Abstract
Cultured neural stem cells (NSCs) provide a powerful means for investigating central nervous system disease, neuron development, differentiation, and regeneration. To obtain sufficient neurospheres, subculturing is essential following establishment of the primary NSC culture. Passaging the primary neurospheres is a key issue that is often ignored. We evaluated the influence of different passaging schedules on primary cultured NSCs. Passaging was performed on day 5, 7 or 9. We observed more neurospheres with diameters of 200–250 μm on day 7 than on day 5 or 9. Prolonging the time of primary culture reduced the cell metabolic activity by the MTT assay and cell proliferation by colony-forming assay and the differentiation to neurons from cells at P2 and later decreased. Additionally, more cells were in G0/G1 phase, and higher expression of p16INK4a and lower expression of cyclin D1 was found when the time of primary culture was prolonged to 9 days compared to 7-days cultures. Thus, in this study, we established that the optimal time for subculturing aggregated NSCs was on day 7 based on the primary culture.
Keywords: Neurosphere, Neural stem cells, Primary culture, Cell passage, Optimal time
Introduction
Neural stem cells (NSCs) offer a unique and powerful in vitro model system for studying and elucidating the molecular and cellular mechanisms underlying development, plasticity, and regeneration. They are the widely used model for studying basic differentiation processes (Favaro et al. 2009), and as recent reports showed, for reprogramming cells into neurosphere-derived induced pluripotent stem cells (Kim et al. 2008, 2009). Furthermore, NSCs cultured in vitro provide an expanding source of cells for transplantation, drug screening studies, gene therapy, and neural development studies (Svendsen and Smith 1999; Prestoz et al. 2001; Goldman 2005; Ourednik et al. 2009).
NSCs can be isolated from the mammalian central nervous system (CNS) and can be maintained in vitro for moderate periods of time without loss of the ability of these cells to be passaged (self-renew) and to differentiate into neurons, astrocytes, and oligodendrocytes, the primary cell types in the CNS (Dhara and Stice 2008; Sun et al. 2008). A major challenge, however, is the expansion to obtain sufficient NSCs for these applications. One option is to extend the culture period for the neurospheres. However, NSCs cultured for prolonged periods of time display elevated proliferative capacity and a declining ability to differentiate into neurons (Vukicevic et al. 2010). Alternatively, it is possible to subculture the primary culture of neurospheres to resolve the problem of the shortage of NSCs. In addition, another challenge is that neurospheres formed in vitro are a mixture of stem cells, progenitor cells, and more differentiated cells such as neuronal and glial cells (Lobo et al. 2002; Suslov et al. 2002; Bez et al. 2003). Thus, while subculture of neurospheres may address that challenge, the proper time of passaging is a key question in culturing NSCs.
As with most cell types in culture, the cell division cycle, which is divided into four distinct phases, G1, S, G2 and M, is the series of events that takes place in proliferating NSCs leading to their differentiation. Proliferating cells traverse the cell cycle from one mitosis to the next. Differentiated cells are arrested in G1, and are often described as passing into G0 representing the differentiated (non-proliferating or quiescent) state. Forcing cells from G1 to S (G1/S transition) may promote expansion of NSCs, which is controlled by cell cycle regulators. Among the family of negative cell cycle regulators, p16INK4a plays a crucial role in growth arrest, which is mediated by inhibiting the activity of the cyclin-dependent kinases CDK4 and CDK6 in cell cycle G1 progression (Salomoni and Calegari 2010). On the other hand, cyclins work as positive cell cycle regulators by forming complexes with CDK4 or CDK6 (Sugimoto et al. 1999) and play a central role in NSC proliferation (Bornfeldt 2003). One of the most important cyclins, cyclin D1, participates in the initiation and progression of the cell cycle. Cyclin D1 is activated during mid-G1 (Bassiouny et al. 2010) and functions in G1/S transition.
This study aimed to clarify the optimal time for passaging the aggregated NSCs. We isolated NSCs from the cerebral cortex of newborn rats and passaged the aggregated NSCs at different time schedules to evaluate the influence of different passaging times on cellular metabolic activity, cell cycle distribution and related gene mRNA expression of NSCs.
Materials and methods
Culture medium and reagents
NSCs were incubated in serum-free medium consisting of equal volumes of Ham’s F12 and Dulbecco’s modified eagle medium (DMEM) (Invitrogen, USA) with the addition of 2% B27 supplement (Invitrogen), 20 ng/mL basic fibroblast growth factor (b-FGF, Sigma, USA), and 20 ng/mL epidermal growth factor (EGF, Sigma). The differentiation culture medium was DMEM/F12 containing 1% fetal bovine serum (FBS, Sigma). Mouse anti-Nestin antibody (Chemicon, USA), rabbit anti-β-III-tubulin antibody (Sigma), mouse anti-GFAP antibody (Chemicon), rabbit anti-CNPase antibody (ABcam, UK), and secondary antibodies labeled with fluorescein isothiocyanate (FITC, goat anti-mouse IgG, Sigma) or tetramethylrhodamine isothiocyanate (TRITC, goat anti-rabbit IgG, Sigma) were used for immunofluorescence.
