Abstract
Mycoplasma contamination is a deleterious event for cell culture laboratories. Plasmocin™ is used to prevent and eradicate mycoplasma infections from cell. In this study, 80 different mammalian cell lines from various sources; human, monkey, mice, hamster and rat were used to study and evaluate plasmocin™ efficiency and compare it to commonly used antibiotics such as BM-cyclin, ciprofloxacin and mycoplasma removal agent (MRA). It was shown that mycoplasma infections were eradicated by plasmocin™, BM-cyclin, ciprofloxacin and MRA in 65%, 66.25%, 20%, and 31.25%, respectively, of infected cell cultures. However, re-infection with mycoplasmas after the period of 4 months occurred in 10–80% of the studied cell lines. Cell cytotoxicity and culture death was observed in 25, 17.5 and 10% of the treated cells, for plasmocin™, BM-cyclin and MRA, respectively. In this study, Plasmocin™ showed strong ability to eradicate mollicutes from our cell lines with minimal percentage of regrowth. However, due to its high cell cytotoxicity it should be used with caution especially when dealing with expensive or hard-to-obtain cell lines. Amongst the antibiotics tested, BM-cyclin was shown to remove mycoplasma with the highest efficiency.
Keywords: Mycoplasma, Plasmocin, Treatment, Cell line, Cytotoxicity
Introduction
Human and animal continuous cell lines are precious and indispensable tools for both biotechnological and biomedical research. In this respect, mycoplasma contamination is a deleterious event for a cell culture laboratory resulting in the production of false data or, in the worst cases, in the loss of cell culture itself (Mariotti et al. 2008). It is well-known that mycoplasma can have adverse effects on cell cultures such as altered levels of protein and of RNA/DNA synthesis, induction of chromosomal aberrations, changes in cell membrane composition and modification of cellular morphology (Drexler et al. 2002; Drexler and Uphoff 2002). It has been highly advised to autoclave and discard infected cultures to minimize the risk of infection transmission to other clean cultures and eradication strategies in a separate quarantine laboratory should be considered as a last resort, if a valuable cell line or primary cell culture is not replaceable (Molla Kazemiha et al. 2009). For eradication purposes, three classes of antibiotics, i.e., tetracyclines, macrolides and quinolones, have been shown to be highly effective against mycoplasmas, both in human/veterinary medicine and in cell culture (Singh et al. 2008; Uphoff and Drexler 2005). Since each antibiotic has its own activity and might not completely treat all the mycoplasma contaminants present in a culture, using a combination of antibiotics have attracted a lot of attentions. To this end, Plasmocin (Macrolid), have been used to prevent and eradicate mycoplasma infections from cell lines. The use of Plasmocin for eliminating mycoplasma from particular cell lines has been studied previously (Bronckaers et al. 2008; Singh et al. 2008; Mnif et al. 2007). There are also some studies on the efficacy of commonly used antibiotics such as Ciprofloxacine (Ridgway et al. 1984; Mowles 1988), BM-Cycline (Shin et al. 2003), and Mycoplasma removal agent (Drexler 1994; Nakai et al. 2000; Souza et al. 2007). However, few studies have reported on the comparison of these four antibiotics (Plasmocin, Ciprofloxacine, BM-Cycline and Mycoplasma removal agent) on the mycoplasma eradication from different cell lines. Previously, we reported the types of contaminants of our cell cultures and eradication strategies using three commonly used antibiotics (Molla Kazemiha et al. 2009). In this study, we aimed to compare the efficacy of Plasmocin™ against three previously studied antibiotics; BM-Cycline, Ciprofloxacine and MRA for decontamination of various mammalian cell lines.
Materials and methods
Cell cultures
A total of 80 different cell types (Table 1) from the National Cell Bank of Iran (Pasteur Institute of Iran, Tehran, Iran) were analyzed in this study. Cell lines were grown at 37 °C in a humidified atmosphere of air containing 5% CO2. The basic growth media were supplemented with 10–20% fetal bovine serum (Sigma, Deisenhofen, Germany). For growth factor-dependent cell lines, specific growth factors or conditioned media containing growth factors were added (Drexler et al. 2001). Fetal Bovine Serum (FBS, Cat No: 10270-106), Roswell Park Memorial Institute medium (RPMI, Cat No: 31800-022), Dulbecco’s Modified Eagle Medium High Glucose (DMEM, Cat No: 12100-046), F12 nutrient mixture (Hams’F12, Cat No: 21700-075), Non Essential Amino Acid (NEAA, Cat No: 11140-050), Penicillin/Streptomycin (Cat No: 15070-063), Horse serum (H.S, Cat No: 16050-130), Trypsin–EDTA (Cat No: 25300) were supplied by Gibco/Invitrogen company. Oxalate, Pyruvate, and Insulin (OPI, Cat No: O 5003), Bovine Insulin (Cat No: I 6634), Human Insulin (Cat No: I 9278), Epidermal Growth Factor (EGF, Cat No: E 9644), Fibroblast Growth Factor—from bovine pituitary (bFGF, Cat No: F 5392), and Human Endothelial Cell Growth Factor (Cat No: E 9640) were purchased from Sigma. All supplements, such as serum, conditioned media and trypsin were mycoplasma negative as indicated by the suppliers. For detection of mycoplasma, the cell lines were cultured initially for at least 1 week after thawing, and samples were taken after a culture period of at least 2 days without medium exchange. No antibiotics were added to the cultures.
