Abstract
Tendon and ligament injuries are very common, requiring some 200,000 reconstructions per year in the USA. Autografting can be used to repair these but donor tissue is limited and harvesting leads to morbidity at the graft sites. Tissue engineering has been used to grow simple tissues such as skin, cartilage and bone and due to their low vascularity and simple structure, tendons should be ideal candidates for such an approach. Scaffolds are essential for tissue engineering as they provide structure and signals that regulate growth. However, they present a physical barrier to cell seeding with the majority of the cells congregating at the scaffold surface. To address this we used centrifugation to enhance penetration of tendon-derived cells to the centres of 3-D scaffolds. The process had no apparent deleterious effects on the cells and both plating efficiency and cell distribution improved. After attachment the cells continued to proliferate and deposit a collagenous matrix. Scaffold penetration was investigated using layers of Azowipes allowing the separation and examination of individual leaves. At relatively low g-forces, cells penetrated a stack of 6 Azowipes leaving cells attached to each leaf. These data suggest that cytocentrifugation improves the penetration and homogeneity of tendon derived cells in 3-D and monolayer cultures.
Keywords: Tissue engineering, Cytocentrifugation, Scaffold, Tendon, Tenocytes, Cell culture
Introduction
It is widely accepted that there is currently a chronic shortage of donated organs for transplantation leading to unnecessary morbidity and loss of life. Furthermore, as life expectancy continues to increase, it is likely that this situation will only become worse. Tissue engineering may provide one solution to this pressing need and in particular, it has been suggested that tissue engineering may be a solution to age-related organ failure (Risbud 2001). Tissue engineering is often described as “an interdisciplinary field that applies the principles of engineering and life sciences toward the development of biological substitutes that restore, maintain, or improve tissue function or a whole organ” (Langer and Vacanti 1993). A typical scenario is that cells are isolated from a patient’s own tissues, expanded in vitro, seeded onto a biocompatible scaffold and then re-implanted into the body. A number of tissue engineered products have now been used successfully in the clinic, most notably bladder (Atala et al. 2006), skin (Zhu et al. 2005) and cartilage (Hollander et al. 2006) demonstrating the validity of tissue engineering as a discipline. Thus far, tissue engineering has been found to work best on tissues with relatively simple structure and when the defect to be repaired is small and where there is relatively little or no need for angiogenesis and enervation within the scaffold. Therefore, due to their low vascularity and simple hierarchical structure, tendons and ligaments should theoretically lend themselves well to this form of repair technology (Calve et al. 2004).
Tissue engineered tendons or ligaments would be highly desirable. Tendon and ligament injuries are very common, requiring surgical repairs to the rotator cuff (51,000 per annum), Achilles tendon (44,000 per annum), patellar tendon (42,000 per annum) and anterior cruciate ligaments (ACL; 50,000 per annum) in the USA (Butler et al. 2004). For ACL repair, typically autografts from the patellar or semitendinous tendon are used however, there is a limited source of donor tissue and the operation leads to pain and morbidity at both the donor and graft site. A number of synthetic or semi-synthetic tendons have been used including Gore-Tex (Giorgetti et al. 2001), Dacron (Nau et al. 2002), carbon fibres and polypropylene (Silver et al. 1991). However, despite excellent initial clinical results their long-term durability is poor and they have been largely abandoned. This lack of success is probably due to the failure to re-create the cellular architecture present in healthy tendon tissue and autografts. This has led to the use of scaffolds that support the growth and development of an appropriate cell system—mainly collagen with or without artificial cross-linking (Koob 2002). A common approach has been to use tendon cells seeded in collagen gels which have then been exposed to cyclic uniaxial strain. This gives rise to constructs that are histologically similar to tendons complete with endotenon and that express a number of genes/proteins typical of tendons (collagen genes I, III, and XII, aggrecan, fibronectin, prolyl hydroxylase, and tenascin) (Garvin et al. 2003). A number of groups have used mesenchymal stem cells (MSC) to generate tissue engineered tendon constructs. MSC are attractive for tissue engineering purposes as they are multipotential and proliferate extensively in culture. MSC grown in culture can differentiate bone, cartilage, fat, tendon and muscle lineages (Pittenger et al. 1999). Tissue engineered preparations derived from MSC have been used successfully to regenerate bone defects in vivo in animals and humans (Petite and Hannouche 2002) and more recently, MSC seeded in either collagen gels or Poly-lactide-co-glycolide scaffolds have been used to generate tendon-like constructs that were then used to repair Achilles and patellar tendon defects in rabbits (Juncosa-Melvin et al. 2006a, b; Ouyang et al. 2003).
Despite being central to the entire tissue engineering process, the successful seeding of donor cells onto scaffolds has received little attention. The cells are required to retain their viability and to be evenly distributed throughout the scaffold and if this is not achieved and then the functional integrity of the tissue engineered construct will be compromised. Historically, the successful coupling of cells to a polymeric scaffold suitable for direct implantation into the body has posed a considerable problem. The scaffold is essential as it mimics the extracellular matrix by providing structural components that can physically support tissue regeneration and chemical signals that direct the proliferation and differentiation of the seeded cells. To be used reliably and successfully in a clinical setting, not only do the most appropriate scaffold materials need to be selected, but also cell seeding and cultivation must be optimised in vitro (Xiao et al. 1999). Although this might seem trivial, the three dimensional nature of the scaffold material presents a physical barrier to the internal cell seeding and typically the majority of the cells will be concentrated at the surface of the constructs with relatively few cells penetrating to the centre (Ohyabu et al. 2009; Dunn et al. 2006; Lee et al. 2008). In the past, gravitational forces have been used to seed cells onto a membrane or into a three-dimensional scaffold. Although this is a cell-friendly procedure, the lack of positive pressure can result in variable cell attachment and a lack of penetration to the centre of the scaffold.
As cytocentrifugation is commonly used for the rapid attachment of cells to microscope slides for fixation and histological analysis, we thought that this might be a possible method to apply a directional force to living cells in vitro and thereby facilitate cell attachment. We hypothesised therefore that the use of cytocentrifugation as a method of attaching cells to membranes or scaffolds would enable more reliable cell seeding to a greater depth of penetration and enable a known quantity of cells to be seeded to a predetermined surface area.