Isolation and cultivation of NSCs
NSCs were cultured from newborn Sprague–Dawley rats (within 24 h of birth), which were provided by the animal center of Nantong University, using previously described methods with minor modifications (Reynolds and Weiss 1992, 1996). All animal experiments were performed in compliance with the US National Institute of Health (NIH) Guide for the Care and Use of Laboratory Animals published by the US National Academy of Sciences (http://www.oacu.od.nih.gov/regs/index.htm) and approved ethically by the Administration committee of experimental animals, Jiangsu Province, China. Briefly, the cerebral cortex was isolated from the whole brain, transferred to ice-cold Hank’s balanced salt solution (Invitrogen), and rinsed twice. The meninges and blood capillaries were carefully removed microscopically. The tissue was mechanically and enzymatically (0.125% trypsin) fragmented. After dissections the cell suspension was transferred into a sterile centrifugation tube and centrifuged subsequently. The single cell suspension was obtained by this procedure and then seeded into a 25-cm2 culture flask (Corning, USA) with 2 mL serum-free NSCs medium at a density of 5 × 105 cells/mL, and cultured in a 37 °C incubator with 95% air, 5% CO2, and 100% humidity. Fresh cell culture medium was added every 2–3 days. After incubation for 5–9 days, cells had proliferated to form neurospheres and were ready for subculture. We set up three groups for every 5 days passage, every 7 days passage and every 9 days passage. The neurospheres were harvested and processed by immunofluorescence by staining for nestin (1:500 mouse anti-nestin).
Passaging neurosphere at different time point for primary culture
Cells proliferate to form spheroids, called neurospheres, which in general, detach from the plastic substrate and float in suspension. Passaging was carried out on day 5, 7 or 9 with three replicate flasks at each time point. After counting the neurospheres that were 100–200 μm, 200–250 μm and more than 250 μm in diameter in the nine flasks, they were washed in phosphate buffered saline (PBS) and collected into nine individual centrifugation tubes and incubated in 0.125% trypsin for 15 min at 37 °C and dissociated mechanically into a single-cell suspension. Then they were centrifuged and re-suspended with serum-free NSC medium and transferred to a Corning T25 culture flask at a cell density of 5 × 105 cells/mL. These cells were collected with the same centrifugation conditions and reseeded in new culture flasks at the same density during the primary culture.
MTT assay of NSCs from three groups of different passaging schedules based on primary culture
The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, Sigma) method (Li et al. 2009) was used to detect cell metabolic activity. One hundred microliter of the single-cell suspension from 5, 7 and 9-days primary cultured neurosheres were inoculated into individual wells of 96-well culture plates (Corning) at a density of 5 × 105 cells/mL. Cell metabolic activity was measured after 24 h culture using EL 800 (Biotek Co.) by measuring the absorbance at 570 nm.
Colony-forming assay of NSCs and diameter of neurospheres at different passages
The colony-forming assay was carried out as previously described (Tropepe et al. 1999). Briefly, NSCs were harvested after subculturing at different time points. Followed by washing twice in PBS, the cells were adjusted to give a starting concentration of 4,000 cells/mL from which serial dilutions were made and final cell dilution was 40 cells/mL. Fifty microliters of cell suspension and 150 μL of serum-free NSCs medium were plated in each well of 96-well plates. Twenty-four hours later, the wells with single cell were counted and kept. After incubating for 5, 7 or 9 days respectively, the fraction of wells containing neurospheres at each time point against the number of total wells was calculated. The diameter of the neurospheres was also measured when cultured for 5, 7, and 9 days in each generation.
Effect of different passaging schedules on the differentiation of NSCs at different passages
The neurospheres at different passages were dissociated into a single cell suspension and plated onto a 24-well plate containing poly-l-lysine-coated cover slips at a density of 3 × 104 cells/mL in 0.5 mL NSC differentiation medium (DMEM/F12 with 1% FBS) per well and cultured for 7 days. Then the cells were processed for double-label immunocytochemistry with antibodies directed against neurons (1:250 rabbit anti-β-III-tubulin), astrocytes (1:400 mouse anti-GFAP) and oligodendrocytes (1:1,000 rabbit anti-CNPase). Antibodies were labeled with species and fragment specific secondary antibodies (1:200 FITC-conjugated goat anti-mouse IgG or 1:600 TRITC-conjugated goat anti-rabbit IgG) and counterstained with Hoechst 33342. The differentiation rate of neurons, astrocytes and oligodendrocyte was calculated based on counts of positively stained cells divided by the total number of cells. For each experiment five fields were counted per three separate wells. All fluorescent cell stainings were viewed under a TCS SP2 confocal laser scanning microscope (Leica, Germany).