Table 1.
PCR results of mycoplasma detection in 80 cell lines
| Noa | Cell line name | Cell type | NCBI Code | Mar | Mfe | Mor | Mhy | Ala | Msa | Mpi | Mho | Mge | Uur |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | MCF-7 | Human breast Adeno carcinoma | C135 | − | − | − | + | − | − | − | − | − | − |
| 2 | T-47D | Human breast ductal carcinoma | C203 | − | + | − | − | − | − | − | − | − | − |
| 3 | RAJI | Human Burkitt’s lymphoma | C127 | − | + | − | − | − | − | − | − | − | − |
| 4 | NSO | Mouse myeloma | C142 | − | + | − | − | − | − | − | − | − | − |
| 5 | HEP G2 | Human hepatocyte carcinoma | C158 | − | − | − | + | − | − | − | − | − | − |
| 6 | A-431 | Human squamous carcinoma | C204 | + | − | − | + | − | − | − | − | − | − |
| 7 | HFFF-PI6 | Human fetal foreskin fibroblast | C170 | − | − | + | − | − | − | − | − | − | − |
| 8 | Hep2 | Human Larynx carcinoma | C144 | − | − | − | + | − | − | − | − | − | − |
| 9 | MRC-5 | Human foetal lung fibroblast | C125 | + | − | − | − | − | − | − | − | − | − |
| 10 | Saos-2 | Human osteogenic sarcoma | C453 | + | + | − | + | − | − | − | − | − | − |
| 11 | McCoy | Synovial tissue fibroblast | C123 | + | − | − | − | − | − | − | − | − | − |
| 12 | CCRF-CEM | Human Acute lymphoblastic leukemia | C105 | − | + | − | − | − | − | − | − | − | − |
| 13 | PANC-1 | Human pancreas duct epithelial carcinoma | C556 | + | + | − | + | − | − | − | − | − | − |
| 14 | B95-8 | Marmoset EBV transformed lymphocytes | C110 | + | + | − | − | − | − | − | − | − | − |
| 15 | CHO | Chinese hamster ovary | C111 | + | − | − | − | − | − | − | − | − | − |
| 16 | DAUDI | Human Burkitt’s lymphoma | C112 | − | − | + | − | − | − | − | − | − | − |
| 17 | BT-474 | Human breast ductal carcinoma | C435 | + | − | − | − | − | − | − | − | − | − |
| 18 | JIYOYE | Human Burkitt’s lymphoma | C117 | − | − | + | − | − | − | − | − | − | − |
| 19 | MDA-MB-468 | Human breast adenocarcinoma | C208 | + | + | − | + | − | − | − | − | − | − |
| 20 | HUT-78 | Human cutaneus T cell lymphoma | C185 | + | + | − | − | + | − | − | − | − | − |
| 21 | J774A.1 | Mouse monocyte/macrophage | C483 | + | − | − | + | − | − | − | − | − | − |
| 22 | LNCap-FGC-10 | Human prostate cancer | C439 | − | − | − | + | − | − | − | − | − | − |
| 23 | F3B6 | Human × mouse heterohybridoma | C197 | + | − | + | − | − | − | − | − | − | − |
| 24 | SP2/0-Ag14 | Mouse myeloma | C129 | − | + | − | + | − | − | − | − | − | − |
| 25 | Vero | African Green Monkey Kidney | C101 | + | + | − | + | − | − | − | − | − | − |
| 26 | K562 | Human CML | C122 | − | − | − | − | + | − | − | − | − | − |
| 27 | WEHI-164 | Mouse BALB/c fibrosarcoma | C200 | − | − | − | + | − | − | − | − | − | − |
| 28 | BCL1 clone 5B1b | Mouse lymphoma | C551 | − | − | − | − | + | − | − | − | − | − |
| 29 | SK-N-MC | Human neuroblastoma | C535 | − | + | − | − | − | − | − | − | − | − |
| 30 | BW5147 | Mouse thymoma | C542 | − | − | − | + | − | − | − | − | − | − |
| 31 | STO | Mouse SIM fetal fibroblast | C537 | − | − | − | + | − | − | − | − | − | − |
| 32 | CT26 | Mouse colon carcinoma | C532 | − | − | − | + | − | − | − | − | − | − |
| 33 | THP-1 | Human acute Monocytic leukemia | C563 | − | − | + | − | − | − | − | − | − | − |
| 34 | PC12 | Rat adrenal fibroblast pheochromocytoma | C153 | − | − | − | + | − | − | − | − | − | − |
| 35 | Seraphina | Human Burkitt’s lymphoma | C102 | − | + | − | − | − | − | − | − | − | − |
| 36 | LCL-PI 12 | Human EBV transformed cord blood B cell | C178 | − | + | − | − | − | − | − | − | − | − |
| 37 | HGF3-PI 53 | Human gingival fibroblast | C502 | − | − | − | + | − | − | − | − | − | − |
| 38 | HSF-PI 17 | Human skin fibroblast | C193 | − | + | − | − | − | − | − | − | − | − |
