Abstract
NCCLS screening and confirmation methods for detecting extended-spectrum β-lactamases (ESBLs) apply only to Escherichia coli and Klebsiella spp., yet ESBLs have been found in other members of the family Enterobacteriaceae. We evaluated the effectiveness of NCCLS methods for detecting ESBLs in 690 gram-negative isolates of Enterobacteriaceae that excluded E. coli, Klebsiella pneumoniae, and Klebsiella oxytoca. Isolates were collected between January 1996 and June 1999 from 53 U.S. hospitals participating in Project ICARE (Intensive Care Antimicrobial Resistance Epidemiology). The antimicrobial susceptibility patterns of the isolates were determined by using the NCCLS broth microdilution method (BMD), and those isolates for which the MIC of ceftazidime, cefotaxime, ceftriaxone, or aztreonam was ≥2 μg/ml or the MIC of cefpodoxime was ≥8 μg/ml (positive ESBL screen test) were further tested for a clavulanic acid (CA) effect by BMD and the disk diffusion method (confirmation tests). Although 355 (51.4%) of the isolates were ESBL screen test positive, only 15 (2.2%) showed a CA effect. Since 3 of the 15 isolates were already highly resistant to the five NCCLS indicator drugs, ESBL detection would have an impact on the reporting of only 1.7% of the isolates in the study. Only 6 of the 15 isolates that showed a CA effect contained a blaTEM, blaSHV, blaCTX-M, or blaOXA β-lactamase gene as determined by PCR (with a corresponding isoelectric focusing pattern). Extension of the NCCLS guidelines for ESBL detection to Enterobacteriaceae other than E. coli and Klebsiella spp. does not appear to be warranted in the United States at present, since the test has poor specificity for this population and would result in changes in categorical interpretations for only 1.7% of Enterobacteriaceae tested.
First described in 1983 (10), extended-spectrum β-lactamases (ESBLs) have contributed to the dramatic increase in resistance to β-lactam agents among gram-negative bacteria in recent years (2, 3, 8). These enzymes, encoded by genes that are typically plasmid borne, hydrolyze penicillins, cephalosporins, and aztreonam and are inhibited by clavulanic acid (CA) (2, 3, 5). Although most commonly produced by Escherichia coli and Klebsiella spp., ESBLs may be harbored by many other gram-negative bacilli as well, including but not limited to many other bacteria in the family Enterobacteriaceae (7, 8, 25).
While most of the currently known ESBLs are derived from the older β-lactamases TEM-1, TEM-2, and SHV-1 (2, 11, 25), CTX-M enzymes are also inhibited by CA and are in the category of ESBLs. In addition, some of the enzymes of the OXA family, although belonging to functional group 2d (5), show a CA effect and are considered ESBLs (2).
While a variety of phenotypic and molecular methods have been successfully employed to confirm the presence of ESBLs in clinical isolates, their use is too labor-intensive to be practical for routine screening in microbiology laboratories. Since routine susceptibility testing does not always detect the presence of ESBLs in pathogenic bacteria (8, 11, 25), the need for a simple yet sensitive screening procedure is readily apparent (9).
The NCCLS has issued guidelines for ESBL screening and confirmation tests that apply to E. coli, Klebsiella pneumoniae, and Klebsiella oxytoca (14, 15, 16). These are based on the MIC or disk diffusion (DD) results for five antimicrobial agents and subsequent testing with ceftazidime (CAZ) and cefotaxime (CTX) in the presence and absence of CA. Uncertainty remains, however, with regard to the need to screen other gram-negative bacilli for ESBLs and the applicability of the existing NCCLS criteria for ESBL detection in non-E. coli and non-Klebsiella spp. Coudron et al. (8) demonstrated the presence of ESBLs in multiple species of enteric gram-negative bacilli by testing for the CA effect with a DD method. A potential problem with this approach, however, is that the presence of resistance mechanisms other than ESBLs, such as AmpC β-lactamases and porin changes, could mask the CA effect by which ESBLs are identified in both DD and broth microdilution (BMD) tests (23).
While existing NCCLS guidelines for ESBL detection in E. coli and Klebsiella spp. employ MIC methods in addition to DD, the sensitivity of these methods too may be limited when they are applied to other pathogenic bacteria, particularly those that are likely to have β-lactam resistance mechanisms other than ESBLs. This study evaluated the suitability of NCCLS guidelines for detection of ESBLs in non-E. coli, non-Klebsiella spp. isolates of Enterobacteriaceae. It also considered whether the prevalence of ESBL-containing isolates justified routine screening in the United States.