Distribution of cell cycle in NSCs determined by flow cytometry
Propidium iodide (PI) staining was performed following the procedure described by Krishan (1975). Briefly, approximately 1 × 106 cells per flask at each time point were dissociated as above mentioned, and a single-cell suspension was harvested and washed with cold PBS. The treated cells were fixed with 1 mL of pre-chilled 70% ethanol (−20 °C) and laid overnight at 4 °C. After ethanol removal, cells were washed with PBS and stained with PI solution (50 μg/mL, Sigma) plus 100 μg/mL RNase (Sigma) at room temperature in the dark for at least 1 h. Cell cycle analysis was performed with a Bio-Rad fluorescence-activated cell-sorting analyzer with CellQuest software (Becton–Dickinson, USA). The fractions of cells in the G0/G1, S, and G2/M phases were quantified with the ModFit LT system.
RT-qPCR for p16Ink4a and cyclin D1
Total RNA was extracted from the cultured NSCs using the RNA Isolation kit according to the manufacturer’s instructions (Takara Bio Co., Ltd., Japan). First-strand complementary DNA (cDNA) was synthesized from 1 μg total RNA with a QuantiTect Reverse Transcription kit (Takara). p16Ink4a or cyclin D1 were co-amplified along with a fragment of the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene, which served as an internal standard. The primer sequences used are listed in Table 1. A single peak of the melting curve confirmed primer specificity. The amplification efficiency of each pair of primers was evaluated with a standard curve that was generated with fluorescent data from a 10-fold dilution series of cDNA (Table 1). Only primers with similar amplification efficiencies were used in this experiment. Real-time PCR was conducted on an ABI 7,500 PCR system (Applied Biosystems, USA). Briefly, each PCR reaction was performed in a 20-μL reaction mixture containing 2 μL cDNA, 10 μL SYBR Green I, 0.4 μL 50 × ROX standard, 1.0 μL 10× PCR forward and reverse primer mix (Takara), and 6.6 μL nuclease-free water. Negative controls (no DNA template) for each primer set were included in each run. The thermal cycle used was comprised of 40 cycles at 95 °C for 15 s and 60 or 62 °C (Table 1) for 1 min. A dissociation curve was calculated and was comprised of 95 °C for 15 s, 60 °C for 1 min, and 95 °C for 10 s. The amount of specific mRNA was quantified by determining the point at which the fluorescence accumulation entered the exponential phase (Ct), and the Ct ratio of the target gene to GAPDH was calculated for each sample. We performed five replicates for each sample of the three times, and all PCR data were analyzed with the Delta–Delta Ct method with the ABI 7,500 system software, version 2.0.4.
Table 1.
Primers used for RT-qPCR in this study
| Gene (GenBank accession) | Sequence (5′–3′) | Amplicon length (bp) | Cycling temperature (°C) | Efficiency (%) |
|---|---|---|---|---|
| p16Ink4a (NM_031550.1) | Sense:tgcggtatttgcggtatctactctc | 181 | 60 | 104.2 |
| Antisense:tagtctcgcgttgccagaagtg | ||||
| CyclinD1 (NM_171992.4) | Sense:tgttcgtggcctctaagatgaag | 174 | 62 | 98.3 |
| Antisense:ggaagtgttcgatgaaatcgtg | ||||
| GAPDH (NM_017008.3) | Sense:ggcacagtcaaggctgagaatg | 143 | 60 | 100 |
| Antisense:atggtggtgaagacgccagta |
Statistical analysis
Independent experiments were performed at least three times. Quantitative data are shown as mean values ± standard error of the mean (SEM). Microscopic counting was from 15 non-overlapping fields. Statistical analysis was performed with GraphPad Prism 5 (GraphPad Software Inc., USA) as described in the figure legends, with significant differences for all tests assumed at the level of p < 0.05. Differences between the groups were analyzed with a one-way analysis of variance (ANOVA) (Newman-Keuls Multiple Comparison Test) when appropriate.