| 39 | B65 | Rat nervous tissue neuronal tumor | C134 | + | − | − | − | − | − | − | − | − | − |
| 40 | P3X63Ag8.653 | Mouse-myeloma | C109 | + | − | − | − | − | − | − | − | − | − |
| 41 | Hela | Human cervix carcinoma | C115 | + | + | − | + | − | − | − | − | − | − |
| 42 | U937 | Human Histiocytic lymphoma | C130 | − | + | − | − | − | − | − | − | − | − |
| 43 | Hek293 | Human embryonic kidney cells | C497 | − | − | − | + | − | − | − | − | − | − |
| 44 | HL60 | Human promyelocytic leukemia | C217 | + | − | − | − | − | + | − | − | − | − |
| 45 | SKBR3 | Human breast adenocarcinoma | C207 | − | − | + | + | − | − | − | − | − | − |
| 46 | Peer | Human acute T cell lymphoblastic leukemia | C511 | − | − | − | − | + | − | − | − | − | − |
| 47 | HL60/mix 1 | Human acute promyelocytic leukemia | C553 | + | − | − | − | − | − | + | − | − | − |
| 48 | HPA/ALL | Human T cell acute lymphoblastic leukemia | C213 | − | + | − | − | − | − | − | − | − | − |
| 49 | MG63 | Human osteosarcoma | C555 | + | − | − | − | − | − | − | − | − | − |
| 50 | LB3.1 (HB-298) | Mouse anti human HLA-DR alpha chain hybridoma | H195 | + | − | − | + | − | − | − | + | − | − |
| 51 | NIH3T3 | Mouse Swiss embryo fibroblast | C156 | − | − | − | + | − | − | − | − | − | − |
| 52 | Caco2 | Human colon adenocarcinoma | C139 | + | − | − | + | − | − | − | − | − | − |
| 53 | CoR-L-105 | Human lung adenocarcinoma | C113 | − | − | + | − | − | − | − | − | − | − |
| 54 | L929 | Mouse connective tissue fibroblast | C161 | − | + | − | − | − | − | − | − | − | − |
| 55 | A3.6B10 (HB-12318) | Mouse anti human CTLA-4 (CD152) hybridoma | H196 | + | − | − | + | − | − | − | + | − | − |
| 56 | Nalm6 | Pre B cell leukemia | C212 | − | + | − | − | − | − | − | − | − | − |
| 57 | KG1 | Human Caucasian bone marrow myeloid leukemia | C119 | − | − | − | − | − | + | − | − | − | − |
| 58 | T45 | Human T cell acute lymphoblastic leukemia | C180 | − | − | − | − | − | − | + | − | − | − |
| 59 | PC3 | Human prostate adenocarcinoma | C427 | − | − | − | + | − | − | − | − | − | − |
| 60 | MDA-MB231 | Human breast adenocarcinoma | C578 | + | − | − | − | − | − | − | − | − | − |
| 61 | CHO DG-44 | Dihydrofolate reductase-deficient CHO | C576 | + | − | − | − | − | − | − | − | − | − |
| 62 | PC12 (Suspension) | Rat adrenal pheochromocytoma | C189 | − | − | − | + | − | − | − | − | − | − |
| 63 | G28 | Mouse anti human CD40 hybridoma | H150 | + | − | − | + | − | + | − | − | − | − |
| 64 | NS1 | Mouse myeloma | C522 | − | − | − | + | − | − | − | − | − | − |
| 65 | MDBK | Bovine normal kidney epithelial cells | C500 | + | − | − | + | − | − | − | − | − | − |
| 66 | Rael | Human Burkitt’s lymphoma | C186 | − | − | + | − | − | − | − | − | − | − |
| 67 | DFW | Human melanoma | C496 | − | + | − | − | − | − | − | − | − | − |
| 68 | LCL PI7 | Human EBV-transformed peripheral blood B cells | C175 | − | + | − | − | − | − | − | − | − | − |
| 69 | B16F10 | Mouse melanoma | C540 | + | + | − | + | − | − | − | − | − | − |
| 70 | ASPC1 | Human pancreas adenocarcinoma | C558 | − | − | − | + | − | − | + | − | − | − |
| 71 | HT29 | Human colon adenocarcinoma | C466 | − | − | − | + | − | − | − | − | − | − |
| 72 | LS180 | Human colon adenocarcinoma | C508 | + | − | − | − | − | − | − | − | − | − |
| 73 | A2780S | Human ovarian carcinoma (sensitive to Cisplatin) | C461 | − | − | − | + | − | − | − | − | − | − |
| 74 | MOLT-17 | Human T cell leukemia | C516 | − | + | − | − | − | − | − | − | − | − |
| 75 | CAOV-4 | Human ovary adenocarcinoma | C595 | − | + | − | − | − | − | − | − | + | − |
| 76 | DND-41 | Human T cell acute lymphoblastic leukemia | C183 | − | − | + | − | − | − | − | − | − | − |