Materials and methods
Reagents and consumables
Unless stated otherwise, all chemicals were purchased from Sigma–Aldrich (Poole, Dorset UK), tissue culture media from Invitrogen (Paisley, UK) and plasticware from Nunc (Nottingham, UK), or Greiner Bio One (Gloucester, UK) and used as supplied.
Tissue samples and cell isolation
Rat tendon samples were collected from male Wistar rats (200–250 g) that were killed according to Home Office regulations by a schedule 1 method. The tails and legs were removed and the tail tendon fascicles and Achilles and patella tendons were dissected from the surrounding tissues under aseptic conditions. The tendons were rinsed in Dulbecco’s Modified Eagle’s Medium supplemented with 10% newborn calf serum, penicillin/streptomycin and Ultraglutamine (DMEM), and then diced into small pieces and digested in filter sterilised DMEM containing crude collagenase (Sigma Cat. No. C0130, 1 mg/mL). The samples were incubated for 18 h at 37 °C with gentle shaking, after which time the majority of the collagen in the sample was digested and the cells freed into the medium. Following digestion, the sample was suspended in medium, filtered through a 70 micron sieve, washed and assessed for cell number. The cells were then either used directly as primary tenocytes or cultured in 75 cm2 flasks and used as first passage (i.e. secondary) tenocytes.
Cytocentrifugation equipment
The cells were cytocentrifuged onto the membranes or tissue culture scaffolds using a Hettich Rotofix 32 benchtop centrifuge adapted for cytocentrifugation using Hettich Cyto-System chambers. All the cytocentrifugation equipment and the surfaces that the cells were required to be spun onto, were first sterilised using 70% industrial methylated spirits. The surface was placed onto the glass microscope slide and then covered with filter card with a disc removed for cell attachment. The cyto-insert funnel was placed over the surface with the mounted adaptor ring screwed firmly into place (Fig. 1a). The slide chamber was placed in the centrifuge and the cells were loaded into the chamber by pipetting 500 μL of cell suspension containing 105 tenocytes into the funnel (Fig. 1b) and centrifuged for 5 min at 64 g (Fig. 1c). Once spun down, the entire Cyto-system chamber was removed intact from the centrifuge and incubated overnight at 37 °C at 5% CO2 to allow the cells to attach. After this overnight incubation, the medium was aspirated and the funnels removed. The surfaces with the cells attached were then transferred to 6 well plates and covered with fresh DMEM, before being returned to the incubator for the duration of the culture period. In parallel to the above, controls were performed in which cells were allowed to settle under the influence of gravity alone but otherwise were treated exactly the same as the cytocentrifuged cells.
Fig. 1.
Loading of cells into cytocentrifuge apparatus. The cytocentrifuge funnel was placed over the material to be seeded and locked into place (a). The cells were then loaded into the chamber (b) and spun down at 64 g for 5 min (c)
Preparation of cell substrates
Monolayer cultures of cells were grown on commercially available 50 μm Polylactic acid (PLA) sheets (average MW 80–100,000; Sidaplax Earthfirst®, Northampton). Before use, the sheets were coated with rat tail collagen dissolved in 0.05 M acetic acid at a density of 10 μg/cm2. Azowipes™ are commercially available nonwoven viscose rayon tissues that can be conveniently adapted for use as a model 3-D scaffold (Sun et al. 2006, 2005). The sheets have a pore size of 80 μm and are 200 μm thick allowing the fabrication of constructs of known thickness by simply stacking the sheets. Before use, the Azowipe™ sheets were exhaustively washed in cell culture medium to remove all traces of alcohol.
Cell analysis
Total cell number
Total cell number was determined by staining with methylene blue which binds stoichiometrically to basic histone proteins in the nuclei thus staining all cells present in the culture (Currie 1981). The medium was removed, the plates washed with phosphate buffered saline (PBS) and then fixed with 70% ethanol for 5 min. After fixation, the plates were washed three times with 10 mM borate buffer pH 8.8. Following this, the cultures were stained with methylene blue (1 mg/mL in borate buffer) for 30 min with gentle agitation. After staining, the dye was removed and the plates were again washed three times with borate buffer to remove any excess dye. The cells were then examined microscopically and if necessary total cell number determined by eluting with 1% HCl in ethanol and measuring the absorbance at 630 nm (Currie 1981).
Collagen deposition
Collagen synthesis and accumulation was demonstrated by staining the cultures with Sirius Red in the presence of saturated picric acid. This assay’s specificity for collagen accumulation is based on the molecules linear nature promoting the binding of its six sulphonic acid groups to the basic amino groups present in collagen. By staining in the presence of saturated picric acid largely eliminates the non-specific binding of this dye to other basic proteins, although the reason for this is unclear (Lopez-De Leon and Rojkind 1985). At the end of the culture period, the wells were washed with PBS and fixed with 70% industrial methylated spirits for 5 min. After fixation, the cultures were washed three times with water, and then stained with a 1 mg/mL solution of Sirius Red in saturated picric acid at room temperature for 30 min with agitation. After this time the dye was removed and the plates washed three times with tap water to remove the unbound dye.
Proliferation
Proliferation was determined by the incorporation of 5-ethynyl-2′-deoxyuridine (EdU) into native DNA. EdU (Invitrogen) was added at a final concentration of 50 μM to cytocentrifuged or gravity-driven cultures of tenocytes attached to collagen coated PLA in DMEM and incubated for 4 h. The cells were washed and fixed with 70% ethanol and then rinsed in Tris-buffered saline TBS. The EdU incorporated into the monolayers was then visualized by staining with 50 μM Alexa Fluor 488 (Invitrogen) for 30 min in 100 mM Tris pH 8.5, 1 mM CuSO4 and 50 mM ascorbic acid. Cells were subsequently washed in TBS and imaged using a dipping lens Zeiss LSM510 NLO upright microscope with an Argon laser to excite the Alexa Fluor at 488 nm.
Data handling and statistical analyses
Data are presented as group means ± standard deviations. At least three replicates of each experiment were performed, and the results presented are representative of these. Where two groups were present, statistical significance was determined using a two-tailed T test. For multiple groups, effects across treatment groups were compared by one-way analysis of variance (ANOVA) using Sigmaplot 11 software. If the overall difference was significant, multiple comparisons were performed between groups using an appropriate ad hoc test. Differences are considered significant at a probability of <0.05 on a two tailed test.