Results
The morphological features of neurospheres at different time points in primary culture
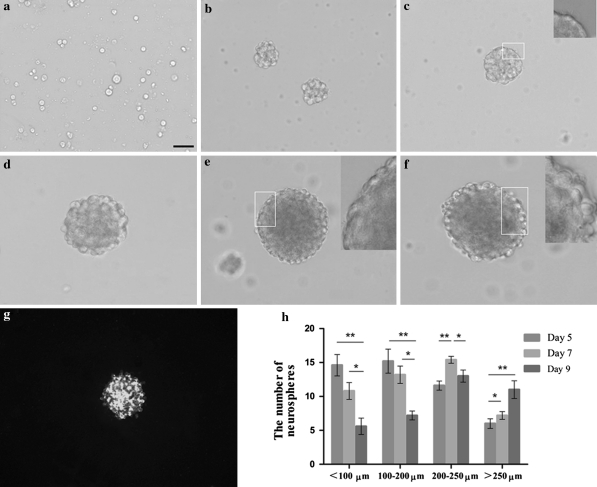
During the first 24 h after isolation from newborn rat cerebral cortex, there were many round and suspended single cells in the medium with clear boundaries that were translucent in appearance (Fig. 1a). At 72 h, a few small floating cell clusters had grown in size, detached from the substrate, and were floating in suspension (Fig. 1b). Over the next few days, the number of NSCs increased rapidly. On day 5, the center of the sphere was light in color and translucent (Fig. 1c). At 7-days, the proliferating NSCs formed neurospheres that mostly measured 100–200 μm in diameter (Fig. 1d) which usually were composed of approximately 0.5–1.0 × 104 cells each. But on the 8th day, the center of the sphere was darkened in color and the outer portion of the sphere is still bright and translucent with light, round healthy cells on the outer edge (Fig. 1e). And when we observed the neurospheres under a microscope on day 9, the dark area was expanded, and the center of the sphere was blackened (Fig. 1f). Figure 1g shows that these cells were immunopositive for nestin, an intermediate filament protein mainly expressed by stem or precursor cells. Before passage, at different time points of 5, 7 and 9 days for primary culture, five observed fields at 20 × objective in every flask were chosen randomly to count the number of neurospheres. As shown in Fig. 1h, at day 5, there were many neurospheres with diameters of 100–200 μm, and there were a large number of neurospheres with diameters of 200–250 μm on day 7. On day 9, the flask contained large cell clusters with dozens of cells, and most of the spheres were more than 250 μm in diameter.
Fig. 1.
The morphological features of neurospheres at different stages in primary culture. a The Cultured NSCs for 24 h showed a clear boundary and were translucent in appearance. b After 72 h, neurospheres could be observed. c A 5-days floating neurosphere, showed the appearance of microspikes that are commonly seen on young healthy neurospheres. d A 7-days neurosphere, showed the absence of dead cells and abundance of large, healthy round cells. e A 8-days neurosphere, showed a few dark area in the center. f On day 9, an unhealthy sphere, showed a high proportion of dead cells in the center. Scale bars: 100 μm for all panels. The inset on the top right corner is the magnification of the chosen white box labeled area. g A neurosphere showed Nestin positive immunostaining. h The number of neurospheres with different diameters at different time points. *p < 0.05, **p < 0.01, n = 15
The effect of different passaging schedule on cell metabolic activity
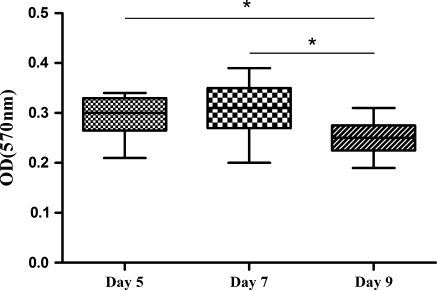
Cell metabolic activity was determined by MTT chromometry assay. Figure 2 shows the MTT reduction reaction results of the neurospheres in the three groups of different passage times. The cell metabolic activity in the 5-days group was not significantly different from that of the 7-days group, but was higher than that of 9-days group (p < 0.05).
Fig. 2.
The MTT reduction reaction results of the NSCs from three groups of different passaging schedules. *p < 0.05, n = 9
The effect of different passaging schedule on colony formation and diameter of colony
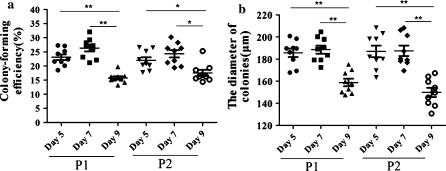
The average colony-forming efficiencies for the NSCs of P1 after subculturing from P0 on days 5, 7 or 9 were 23.11% ± 1.02%, 26.36% ± 1.19% and 15.74% ± 0.60%, respectively. For P2, they were 21.99% ± 1.13%, 23.76% ± 1.07% and 17.53% ± 1.10% for every 5, 7 and 9 days passage group. As shown in Fig. 3a, NSC colony formation was decreased greatly in every 9 days passage group, whereas there was no significant difference between day 5 and 7 groups in NSC colony number at P1 and P2. As in the conventional neurosphere assay, the colony number is an indication of the number of viable NSCs in the original population. When cultured for 5 days at P1 and P2, the diameter of the colonies in day 9 group was significantly smaller than the two other groups (Fig. 3b).
Fig. 3.
The effect of different passaging schedules on the formation of colony (Fig. 3a) and diameter of colony (Fig. 3b). a shows a reduced the colony-forming efficiency for the 9 days passage of all groups. b shows reduced the size of colony for the 9 days passage of all groups. P1 first passage cells, P2 second passage cells, *p < 0.05, **p < 0.01, n = 9
The time of primary culture affects the differentiation of NSCs
The primary cultured and passaged neurospheres at P1 and P2 are able to produce neurons, astrocytes and oligodendrocytes when allowed to differentiate in the presence of differentiation supplements. The neurospheres at P1 generated similar proportions of β-III-tubulin-positive neurons, GFAP-positive astrocytes and CNPase-positive oligodendrocytes as compared to those of P0. However, prolonging the primary culture time reduced the differentiation of NSCs into neurons and oligodendrocytes at P2 (Fig. 4).