| 77 | RPMI 8402 | Human T cell acute lymphoblastic leukemia | C184 | − | + | − | − | − | − | − | − | − | − |
| 78 | LL/2(LLC1) | Mouse Lewis lung carcinoma | C587 | + | − | − | + | + | − | − | − | − | − |
| 79 | BHK21-2PCLone13 | Baby hamster kidney from Syrian hamster (suspension culture) | C108 | + | + | − | − | − | − | − | − | − | − |
| 80 | HFIF PI4 | Human fetal liver fibroblast | C168 | − | + | − | + | − | − | − | − | − | + |
aCell lines 1–40 (Molla Kazemiha et al. 2009), Cell lines 41–80 (Present study)
Mar, M. arginini; Mfe, M. fermentans; Mor, M. orale; Mhy, M. hyorhinis; Ala, A. laidlawii; Msa, M. salivarium; Mpi, M. pirum; Mho, M. hominis; Mge, M. genitalium; Uur, U. urealyticum; (−), non-detected mycoplasma species; (+), detected species-specific mycoplasma; NCBI, National Cell Bank of Iran
Detection of mollicutes
The mycoplasma contamination status in 80 cell lines was determined using PCR-based method as described by Molla kazemiha et al. (2009). Table 2 shows the primer sequences with melting temperature (Tm) and guanine-cytosine content (GC %). All primers were obtained from CinnaGene (Iran) company.
Table 2.
The sequences of oligonucleotide primers used for detection of mycoplasmas
| Primers of species-specific mycoplasmas | Primer sequence | Tm | GC | Amplicon size |
|---|---|---|---|---|
| Universal primer | S: GTG GGG AGG AAA YAG GAT TAG A | 53–54.8 | 45–50 | 425 bp |
| AS: GGC ATG ATG ATT TGA CGT CRT | 50.5–52.4 | 45–48 | ||
| M. Arginini | S: TGA TCA TTA GTC GGT GGA GAG TTC | 55.7 | 46 | 326 bp |
| AS: TAT CTC TAG AGT CCT CGA CAT GAC TC | 58 | 46 | ||
| M. Orale | S: TGA TCA TTA GTC GGT GGA AAA CTA | 52.3 | 38 | 325 bp |
| AS: TAT CTC TAG AGT CCT CGA CAT GAC TC | 58 | 46 | ||
| M. Hyorhinis | S: CGA TGA TCA TTA GTT GGT GGA ATA AAT | 53.7 | 33 | 334 bp |
| AS: AGG CAG TAT CTC TAG AGT CCT TAA CTT A | 57 | 39 | ||
| M. Fermentans | S: TGA TCA TTA GCT GAT GGG GAA CT | 53.5 | 43 | 324 bp |
| AS: TCT CTT AGA GTC CTC AAC TAA ATG | 52.3 | 38 | ||
| M. Genitalium | S: ATA GAT ACT AGC TGT CGG AGC GAT | 55.7 | 46 | 335 bp |
| AS: CCA ATT TAC ATT AGC AGT CTC GTT AA | 53.2 | 35 | ||
| A. Laidlawii | S: GAT GAG AAC TAA GTG TTG GCC ATA A | 54.4 | 40 | 300 bp |
| AS: CGC TAG AGT CCC CAA CTT AAT GA | 55.3 | 48 | ||
| M. Hominis | S: ATC ATT AGT CGG TGG AGA ATC A | 55.1 | 41 | 301 bp |
| AS: GCA GTA TCT CTA CTA GAG TCC TCA ACT TAAT | 59.1 | 39 | ||
| M. Pirum | S: TGG ATG TTA GAT GTC GGG GTA AA | 53.5 | 43 | 324 bp |
| AS: GTT GGC AGT ATC GCT AGA CAA A | 56.7 | 41 | ||
| M. Pneumoniae | S: GAT ACT AGC TGT CGG GGC GAT | 56.3 | 57 | 329 bp |
| AS: AAT TTG CAT TAG TAG CAG TCT CGC TAG | 56.7 | 41 | ||
| M. Salivarium | S: GAT CAT TAG TCG GCA GAG AAC TCG | 57.4 | 50 | 324 bp |
| AS: TAT CTC TAG AGT CCT CGA CAT GAC TC | 58 | 46 | ||
| U. Urealyticum | S: CAT CAT TAA ATG TCG GCT CGA A | 51.1 | 41 | 323 bp |
| AS: CGG TAG CAG TAT CGC TAG AAA AGC | 57.4 | 50 |
Previous studied cell lines (Molla kazemiha et al. 2009) were treated with Plasmocin™ (Invivogen, USA, cat. No 04J05-SV) and the Plasmocin™ efficacy compared with previously used antibiotics, BM-Cyclin (Roche, Mannheim, Germany, cat. No.799050), Ciprofloxacin (ICN, USA, cat. No. 199020) and MRA (Serotec,UK, cat. No, Buf035). Moreover, another 40 new cell lines were screened for their mycoplasma contamination status and treated with above mentioned four antibiotics. The effects of each antibiotic on culture death as well as eradication and regrowth of mycoplasma were evaluated. Samples were treated with the antibiotics with doses and duration as stated below:
Plasmocin™ was added for 14 days at a final concentration of 25 μg/mL.