Results
Rat tail tendon cells cytocentrifuged onto tissue culture plastic
Initial studies to determine whether the cells could survive the considerable forces exerted during the process were carried out by cytocentrifuging primary tenocytes for 5 min onto standard tissue culture plastic. 24 h after cytocentrifugation, it was found that the tenocytes had survived the cytocentrifugation process, attaching to the tissue culture plastic, spreading and adopting a fibroblastic morphology typical of tenocytes in culture (Fig. 2). Plating efficiency, defined as the proportion of cells adhering to the matrix 24 h after plating, was significantly increased in the cytocentrifuged cultures by almost twofold, increasing from 44% in the gravity driven cultures to 85% in the cytocentrifuged cultures (data not shown) and, as might be expected, varying the surface area of the funnel altered the resultant cell density with larger funnels producing lower cell densities (Fig. 2).
Fig. 2.
Primary tenocytes deposited onto tissue culture plastic after cytocentrifugation—a 105 cells seeded into a 3 mm funnel b 105 cells seeded into a 5 mm funnel
Rat tenocytes cytocentrifuged onto collagen-coated polylactic acid
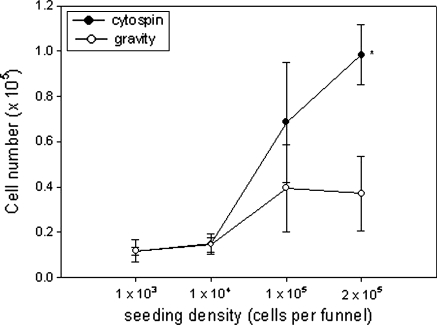
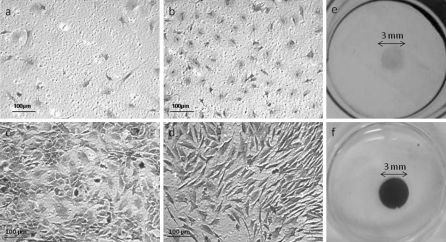
Because of their biocompatibility and biodegradability, a large proportion of tissue engineering scaffolds are synthesised from polylactic acid (PLA), polyglycolic acid or mixtures of the two (Athanasiou et al. 1998; Zwingmann et al. 2007; Liu et al. 2010). Furthermore, due to its inherent flexibility, PLA membrane lends itself to the study of biomechanical effects on cell growth. However, despite the widespread use of these polymers in tissue engineering, it was found that the cells did not readily attach to PLA membranes in monolayer culture. This was improved to some extent by coating the PLA membranes with collagen although plating efficiency was still low. By applying the secondary tenocytes under centrifugal force, the cells rapidly adhered to the membranes with high efficiency and remained attached (Fig. 3). Figure 4 shows microscopic views of secondary rat Achilles and patella tendon cells seeded under gravity driven conditions (Fig. 4a–d). Also shown are macroscopic views of the Achilles tendon cell cultures (Fig. 4e, f). Together these data clearly demonstrate that the cells attached to the membranes at high density and in a uniform manner and that by using cytocentrifugation, the number of cells attaching is significantly increased.
Fig. 3.
A comparison of cell seeding by cytocentrifugation as compared to gravity. Secondary tenocytes were seeded onto collagen coated PLA at increasing densities, allowed to adhere and spread for 24 h and then cell number determined using the methylene blue assay. Data are presented as mean ± SD. *Denotes significance p < 0.05
Fig. 4.
Photomicrographs of secondary tenocytes attached to collagen coated PLA after attachment under gravity and cytocentrifugation—105 cells were seeded into a 3 mm funnel and allowed to attach either under the influence of gravity a patella tendon cells, b Achilles tendon cells or by cytocentrifugation c patella tendon cells, d Achilles tendon cells. Macroscopic views of secondary Achilles tendon cells seeded by e gravity or f cytocentrifugation
It was found that cytocentrifugation significantly increased plating efficiency of primary tenocytes from ~40 to 88% with some individual cultures showing efficiencies approaching 100% (Fig. 5a). In addition to this, cytocentrifugation reduced variability in the distribution of cells after plating out. By dividing the culture surface into grids, cell distribution could be determined and the Coefficient of Variation calculated. It was found that after cytocentrifugation both the magnitude and range of the grid standard deviation was reduced significantly (by ~60%) (Fig. 5b) and the mean coefficient of Variation reduced by ~70% (Fig. 5c).
Fig. 5.
Effect of cytocentrifugation on primary tenocytes plating efficiency and distribution variability. After plating out, the cultures were divided into grids and the total cells and numbers of cells in each square determined. From this the plating efficiency (a) the intra-grid standard deviation (b) and the mean grid coefficient of variation (c) were determined
Time course of attachment
It was expected that cells cytocentrifuged onto culture surfaces would attach and begin to grow considerably sooner than those allowed to attach under the influence of gravity alone and this was indeed the case. To characterise this further, secondary tendon cells were plated out either with or without cytocentrifugation, the cell supernatant removed after increasing periods of time and the cells were washed, fixed, stained and counted. It was found that the cytospin cells attached rapidly with the majority of the cells attaching during the initial 10 min cytospin period. Allowing the cells to settle further continued to increase the plating efficiency but only by a further 50%. In contrast, the gravity driven cells attached and spread at a much slower rate with less than 25% of cells attaching in the first 10 min after plating. The cells continued to attach over the next 2 h reaching plating almost as high as the cytocentrifuged cells (Fig. 6).
Fig. 6.
