells compared to Ag-GILT+/+Tg mice (Fig. 5a and b) (p < 0.001). However, no difference was observed in the absolute number of TRP1-specific CD25+Foxp3+ Treg cells between these strains (Fig. 5c), given the decreased percentage of CD4+Vβ14+ T cells in Ag+GILT-/-Tg mice (Fig. 2b).
Figure 5. GILT-deficient Tg mice have an increased percentage of Foxp3+ Treg cells.

(a) Lymph node cells were collected from Ag-GILT+/+Tg and Ag+GILT-/-Tg mice, stained with antibodies to CD4, Vβ14, CD25, and Foxp3, and analyzed by flow cytometry. Samples were gated on CD4+Vβ14+ cells. (b) Percentage of TRP1-specific CD25+Foxp3+ cells from Ag- GILT+/+Tg and Ag+GILT-/-Tg mice compared using an unpaired t test (***, p<0.001). (c) Absolute number of TRP1-specific CD25+Foxp3+ cells from Ag-GILT+/+Tg and Ag+GILT-/-Tg mice. Data shown in (b) and (c) are from three pooled experiments with 3 mice per group.
To determine whether the increased percentage of TRP1-specific Treg cells observed in Ag+GILT-/-Tg mice contributes to TRP1 tolerance, CD4+CD25- or total CD4+ T cells from Ag+GILT-/-Tg mice were adoptively transferred into RAG-/- mice. Transfer of Treg cell depleted CD4+ T cells from Ag+GILT-/-Tg mice induced mild vitiligo in all recipient mice with a median onset of 9 weeks (Fig. 6a). Vitiligo induced by CD4+CD25- TRP1-specific T cells was characterized by sparse individual white hairs which progressed to involve nearly the entire dorsum of the mice; ocular damage was not observed (Fig. 6b, left side). As in Figure 3, transfer of sorted total CD4+ TRP1-specific T cells from Ag+GILT-/-Tg mice did not induce autoimmunity after 15 weeks (Fig. 6b, right side). These data demonstrate that Treg cell depletion partially restores the ability of CD4+ TRP1-specific T cells that develop in the absence of GILT to induce autoimmunity.
Figure 6. Depletion of CD25+ Treg cells partially restores the ability of CD4+ TRP1-specific T cells from GILT-deficient mice to induce autoimmunity.
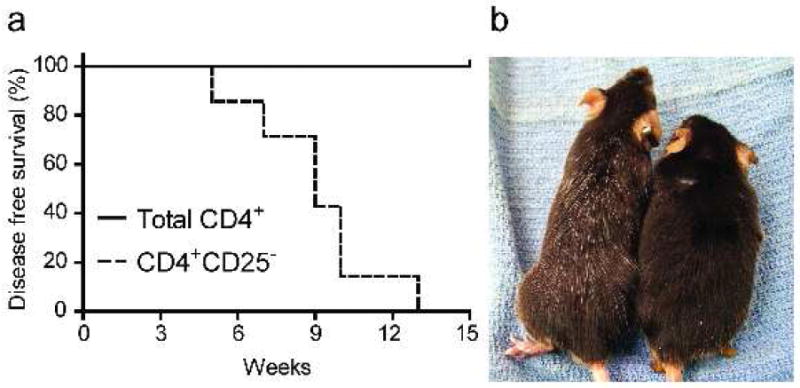
(a) Total CD4+ (n = 3) and CD4+CD25- (n = 7) TRP1-specific T cells were FACS sorted from Ag+GILT-/-Tg mice and adoptively transferred into RAG-/- recipients by tail vein injection. Mice were followed for vitiligo onset. Disease free survival curves were compared by the log rank test. Data is from two pooled experiments. (b) Adoptive transfer of CD4+CD25- T cells induced the appearance of individual white hairs in recipient mice (left). Mice that received total CD4+ T cells did not develop vitiligo (right).