Fig. 4.
Differentiation of neurospheres from primary culture and sub-culture passages. a, b, c Representative double staining with Hoechst 33342 counterstain (blue), β-III-tubulin (red) and GFAP (green) antibodies for differentiated P2 NSCs in the groups of day 5, 7, or 9, respectively. d, e, f Presentative immunostaining with Hoechst 33342 counterstain (blue) and CNPase (red) antibody for differentiated P2 NSCs in the groups of day 5, 7, or 9, respectively. g Differentiation rate of neurospheres at different passage time schedules. *p < 0.05, n = 9. Scale bars: 100 μm for all panels
The time of primary culture affects the cell cycle in NSCs
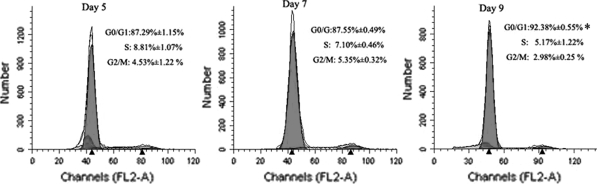
The G1 phase is the important checkpoint in the process of cell proliferation. We used flow cytometry to investigate the proportion of NSCs in the G1 phase following different time points of primary culture. The PI cell-cycle analysis revealed that the percentage of cells in the G0/G1 phase of 9-days group was significantly increased in comparison with the two other groups (p < 0.05), whereas no difference was seen between 5- and 7-day groups (p > 0.05). The average percentages in the G0/G1 phase from three independent experiments were 87.29% ± 1.15%, 87.55% ± 0.49%, 92.38% ± 0.55% on day 5, 7, and 9, respectively (Fig. 5).
Fig. 5.
The effect of primary culture time on cell cycle progression. NSCs were cultured for different times, and the DNA was stained with PI and analyzed with flow cytometry. Representative diagrams are shown for day 5, 7 and 9. The percentages of cells in each phase of the cell cycle from three independent experiments are shown. Compared with day 5 or 7, the percentage of cells in G0/G1 phase on day 9 was greater, *p < 0.05, n = 9
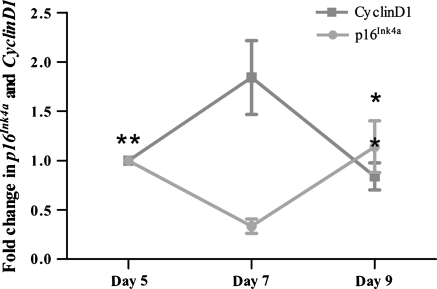
Relative expression of p16Ink4a and cyclin D1 at different time points for primary culture
To investigate the expression levels of p16Ink4a and cyclin D1, relative expression of genes at different time points was analyzed with relative expression software tool. Comparing with 7-day group, modest but significantly lower expression was recorded for cyclin D1 on day 5 and 9 (0.56- and 0.45-fold respectively; p < 0.05). Moreover, the corresponding values were higher on day 9 than on day 5, but there were no significant differences between the two groups. On the other hand, we observed a significant increase (p < 0.05) in the expression of p16INK4a on day 9 (3.4-fold greater than that on day 7). The mean normalized expression ratio was 0.3-fold on day 7 compared with day 5. However, there was no significant difference between day 5 and 9. Side-by-side comparisons of the expression profiles of these genes by RT-qPCR are shown in Fig. 6.
Fig. 6.
Analysis of expression of cell cycle regulator genes in primary cultured NSCs at days 5, 7, and 9 with RT-qPCR (values are normalized to day 5). *p < 0.05 compared to day 7, n = 9
Discussion
Culture systems for the isolation, expansion, and differentiation of NSCs provide a unique and powerful in vitro model system for studying and elucidating the molecular and cellular mechanisms underlying development, plasticity, and regeneration. NSCs have been isolated from the mammalian CNS and can be maintained in vitro for extended periods of time without losing their properties of proliferation and potential for differentiation (Reynolds and Weiss 1996). Stem cells isolated from the newborn rat CNS can be maintained in an undifferentiated proliferative state in a defined serum-free medium supplemented with EGF and bFGF. After approximately 7 days in this growth medium, the proliferating EGF- and bFGF-responsive NSCs generate non-adherent spherical clusters of cells, commonly termed neurospheres (Bez et al. 2003), which measure 100–200 μm (Fig. 1d) in diameter. At this stage, the neurospheres can be passaged. This procedure can be repeated weekly, resulting in an exponential increase in total cell numbers. In our study, newborn rat-derived neurospheres cultured in this manner have been passaged for 10 weeks with no loss in their proliferative ability, resulting in a 107-fold increase in cell number (data not shown).
