Abstract
Severe acute respiratory syndrome (SARS) has caused major outbreaks worldwide, resulting in an urgent need to obtain sensitive and accurate diagnosis of this disease. PCR-based detection methods were developed for use on a variety of samples, including blood. Eighteen subjects were investigated, and results indicated that blood samples contain sufficient virus for detection by using quantitative real-time PCR.
Severe acute respiratory syndrome (SARS) was first identified in late November 2002 in Guangdong Province, China. In the ensuing months, major outbreaks were reported from Vietnam, Hong Kong, Canada, Singapore, other parts of China, Taiwan, and elsewhere in the world. The disease is unusual in its high level of infectivity, as demonstrated among the health care workers and family members that were in close contact with infected individuals. In addition, it was reported that infected patients do not respond to empirical antimicrobial treatment for acute community-acquired typical or atypical pneumonia (4, 7). The cause of SARS was identified as a novel coronavirus (CoV) (3, 4) because clinical specimens from patients infected with SARS revealed the presence of crown-shaped CoV particles. This new CoV was thus referred to as SARS CoV. The full-length genome sequence of the SARS CoV was reported from different isolates (5, 10), and the genome organization of SARS CoV was found to be similar to that of other CoVs (5, 10).
CoVs are a family of positive-strand RNA-enveloped viruses called Coronaviridae, which are now categorized under the newly established order Nidovirales. This order comprises the families Coronaviridae and Arteriviridae. The name Nidovirales comes from the Latin word nidus, for nest, referring to the 3′-coterminal “nested” set of subgenomic mRNAs produced during viral infection (2). The SARS CoV genome is very large, 29.7 kb (5, 10), and encodes 23 putative proteins. Major structural proteins include nucleocapsid, spike, membrane, and small envelope. Nonstructural proteins include the papain-like proteinase, 3C-like proteinase, RNA-dependent RNA polymerase (RdRp), helicase, and many other proteins involved with viral replication and transcription (2, 6). In other CoVs, many of the nonstructural proteins are only slightly conserved in the viral sequence, the exception being RdRp, which is highly conserved in many CoVs. In previous studies, primer pairs have been designed against different regions along the SARS CoV (3, 8, 9) and have managed to detect SARS CoV in a variety of clinical samples (3, 8, 9). In one report, the earliest detection observed was in sputum at day 3 (3), while in a different study, detection was found only at day 5 in nasopharyngeal aspirate (NPA), peaking at day 10 (8). In the same study, no association was found with the NPA viral load and clinical progression (8). For this report, we have investigated the use of blood as a means of detecting viral load in SARS patients, a method which in the future may allow improved estimation of disease progression.
Specific primers were designed against the highly conserved polymerase gene of the SARS CoV genome, as sequence comparison among the 14 SARS isolates demonstrated no variations in the SARS CoV RdRp region (10), making the RdRp an ideal region for designing specific diagnostic PCR primers in order to ensure that they will not quickly become obsolete due to sequence mutation. The performance of these primers was assessed in two assays using in vitro-transcribed RNA and virus-spiked samples. In the first PCR assay, 2 μl of RNA was reverse transcribed with Expand reverse transcriptase (Roche, Mannheim, Germany) using primer 5′-GGCATCATCAGAAAGAATCATCAT-3′, thereby generating 20 μl of cDNA. This was amplified with primers 5′-GGTTGGGATTATCCAAAATGTGA-3′ and 5′-GGCATCATCAGAAAGAATCATCAT-3′ using 2.5 μl in 25-μl reaction mixtures (94°C for 10 s; 35 cycles of 94°C for 10 s, 50°C for 30 s, and 72°C for 1 min; and finally, 72°C for 7 min) with an Expand High-Fidelity PCR system (Roche). Nested primers (5′-ACTATATGTTAAACCAGGTGG-3′ and 5′-ATTTACATTGGCTGTAACAGC-3′) were used in a second round of PCR, which used 2.5 μl of first-round PCR product as a template in a 25-μl reaction mixture. The size of this nested PCR product was 110 bp and was resolved in 1.5 to 2% agarose gels. PCR products were sequenced directly to confirm the identity of the products. Results are shown in Table 1.
TABLE 1.