Discussion
The TRP1-specific TCR Tg mouse strain (Muranski et al., 2008) is an ideal model to investigate the development of CD4+ T cell-mediated immunity to a skin-restricted Ag relevant to immunosurveillance of cutaneous malignancy and the pathogenesis of autoimmunity. The Tg T cells are specific for a naturally occurring epitope of a melanocyte differentiation Ag. Another advantage is that T cells are specific for a self Ag expressed in its native genetic context rather than a foreign Ag expressed under a tissue-specific promoter which may not fully recapitulate thymic and tissue-specific expression and development of tolerance.
Negative selection of TRP1-specific thymocytes in Ag+GILT+/+Tg mice, but not in Ag+GILT-/-Tg mice, demonstrates that GILT is required for central tolerance to this skin-restricted Ag (Fig. 1). Similar to GILT-facilitated class II-restricted presentation of TRP1 in B cells and bone marrow-derived DCs (Rausch et al., 2010), we hypothesize that GILT improves the efficiency of TRP1 processing and presentation by the thymic APCs that mediate negative selection, namely thymic DCs and mTECs. Our findings are consistent with prior studies demonstrating diminished negative selection with reduced or absent class II on thymic APCs or diminished self Ag expression. In OT-II Tg mice that express OVA, a lack of MHC class II expression on thymic DCs results in diminished negative selection of CD4+ OVA-specific T cells (Gallegos and Bevan, 2004). Similarly, inhibition of MHC class II expression on mTECs results in diminished negative selection of autoreactive CD4+ T cells in several TCR Tg systems (Hinterberger et al., 2010). Mice lacking expression of autoimmune regulator (Aire), a transcription factor that controls the expression of a large number of tissue-restricted self Ags in the thymus (Anderson et al., 2002), also display impaired negative selection of autoreactive CD4+ T cells (Liston et al., 2003).
Central deletion of TRP1-specific thymocytes likely explains why Ag+GILT+/+Tg mice do not develop spontaneous vitiligo. However, Ag+GILT-/-Tg mice also failed to develop vitiligo despite the presence of significant numbers of TRP1-specific T cells in peripheral lymphoid organs (Fig. 2). Deficient Ag presentation in GILT-deficient animals resulting in reduced ability to activate TRP1-specific T cells is unlikely to fully account for the inability to develop vitiligo for several reasons. RAG-expressing TRP1-specific TCR Tg mice eventually develop spontaneous vitiligo in the absence of GILT expression (Rausch et al., 2010). In addition, TRP1-specific T cells from Ag+GILT-/-Tg mice failed to induce vitiligo following adoptive transfer into GILT-expressing hosts (Fig. 3). Furthermore, some TRP1-specific T cells from Ag+GILT-/-Tg mice had an effector memory phenotype (CD62L-CD44+) (Fig. 2c), demonstrating that TRP1-specific T cell activation can take place in the absence of GILT. The inability of these T cells to induce vitiligo is likely due to other tolerance mechanisms.
Although CD4+ TRP1-specific T cells escaped negative selection in the absence of GILT, these cells were tolerant to TRP1 and did not mediate vitiligo (Fig. 3). Consistent with this finding, TRP1-specific T cells from Ag+GILT-/-Tg mice had diminished cytokine production following Ag exposure (Fig. 4). The diminished activity of TRP1-specific T cells from Ag+GILT-/- Tg mice is partially due to increased TRP1-specific Treg cells (Fig. 5), as adoptive transfer of CD4+CD25- T cells from Ag+GILT-/-Tg mice induced mild vitiligo in RAG-/- recipients (Fig. 6). This finding suggests that GILT may modulate the development of Treg cells.