Multiple methods have been developed to isolate and expand NSCs, two of which have been recently published (Chen et al. 2007; Brewer and Torricelli 2007). Each method has its own advantages. The neurosphere culture system is straightforward, reproducible, and not technically demanding, making it an ideal system to explore the characteristics of stem cells. The original culture systems and parameters were based on the work of Reynolds and Weiss (1992, 1996). However, several factors were reported to impact the culture system, including the centrifugation parameters (Ye et al. 2008) and the time of passaging. In our study, we evaluated the influence of different passaging schedules on primary cultured NSCs and found that there would be different results after subculturing at different stages. When passaged at the stage of 100–200 μm in diameter, we acquired a large number of translucent single cells with the least amount of debris. This phenomenon could be seen by flow cytometry. On day 9, an apoptosis or debris peak appeared before G0/G1 peak. But this peak didn’t obviously appear on day 5 or day 7 (Fig. 5).
In detail, during the first few days, single cells proliferated to form small clusters of cells that sometimes adhered slightly to the culture vessel. These lifted off from the substratum as the density of the sphere increased. Viable neurospheres were mostly semitransparent, with many cells on the outer surface displaying microspikes (Fig. 1c). Cells should be passaged earlier rather than later and before the neurospheres grow too large (>250 μm in diameter). If the neurospheres are allowed to grow too large, the cells in the inside of the neurospheres are unable to neither acquire oxygen and nutrients nor eliminate their metabolites (Fig. 1d, e). Larger neurospheres are also more difficult to dissociate. After repeated mechanical pipetting, there will be more debris and necrotic cells. Therefore, it is important that cultures be monitored each day to determine the conditions of the neurospheres (round phase-bright spheres with a smooth periphery) and medium.
The cell metabolic activity in the 5-days group was not significantly different from that of the 7-days group, but higher than that of the 9-days groups (Fig. 2). The colony-forming efficiency is an indication of the number of viable NSCs in the original population. In our study, when the time of primary culture was prolonged to 9 days, the colony-forming efficiency was significantly decreased. This feature was seen in both P1 and P2. In addition, the proliferative capacity of NSCs was also inhibited, which was elucidated by the investigation of the colony size. The differentiation assay demonstrated that the neuropsheres at P0, P1 or P2 are able to produce neurons, astrocytes and oligodendrocytes. The proportion of the three lineage differentiated cells has no difference at P0 and P1. However, when cultured for 9 days there were fewer NSCs at P2 differentiated into neurons and oligodendrocytes than the other two groups, and most of them differentiated into astrocytes.
Although much is known about the biology and behavior of NSCs, efforts have only recently focused on characterizing the morpho-functional features and metabolic properties of the neurospheres (Bez et al. 2003), which are largely unknown. It has been suggested that the more number of cells reside in G0/G1, the more cells are quiescent. In our study, on day 9 most of cells in the neurospheres reside in G0/G1 and the number of cells in the G0/G1 phase on day 9 was higher than that on days 5 or 7. Prolonging the time of primary culture would induce most of the cells in the neurospheres to reside in the state of non-differentiation. Forcing the cells from G1 to S may promote the expansion of NSCs. CDK4/6-cyclin D, CDK2-cyclin E, and the transcription complex that includes Rb and E2F are pivotal in controlling the checkpoint of passage of NSCs from G1 phase into S phase (Salomoni and Calegari 2010). Passing through the restriction point and transition to S phase are triggered by activation of the cyclin D/cdk complex, which phosphorylates Rb. Phosphorylated Rb dissociates from E2F, which is then free to initiate DNA replication (Bartek and Lukas 2001). In this study, cyclin D1 expression was found to be high at days 5 and 7. However, the expression significantly decreased when the incubation time was extended to 9 days, emphasizing the decreased NSC turnover at day 9. This observation is consistent with the findings of other investigators (Sicinski et al. 1995) who reported that downregulation of cyclin D1 delayed neural development induced by nutritional deprivation. These data appear to be consistent with the results of previous studies (Lange et al. 2009).
p16Ink4a is considered to be a tumor suppressor (Sherr 2001) that limits cell proliferation and is a negative regulator of NSC function in aging animals (Molofsky et al. 2003). In addition, p16Ink4a inhibits NSC self-renewal in culture (Molofsky et al. 2003). To examine changes in gene expression of p16INK4a when extending the time of primary culture, we examined the level of p16INK4a at different stages with RT-qPCR. We observed a 3.4-fold increase in p16Ink4a expression in long-term primary cultured neurospheres compared to that of on day 7 (Fig. 6). The elevated p16Ink4a expression reduced the proliferation of NSCs in vitro, although the magnitude of this effect may depend on culture conditions to some extent, because p16Ink4a can be induced in response to stress (Sherr 2001). This observation is further supported by the significantly decreased proliferation rate observed in the in vitro (Vukicevic et al. 2010) and in vivo (Molofsky et al. 2006) experiments done by other investigators.
p16Ink4a is a cyclin-dependent kinase inhibitor that promotes Rb activation, slowing cell-cycle progression and inducing cellular senescence (Lowe and sherr 2003). In our study, we found stage-related differences in cyclin D1 and p16Ink4a, which are the two important regulators of G1. With a delay in passaging, the number of cells in G1 increased (Fig. 5). Moreover, we also observed a decrease in cyclin D1 expression and an increase in p16Ink4a expression when the time of primary culture was extended to 9 days (Fig. 6).