Time course of attachment of secondary tenocytes to PLA membrane. Cells were plated out either with or without cytocentrifugation, the cell supernatant removed after increasing periods of time and the cells were washed, fixed, stained and counted. ***denotes significance p < 0.001
Rat tail tendon cells cytocentrifuged onto 3-D Azowipe® scaffolds
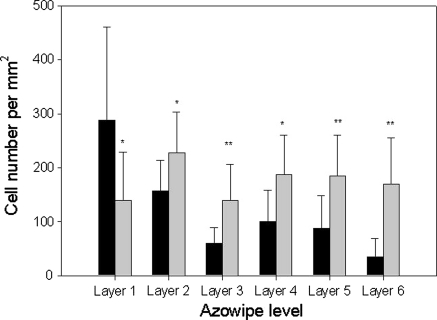
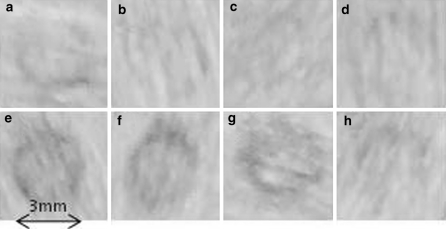
To generate a viable 3-D construct, cells need to be seeded onto a scaffold such that they reach and attach to the centre of the construct whilst remaining viable. Azowipes® are a commercially available nonwoven viscose rayon tissue bonded with a styrene butadiene copolymer. Normally these are impregnated with alcohol and used as bactericidal wipes, however, if the alcohol is removed by exhaustive washing they can be used without further treatment to support fibroblastic cell growth and, if used as a stack, can be easily used to demonstrate penetration through a 3-D scaffold by separating and examining the leaves individually (Sun et al. 2006, 2005). Furthermore, the Azowipes® are 200 μ thick and scaffolds of known thickness can easily be fabricated by stacking the Azowipes®. By using the Azowipes® combined in layers as a model, cells could be seeded onto the scaffold and the sheets could subsequently be separated easily for analysis. These cultures were stained with Sirius Red (i.e. for collagen production) instead of methylene blue as, in this case, the methylene blue stains the entire scaffold material rendering the cells invisible. As shown in Fig. 7, cytocentrifuged primary tendon cells penetrated and attached through to the sixth layer of the Azowipes®, and a clear ring of cells is evident on each of the layers. Cell distribution on the Azowipes was determined by dividing the individual sheets into 10 grids and determining the cell density in each of the grids. It was found that in the gravity seeded constructs the majority of the cells were localised to the top layer and the number then progressively decreased from layer 1 through to 6. In contrast, the cytocentrifuged cells were distributed evenly between the individual layers (Fig. 8). ANOVA revealed no significant difference between the different layers of the cytocentrifuged Azowipes whereas a significant difference was found in the gravity seeded Azowipes (p ≤ 0.001). Furthermore, the mean Coefficient of Variation was reduced from 0.62 ± 0.2 for the gravity seeded cells to 0.45 ± 0.1 for the cytocentrifuged cells indicating a reduced grid to grid. i.e. lateral, variability. Microscopic analysis of the Azowipe layers revealed the cells to have become firmly associated with the individual fibres of the Azowipes; this being the case throughout the entire thickness of the construct (Fig. 9).
Fig. 7.
Depth of cell penetration after cytocentrifugation. A ring of tenocytes is evident on the 1st to 6th layers of Azowipe after staining with Sirius Red (labelled a–f respectively). This demonstrates adherence and growth after penetrating up to 3 mm of Azowipe after centrifugation
Fig. 8.
Cell distribution after seeding onto Azowipes by gravity (black bars) or cytocentrifugation (grey bars). Individual sheets were divided into grids and the cell density in each of the grids determined microscopically. In the gravity seeded constructs the majority of the cells were localised to the top layer with decreasing numbers thereafter. In contrast, the cytocentrifuged cells were distributed evenly between the individual layers. No significant difference was found between the cytocentrifuged Azowipes whereas a significant difference was found between the gravity seeded Azowipes (ANOVA, p ≤ 0.001). *Denote statistical significance from corresponding gravity control (*p < 0.05; **p < 0.01)
Fig. 9.
Multiphoton image of primary tenocytes attached to Azowipe fibres after cytocentrifugation
Viability of cytocentrifuged cells
It was apparent that collagen continued to be produced and deposited on the Azowipes when the cultures were allowed to continue thus confirming the viability of the cytocentrifuged cells. A comparison of cultures of primary tenocytes grown for 6 or 8 days on Azowipes shows that the cells remain viable on all layers and that more intense collagen staining is seen after the longer culture period, suggesting that the cells continue to produce extracellular matrix once spun onto the scaffold (Fig. 10). Furthermore, EdU staining of secondary tenocytes seeded onto PLA membranes confirmed that both gravity-seeded and cytocentrifuged cells continued to proliferate at similar rates with 14.7 ± 3.1% and 15.1 ± 1.6% of gravity or cytocentrifugation seeded cells undergoing cell division at any one time, respectively (Fig. 11).
Fig. 10.
Continued cell viability after cytocentrifugation. Tenocytes were seeded on to Azowipes as described in the text and then fixed and stained with Sirius Red after 6 or 8 days in culture. a–d Shows layers 2–5 respectively after 6 days in culture and e–h show layers 2–5 respectively after 8 days in culture. It was noted that more collagen was deposited after day 8 suggesting continued viability and collagen synthesis
Fig. 11.
Proliferation of tenocytes after cytocentrifugation. Tenocytes were seeded on to collagen coated PLA either by cytocentrifugation (d–f) or gravity alone (a–c) and then proliferation determined by EdU incorporation (a, d). The cells were also visualised by phase contrast microscopy (c, f) and after staining with DAPI (b, e). The scale bars (bottom/rightc, f) represent 20 μm
Discussion
In this manuscript we have shown that a commercially available cytospin apparatus can be used to facilitate the attachment of tendon-derived cells on to tissue culture plastic, PLA sheet and a 3-D cell culture scaffold. The cells attached with considerably higher efficiency and much more rapidly than when allowed to attach under the influence of gravity alone. After attachment the cells were viable and continued to grow and deposit collagen on the matrix for up to 8 days in culture. The cells were found to penetrate a 3-D matrix (Azowipe) effectively and continued to grow throughout the matrix. Taken together these data suggest that this might well be an appropriate method for the initiation of standard monolayer or 3-D cultures and might also be used to some advantage in more conventional cell culture models.