Intrathymic development of Treg cells involves presentation of self peptides by thymic APCs. In a hemagglutinin (HA)-specific TCR Tg mouse model that expresses HA, Treg cell frequency is increased in animals expressing a high affinity TCR, but the Treg cell compartment is unchanged in animals expressing a lower affinity TCR, suggesting that TCR affinity influences Treg cell development (Jordan et al., 2001). Recent evidence also demonstrates that the overall avidity of the interaction between self-reactive thymocytes and their cognate self-peptide:MHC complexes may direct T cell fate (Hinterberger et al., 2010). Reduced MHC class II expression on mTECs in OVA-specific TCR Tg mice in which OVA expression is restricted to mTECs results in diminished negative selection concomitant with an expansion of OVA-specific Treg cells (Hinterberger et al., 2010). In our model, although MHC class II expression is unchanged on thymic APCs (unpublished data), the number of TRP1 peptide:MHC complexes is likely reduced in the absence of GILT. Therefore, the absence of GILT may similarly reduce the avidity of TCR interaction with peptide:MHC complexes and shift the fate of TRP1-specific thymocytes from central deletion to Treg cell development.
Treg cells can also be induced in the periphery following suboptimal Ag stimulation (Bruder et al., 2005; Kretschmer et al., 2005). Peripheral Treg cell conversion is induced by targeting low doses of HA peptide to DCs under conditions of suboptimal costimulation in an HA-specific TCR Tg model (Kretschmer et al., 2005). Similarly, targeting of HA peptide to immature DCs in an HA-specific TCR Tg mouse model in which HA is expressed in pancreatic β cells led to an increase in peripheral conversion of CD4+ T cells to Treg cells and protected animals from developing diabetes (Bruder et al., 2005). GILT-deficient APCs are capable of low level presentation of TRP1 (Rausch et al., 2010), and this suboptimal presentation may induce TRP1-specific T cells to undergo peripheral conversion to Treg cells.
Although Treg cell depletion restores the ability of TRP1-specific T cells from Ag+GILT-/- Tg mice to induce vitiligo (Fig. 6b), the severity of autoimmunity induced by these cells is diminished in comparison to disease induced by TRP1-specific T cells from Ag-GILT+/+Tg mice (Fig. 3b). This result suggests the contribution of other tolerance mechanisms. Decreased numbers of TRP1-specific T cells in Ag+GILT-/-Tg mice compared to Ag-GILT+/+Tg mice may reflect reduced proliferation or increased susceptibility to peripheral deletion. Recent studies have characterized specialized populations of lymph node stromal cells that express tissue-restricted Ags and are capable of mediating peripheral deletion of autoreactive T cells (Cohen et al., 2010; Gardner et al., 2008; Lee et al., 2007; Nichols et al., 2007). These lymph node stromal cells may be involved in maintenance of tolerance to TRP1.
Using a Tg mouse model we have uncovered a novel role for GILT in modulating tolerance to the melanocyte differentiation Ag TRP1. Presentation of self Ag in the thymus plays an essential role in the negative selection of autoreactive T cells and the intrathymic generation of Treg cells. GILT facilitates the class II-restricted processing of TRP1 (Rausch et al., 2010). Enhanced presentation of TRP1 by the thymic APCs likely modulates the development of TRP1-specific T cells. These findings highlight a critical role for GILT in shaping the CD4+ T cell repertoire to tissue-restricted self Ags capable of mediating autoimmune disease and anti-tumor immunity.
Materials and Methods
Mice
C57BL/6 (wild-type) and RAG1-/- mice were obtained from Jackson Laboratory (Bar Harbor, ME). GILT-/- mice were kindly provided by Dr. Peter Cresswell (Maric et al., 2001). RAG1-/- TRP1-specific TCR Tg mice were generously provided by Dr. Nicholas Restifo (Muranski et al., 2008) and were backcrossed onto the GILT-/- background. Thymuses, spleens, and inguinal, axillary and cervical lymph nodes were isolated as described (Rausch et al., 2010). All animals were housed in microisolator cages. These studies were approved by the institutional review board.