Conclusion
The culture system we used provides an in vitro assay to address the question why passaging primary NSCs at different time schedules leads to different results. There were more neurospheres with diameters of 200–250 μm on day 7. Prolonging the time of primary culture reduced the cell metabolic activity and colony-forming efficiency and the number of neurons differentiated from P2 cells gradually decreased. In addition, cell cycle-associated regulators were examined at the molecular level, and their expression corroborated that passaging neurospheres on the 7th day of primary culture provided favorable NSCs growth.
Acknowledgments
This study was supported by a grant from the National Natural Science Foundation of China (No. 81070992), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and the Foundation of the Graduate Natural Science Innovation Project of Nantong University (No. YKC10050).
Conflict of interest This study was supported by a grant from the National Natural Science Foundation of China (No. 81070992), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and the Foundation of the Graduate Natural Science Innovation Project of Nantong University (No. YKC10050). The authors have declared that no conflict of interest exists.
Footnotes
Fangling Xiong and Huasong Gao contributed equally to this work.
Contributor Information
Mei Liu, Phone: +86-513-85051852, Email: liumei@ntu.edu.cn.
Yilu Gao, Phone: +86-513-81160807, Email: yilugao@hotmail.com.
References
- Bartek J, Lukas J. Pathways governing G1/S transition and their response to DNA damage. FEBS Lett. 2001;490:117–122. doi: 10.1016/S0014-5793(01)02114-7. [DOI] [PubMed] [Google Scholar]
- Bassiouny AE, Nosseir MM, Zoheiry MK, Ameen NA, Abdel-Hadi AM, Ibrahim IM, Zada S, El-Deen AH, El-Bassiouni NE. Differential expression of cell cycle regulators in HCV-infection and related hepatocellular carcinoma. World J Hepatol. 2010;2:32–41. doi: 10.4254/wjh.v2.i1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bez A, Corsini E, Curti D, Biggiogera M, Colombo A, Nicosia RF, Pagano SF, Parati EA. Neurosphere and neurosphere-forming cells: morphological and ultrastructural characterization. Brain Res. 2003;993:18–29. doi: 10.1016/j.brainres.2003.08.061. [DOI] [PubMed] [Google Scholar]
- Bornfeldt KE. The cyclin-dependent kinase pathway moves forward. Circ Res. 2003;92:345–347. doi: 10.1161/01.RES.0000061765.06145.10. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR. Isolation and culture of adult neurons and neurospheres. Nat Protoc. 2007;2:1490–1498. doi: 10.1038/nprot.2007.207. [DOI] [PubMed] [Google Scholar]
- Chen Y, Balasubramaniyan V, Peng J, Hurlock EC, Tallquist M, Li J, Lu QR. Isolation and culture of rat and mouse oligodendrocyte precursor cells. Nat Protoc. 2007;2:1044–1051. doi: 10.1038/nprot.2007.149. [DOI] [PubMed] [Google Scholar]
- Dhara SK, Stice SL. Neural differentiation of human embryonic stem cells. J Cell Biochem. 2008;105:633–640. doi: 10.1002/jcb.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaro R, Valotta M, Ferri AL, Latorre E, Mariani J, Giachino C, Lancini C, Tosetti V, Ottolenghi S, Taylor V, Nicolis SK. Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nat Neurosci. 2009;12:1248–1256. doi: 10.1038/nn.2397. [DOI] [PubMed] [Google Scholar]
- Goldman S. Stem and progenitor cell-based therapy of the human central nervous system. Nat Biotechnol. 2005;23:862–871. doi: 10.1038/nbt1119. [DOI] [PubMed] [Google Scholar]
- Kim JB, Zaehres H, Wu G, Gentile L, Ko K, Sebastiano V, Arauzo-Bravo MJ, Ruau D, Han DW, Zenke M, Scholer HR. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature. 2008;454:646–650. doi: 10.1038/nature07061. [DOI] [PubMed] [Google Scholar]
- Kim JB, Sebastiano V, Wu G, Arauzo-Bravo MJ, Sasse P, Gentile L, Ko K, Ruau D, Ehrich M, Boom D, Meyer J, Hubner K, Bernemann C, Ortmeier C, Zenke M, Fleischmann BK, Zaehres H, Scholer HR. Oct4-induced pluripotency in adult neural stem cells. Cell. 2009;136:411–419. doi: 10.1016/j.cell.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Krishan A. Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J Cell Biol. 1975;66:188–193. doi: 10.1083/jcb.66.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange C, Huttner WB, Calegari F. Cdk4/cyclinD1 overexpression in neural stem cells shortens G1, delays neurogenesis, and promotes the generation and expansion of basal progenitors. Cell Stem Cell. 2009;5:320–331. doi: 10.1016/j.stem.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Li XG, Yang ZY, Zhang AF. The effect of neurotrophin-3/chitosan carriers on the proliferation and differentiation of neural stem cells. Biomaterials. 2009;30:4978–4985. doi: 10.1016/j.biomaterials.2009.05.047. [DOI] [PubMed] [Google Scholar]