BM-Cyclin I (10 μg/mL) and BM-Cyclin II (5 μg/mL) were used in three alternating cycle of 3 and 4 days, respectively.
Ciprofloxacin was used for 14 days at10 μg/mL.
MRA was added to the culture medium for 10 days at a final concentration of 0.5 μg/mL.
The concentration of Plasmocin, BM cyclin and MRA were chosen according to the manufacturer’s instructions. Ciprofloxacin concentration was specified according to the published report (Fleckenstein and Drexler 1996). Following the treatment with these reagents, cells were cultured in antibiotic-free medium (also without penicillin, streptomycin or other commonly used antibiotics) for at least another 2 weeks prior to testing for residual mycoplasmal contamination. All cured cultures were retested for regrowth of mycoplasmas for up to 4 months following the treatment.
Statistical analysis
All statistical analyses were performed with SPSS 16.0 (IBM® SPSS® Statistics, USA). Since the data were ordinal and non-normally distributed, non-parametric Kruskal–Wallis analysis was used for comparison of the 4 groups, and two-by-two comparisons were made using the Mann–Whitney U test. Differences at P < 0.05 were considered to be statistically significant.
Results
Determination of mycoplasma contamination status
Table 1 summarizes types of mollicutes in each cell lines determined by PCR-based method. It can be seen that 55/80 (68.75%) of the infected cell cultures contained one mycoplasma species. Other samples were infected with two 13/80 (16.25%) or three 12/80 (15%) different species. M. hyorhinis was detected in 35/80 (43.75%) of the studied samples, M. arginini in 30/80 (37.5%), M. fermentans in 28/80 (35%), M. orale in 9/80 (11.25%), A. laidlawii in 5/80 (6.25%), M. salivarium and M. pirum in 3/80 (3.75%), M. hominis in 2/80 (2.5%), M. genitalium and U. urealyticum in 1/80 (1.25%).
Eradication of mycoplasmal contamination
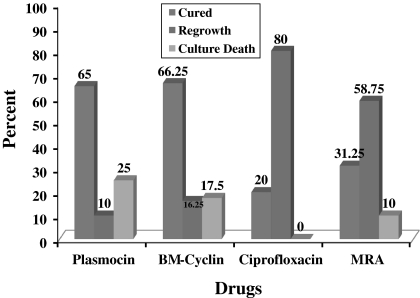
The results obtained from eradication experiments (mycoplasma removal) are summarized in Fig. 1 and Table 3. Mycoplasma infections were eliminated by BM-Cyclin, Plasmocin™, MRA and Ciprofloxacin and in 66.25, 65, 31.25 and 20% of the infected cell cultures, respectively. Furthermore, the decontamination was confirmed by PCR, as no mycoplasma was detected in treated cultures 14 days after completion of antibiotic treatment (Fleckenstein and Drexler 1996). Mycoplasma regrowth was observed in 10, 16.25, 80 and 58.75% of the cured cell lines 4 months after treatment with Plasmocin™, BM-cyclin, ciprofloxacin and MRA, respectively. In spite of the absence of Plasmocin™—targets in eukaryotic cells, the highest level (25%) of cell cytotoxicity was observed among Plasmocin™ treated cell lines. While BM-cyclin, ciprofloxacin and MRA were cytotoxic up to 17.5, 0, and 10% of the studied cell lines (Fig. 1). Notably, except in one case, regrowth problem for BM-cyclin treated cell lines could be solved by its replacement with Plasmocin™ and vice versa. The statistical results of two-by-two comparisons are indicated in Table 4 with significant differences between four groups but not between the Plasmocin™/BM-cyclin and MRA/Ciprofloxacin.
Fig. 1.
Results of the treatment of mycoplasma-positive cell cultures with four antibiotics including Plasmocin, BM-cyclin, Ciprofloxacin and MRA
Table 3.