Flow cytometry
Cells were stained with FITC, PE, PE-Cy7, PerCP or APC-conjugated mAbs against murine Vβ14 (clone 14-2), CD3 (145-2C11), CD4 (RM4-5), CD8 (53-6.7), CD25 (PC61.5), CD49b (DX5), Foxp3 (FJK-16s), IL-2 (JES6-5H4), IL-10 (JES5-16E3), IL-17A (eBio17B7), IFN-γ (XMG1.2), and corresponding isotype controls (BD Biosciences; eBioscience, San Diego, CA), as described (Rausch et al., 2010). When indicated, dead cell exclusion was performed by staining with 7-AAD (10 μg/ml) (Sigma, St. Louis, MO). For intracellular cytokine staining, cells were stimulated for 3 hours with 50 ng/ml PMA (Sigma) and 1 μg/ml ionomycin (Calbiochem, San Diego, CA) in the presence of monensin (eBioscience); cells were fixed and permeabilized with IC Fixation/Permeabilization buffer (eBioscience). Cell sorting was performed using a FACSAria-II cell sorter (BD Biosciences).
Adoptive transfer
CD4+ TRP1-specific T cells were isolated from pooled lymph node and spleen cells from Ag- GILT+/+Tg and Ag+GILT-/-Tg mice using the EasySep mouse CD4 positive selection kit (Stemcell Technologies, Vancouver, Canada). CD4+ T cell purity was 90-95%. Both groups in Figure 3 were conducted at the same time; the control group was previously reported and is reproduced with permission (Rausch et al., 2010) (Copyright 2010. The American Association of Immunologists, Inc.). For Treg cell depletion, total CD4+ and CD4+CD25- T cells were FACS-sorted from Ag+GILT-/- Tg pooled lymph node and spleen cells. The purity of sorted CD4+CD25- T cells was >95%. In all cases, 2.5×105 CD4+ T cells were injected intravenously into RAG-/- mice. Mice were visually inspected each week for the development of depigmented fur and eye changes. The minimum criteria used to establish vitiligo onset was either a 2-mm2 patch of white fur or a 1-cm2 patch with scattered individual white hairs on the dorsum of the animal.
In vitro stimulation assay
CD4+ TRP1-specific T cells (1×105) were cocultured for 48 hours with 5×105 wild-type bone marrow-derived DCs and B16.F10 melanoma lysate, murine TRP1109-130 peptide NCGTCRPGWRGAACNQKILTVR (10 μg/mL) or PDV squamous cell carcinoma lysate, as described (Rausch et al., 2010). Some T cells were stimulated with plate-bound anti-CD3ε (145-2C11) (10 μg/ml) and soluble anti-CD28 (37.51) (2 μg/ml). CD4+ T cells were positively selected as above. The IL-2 concentration in culture supernatants was determined by ELISA (BD Biosciences).
Acknowledgments
This work was supported by the National Institutes of Health grants K08-AR054388 (to K.T.H.) and T32-CA09213 (to M.P.R.) and the Melanoma Research Foundation Career Development Research Award (to K.T.H.).
Abbreviations used
- Ag
antigen
- APC
antigen presenting cell
- CD4SP
CD4 single positive
- DC
dendritic cell
- DP
double positive
- GILT
gamma-interferon-inducible lysosomal thiol reductase
- HA
influenza virus hemagglutinin
- mTEC
medullary thymic epithelial cell
- TCR
T cell receptor
- Tg
transgenic
- Treg
regulatory T
- TRP1
tyrosinase-related protein 1
Footnotes
Conflict of Interest: The authors state no conflict of interest.