Experiments which use sufficiently high centrifugal force to separate cellular components based on their relative densities have been used in biological work since used by Gurwitsch in 1904 (Beams et al. 1960). These pioneering studies demonstrated that this displacement did not noticeably injure the cells or alter their normal development (Beams et al. 1960). Numerous studies have demonstrated that many cell types are sensitive to external mechanical stimulation, it is therefore intuitive, to assume that top–bottom axial stress exerted on cells during centrifugation also causes changes in cell behaviour. However, all cells are routinely subjected to centrifugal and shear stress daily during the course of normal cell culture manipulation. Li et al. have presented the most up-to-date research into the effects of centrifugal force on osteoblastic cells. They demonstrated that “usually-used” magnitudes of centrifugation caused a temporary and reversible change in cell proliferation and gene expression. After 24 h the cells had returned to their pre-centrifugation profiles and no lasting effect on proliferation or gene expression were seen (Li et al. 2009). Similarly, Godbey et al. (2004) found that centrifugation at high rotation speeds of around 670 g resulted in cell lysis whereas speeds of up to 280 g produced no lasting damage to the cells. Previously, this group has used centrifugal force to harvest bone marrow cells from rat long bones with no apparent loss of viability (Dobson et al. 1999). In the case of the work presented here, rotational speeds were kept to 64 g and so it would seem highly unlikely that the cells would be affected biologically or suffer any damage.
Dynamic seeding of cells is not a totally new concept; research by Xiao et al. (1999) supports our work by suggesting that dynamic seeding followed by static cultivation is an appropriate and efficient method of seeding human dermal fibroblasts, by optimising in-growth, homogenous attachment and extracellular matrix production. In their studies, dynamic seeding was achieved by the use of a non-heated magnetic stirrer. Similarly, Godbey et al. (2004) found that the use of centrifugal force via a novel rotor system resulted in superior seeding depths and densities without reducing cellular activity. However, the authors only considered the seeding efficiency over a period of 24 h and did not present data regarding the long-term viability of cultured cells after centrifugal seeding. Here we have shown that the cells do indeed continue to proliferate and synthesise matrix for up to 8 days following centrifugation. Perhaps related to this, Funatsu et al. used cytocentrifugation to seed hepatocytes into holofibres to form hepatocyte-organoids. These were then maintained as part of a continuous flow cultures system and retained viability and liver related function for up to 4 months (Funatsu et al. 2001). Similarly porcine chondrocytes were seeded centrifugally onto scaffolds to produce meniscus like constructs. This also improved cell penetration and preserved a chondrocytic phenotype (Weinand et al. 2009). A major benefit of using a modified Cytosystem is that medium continues to flow through the construct (preliminary results suggest ~1.5 mL/min) promoting cell penetration. In contrast, where a simple adaptation of a centrifuge tube is used this is not the case limiting penetration and, in the case of Weinand et al. (2009) the constructs were “flipped” to assist with penetration.
The successful generation of tissue engineered tendons will rely on the ability of tenocytes to adhere to a scaffold, proliferate, and finally organize the scaffold material or extracellular matrix molecules into a functional tendon. Unfortunately many scaffold materials do not support optimal cell adhesion and therefore need to be modified either by chemical derivatisation or by coating. Collagen coating has been used for many years to promote fibroblastic cell attachment to a variety of supports including titanium (Lowenberg et al. 1988), hydrogels (Toselli et al. 1984) and tissue culture plastic (Srivastava et al. 1990). Qin et al. (2005) suggested that the use of extracellular matrix components as coating proteins ‘could be used to promote cell adhesion and enhance (tissue engineering) scaffold performance’ and for this reason we looked at the viability of cells cytocentrifuged onto a collagen coated PLA membrane. Fibronectin and type I collagen are both major ECM components in tendons, mediating cell attachment and providing substrates for cell growth in regenerative tendons. The adhesion of tenocytes is determined by their interaction with the ECM and their expression of cell adhesion proteins. Therefore, the coating of tissue engineering scaffolds with ECM components aims at enhancing tenocyte adhesion and proliferation (Qin et al. 2005). Numerous studies have looked at the effect of different surface coatings for various cell types and this was not the purpose of the current study.
The use of cytocentrifugation dramatically improved the plating efficiency of tenocytes on PLA over and above the effects of collagen coating. This is obviously of major importance both in an academic or clinical setting as the accurate delivery of known numbers of cells is essential for both reproducible experimental procedures and the generation of clinical constructs. When transformed cell lines are used this is not normally an issue as seeding/plating efficiencies are usually very high. This is however, not the case for primary cells where, depending on the cell type and culture conditions, efficiencies of 50–60% are typical and lower efficiencies not unusual (Tanswell et al. 1991; Seo et al. 2002). With such low plating efficiencies, the number of seeded cells that actually adhere and proliferate under standard culture conditions can at best be estimated. For many applications, this is unimportant however, where absolute cell numbers are calculated, such as in the generation of growth curves, this may significantly affect the outcomes. By applying the cells using centrifugal force the plating efficiency was approximately doubled increasing from 40.2 to 78.9% with some cultures achieving close to 100%. Furthermore, the variability in overall plating efficiency was reduced by 36% and the homogeneity of cell distribution considerably improved with the coefficient of variation reduced by 67%. Taken together this would indicate that cytocentrifugation would improve the reliability and reproducibility of cell cultures by ensuring that a standardised number of cells initiating a cell culture and by ensuring that the spatial distribution of the cells is homogeneous across the culture vessel.
A major aim in the initiation of any 3-D cell culture or tissue engineered construct is the achievement of a uniform distribution of cells in all 3 dimensions. In the past this has presented major problems as cells have tended to be concentrated on the surface of scaffolds and penetration to the centre of the scaffold has been minimal. As tissue engineered constructs become larger this will become more and more of an issue. A number of approaches have been used to address this problem all with some degree of success. These include the use of magnetic particles (Shimizu et al. 2006, 2007), perfusion (Dai et al. 2009; Maidhof et al. 2010), acoustic waves (Bok et al. 2009; Li et al. 2007) and, similar to the approach of this laboratory, centrifugation (Godbey et al. 2004; Roh et al. 2007; Ng et al. 2010). All of these have demonstrated an improved ability to deliver cells to the centre of 3-D cell culture scaffolds and the choice of method would probably be dependent on other factors including cost, simplicity, selectivity and sterility. Although working on similar principles, the cytospin apparatus that we have chosen to use offers a number of advantages over the other centrifugal approaches described. The components are readily available and constructed to a high standard. The cytospin apparatus used in this investigation offers a number of distinct advantages chiefly in that the funnel used to load the cells is removable and comes in a variety of sizes. This enables the easy removal of scaffolds after cell seeding and some degree of control of seeding density and seeding area. Furthermore, the ease of assembly and disassembly helps to maintain sterility of the construct.