MATERIALS AND METHODS
Bacterial isolates.
The isolates collected for this study were 690 non-E. coli, non-K. pneumoniae, and non-K. oxytoca bacterial strains comprising over 30 species from nine genera of the family Enterobacteriaceae. The isolates were received from 53 hospital laboratories throughout the United States that participated in phases 2 and 3 of Project ICARE (Intensive Care Antimicrobial Resistance Epidemiology) from January 1996 until June 1999. Enterobacteriaceae demonstrating resistance to any extended-spectrum cephalosporin, fluoroquinolone, or carbapenem, from both intensive care unit patients and other inpatient services, were sent to the central ICARE laboratory from participating laboratories. Duplicate isolates from the same patient were excluded by the submitting laboratory. The bacterial isolates were subcultured from −70°C storage onto trypticase soy agar plates containing 5% defibrinated sheep's blood (Difco; BD BioSciences, Sparks, Md.) and then subcultured once again prior to testing. The quality control strains used in this study for antimicrobial susceptibility testing included E. coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, and the ESBL control strain, K. pneumoniae ATCC 700603 (14, 15, 16).
Antimicrobial susceptibility testing.
All isolates were screened for ESBLs by BMD using NCCLS guidelines (15). MIC plates containing eight twofold dilutions of CAZ, CTX, ceftriaxone (CRO), aztreonam (ATM), and cefpodoxime (CPD) were prepared in-house. Isolates for which the MIC of CAZ, CTX, CRO, or ATM was ≥2 μg/ml or for which the MIC of CPD was ≥8 μg/ml were further tested by using MIC plates containing each of the above antimicrobials alone and in combination with 4 μg of CA/ml. In addition, the antimicrobial susceptibility patterns of the isolates were determined by the NCCLS DD method (14) using the same bacterial suspension that was used for BMD for the CA MIC confirmation procedure. Mueller-Hinton agar plates (BD Biosciences) and disks containing 30 μg of CAZ or CTX (BD Biosciences) with and without 10 μg of CA were used. For the ESBL confirmation test, isolates were considered positive for ESBL production if MICs for either CAZ or CTX decreased by three or more doubling dilutions when tested in the presence of CA or if zone diameters increased by ≥5 mm for either CAZ or CTX when tested alone and with CA. BMD and DD tests were repeated for strains showing discrepant results. Isolates with positive screen test results for the presence of the ESBL phenotype that demonstrated a CA effect were further evaluated by isoelectric focusing (IEF) and PCR.
IEF.
IEF was performed with crude lysates of isolates demonstrating a CA effect to determine the number and isoelectric points (pIs) of β-lactamases (4, 13). We considered bands with pIs of 5.2 to 6.5 to be suggestive of TEM and those with pIs of 7.0 to 8.2 to be suggestive of SHV. Bands with pIs of 7.5 to 8.9 were considered as indicating potential CTX-M enzymes, while any band with a pI of 5.5 to 9.0 was considered as indicating a possible OXA-type β-lactamase (23). Enzyme extracts requiring more than 10 min to produce a color change after being mixed with nitrocephin (100 μg/ml) were concentrated (Centricon; Amicon, Inc., Beverly, Mass.).
PCR.
The presence of the blaTEM, blaSHV, blaOXA, and blaCTX-M genes in isolates that demonstrated a CA effect was determined by PCR as previously described (12, 21, 23). Three sets of oligonucleotides to detect the presence of genes encoding OXA-type β-lactamases were used in individual reactions according to the protocol described by Steward et al. (23). Detection of blaCTX-M genes was performed in the following sequence: isolates were first amplified with the primers CTX-MA and CTX-MB, which correspond to conserved areas of blaCTX-M-type genes and result in a 550-bp product (1); isolates containing genes encoding CTX-M-type β-lactamases were evaluated further with three sets of oligonucleotides that can distinguish several of the major groups within the blaCTX-M-type gene family and do not amplify the closely related blaK-1 gene of K. oxytoca. The primer set CTX-M-9 (Up/4)-CTX-M-9 (Dn/860) was used to amplify blaCTX-M-9 and related genes (22); the primer set CTX-M-2F-CTX-M-2R, based on the report by Petroni et al. (19), was used to amplify blaCTX-M-2 and related genes; and primer set CTX-M-F3-CTX-M-R3 (6) was used to amplify blaCTX-M-10 and related genes. Control organisms included strains of E. coli or Salmonella that possessed either blaTEM-1, blaSHV-3, blaOXA-3, blaOXA-4, blaOXA-7, blaCTX-M-5, blaCTX-M-9, or the blaCTX-M-10 gene. E. coli ATCC 25922 was used as a negative control.