References
- Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3:756–63. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- Arunachalam B, Phan UT, Geuze HJ, Cresswell P. Enzymatic reduction of disulfide bonds in lysosomes: characterization of a gamma-interferon-inducible lysosomal thiol reductase (GILT) Proc Natl Acad Sci U S A. 2000;97:745–50. doi: 10.1073/pnas.97.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson JF, Harper DC, Tenza D, Raposo G, Marks MS. Pmel17 initiates premelanosome morphogenesis within multivesicular bodies. Mol Biol Cell. 2001;12:3451–64. doi: 10.1091/mbc.12.11.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder D, Westendorf AM, Hansen W, Prettin S, Gruber AD, Qian Y, et al. On the edge of autoimmunity: T-cell stimulation by steady-state dendritic cells prevents autoimmune diabetes. Diabetes. 2005;54:3395–401. doi: 10.2337/diabetes.54.12.3395. [DOI] [PubMed] [Google Scholar]
- Bryant P, Ploegh H. Class II MHC peptide loading by the professionals. Curr Opin Immunol. 2004;16:96–102. doi: 10.1016/j.coi.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Cohen JN, Guidi CJ, Tewalt EF, Qiao H, Rouhani SJ, Ruddell A, et al. Lymph node-resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation. J Exp Med. 2010;207:681–8. doi: 10.1084/jem.20092465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Gallegos AM, Bevan MJ. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J Exp Med. 2004;200:1039–49. doi: 10.1084/jem.20041457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Borron JC, Solano F. Molecular anatomy of tyrosinase and its related proteins: beyond the histidine-bound metal catalytic center. Pigment Cell Res. 2002;15:162–73. doi: 10.1034/j.1600-0749.2002.02012.x. [DOI] [PubMed] [Google Scholar]
- Gardner JM, Devoss JJ, Friedman RS, Wong DJ, Tan YX, Zhou X, et al. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science. 2008;321:843–7. doi: 10.1126/science.1159407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque MA, Li P, Jackson SK, Zarour HM, Hawes JW, Phan UT, et al. Absence of gamma-interferon-inducible lysosomal thiol reductase in melanomas disrupts T cell recognition of select immunodominant epitopes. J Exp Med. 2002;195:1267–77. doi: 10.1084/jem.20011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings KT, Lackman RL, Cresswell P. Functional requirements for the lysosomal thiol reductase GILT in MHC class II-restricted antigen processing. J Immunol. 2006;177:8569–77. doi: 10.4049/jimmunol.177.12.8569. [DOI] [PubMed] [Google Scholar]
- Hinterberger M, Aichinger M, da Costa OP, Voehringer D, Hoffmann R, Klein L. Autonomous role of medullary thymic epithelial cells in central CD4(+) T cell tolerance. Nat Immunol. 2010;11:512–9. doi: 10.1038/ni.1874. [DOI] [PubMed] [Google Scholar]
- Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–6. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333:742–6. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- Klein L, Hinterberger M, Wirnsberger G, Kyewski B. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat Rev Immunol. 2009;9:833–44. doi: 10.1038/nri2669. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Kokubo T, Sato K, Kimura S, Asano K, Takahashi H, et al. CD4+ T cells from peripheral blood of a melanoma patient recognize peptides derived from nonmutated tyrosinase. Cancer Res. 1998;58:296–301. [PubMed] [Google Scholar]
- Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–27. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- Lang KS, Caroli CC, Muhm A, Wernet D, Moris A, Schittek B, et al. HLA-A2 restricted, melanocyte-specific CD8(+) T lymphocytes detected in vitiligo patients are related to disease activity and are predominantly directed against MelanA/MART1. J Invest Dermatol. 2001;116:891–7. doi: 10.1046/j.1523-1747.2001.01363.x. [DOI] [PubMed] [Google Scholar]