The use of cytocentrifugation as a method of cell seeding may also offer some benefits in conventional monolayer cultures. Although traditional monolayer cell culture has been used successfully for many decades and generated much useful data, there are obvious shortcomings. In the case of tendon cells, the rapid proliferation seen in vitro is in stark contrast to the low proliferative (~5%) index seen under normal physiological situations in vivo (Chuen et al. 2004; Lui et al. 2007; Scott et al. 2007; Wu et al. 2010). This may be due to number of factors such as the lack of a matrix/scaffold or quite simply the effect of two-dimensional growth. It is well established that monolayer culture has a profound effect on cell growth that can to some extent be reversed by adopting 3-D culture models (LaRue et al. 2004; Mueller-Klieser 1987). Similarly, preliminary investigations in this laboratory show that monolayer cultures of tendon cells express different cell surface markers compared to those seen in vivo. Another possibility is the inherent selectivity of adherent rapidly proliferating cells in standard monolayer cultures. It is well known in other tissues such as bone marrow that non-adherent cells are necessary for the correct function of the mesenchymal-derived cells (Scutt and Bertram 1995; Scutt et al. 1995; Friedenstein et al. 1992; Rickard et al. 1995). By inducing the cells to adhere by cytocentrifugation this possible culture artefact can be avoided and the behaviour of either adherent cells or adherent plus non-adherent cells observed.
As long as experiments are performed at a small scale (1 cm3 or smaller) existing equipment can be easily adapted thus minimising costs. It would be expected however, that for large animal or clinic purposes where tissue engineered constructs with dimensions of 2.5–10 cm or more, a suitable custom built apparatus would need to be fabricated. The challenge here would be that cytocentrifugation combines both centrifugation and fluid flow, as the culture medium flows through the construct and the filter to be deposited in the bucket. The centrifugation aspect of this presents few difficulties as centrifugation in litre quantities is relatively commonplace. However, a mechanism would need to be designed allowing the passage culture medium through the construct at a controlled rate. If this could be overcome, the system would present a major advantage over more conventional fluid flow devices as the entire system is self-contained enabling easy and efficient sterilisation. The system can be assembled and sealed under sterile conditions and then run with minimal risk of contamination. It should be noted however that upscaling would require considerable optimisation with regards to the parameters described above. In particular, it is likely that larger rotors will be used and therefore slower speeds will be necessary to avoid excessive g-forces. Furthermore, seeding onto a 2–5 mm scaffold requires up to 30 min centrifugation. Obviously larger constructs will require considerably longer and conditions will need to be carefully optimised to reduce this to a minimum whilst retaining the viability of cells.
References
- Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- Athanasiou KA, Agrawal CM, Barber FA, Burkhart SS. Orthopaedic applications for PLA-PGA biodegradable polymers. Arthroscopy. 1998;14:726–737. doi: 10.1016/S0749-8063(98)70099-4. [DOI] [PubMed] [Google Scholar]
- Beams HW, Tahmisian TN, Anderson E, Devine R. Studies on the fine structure of ultracentrifuged spinal ganglion cells. J Biophys Biochem Cytol. 1960;8:793–811. doi: 10.1083/jcb.8.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok M, Li H, Yeo LY, Friend JR. The dynamics of surface acoustic wave-driven scaffold cell seeding. Biotechnol Bioeng. 2009;103:387–401. doi: 10.1002/bit.22243. [DOI] [PubMed] [Google Scholar]
- Butler DL, Juncosa N, Dressler MR. Functional efficacy of tendon repair processes. Annu Rev Biomed Eng. 2004;6:303–329. doi: 10.1146/annurev.bioeng.6.040803.140240. [DOI] [PubMed] [Google Scholar]
- Calve S, Dennis RG, Kosnik PE, 2nd, Baar K, Grosh K, Arruda EM. Engineering of functional tendon. Tissue Eng. 2004;10:755–761. doi: 10.1089/1076327041348464. [DOI] [PubMed] [Google Scholar]
- Chuen FS, Chuk CY, Ping WY, Nar WW, Kim HL, Ming CK. Immunohistochemical characterization of cells in adult human patellar tendons. J Histochem Cytochem. 2004;52:1151–1157. doi: 10.1369/jhc.3A6232.2004. [DOI] [PubMed] [Google Scholar]
- Currie GA. Platelet-derived growth-factor requirements for in vitro proliferation of normal and malignant mesenchymal cells. Br J Cancer. 1981;43:335–343. doi: 10.1038/bjc.1981.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W, Dong J, Chen G, Uemura T. Application of low-pressure cell seeding system in tissue engineering. Biosci Trends. 2009;3:216–219. [PubMed] [Google Scholar]
- Dobson KR, Reading L, Haberey M, Marine X, Scutt A. Centrifugal isolation of bone marrow from bone: an improved method for the recovery and quantitation of bone marrow osteoprogenitor cells from rat tibiae and femurae. Calcif Tissue Int. 1999;65:411–413. doi: 10.1007/s002239900723. [DOI] [PubMed] [Google Scholar]
- Dunn JC, Chan WY, Cristini V, Kim JS, Lowengrub J, Singh S, Wu BM. Analysis of cell growth in three-dimensional scaffolds. Tissue Eng. 2006;12:705–716. doi: 10.1089/ten.2006.12.705. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Latzinik NV, Gorskaya Yu F, Luria EA, Moskvina IL. Bone marrow stromal colony formation requires stimulation by haemopoietic cells. Bone Miner. 1992;18:199–213. doi: 10.1016/0169-6009(92)90807-P. [DOI] [PubMed] [Google Scholar]