Investigation of subjects for presence of SARS CoVa
| Subject no. | Diagnosis | Days from onset | Days from illness to discharge or result | Result of quantitative real-time PCR (copies/ml of blood) | Result of gel-based nested PCR |
|---|---|---|---|---|---|
| 1 | Probable SARS | 2 | 9 | 2,020 | + |
| 2 | Probable SARS | 4 | Died | 9,100 | + |
| 3 | Probable SARS | 4 | >30 | 3,120 | + |
| 4 | Probable SARS | 5 | 13 | 9,600 | + |
| 5 | Probable SARS | 12 | 18 | Negative | − |
| 6 | Probable SARS | 13 | 18 | Negative | − |
| 7 | Probable SARS | 14 | >30 | Negative | − |
| 8 | Probable SARS | 14 | 36 | Negative | − |
| 9 | Non-SARS | 0 | 0 | Negative | − |
| 10 | Non-SARS | 2 | 1 | Negative | − |
| 11 | Non-SARS | 3 | 12 | Negative | − |
| 12 | Non-SARS | 10 | 9 | Negative | − |
| 13 | Control | Negative | − | ||
| 14 | Control | Negative | − | ||
| 15 | Control | Negative | − | ||
| 16 | Control | Negative | − | ||
| 17 | Control | Negative | − | ||
| 18 | Control | Negative | − |
Eighteen subjects were tested by both nested PCR and quantitative real-time PCR (LightCycler). Four out of eight subjects with probable SARS cases tested positive. Specimens from non-SARS cases and controls from normal healthy individuals consistently tested negative.
Next, we used the LightCycler SARS CoV quantification kit (Roche) in a one-step reverse transcriptase PCR for the real-time quantitative PCR that was also designed for the polymerase region and utilized sequence-specific hybridization probes as the detection format. Five microliters of RNA was reverse transcribed and amplified in a 20-μl reaction mixture according to the manufacturer's recommendations.
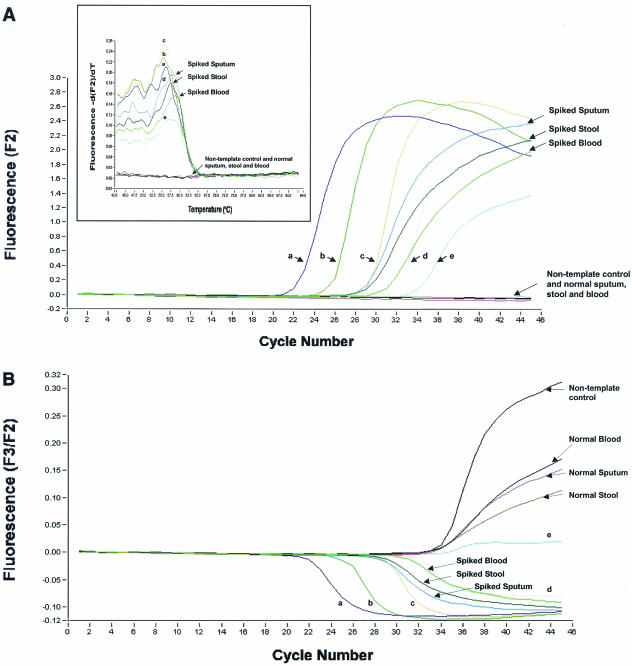
In order to test the sensitivities of this assay, virus grown in Vero E6 cells was harvested, titrated, and spiked into 200 μl of Tris-EDTA buffer, sputum, stool suspension, and blood. RNA was extracted from spiked samples by using the HighPure nucleic acid viral kit (Roche) and the QIAamp viral RNA Mini kit (Qiagen) according to the manufacturers' instructions. Comparable results were achieved. Quantitated single-stranded RNA standards (provided by Roche) showed the sensitivity of the assay to be less than 85 copies per reaction (Fig. 1A). The melting curves (Fig. 1A, inset) also confirmed the specificity of the PCR. The internal control showed that there was no inhibition in the samples that had negative signals (Fig. 1B). The detected viral load in the spike samples varied from 1 × 103 to 5 × 103 copies per reaction (Fig. 1A). This suggests that the extraction protocol used was approximately 10% efficient, with sputum having the greatest efficiency for the clinical samples.
FIG. 1.
Detection of SARS CoV by real-time quantitative PCR. (A) Amplification of single-stranded RNA standards (indicated as a to e) and RNA extracted from sputum, stool, and blood spiked with virus grown in Vero E6 cells. The x axis denotes the cycle number of the quantitative PCR assay, and the y axis denotes fluorescence intensity (F2) over the background level. The nontemplate control (water) is indicated. The viral load is indicated as the number of copies per reaction: spiked sputum, 5 × 103; spiked stool, 4 × 103; spiked blood, 1 × 103. RNA standards were as follows: (a) 1 × 106 copies per reaction, (b) 9.5 × 104 copies per reaction, (c) 8.7 × 103 copies per reaction, (d) 1.1 × 103 copies per reaction, and (e) 8.5 × 10 copies per reaction. The inset graph represents melting-curve analysis of the PCR products. Signals from RNA standards (a to e), spiked samples, normal samples, and nontemplate control (water) are shown. The x axis denotes the temperature (oC), and the y axis denotes the fluorescence intensity (F2) over the background level. (B) Detection of the internal control in fluorometer channel F3 in parallel with the simultaneous amplification of the RNA standards (a to e), spiked samples, and nontemplate control is shown.