RESULTS
Phenotypic results.
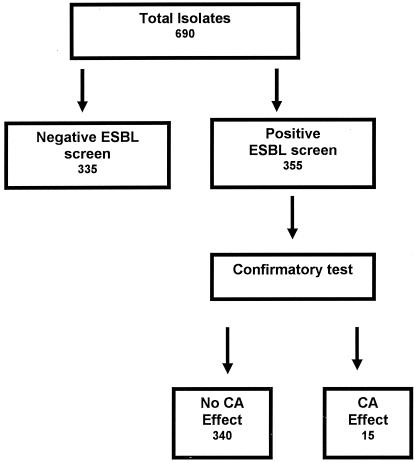
The species distribution of the 690 Enterobacteriaceae isolates included in this study is shown in Table 1. Of the 690 isolates, 355 (51.4%) were screen test positive by NCCLS guidelines for ESBL detection (Fig. 1). Fifteen (2.2% of the total) isolates demonstrated a CA effect with CTX, CAZ, or both agents. The MIC ranges for the five ESBL indicator drugs for the ESBL screen test-positive isolates and the numbers of isolates that were confirmed to harbor ESBLs are shown in Table 1. For approximately 80% of the isolates that were ESBL screen test positive, high cephalosporin MICs were demonstrated, with concomitant resistance to cefoxitin and susceptibility to cefepime suggestive of the presence of an AmpC β-lactamase (data not shown), while for the remaining isolates, including several of the less commonly isolated species, cephalosporin MICs of 4 μg/ml or less, which were not reduced in the presence of CA, were demonstrated.
TABLE 1.
Study isolates grouped by species and ESBL phenotype (n = 690)
| Organism | Total no. of isolates | No. of ESBL screen test-positive isolates | MIC range (μg/ml) for screen test-positive isolates
|
No. of confirmatory test-positive isolates | ||||
|---|---|---|---|---|---|---|---|---|
| CAZ | CTX | CRO | ATM | CPD | ||||
| Citrobacter amalonaticus | 1 | 1 | ≤2 | 8 | >64 | >64 | 16 | 1 |
| Citrobacter braakii | 2 | 2 | >128 | >64 | >64 | >64 | >16 | 0 |
| Citrobacter farmeri | 3 | 2 | ≤2->128 | ≤1-32 | ≤1-16 | 4->64 | 1->16 | 0 |
| Citrobacter freundii | 52 | 40 | ≤1->128 | ≤1->64 | ≤1->64 | ≤1->64 | 4->16 | 1 |
| Citrobacter freundii complex | 1 | 1 | >128 | >64 | >64 | >64 | >16 | 0 |
| Citrobacter freundii or youngae | 17 | 16 | ≤2 | >128 | 2->64 | ≤1->64 | 8->16 | 0 |
| Citrobacter koseri | 1 | 0 | 0 | |||||
| Citrobacter species | 14 | 14 | ≤2->128 | ≤1->64 | ≤1->64 | ≤1->64 | 16->16 | 0 |
| Citrobacter werkmanii | 3 | 2 | 128->128 | 64->64 | >64 | >64 | >16 | 0 |
| Enterobacter aerogenes | 47 | 37 | ≤1->128 | ≤1->64 | ≤1->64 | ≤1->64 | 1->16 | 1 |
| Enterobacter amnigenus | 1 | 1 | 128 | >64 | >64 | >64 | >16 | 0 |
| Enterobacter asburiae | 1 | 1 | 128 | >64 | >64 | >64 | >16 | 0 |
| Enterobacter cloacae | 98 | 83 | ≤1->128 | ≤1->64 | ≤1->64 | ≤1->64 | 8->16 | 2 |
| Enterobacter gergoviae | 1 | 0 | 0 | |||||
| Enterobacter hormaechei | 2 | 2 | ≤1-4 | ≤1-16 | ≤1-8 | ≤1-2 | 8->16 | 0 |
| Enterobacter intermedius | 1 | 1 | 128 | >64 | >64 | >64 | >16 | 0 |
| Enterobacter taylorae | 1 | 1 | ≤2 | ≤1 | ≤1 | ≤1 | >16 | 0 |
| Hafnia alvei | 1 | 1 | 4 | ≤1 | 2 | ≤1 | 16 | 0 |
| Klebsiella rhinoscleromatis | 1 | 1 | 2 | ≤1 | ≤1 | ≤1 | >16 | 0 |
| Morganella morganii | 59 | 47 | ≤1 | >128 | ≤1-32 | ≤1-32 | 8->16 | 0 |
| Proteus mirabilis | 171 | 6 | 2-64 | ≤1 | ≤1 | ≤1 | ≤0.25-4 | 1 |
| Proteus penneri | 1 | 1 | ≤2 | >64 | 64 | ≤1 | 16 | 1 |