- Funatsu K, Ijima H, Nakazawa K, Yamashita Y, Shimada M, Sugimachi K. Hybrid artificial liver using hepatocyte organoid culture. Artif Organs. 2001;25:194–200. doi: 10.1046/j.1525-1594.2001.025003194.x. [DOI] [PubMed] [Google Scholar]
- Garvin J, Qi J, Maloney M, Banes AJ. Novel system for engineering bioartificial tendons and application of mechanical load. Tissue Eng. 2003;9:967–979. doi: 10.1089/107632703322495619. [DOI] [PubMed] [Google Scholar]
- Giorgetti M, Giannessi E, Ricciardi MP. Expanded polytetrafluoroethylene as tendon replacement: an experimental study in chickens. Scand J Plast Reconstr Surg Hand Surg. 2001;35:23–27. doi: 10.1080/02844310151032493. [DOI] [PubMed] [Google Scholar]
- Godbey WT, Hindy SB, Sherman ME, Atala A. A novel use of centrifugal force for cell seeding into porous scaffolds. Biomaterials. 2004;25:2799–2805. doi: 10.1016/j.biomaterials.2003.09.056. [DOI] [PubMed] [Google Scholar]
- Hollander AP, Dickinson SC, Sims TJ, Brun P, Cortivo R, Kon E, Marcacci M, Zanasi S, Borrione A, Luca C, Pavesio A, Soranzo C, Abatangelo G. Maturation of tissue engineered cartilage implanted in injured and osteoarthritic human knees. Tissue Eng. 2006;12:1787–1798. doi: 10.1089/ten.2006.12.1787. [DOI] [PubMed] [Google Scholar]
- Juncosa-Melvin N, Boivin GP, Galloway MT, Gooch C, West JR, Butler DL. Effects of cell-to-collagen ratio in stem cell-seeded constructs for Achilles tendon repair. Tissue Eng. 2006;12:681–689. doi: 10.1089/ten.2006.12.681. [DOI] [PubMed] [Google Scholar]
- Juncosa-Melvin N, Boivin GP, Gooch C, Galloway MT, West JR, Dunn MG, Butler DL. The effect of autologous mesenchymal stem cells on the biomechanics and histology of gel-collagen sponge constructs used for rabbit patellar tendon repair. Tissue Eng. 2006;12:369–379. doi: 10.1089/ten.2006.12.369. [DOI] [PubMed] [Google Scholar]
- Koob TJ. Biomimetic approaches to tendon repair. Comp Biochem Physiol . 2002;133:1171–1192. doi: 10.1016/S1095-6433(02)00247-7. [DOI] [PubMed] [Google Scholar]
- Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- LaRue KE, Khalil M, Freyer JP. Microenvironmental regulation of proliferation in multicellular spheroids is mediated through differential expression of cyclin-dependent kinase inhibitors. Cancer Res. 2004;64:1621–1631. doi: 10.1158/0008-5472.CAN-2902-2. [DOI] [PubMed] [Google Scholar]
- Lee M, Wu BM, Dunn JC. Effect of scaffold architecture and pore size on smooth muscle cell growth. J Biomed Mater Res A. 2008;87:1010–1016. doi: 10.1002/jbm.a.31816. [DOI] [PubMed] [Google Scholar]
- Li H, Friend JR, Yeo LY. A scaffold cell seeding method driven by surface acoustic waves. Biomaterials. 2007;28:4098–4104. doi: 10.1016/j.biomaterials.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Li J, Jiang L, Liao G, Chen G, Liu Y, Wang J, Zheng Y, Luo S, Zhao Z. Centrifugal forces within usually-used magnitude elicited a transitory and reversible change in proliferation and gene expression of osteoblastic cells UMR-106. Mol Biol Rep. 2009;36:299–305. doi: 10.1007/s11033-007-9179-y. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang L, Zhou G, Li Q, Liu W, Yu Z, Luo X, Jiang T, Zhang W, Cao Y. In vitro engineering of human ear-shaped cartilage assisted with CAD/CAM technology. Biomaterials. 2010;31:2176–2183. doi: 10.1016/j.biomaterials.2009.11.080. [DOI] [PubMed] [Google Scholar]
- Lopez-De Leon A, Rojkind M. A simple micromethod for collagen and total protein determination in formalin-fixed paraffin-embedded sections. J Histochem Cytochem. 1985;33:737–743. doi: 10.1177/33.8.2410480. [DOI] [PubMed] [Google Scholar]
- Lowenberg BF, Pilliar RM, Aubin JE, Sodek J, Melcher AH. Cell attachment of human gingival fibroblasts in vitro to porous-surfaced titanium alloy discs coated with collagen and platelet-derived growth factor. Biomaterials. 1988;9:302–309. doi: 10.1016/0142-9612(88)90023-3. [DOI] [PubMed] [Google Scholar]
- Lui PP, Cheuk YC, Hung LK, Fu SC, Chan KM. Increased apoptosis at the late stage of tendon healing. Wound Repair Regen. 2007;15:702–707. doi: 10.1111/j.1524-475X.2007.00276.x. [DOI] [PubMed] [Google Scholar]
- Maidhof R, Marsano A, Lee EJ, Vunjak-Novakovic G. Perfusion seeding of channeled elastomeric scaffolds with myocytes and endothelial cells for cardiac tissue engineering. Biotechnol Prog. 2010;26:565–572. doi: 10.1002/btpr.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Klieser W. Multicellular spheroids. A review on cellular aggregates in cancer research. J Cancer Res Clin Oncol. 1987;113:101–122. doi: 10.1007/BF00391431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nau T, Lavoie P, Duval N. A new generation of artificial ligaments in reconstruction of the anterior cruciate ligament. Two-year follow-up of a randomised trial. J Bone Joint Surg Br. 2002;84:356–360. doi: 10.1302/0301-620X.84B3.12400. [DOI] [PubMed] [Google Scholar]
- Ng R, Gurm JS, Yang ST. Centrifugal seeding of mammalian cells in nonwoven fibrous matrices. Biotechnol Prog. 2010;26:239–245. doi: 10.1002/btpr.281. [DOI] [PubMed] [Google Scholar]
- Ohyabu Y, Adegawa T, Yoshioka T, Ikoma T, Shinozaki K, Uemura T, Tanaka J. A collagen sponge incorporating a hydroxyapatite/chondroitinsulfate composite as a scaffold for cartilage tissue engineering. J Biomater Sci Polym Ed. 2009;20:1861–1874. doi: 10.1163/156856208X386462. [DOI] [PubMed] [Google Scholar]