The results of antibiotic treatment of each cell line
| No* | Cell line name | Cell type | NCBI code | Pla | BM | MRA | Cip | Curing antibiotic | Mycoplasma species |
|---|---|---|---|---|---|---|---|---|---|
| 1 | MCF-7 | Human breast Adeno carcinoma | C135 | Ο | Ο | Ο | ∆ | Pla, BM, MRA | Mhy |
| 2 | T-47D | Human breast ductal carcinoma | C203 | Ο | Ο | ∆ | ∆ | Pla, BM | Mfe |
| 3 | RAJI | Human Burkitt’s lymphoma | C127 | Ο | X | X | ∆ | Pla | Mfe |
| 4 | NSO | Mouse myeloma | C142 | Ο | X | Ο | Ο | Pla, MRA, Cip | Mfe |
| 5 | HEP G2 | Human hepatocyte carcinoma | C158 | Ο | Ο | X | ∆ | Pla, BM | Mhy |
| 6 | A-431 | Human squamous carcinoma | C204 | Ο | ∆ | ∆ | ∆ | Pla | Mar, Mhy |
| 7 | HFFF-PI6 | Human fetal foreskin fibroblast | C170 | X | Ο | ∆ | ∆ | BM | Mor |
| 8 | Hep2 | Human Larynx carcinoma | C144 | Ο | Ο | ∆ | ∆ | Pla, BM | Mhy |
| 9 | MRC-5 | Human foetal lung fibroblast | C125 | X | Ο | Ο | Ο | BM, MRA, Cip | Mar |
| 10 | Saos-2 | Human osteogenic sarcoma | C453 | Ο | ∆ | ∆ | ∆ | Pla | Mar, Mfe, Mhy |
| 11 | McCoy | Synovial tissue fibroblast | C123 | Ο | Ο | ∆ | ∆ | Pla, BM | Mar |
| 12 | CCRF-CEM | Human Acute lymphoblastic leukemia | C105 | X | Ο | Ο | Ο | BM, MRA, Cip | Mfe |
| 13 | PANC-1 | Human pancreas duct epithelial carcinoma | C556 | Ο | Ο | ∆ | ∆ | Pla, BM | Mar, Mfe, Mhy |
| 14 | B95-8 | Marmoset EBV transformed lymphocytes | C110 | ∆ | Ο | ∆ | ∆ | BM | Mar, Mfe |
| 15 | CHO | Chinese hamster ovary | C111 | Ο | Ο | Ο | Ο | Pla, BM, MRA, Cip | Mar |
| 16 | DAUDI | Human Burkitt’s lymphoma | C112 | X | Ο | ∆ | ∆ | BM | Mor |
| 17 | BT-474 | Human breast ductal carcinoma | C435 | Ο | Ο | Ο | ∆ | Pla, BM, MRA | Mar |
| 18 | JIYOYE | Human Burkitt’s lymphoma | C117 | Ο | X | Ο | ∆ | Pla, MRA | Mor |
| 19 | MDA-MB-468 | Human breast adenocarcinoma | C208 | Ο | ∆ | ∆ | ∆ | Pla | Mar, Mfe, Mhy |
| 20 | HUT-78 | Human cutaneus T cell lymphoma | C185 | Ο | Ο | ∆ | ∆ | Pla, BM | Mar, Mfe, Ala |
| 21 | J774A.1 | Mouse monocyte/macrophage | C483 | ∆ | ∆ | ∆ | ∆ | Mar, Mhy | |
| 22 | LNCap-FGC-10 | Human prostate cancer | C439 | Ο | Ο | ∆ | ∆ | Pla, BM | Mhy |
| 23 | F3B6 | Human × mouse heterohybridoma | C197 | X | X | ∆ | ∆ | Mar, Mor | |
| 24 | SP2/0-Ag14 | Mouse myeloma | C129 | ∆ | Ο | ∆ | ∆ | BM | Mfe, Mhy |
| 25 | Vero | African Green Monkey Kidney | C101 | Ο | Ο | ∆ | ∆ | Pla, BM | Mar, Mfe, Mhy |
| 26 | K562 | Human CML | C122 | Ο | Ο | ∆ | ∆ | Pla, BM | Ala |
| 27 | WEHI-164 | Mouse BALB/c fibrosarcoma | C200 | Ο | Ο | ∆ | ∆ | Pla, BM | Mhy |
| 28 | BCL1 clone 5B1b | Mouse lymphoma | C551 | X | X | Ο | Ο | MRA, Cip | Ala |
| 29 | SK-N-MC | Human neuroblastoma | C535 | Ο | ∆ | ∆ | ∆ | Pla | Mfe |
| 30 | BW5147 | Mouse thymoma | C542 | X | Ο | X | ∆ | BM | Mhy |
| 31 | STO | Mouse SIM fetal fibroblast | C537 | ∆ | Ο | ∆ | ∆ | BM | Mhy |
| 32 | CT26 | Mouse colon carcinoma | C532 | ∆ | Ο | ∆ | ∆ | BM | Mhy |
| 33 | THP-1 | Human acute Monocytic leukemia | C563 | Ο | Ο | ∆ | ∆ | Pla, BM | Mor |
| 34 | PC12 | Rat adrenal fibroblast pheochromocytoma | C153 | ∆ | Ο | ∆ | ∆ | BM | Mhy |
| 35 | Seraphina | Human Burkitt’s lymphoma | C102 | Ο | Ο | Ο | Ο | Pla, BM, MRA, Cip | Mfe |