- Lobo MVT, Alonso FJM, Redondo C, Lopez-Toledano MA, Caso E, Herranz AS, Paino CL, Reimers D, Bazan E. Cellular characterization of epidermal growth factor-expanded free-floating neurospheres. J Histochem Cytochem. 2002;51:89–103. doi: 10.1177/002215540305100111. [DOI] [PubMed] [Google Scholar]
- Lowe SW, Sherr CJ. Tumor suppression by Ink4a-Arf: progress and puzzles. Curr Opin Genet Dev. 2003;13:77–83. doi: 10.1016/S0959-437X(02)00013-8. [DOI] [PubMed] [Google Scholar]
- Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, Sharpless NE, Morrison SJ. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ourednik V, Ourednik J, Xu YF, Zhang Y, Lynch WP, Snyder EY, Schachner M. Cross-talk between stem cells and the dysfunctional brain is facilitated by manipulating the niche: evidence from an adhesion molecule. Stem Cells. 2009;27:2846–2856. doi: 10.1002/stem.227. [DOI] [PubMed] [Google Scholar]
- Prestoz L, Relvas JB, Hopkins K, Patel S, Sowinski P, Price J, ffrench-Constant C. Association between integrin-dependent migration capacity of neural stem cells in vitro and anatomical repair following transplantation. Mol Cell Neurosci. 2001;18:473–484. doi: 10.1006/mcne.2001.1037. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1701–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev Biol. 1996;175:1–13. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- Salomoni P, Calegari F. Cell cycle control of mammalian neural stem cells: putting a speed limit on G1. Trends Cell Biol. 2010;205:233–243. doi: 10.1016/j.tcb.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. The INK4a/ARF network in tumour suppression. Nat Rev Mol Cell Biol. 2001;2:731–737. doi: 10.1038/35096061. [DOI] [PubMed] [Google Scholar]
- Sicinski P, Donaher JL, Parker SB, Li T, Fazeli A, Gardner H, Haslam SZ, Bronson RT, Elledge SJ, Weinberg RA. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell. 1995;82:621–630. doi: 10.1016/0092-8674(95)90034-9. [DOI] [PubMed] [Google Scholar]
- Sugimoto M, Nakamura T, Ohtani N, Hampson L, Hampson IN, Shimamoto A, Furuichi Y, Okumura K, Niwa S, Taya Y, Hara E. Regulation of CDK4 activity by a novel CDK4-binding protein, p34(SEI-1) Genes Dev. 1999;13:3027–3033. doi: 10.1101/gad.13.22.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Pollard S, Conti L, Toselli M, Biella G, Parkin G, Willatt L, Falk A, Cattaneo E, Smith A. Long-term tripotent differentiation capacity of human neural stem (NS) cells in adherent culture. Mol Cell Neurosci. 2008;38:245–258. doi: 10.1016/j.mcn.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Suslov ON, Kukekov VG, Ignatova TN, Steindler DA. Neural stem cell heterogeneity demonstrated by molecular phenotyping of clonal neurospheres. Proc Natl Acad Sci USA. 2002;99:14506–14511. doi: 10.1073/pnas.212525299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svendsen CN, Smith AG. New prospects for human stem cell therapy in the nervous system. Trends Neurosci. 1999;22:357–364. doi: 10.1016/S0166-2236(99)01428-9. [DOI] [PubMed] [Google Scholar]
- Tropepe V, Sibilia M, Ciruna BG, Rossant J, Wagner EF, Kooy D. Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Dev Biol. 1999;208:166–188. doi: 10.1006/dbio.1998.9192. [DOI] [PubMed] [Google Scholar]
- Vukicevic V, Jauch A, Dinger TC, Gebauer L, Hornich V, Bornstein SR, Ehrhart-Bornstein M, Müller AM. Genetic instability and diminished differentiation capacity in long-term cultured mouse neurosphere cells. Mech Ageing Dev. 2010;131:124–132. doi: 10.1016/j.mad.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Ye S, Su ZP, Zhang J, Qian X, Zhuge QC, Zeng YJ. Differential centrifugation in culture and differentiation of rat neural stem cells. Cell Mol Neurobiol. 2008;28:511–517. doi: 10.1007/s10571-007-9194-5. [DOI] [PMC free article] [PubMed] [Google Scholar]