| Proteus vulgaris | 10 | 6 | ≤1-4 | ≤1->64 | 2->64 | ≤1->64 | ≤0.25->16 | 4 |
| Providencia rettgeri | 7 | 2 | 4 | ≤1 | ≤1 | ≤1 | ≤0.25-0.5 | 0 |
| Providencia stuartii | 114 | 41 | ≤1-32 | ≤1->64 | ≤1-32 | ≤1-4 | ≤0.25->16 | 0 |
| Serratia fonticola | 1 | 1 | 64 | 64 | >64 | 32 | >16 | 0 |
| Serratia marcescens | 74 | 42 | ≤1->128 | ≤1->64 | ≤1->64 | ≤1->64 | 2->16 | 2 |
| Serratia plymuthica | 1 | 1 | 2 | 4 | 2 | ≤1 | 8 | 0 |
| Serratia rubidaea | 2 | 2 | 32-64 | ≤1 | ≤1 | 2-4 | 4-8 | 2 |
| Serratia (species unspecified) | 1 | 0 | 0 | |||||
| Shigella group D | 1 | 0 | 0 | |||||
| Total | 690 | 355 | 15 | |||||
FIG. 1.
Summary of phenotypic profiles of study isolates.
The MIC results (when tested with and without CA) and categorical interpretations (susceptible, intermediate, or resistant) for the five screening drugs determined by the traditional NCCLS breakpoints for the 15 Enterobacteriaceae isolates confirmed to harbor ESBLs are shown in Table 2. All of the ESBL-producing isolates were detected by testing CAZ and CTX with and without CA, as recommended by NCCLS, although in some cases the MIC results with CA were below the lowest concentration of the antimicrobial agent recommended by NCCLS for testing (i.e., 0.25 μg/ml). Since three of the isolates (Citrobacter freundii 3757, Serratia marcescens 91, and S. marcescens 167) were already classified as resistant to all five antimicrobial agents, the interpretations for the cephalosporin and penicillin results would have changed from susceptible (or intermediate) to resistant only for the remaining 12 isolates (1.7% of the total isolates studied) based on the positive ESBL confirmation test.
TABLE 2.
Susceptibility results for ESBL-positive isolates by BMD
| Isolate | MIC in μg/ml (isolate resistance categorya)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CAZ | CAZ+CAb | CTX | CTX+CA | CRO | CRO+CAc | ATM | ATM+CAc | CPD | CPD+CAc | |
| Citrobacter amalonaticus 4026 | 2 (S) | 0.5 | 8 (S) | 0.5 | >64 (R) | 0.5 | >64 (R) | 16 | 8 (R) | 2 |
| Citrobacter freundii 3757 | 128 (R) | 2 | 64 (R) | 0.5 | 64 (R) | 2 | >64 (R) | 0.25 | 128 (R) | 8 |
| Enterobacter aerogenes 3701 | 4 (S) | 0.25 | 4 (S) | ≤0.03 | 4 (S) | ≤0.06 | 4 (S) | ≤0.03 | 32 (R) | ≤0.06 |
| Enterobacter cloacae 53 | 32 (R) | 0.12 | 16 (I) | 0.06 | 16 (I) | ≤0.06 | 64 (R) | 0.5 | 32 (R) | 0.5 |
| Enterobacter cloacae 3146 | 128 (R) | 0.5 | 16 (I) | 0.5 | 32 (I) | 0.5 | >64 (R) | 0.25 | 64 (R) | 2 |
| Proteus mirabilis 3750 | 32 (R) | 0.12 | 0.5 (S) | ≤0.03 | ≤0.06 (S) | ≤0.06 | 0.25 (S) | ≤0.03 | 8 (R) | ≤0.06 |
| Proteus penneri 1767 | 0.12 (S) | 0.12 | 16 (I) | ≤0.03 | >64 (R) | ≤0.06 | 0.06 (S) | ≤0.03 | 32 (R) | ≤0.06 |
| Proteus vulgaris 1781 | ≤0.06 (S) | ≤0.06 | 1 (S) | ≤0.03 | 64 (R) | ≤0.06 | ≤0.03 (S) | ≤0.03 | 1 (S) | 0.12 |
| Proteus vulgaris 1405 | ≤0.06 (S) | ≤0.06 | 8 (S) | ≤0.03 | >64 (R) | ≤0.06 | ≤0.03 (S) | ≤0.03 | 128 (R) | ≤0.06 |