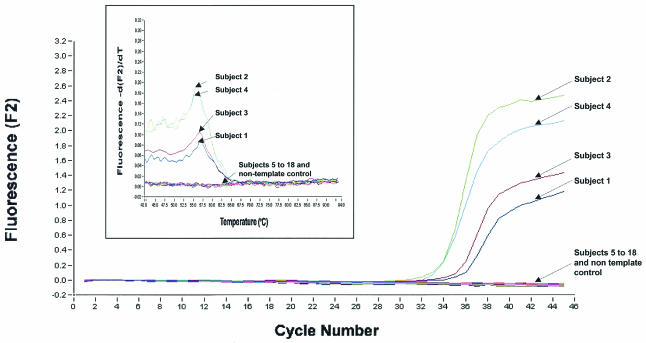
We noted that during the outbreak, most clinical specimens collected for SARS detection (including sputum, NPA, and endotracheal tube samples) involved considerable risk to the health care worker; thus, in order to reduce this risk, we have evaluated the suitability of blood for SARS CoV detection. We investigated 18 subjects comprising eight probable SARS patients and four patients who initially had symptoms similar to SARS but were later diagnosed otherwise. Two of the four subjects who were initially “suspect” for SARS were later diagnosed as having pulmonary tuberculosis and Escherichia coli urinary tract infection. The diagnosis of the other two patients was not determined. Four out of eight probable SARS patients had detectable virus in their blood, while the four non-SARS patients and six control samples from healthy individuals showed no virus (Fig. 2 and Table 1). In addition, control samples spiked with other human CoVs showed no signals (data not shown). Once again, melting-curve analysis confirmed the specificity of the PCR products (Fig. 2, inset). Viral load in the four positive SARS patients varied from 2 × 103 copies to 1 × 104 copies per ml of blood (Fig. 2; Table 1). It is unclear from these data whether this variation predicts clinical outcome; however, work from other studies has shown that blood viral load is a good indicator of disease progression (1). The two PCR methods described herein showed equal sensitivity in terms of detection. However, the gel-based assay method is laborious and nonquantitative.
FIG. 2.
Detection of SARS CoV from 18 subjects by real-time quantitative PCR. Data for RNA from 18 subjects are shown. The viral load is indicated as number of copies per ml of blood. Subject 1, 2,020 copies; subject 2, 9,100 copies; subject 3, 3,120 copies; subject 4, 9,600 copies. A sample would be considered positive if it generated a typical amplification curve within the 45 cycles. Negative signals from subjects 5 to 18 and the nontemplate control (water) are shown. The inset graph shows melting-curve analysis of the PCR products from the 18 subjects. The x axis denotes the temperature (oC), and the y axis denotes the fluorescence intensity (F2) over the background level.
We detected virus in blood at 2 days after the onset of symptoms, which is earlier than previously reported (3, 8, 9). Although it is important to further define the window period for detection of SARS CoV in blood, it is interesting to speculate that the time course of SARS CoV viremia may be relatively short, with our data suggesting that, at days 12 to 14, viral load is too low to detect (Table 1). More patients and sequential sampling would be required to confirm this supposition.
In conclusion, we have shown that the SARS CoV viral load can be determined in patients' blood by using PCR methods, and our data suggest that SARS CoV can be detected early in blood, i.e., within the first week of symptom onset. This fact allows early diagnosis and determination of viral load, both of which are useful in clinical and public health management settings. Further work is under way to further define the role of blood in diagnosis.
Acknowledgments
This work was funded by the Agency of Science and Technology (A*STAR), Singapore, Republic of Singapore.
We thank Loh Gek Kee and Tan Yian Kim (DSO National Laboratories), Patricia Tay and Khoo Chen Ai (Genome Institute of Singapore), Kamal Singh (National University Hospital), and the doctors and staff at Tan Tock Seng Hospital.