- Lee JW, Epardaud M, Sun J, Becker JE, Cheng AC, Yonekura AR, et al. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nat Immunol. 2007;8:181–90. doi: 10.1038/ni1427. [DOI] [PubMed] [Google Scholar]
- Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4:350–4. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- Lo WL, Felix NJ, Walters JJ, Rohrs H, Gross ML, Allen PM. An endogenous peptide positively selects and augments the activation and survival of peripheral CD4+ T cells. Nat Immunol. 2009;10:1155–61. doi: 10.1038/ni.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster AD, Weinshank RL, Feinman R, Ravetch JV. Molecular and biochemical characterization of a novel gamma-interferon-inducible protein. J Biol Chem. 1988;263:12036–43. [PubMed] [Google Scholar]
- Mamalaki C, Murdjeva M, Tolaini M, Norton T, Chandler P, Townsend A, et al. Tolerance in TCR/cognate antigen double-transgenic mice mediated by incomplete thymic deletion and peripheral receptor downregulation. Dev Immunol. 1996;4:299–315. doi: 10.1155/1995/54219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelcorn-Monson RL, Shear NH, Yau E, Sambhara S, Barber BH, Spaner D, et al. Cytotoxic T lymphocyte reactivity to gp100, MelanA/MART-1, and tyrosinase, in HLA-A2-positive vitiligo patients. J Invest Dermatol. 2003;121:550–6. doi: 10.1046/j.1523-1747.2003.12413.x. [DOI] [PubMed] [Google Scholar]
- Maric M, Arunachalam B, Phan UT, Dong C, Garrett WS, Cannon KS, et al. Defective antigen processing in GILT-free mice. Science. 2001;294:1361–5. doi: 10.1126/science.1065500. [DOI] [PubMed] [Google Scholar]
- McCaughtry TM, Baldwin TA, Wilken MS, Hogquist KA. Clonal deletion of thymocytes can occur in the cortex with no involvement of the medulla. J Exp Med. 2008;205:2575–84. doi: 10.1084/jem.20080866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–73. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton GK, Eisinger M, Bystryn JC. Antibodies to normal human melanocytes in vitiligo. J Exp Med. 1983;158:246–51. doi: 10.1084/jem.158.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negroiu G, Dwek RA, Petrescu SM. Folding and maturation of tyrosinase-related protein-1 are regulated by the post-translational formation of disulfide bonds and by N-glycan processing. J Biol Chem. 2000;275:32200–7. doi: 10.1074/jbc.M005186200. [DOI] [PubMed] [Google Scholar]
- Nichols LA, Chen Y, Colella TA, Bennett CL, Clausen BE, Engelhard VH. Deletional self-tolerance to a melanocyte/melanoma antigen derived from tyrosinase is mediated by a radio-resistant cell in peripheral and mesenteric lymph nodes. J Immunol. 2007;179:993–1003. doi: 10.4049/jimmunol.179.2.993. [DOI] [PubMed] [Google Scholar]
- Nikolic-Zugic J, Bevan MJ. Role of self-peptides in positively selecting the T-cell repertoire. Nature. 1990;344:65–7. doi: 10.1038/344065a0. [DOI] [PubMed] [Google Scholar]
- Ogg GS, Rod Dunbar P, Romero P, Chen JL, Cerundolo V. High frequency of skin-homing melanocyte-specific cytotoxic T lymphocytes in autoimmune vitiligo. J Exp Med. 1998;188:1203–8. doi: 10.1084/jem.188.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overwijk WW, Lee DS, Surman DR, Irvine KR, Touloukian CE, Chan CC, et al. Vaccination with a recombinant vaccinia virus encoding a “self” antigen induces autoimmune vitiligo and tumor cell destruction in mice: requirement for CD4(+) T lymphocytes. Proc Natl Acad Sci U S A. 1999;96:2982–7. doi: 10.1073/pnas.96.6.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo B, Campanelli R, Garbelli S, Mantovani S, Lantelme E, Brazzelli V, et al. Specific cytotoxic T lymphocyte responses against Melan-A/MART1, tyrosinase and gp100 in vitiligo by the use of major histocompatibility complex/peptide tetramers: the role of cellular immunity in the etiopathogenesis of vitiligo. J Invest Dermatol. 2001;117:326–32. doi: 10.1046/j.1523-1747.2001.01408.x. [DOI] [PubMed] [Google Scholar]