- Ouyang HW, Goh JC, Thambyah A, Teoh SH, Lee EH. Knitted poly-lactide-co-glycolide scaffold loaded with bone marrow stromal cells in repair and regeneration of rabbit Achilles tendon. Tissue Eng. 2003;9:431–439. doi: 10.1089/107632703322066615. [DOI] [PubMed] [Google Scholar]
- Petite H, Hannouche D. Marrow stromal stem cells for repairing the skeleton. Biotechnol Genet Eng Rev. 2002;19:83–101. [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Qin TW, Yang ZM, Wu ZZ, Xie HQ, Qin J, Cai SX. Adhesion strength of human tenocytes to extracellular matrix component-modified poly(DL-lactide-co-glycolide) substrates. Biomaterials. 2005;26:6635–6642. doi: 10.1016/j.biomaterials.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Rickard DJ, Kazhdan I, Leboy PS. Importance of 1,25-dihydroxyvitamin D3 and the nonadherent cells of marrow for osteoblast differentiation from rat marrow stromal cells. Bone. 1995;16:671–678. doi: 10.1016/8756-3282(95)00099-Y. [DOI] [PubMed] [Google Scholar]
- Risbud M. Tissue engineering: implications in the treatment of organ and tissue defects. Biogerontology. 2001;2:117–125. doi: 10.1023/A:1011585117310. [DOI] [PubMed] [Google Scholar]
- Roh JD, Nelson GN, Udelsman BV, Brennan MP, Lockhart B, Fong PM, Lopez-Soler RI, Saltzman WM, Breuer CK. Centrifugal seeding increases seeding efficiency and cellular distribution of bone marrow stromal cells in porous biodegradable scaffolds. Tissue Eng. 2007;13:2743–2749. doi: 10.1089/ten.2007.0171. [DOI] [PubMed] [Google Scholar]
- Scott A, Cook JL, Hart DA, Walker DC, Duronio V, Khan KM. Tenocyte responses to mechanical loading in vivo: a role for local insulin-like growth factor 1 signaling in early tendinosis in rats. Arthritis Rheum. 2007;56:871–881. doi: 10.1002/art.22426. [DOI] [PubMed] [Google Scholar]
- Scutt A, Bertram P. Bone marrow cells are targets for the anabolic actions of prostaglandin E2 on bone: induction of a transition from nonadherent to adherent osteoblast precursors. J Bone Miner Res. 1995;10:474–487. doi: 10.1002/jbmr.5650100320. [DOI] [PubMed] [Google Scholar]
- Scutt A, Zeschnigk M, Bertram P. PGE2 induces the transition from non-adherent to adherent bone marrow mesenchymal precursor cells via a cAMP/EP2-mediated mechanism. Prostaglandins. 1995;49:383–395. doi: 10.1016/0090-6980(95)00070-Q. [DOI] [PubMed] [Google Scholar]
- Seo YR, Sweeney C, Smith ML. Selenomethionine induction of DNA repair response in human fibroblasts. Oncogene. 2002;21:3663–3669. doi: 10.1038/sj.onc.1205468. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Ito A, Honda H. Enhanced cell-seeding into 3D porous scaffolds by use of magnetite nanoparticles. J Biomed Mater Res B Appl Biomater. 2006;77:265–272. doi: 10.1002/jbm.b.30443. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Ito A, Honda H. Mag-seeding of rat bone marrow stromal cells into porous hydroxyapatite scaffolds for bone tissue engineering. J Biosci Bioeng. 2007;104:171–177. doi: 10.1263/jbb.104.171. [DOI] [PubMed] [Google Scholar]
- Silver FH, Tria AJ, Zawadsky JP, Dunn MG. Anterior cruciate ligament replacement: a review. J Long Term Eff Med Implants. 1991;1:135–154. [PubMed] [Google Scholar]
- Srivastava S, Gorham SD, Courtney JM. The attachment and growth of an established cell line on collagen, chemically modified collagen, and collagen composite surfaces. Biomaterials. 1990;11:162–168. doi: 10.1016/0142-9612(90)90149-K. [DOI] [PubMed] [Google Scholar]
- Sun T, Norton D, Haycock JW, Ryan AJ, MacNeil S. Development of a closed bioreactor system for culture of tissue-engineered skin at an air-liquid interface. Tissue Eng. 2005;11:1824–1831. doi: 10.1089/ten.2005.11.1824. [DOI] [PubMed] [Google Scholar]
- Sun T, Jackson S, Haycock JW, MacNeil S. Culture of skin cells in 3D rather than 2D improves their ability to survive exposure to cytotoxic agents. J Biotechnol. 2006;122:372–381. doi: 10.1016/j.jbiotec.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Tanswell AK, Byrne PJ, Han RN, Edelson JD, Han VK. Limited division of low-density adult rat type II pneumocytes in serum-free culture. Am J Physiol. 1991;260:L395–L402. doi: 10.1152/ajplung.1991.260.6.L395. [DOI] [PubMed] [Google Scholar]
- Toselli P, Mogayzel PJ, Jr., Faris B, Ferrera R, Franzblau C (1984) Mammalian cell growth on collagen-hydrogels. Scan Electron Microsc (Pt 3):1301–1312 [PubMed]
- Weinand C, Xu JW, Peretti GM, Bonassar LJ, Gill TJ. Conditions affecting cell seeding onto three-dimensional scaffolds for cellular-based biodegradable implants. J Biomed Mater Res B Appl Biomater. 2009;91:80–87. doi: 10.1002/jbm.b.31376. [DOI] [PubMed] [Google Scholar]
- Wu YF, Chen CH, Cao Y, Avanessian B, Wang XT, Tang JB. Molecular events of cellular apoptosis and proliferation in the early tendon healing period. J Hand Surg Am. 2010;35:2–10. doi: 10.1016/j.jhsa.2009.10.021. [DOI] [PubMed] [Google Scholar]
- Xiao YL, Riesle J, Blitterswijk CA. Static and dynamic fibroblast seeding and cultivation in porous PEO/PBT scaffolds. J Mater Sci Mater Med. 1999;10:773–777. doi: 10.1023/A:1008946832443. [DOI] [PubMed] [Google Scholar]
- Zhu N, Warner RM, Simpson C, Glover M, Hernon CA, Kelly J, Fraser S, Brotherston TM, Ralston DR, MacNeil S. Treatment of burns and chronic wounds using a new cell transfer dressing for delivery of autologous keratinocytes. Eur J Plast Surg. 2005;28:319–330. doi: 10.1007/s00238-005-0777-4. [DOI] [Google Scholar]
- Zwingmann J, Mehlhorn AT, Sudkamp N, Stark B, Dauner M, Schmal H. Chondrogenic differentiation of human articular chondrocytes differs in biodegradable PGA/PLA scaffolds. Tissue Eng. 2007;13:2335–2343. doi: 10.1089/ten.2006.0393. [DOI] [PubMed] [Google Scholar]