| Proteus vulgaris 1699 | ≤0.06 (S) | ≤0.06 | 32 (I) | ≤0.03 | >64 (R) | ≤0.06 | ≤0.03 (S) | ≤0.03 | 128 (R) | ≤0.06 |
| Proteus vulgaris 1765 | ≤0.06 (S) | ≤0.06 | 2 (S) | 0.06 | >64 (R) | ≤0.06 | ≤0.03 (S) | ≤0.03 | >128 (R) | 0.12 |
| Serratia marcescens 91 | 128 (R) | 0.25 | >64 (R) | 2 | >64 (R) | 2 | >64 (R) | 1 | >128 (R) | 4 |
| Serratia marcescens 167 | 64 (R) | 0.25 | 64 (R) | 1 | >64 (R) | 0.5 | >64 (R) | 1 | >128 (R) | 4 |
| Serratia rubidaea 3139 | 64 (R) | 1 | 0.25 (S) | 0.5 | 0.5 (S) | 0.12 | 2 (S) | 0.25 | 4 (I) | 0.25 |
| Serratia rubidaea 3134 | 32 (R) | 1 | 0.5 (S) | 0.25 | 0.5 (S) | 0.25 | 2 (S) | 0.25 | 2 (S) | 0.5 |
S, susceptible; I, intermediate; R, resistant.
A fixed concentration of 4 μg of CA per ml was used in conjunction with each antimicrobial agent.
CRO+CA, ATM+CA, and CPD+CA are not recommended by NCCLS as ESBL confirmatory tests.
Comparison of BMD and DD.
A comparison of results for the NCCLS ESBL confirmatory tests using the BMD and the DD methods for the 15 isolates that demonstrated a CA effect is shown in Table 3. The most effective confirmation method was BMD with cefotaxime (13 confirmed of 15 positive, if the two Proteus vulgaris isolates showing variably positive reactions are included). Conversely, DD using cefotaxime was the least effective method of confirming ESBL production (5 confirmed of 15 positive). Ceftazidime gave much more consistent results between BMD and DD testing but detected fewer ESBL-producing isolates.
TABLE 3.
Comparison of results of NCCLS ESBL confirmatory tests by BMD and DD for 15 isolates showing a CA effect
| Isolate | Results of confirmatory tests using CAZ/CTX bya:
|
|
|---|---|---|
| BMD | DD | |
| Citrobacter amalonaticus 4026 | −/+ | −/− |
| Citrobacter freundii 3757 | +/+ | +/+ |
| Enterobacter aerogenes 3701 | +/+ | −/− |
| Enterobacter cloacae 53 | +/+ | +/+ |
| Enterobacter cloacae 3146 | +/+ | +/+ |
| Proteus mirabilis 3750 | +/+ | +/− |
| Proteus penneri 1767 | −/+ | −/− |
| Proteus vulgaris 1781 | −/var | −/− |
| Proteus vulgaris 1405 | −/var | −/− |
| Proteus vulgaris 1699 | var/+ | −/− |
| Proteus vulgaris 1765 | −/+ | −/− |
| Serratia marcescens 91 | +/+ | +/+ |
| Serratia marcescens 167 | +/+ | +/+ |
| Serratia rubidaea 3139 | +/− | +/− |
| Serratia rubidaea 3134 | +/− | +/− |
+, positive test result; −, negative test result; var, variable results.
PCR and IEF results.
By PCR, 4 of 15 ESBL-producing isolates were positive for both blaTEM and blaSHV, 1 was positive for blaTEM only, and 1 was positive for blaSHV only. Each of these six isolates had a corresponding IEF pattern consistent with the presence of TEM and SHV β-lactamases (Table 4). No ESBL genes, including blaOXA or blaCTX-M, were identified by PCR in the other nine ESBL-positive isolates. However, all nine isolates showed β-lactamase bands by IEF.
TABLE 4.