- Parkhurst MR, Riley JP, Robbins PF, Rosenberg SA. Induction of CD4+ Th1 lymphocytes that recognize known and novel class II MHC restricted epitopes from the melanoma antigen gp100 by stimulation with recombinant protein. J Immunother. 2004;27:79–91. doi: 10.1097/00002371-200403000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207:637–50. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch MP, Irvine KR, Antony PA, Restifo NP, Cresswell P, Hastings KT. GILT accelerates autoimmunity to the melanoma antigen tyrosinase-related protein 1. J Immunol. 2010;185:2828–35. doi: 10.4049/jimmunol.1000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins PF, El-Gamil M, Li YF, Zeng G, Dudley M, Rosenberg SA. Multiple HLA class II-restricted melanocyte differentiation antigens are recognized by tumor-infiltrating lymphocytes from a patient with melanoma. J Immunol. 2002;169:6036–47. doi: 10.4049/jimmunol.169.10.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealy R, Chaka W, Surman S, Brown SA, Cresswell P, Hurwitz JL. Target peptide sequence within infectious human immunodeficiency virus type 1 does not ensure envelope-specific T-helper cell reactivation: influences of cysteine protease and gamma interferon-induced thiol reductase activities. Clin Vaccine Immunol. 2008;15:713–9. doi: 10.1128/CVI.00412-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth IM, Wilming L, Lee AW, Taylor MS, Gautier P, Barlow K, et al. Genomic anatomy of the Tyrp1 (brown) deletion complex. Proc Natl Acad Sci U S A. 2006;103:3704–9. doi: 10.1073/pnas.0600199103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swat W, Ignatowicz L, von Boehmer H, Kisielow P. Clonal deletion of immature CD4+8+ thymocytes in suspension culture by extrathymic antigen-presenting cells. Nature. 1991;351:150–3. doi: 10.1038/351150a0. [DOI] [PubMed] [Google Scholar]
- Topalian SL, Gonzales MI, Parkhurst M, Li YF, Southwood S, Sette A, et al. Melanoma-specific CD4+ T cells recognize nonmutated HLA-DR-restricted tyrosinase epitopes. J Exp Med. 1996;183:1965–71. doi: 10.1084/jem.183.5.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touloukian CE, Leitner WW, Robbins PF, Li YF, Kang X, Lapointe R, et al. Expression of a “self-”antigen by human tumor cells enhances tumor antigen-specific CD4(+) T-cell function. Cancer Res. 2002;62:5144–7. [PMC free article] [PubMed] [Google Scholar]
- Touloukian CE, Leitner WW, Topalian SL, Li YF, Robbins PF, Rosenberg SA, et al. Identification of a MHC class II-restricted human gp100 epitope using DR4-IE transgenic mice. J Immunol. 2000;164:3535–42. doi: 10.4049/jimmunol.164.7.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Geel NA, Mollet IG, De Schepper S, Tjin EP, Vermaelen K, Clark RA, et al. First histopathological and immunophenotypic analysis of early dynamic events in a patient with segmental vitiligo associated with halo nevi. Pigment Cell Melanoma Res. 2010;23:375–84. doi: 10.1111/j.1755-148X.2010.00703.x. [DOI] [PubMed] [Google Scholar]
- Wang S, Bartido S, Yang G, Qin J, Moroi Y, Panageas KS, et al. A role for a melanosome transport signal in accessing the MHC class II presentation pathway and in eliciting CD4+ T cell responses. J Immunol. 1999;163:5820–6. [PubMed] [Google Scholar]
- Xie Y, Akpinarli A, Maris C, Hipkiss EL, Lane M, Kwon EK, et al. Naive tumor-specific CD4(+) T cells differentiated in vivo eradicate established melanoma. J Exp Med. 2010;207:651–67. doi: 10.1084/jem.20091921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zal T, Weiss S, Mellor A, Stockinger B. Expression of a second receptor rescues self-specific T cells from thymic deletion and allows activation of autoreactive effector function. Proc Natl Acad Sci U S A. 1996;93:9102–7. doi: 10.1073/pnas.93.17.9102. [DOI] [PMC free article] [PubMed] [Google Scholar]