| 36 | LCL-PI 12 | Human EBV transformed cord blood B cell | C178 | Ο | Ο | Ο | Ο | Pla, BM, MRA, Cip | Mfe |
| 37 | HGF3-PI 53 | Human gingival fibroblast | C502 | X | Ο | ∆ | ∆ | BM | Mhy |
| 38 | HSF-PI 17 | Human skin fibroblast | C193 | X | Ο | X | ∆ | BM | Mfe |
| 39 | B65 | Rat nervous tissue neuronal tumor | C134 | X | X | Ο | ∆ | MRA | Mar |
| 40 | P3X63Ag8.653 | Mouse-myeloma | C109 | X | X | ∆ | ∆ | Mar | |
| 41 | Hela | Human cervix carcinoma | C115 | Ο | ∆ | ∆ | ∆ | Pla | Mar, Mfe, Mhy |
| 42 | U937 | Human Histiocytic lymphoma | C130 | Ο | Ο | ∆ | ∆ | Pla, BM | Mfe |
| 43 | Hek293 | Human embryonic kidney cells | C497 | ∆ | Ο | ∆ | ∆ | BM | Mhy |
| 44 | HL60 | Human promyelocytic leukemia | C217 | Ο | Ο | ∆ | ∆ | Pla, BM | Mar, Msa |
| 45 | SKBR3 | Human breast adenocarcinoma | C207 | Ο | ∆ | ∆ | ∆ | Pla | Mor, Mhy |
| 46 | Peer | Human acute T cell lymphoblastic leukemia | C511 | X | Ο | X | Ο | BM, Cip | Ala |
| 47 | HL60/mix 1 | Human acute promyelocytic leukemia | C553 | X | Ο | ∆ | ∆ | BM | Mar, Mpi |
| 48 | HPA/ALL | Human T cell acute lymphoblastic leukemia | C213 | X | X | X | Ο | Cip | Mfe |
| 49 | MG63 | Human osteosarcoma | C555 | Ο | Ο | Ο | ∆ | Pla, BM, MRA | Mar |
| 50 | LB3.1 (HB-298) | Mouse anti human HLA-DR alpha chain hybridoma | H195 | Ο | ∆ | ∆ | ∆ | Pla | Mar, Mhy, Mho |
| 51 | NIH3T3 | Mouse Swiss embryo fibroblast | C156 | Ο | Ο | Ο | ∆ | Pla, BM, MRA | Mhy |
| 52 | Caco2 | Human colon adenocarcinoma | C139 | Ο | ∆ | ∆ | ∆ | Pla | Mar, Mhy |
| 53 | CoR-L-105 | Human lung adenocarcinoma | C113 | Ο | Ο | ∆ | ∆ | Pla, BM | Mor |
| 54 | L929 | Mouse connective tissue fibroblast | C161 | Ο | Ο | ∆ | ∆ | Pla, BM | Mfe |
| 55 | A3.6B10 (HB-12318) | Mouse anti human CTLA-4 (CD152) hybridoma | H196 | Ο | ∆ | ∆ | ∆ | Pla | Mar, Mhy, Mho |
| 56 | Nalm6 | Pre B cell leukemia | C212 | Ο | Ο | Ο | Ο | Pla, BM, MRA, Cip | Mfe |
| 57 | KG1 | Human Caucasian bone marrow myeloid leukemia | C119 | Ο | Ο | Ο | Ο | Pla, BM, MRA, Cip | Msa |
| 58 | T45 | Human T cell acute lymphoblastic leukemia | C180 | X | X | Ο | ∆ | MRA | Mpi |
| 59 | PC3 | Human prostate adenocarcinoma | C427 | Ο | Ο | ∆ | ∆ | Pla, BM | Mhy |
| 60 | MDA-MB231 | Human breast adenocarcinoma | C578 | Ο | Ο | ∆ | ∆ | Pla, BM | Mar |
| 61 | CHO DG-44 | Dihydrofolate reductase-deficient CHO | C576 | Ο | Ο | Ο | Ο | Pla, BM, MRA, Cip | Mar |
| 62 | PC12 (Suspension) | Rat adrenal pheochromocytoma | C189 | X | ∆ | Ο | ∆ | MRA | Mhy |
| 63 | G28 | Mouse anti human CD40 hybridoma | H150 | ∆ | X | Ο | ∆ | MRA | Mar, Mhy, Msa |
| 64 | NS1 | Mouse myeloma | C522 | Ο | Ο | Ο | Ο | Pla, BM, MRA, Cip | Mhy |
| 65 | MDBK | Bovine normal kidney epithelial cells | C500 | Ο | X | ∆ | ∆ | Pla | Mar, Mhy |
| 66 | Rael | Human Burkitt’s lymphoma | C186 | Ο | X | ∆ | ∆ | Pla | Mor |
| 67 | DFW | Human melanoma | C496 | Ο | Ο | Ο | Ο | Pla, BM, MRA, Cip | Mfe |
| 68 | LCL PI7 | Human EBV-transformed peripheral blood B cells | C175 | Ο | Ο | Ο | ∆ | Pla, BM, MRA | Mfe |
| 69 | B16F10 | Mouse melanoma | C540 | Ο | ∆ | ∆ | ∆ | Pla | Mar, Mfe, Mhy |