PCR and IEF results for ESBL-positive strains
| Isolate | Result for indicated β-lactamase gene by PCRa
|
pI(s) of β-lactamases detected by IEF | |||
|---|---|---|---|---|---|
| TEM | SHV | OXA | CTX-M | ||
| Citrobacter amalonaticus 4026 | − | − | − | − | 5.6, 6.1 |
| Citrobacter freundii 3757 | − | + | − | − | 8.2, 8.3 |
| Enterobacter aerogenes 3701 | − | − | − | − | 7.3, 7.6, 7.9, 8.0 |
| Enterobacter cloacae 53 | + | + | − | − | 5.5, 7.9 |
| Enterobacter cloacae 3146 | + | + | − | − | 5.5, 8.2 |
| Proteus mirabilis 3750 | + | − | − | − | 6.3 |
| Proteus penneri 1767 | − | − | − | − | 6.6 |
| Proteus vulgaris 1781 | − | − | − | − | 8.1 |
| Proteus vulgaris 1405 | − | − | − | − | 7.3, 7.5 |
| Proteus vulgaris 1699 | − | − | − | − | 7.3 |
| Proteus vulgaris 1765 | − | − | − | − | 6.6 |
| Serratia marcescens 91 | − | − | − | − | 6.1 |
| Serratia marcescens 167 | − | − | − | − | 6.1, 6.2 |
| Serratia rubidaea 3139 | + | + | − | − | 5.3, 5.4, 6.7, 7.4 |
| Serratia rubidaea 3134 | + | + | − | − | 5.4, 5.5, 6.7, 7.4 |
−, negative test result; +, positive test result.
DISCUSSION
Broad-spectrum β-lactam resistance among gram-negative pathogens is associated with increased mortality, increased length of hospitalization, and elevated medical costs (7). For E. coli and Klebsiella spp., a primary cause of such resistance is the presence of broad-spectrum β-lactamases, including ESBLs. Because of the importance of ESBLs in managing anti-infective therapy and the inability of some susceptibility testing methods to detect ESBL-mediated resistance, the NCCLS developed screening and confirmation methods for ESBLs (16). NCCLS guidelines have proven useful for K. pneumoniae (23), although shortcomings have been observed for E. coli (24).
The NCCLS guidelines do not apply, however, to isolates of Enterobacteriaceae other than E. coli, K. pneumoniae, and K. oxytoca, even though several other species may carry ESBLs (8, 18, 20). Thus, the question of whether to apply NCCLS guidelines to other members of the family Enterobacteriaceae has been looming for several years (8, 25).
We applied the NCCLS ESBL procedures to a nationwide cohort of non-E. coli, non-K. pneumoniae, and non-K. oxytoca hospital isolates of Enterobacteriaceae. While slightly more than one-half of the isolates tested positive for ESBL activity on the initial screen, only 2.2% had positive results on confirmatory testing. Since three of the isolates were already classified as resistant to the extended-spectrum cephalosporins and aztreonam, categorical interpretations would have changed (i.e., from susceptible or intermediate to resistant) for only 1.7% of the isolates examined.
Among the 340 isolates that were ESBL screen test positive but did not show a CA effect, approximately 80% had a β-lactam resistance profile consistent with AmpC enzymes (i.e., high cephalosporin and cefoxitin MICs and low cefepime MICs). It is possible that the presence of an AmpC β-lactamase may have masked the production of an ESBL in these isolates, although the low cefepime MICs argue against the presence of several types of ESBLs, such as CTX-Ms and some TEMs and SHVs, since they have activity against cefepime (11, 26). In a previous study with K. pneumoniae (23), testing cefepime with and without CA had limited utility in identifying ESBLs in isolates that also contained an AmpC β-lactamase. However, this approach may deserve further investigation.
With regard to the isolates that were ESBL confirmation test positive but for which no mechanism could be discerned, there are several β-lactamases that may be present. For example, the Form I and Form II enzymes of Citrobacter diversus (5) are broad-spectrum β-lactamases of chromosomal origin that are inhibited by CA but not formally classified as ESBLs. The plasmid-mediated OHIO-1 enzyme of S. marcescens and Enterobacter cloacae (class 2b) and the chromosomal and plasmid-mediated FPM-1 genes of Proteus spp. (class 2e) are other examples of β-lactamases that are inhibited by CA (5). Some of the group 1 plasmid-encoded AmpC enzymes, such as FEC-1 and MOX-1, are also inhibited by CA (11) and could be responsible for the resistance profiles exhibited by some of our study isolates. Lack of defined PCR primer sets and our inability to access the necessary control strains prevented us from testing our isolates for the genes encoding these β-lactamases.