REFERENCES
- 1.Candotti, D., J. Temple, F. Sarkodie, and J. P. Allain. 2003. Frequent recovery and broad genotype 2 diversity characterize hepatitis C virus infection in Ghana, West Africa. J. Virol. 77:7914-7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cavanagh, D. 2003. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch. Virol. 14:629-633. [PubMed] [Google Scholar]
- 3.Drosten, C., S. Gunther, W. Preiser, S. van der Werf, H. R. Brodt, S. Becker, H. Rabenau, M. Panning, L. Kolesnikova, R. A. Fouchier, A. Berger, A. M. Burguiere, J. Cinatl, M. Eickmann, N. Escriou, K. Grywna, S. Kramme, J. C. Manuguerra, S. Muller, V. Rickerts, M. Sturmer, S. Vieth, H. D. Klenk, A. D. Osterhaus, H. Schmitz, and H. W. Doerr. 2003. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 348:1967-1976. [DOI] [PubMed] [Google Scholar]
- 4.Kiazek, T. G., D. Erdman, C. S. Goldsmith, S. R. Zaki, T. Peret, S. Emery, S. Tong, C. Urbani, J. A. Comer, W. Lim, P. E. Rollin, S. F. Dowell, A. E. Ling, C. D. Humphrey, W. J. Shieh, J. Guarner, C. D. Paddock, P. Rota, B. Fields, J. DeRisi, J. Y. Yang, N. Cox, J. M. Hughes, J. W. LeDuc, W. J. Bellini, L. J. Anderson, and the SARS Working Group.2003. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 348:1953-1966. [DOI] [PubMed] [Google Scholar]
- 5.Marra, M. A., S. J. M. Jones, C. R. Astell, R. A. Holt, A. Brooks-Wilson, Y. S. Butterfield, J. Khattra, J. K. Asano, S. A. Barber, S. Y. Chan, A. Cloutier, S. M. Coughlin, D. Freeman, N. Girn, O. L. Griffith, S. R. Leach, M. Mayo, H. McDonald, S. B. Montgomery, P. K. Pandoh, A. S. Petrescu, A. G. Robertson, J. E. Schein, A. Siddiqui, D. E. Smailus, J. M. Stott, G. S. Yang, F. Plummer, A. Andonov, H. Artsob, N. Bastien, K. Bernard, T. F. Booth, D. Bowness, M. Czub, M. Drebot, L. Fernando, R. Flick, M. Garbutt, M. Gray, A. Grolla, S. Jones, H. Feldmann, A. Meyers, A. Kabani, Y. Li, S. Normand, U. Stroher, G. A. Tipples, S. Tyler, R. Vogrig, D. Ward, B. Watson, R. C. Brunham, M. Krajden, M. Petric, D. M. Skowronski, C. Upton, and R. L. Roper. 2003. The genome sequence of the SARS-associated coronavirus. Science 300:1399-1404. (First published online 1 May 2003; 10.1126/science.1085963.) [DOI] [PubMed] [Google Scholar]
- 6.Ng, L. F. P., and D. X. Liu. 2002. Membrane association and dimerization of a cysteine-rich, 16-kilodalton polypeptide released from the C-terminal region of the coronavirus infectious bronchitis virus 1a polyprotein. J. Virol. 76:6257-6267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peiris, J. S. M., S. T. Lai, L. L. Poon, Y. Guan, L. Y. Yam, W. Lim, J. Nicholls, W. K. Yee, W. W. Yan, M. T. Cheung, V. C. Cheng, K. H. Chan, D. N. Tsang, R. W. Yung, T. K. Ng, K. Y. Yuen, and the SARS Study Group. 2003. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 361:1319-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peiris, J. S. M., C. M. Chu, V. C. C. Cheng, K. S. Chan, I. F. N. Hung, L. L. Poon, K. I. Law, B. S. F. Tang, T. Y. W. Hon, C. S. Chan, K. H. Chan, J. S. C. Ng, B. J. Zheng, W. L. Ng, R. W. M. Lai, Y. Guan, K. Y. Yuen, and the HKU/UCH SARS Study Group. 2003. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet 361:628-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poon, L. L. M., O. K. Wong, W. Luk, Y. Y. Kwok, J. S. M. Peiris, and Y. Guan. 2003. Rapid diagnosis of a coronavirus associated with severe acute respiratory syndrome (SARS). Clin. Chem. 49:953-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruan, Y. J., C. L. Wei, A. E. Ling, V. B. Vega, H. Thoreau, S. Y. Se Thoe, J. M. Chia, P. Ng, K. P. Chiu, L. Lim, T. Zhan, K. P. Chan, L. L. E. Oon, M. L. Ng, Y. S. Leo, L. F. P. Ng, E. C. Ren, L. W. Stanton, P. M. Long, and E. T. Liu. 2003. Comparative full-length genome sequence analysis of 14 SARS coronavirus isolates and common mutations associated with putative origins of infection. Lancet 361:1779-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]