In addition to group 1 β-lactamases, changes in porin profiles could also lead to false-positive screening tests using NCCLS methods for ESBL detection. Rasheed et al. have described a porin change that combined with a non-ESBL β-lactamase to produce a false-positive ESBL phenotype (21). Conversely, it is conceivable that porin changes may mask a CA effect in an ESBL-producing strain by failing to allow the entrance of sufficient quantities of cephalosporin into the bacteria for the effect to be evident (17).
In summary, our study demonstrates that ESBLs are detected infrequently in non-E. coli, non-K. pneumoniae, and non-K. oxytoca isolates of Enterobacteriaceae from 53 U.S. hospitals. Moreover, NCCLS detection methods are based on a phenotypic profile that has the potential to yield false-positive and false-negative results among isolates in which other β-lactamases are common. Given the infrequency of a positive confirmation test, the labor-intensiveness and cost of the ESBL testing, and the pitfalls regarding sensitivity and specificity inherent in the test, evaluation of non-E. coli and non-Klebsiella spp. isolates by NCCLS ESBL detection methods should not be undertaken as a routine practice in the United States at this time.
Acknowledgments
We thank the microbiology personnel at the Project ICARE hospital laboratories for sending the isolates included in this study.
Phase 4 of Project ICARE is supported in part by unrestricted grants to the Rollins School of Public Health of Emory University by Abbott Laboratories (North Chicago, Ill.), Astra-Zeneca Pharmaceuticals (Wilmington, Del.), Pharmaceuticals Division, Bayer Corporation (West Haven, Conn.), Cubist Pharmaceuticals Inc. (Lexington, Mass.), Elan Pharmaceuticals (San Diego, Calif.), Pfizer Incorporated (New York, N.Y.), and Roche Laboratories (Nutley, N.J.). M.J.S. was funded in part through the Centers for Disease Control and Prevention Postdoctoral Fellowship Training Program in Infectious Diseases (grant number T01/CCU111438) and through a fellowship grant from the American Physicians Fellowship for Medicine in Israel.
REFERENCES
- 1.Bonnet, R., C. Dutour, J. L. M. Sampaio, C. Chanal, D. Sirot, R. Labia, C. De Champs, and J. Sirot. 2001. Novel cefotaximase (CTX-M-16) with increased catalytic efficiency due to substitution Asp-240→Gly. Antimicrob. Agents Chemother. 45:2269-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradford, P. A. 2001. Extended-spectrum β-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 14:933-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bush, K. 2001. New β-lactamases in gram-negative bacteria: diversity and impact on the selection of antimicrobial therapy. Clin. Infect. Dis. 32:1085-1089. [DOI] [PubMed] [Google Scholar]
- 4.Bush, K., and S. B. Singer. 1989. Effective cooling allows sonication to be used for liberation of β-lactamases from Gram-negative bacteria. J. Antimicrob. Chemother. 24:82-84. [DOI] [PubMed] [Google Scholar]
- 5.Bush, K., G. A. Jacoby, and A. A. Medeiros. 1995. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 39:1211-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coque, T. M., A. Oliver, J. C. Pérez-Díaz, F. Baquero, and R. Cantón. 2002. Genes encoding TEM-4, SHV-2, and CTX-M-10 extended-spectrum β-lactamases are carried by multiple Klebsiella pneumoniae clones in a single hospital (Madrid, 1989 to 2000). Antimicrob. Agents Chemother. 46:500-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cosgrove, S. E., K. S. Kaye, G. M. Eliopoulos, and Y. Carmeli. 2002. Health and economic outcomes of the emergence of third-generation cephalosporin resistance in Enterobacter species. Arch. Intern. Med. 162:185-190. [DOI] [PubMed] [Google Scholar]
- 8.Coudron, P. E., E. S. Moland, and C. C. Sanders. 1997. Occurrence and detection of extended-spectrum β-lactamases in members of the family Enterobacteriaceae at a veterans medical center: seek and you may find. J. Clin. Microbiol. 35:2593-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Essack, S. Y. 2000. Laboratory detection of extended-spectrum β-lactamases (ESBLs)—the need for a reliable, reproducible method. Diagn. Microbiol. Infect. Dis. 37:293-295. [DOI] [PubMed] [Google Scholar]
- 10.Knothe, H., P. Shah, V. Krcmery, M. Antal, and S. Mitsuhashi. 1983. Transferable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates of Klebsiella pneumoniae and Serratia marcescens. Infection 11:315-317. [DOI] [PubMed] [Google Scholar]
- 11.Livermore, D. M. 1995. β-Lactamases in laboratory and clinical resistance. Clin. Microbiol. Rev. 8:557-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mabilat, C., and S. Goussard. 1993. PCR detection and identification of genes for extended-spectrum β-lactamases, p. 553-559. In D. H. Persing, T. F. Smith, F. C. Tenover, and T. J. White (ed.), Diagnostic molecular microbiology: principles and applications. American Society for Microbiology, Washington, D.C.
- 13.Matthew, M., A. M. Harris, M. J. Marshall, and G. W. Ross. 1975. The use of analytical isoelectric focusing for detection and identification of β-lactamases. J. Gen. Microbiol. 88:169-178. [DOI] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. 2000. Performance standards for antimicrobial disk susceptibility tests, 7th ed. Approved standard M2-A7. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 15. National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 16. National Committee for Clinical Laboratory Standards. 2002. Performance standards for antimicrobial susceptibility testing. Twelfth informational supplement M100-S12. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 17.Pangon, B., C. Bizet, A. Buré, F. Pichon, A. Philippon, B. Regnier, and L. Gutmann. 1989. In vivo selection of a cephamycin-resistant, porin-deficient mutant of Klebsiella pneumoniae producing a TEM-3 β-lactamase. J. Infect. Dis. 159:1005-1006. [DOI] [PubMed] [Google Scholar]
- 18.Perilli, M., A. Felici, N. Franceschini, A. De Santis, L. Pagani, F. Luzzaro, A. Oratore, G. M. Rossolini, J. R. Knox, and G. Amicosante. 1997. Characterization of a new TEM-derived β-lactamase produced in a Serratia marcescens strain. Antimicrob. Agents Chemother. 41:2374-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petroni, A., A. Corso, R. Melano, M. L. Cacace, A. M. Bru, A. Rossi, and M. Galas. 2002. Plasmidic extended-spectrum β-lactamases in Vibrio cholerae 01 El Tor isolates in Argentina. Antimicrob. Agents Chemother. 46:1462-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pitout, J. D. D., K. S. Thomson, N. D. Hanson, A. F. Ehrhardt, E. S. Moland, and C. C. Sanders. 1998. β-Lactamases responsible for resistance to expanded-spectrum cephalosporins in Klebsiella pneumoniae, Escherichia coli, and Proteus mirabilis isolates recovered in South Africa. Antimicrob. Agents Chemother. 42:1350-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rasheed, J. K., C. Jay, B. Metchock, F. Berkowitz, L. Weigel, J. Crellin, C. Steward, B. Hill, A. A. Medeiros, and F. C. Tenover. 1997. Evolution of extended-spectrum β-lactam resistance (SHV-8) in a strain of Escherichia coli during multiple episodes of bacteremia. Antimicrob. Agents Chemother. 41:647-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabaté, M., R. Tarragó, F. Navarro, E. Miró, C. Vergés, J. Barbé, and G. Prats. 2000. Cloning and sequence of the gene encoding a novel cefotaxime-hydrolyzing β-lactamase (CTX-M-9) from Escherichia coli in Spain. Antimicrob. Agents Chemother. 44:1970-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steward, C. D., J. K. Rasheed, S. K. Hubert, J. W. Biddle, P. M. Raney, G. J. Anderson, P. P. Williams, K. L. Brittain, A. Oliver, J. E. McGowan, Jr., and F. C. Tenover. 2001. Characterization of clinical isolates of Klebsiella pneumoniae from 19 laboratories using the National Committee for Clinical Laboratory Standards extended-spectrum β-lactamase detection methods. J. Clin. Microbiol. 39:2864-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tenover, F. C., P. M. Raney, P. P. Williams, J. K. Rasheed, J. W. Biddle, A. Oliver, S. K. Fridkin, L. Jevitt, and J. E. McGowan, Jr. 2003. Evaluation of the NCCLS extended-spectrum β-lactamase confirmation methods for Escherichia coli with isolates collected during Project ICARE. J. Clin. Microbiol. 41:3142-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomson, K. S. 2001. Controversies about extended-spectrum and AmpC beta-lactamases. Emerg. Infect. Dis. 7:333-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu, W. L., M. A. Pfaller, P. L. Winokur, and R. N. Jones. 2002. Cefepime MIC as a predictor of the extended-spectrum β-lactamase type in Klebsiella pneumoniae, Taiwan. Emerg. Infect. Dis. 8:522-524. [DOI] [PMC free article] [PubMed] [Google Scholar]