| 70 | ASPC1 | Human pancreas adenocarcinoma | C558 | Ο | Ο | ∆ | ∆ | Pla, BM | Mhy, Mpi |
| 71 | HT29 | Human colon adenocarcinoma | C466 | Ο | Ο | ∆ | ∆ | Pla, BM | Mhy |
| 72 | LS180 | Human colon adenocarcinoma | C508 | X | Ο | Ο | ∆ | BM, MRA | Mar |
| 73 | A2780S | Human ovarian carcinoma (sensitive to Cisplatin) | C461 | Ο | X | Ο | ∆ | Pla, MRA | Mhy |
| 74 | MOLT-17 | Human T cell leukemia | C516 | Ο | Ο | Ο | Ο | Pla, BM, MRA, Cip | Mfe |
| 75 | CAOV-4 | Human ovary adenocarcinoma | C595 | Ο | Ο | ∆ | ∆ | Pla, BM | Mfe,Mge |
| 76 | DND-41 | Human T cell acute lymphoblastic leukemia | C183 | X | Ο | X | ∆ | BM | Mor |
| 77 | RPMI 8402 | Human T cell acute lymphoblastic leukemia | C184 | Ο | X | X | Ο | Pla, Cip | Mfe |
| 78 | LL/2(LLC1) | Mouse Lewis lung carcinoma | C587 | Ο | ∆ | ∆ | ∆ | Pla | Mar, Mhy, Ala |
| 79 | BHK21-2PCLone13 | Baby hamster kidney from Syrian hamster (suspension culture) | C108 | X | Ο | ∆ | ∆ | BM | Mar, Mfe |
| 80 | HFIF PI4 | Human fetal liver fibroblast | C168 | X | Ο | ∆ | ∆ | BM | Mfe, Mhy, Uur |
Ο, cured; ∆, regrowth; X, culture death; Pla, Plasmocin™; BM, BM-cyclin; Cip, Ciprofloxacin; NCBI, National Cell Bank of Iran; Mar, M. arginini; Mfe, M. fermentans; Mor, M. orale; Mhy, M. hyorhinis; Ala, A. laidlawii; Msa, M. salivarium; Mpi, M. pirum; Mho, M. hominis; Mge, M. genitalium; Uur, U. urealyticum; (−), non-detected mycoplasma species; (+), detected species-specific mycoplasma
Table 4.
Statistical results of two-by-two comparisons
| Antibiotic 1 | Antibiotic 2 | P value |
|---|---|---|
| Plasmocin™ | BM-cyclin | 0.643 |
| Plasmocin™ | MRA | 0.017 |
| Plasmocin™ | Ciprofloxacin | 0.003 |
| BM-cyclin | MRA | 0.002 |
| BM-cyclin | Ciprofloxacin | 0.000 |
| MRA | Ciprofloxacin | 0.660 |
Discussion
Methods of elimination should ideally be simple, easy, rapid, efficient, reliable and inexpensive and have minimal effect on the eukaryotic cells. However, there is clearly not a single method available that is both 100% effective and fulfills all the ideal requirements. To this end, administration of antibiotics is the most common and efficient approach (Drexler and Uphoff 2002). Obviously it is important to know the efficacy of the antibiotics for the eradication of mycoplasma in cultures, as well as the practicality of the approach, and the potential side-effects on the eukaryotic cells (Uphoff et al. 2002). The antibiotic effectiveness for eradication of each strain of mycoplasma is related to several parameters including cell types, cell species (human or animal) and severity of infection. In addition, some cell types may be infected with several species making it difficult to draw an accurate conclusion.
The mechanism of action of each antibiotic is different. Plasmocin (Macrolid and quinolone) acts on the protein machinery and also on DNA replication by interfering with ribosomal translation and replication fork, respectively. BM-Cyclin binds to the 30S and 50S ribosomal subunits and inhibits protein synthesis. According to the manufacturer’s information, the bactericidal components of BM cyclin (Roche) are composed of pleuromutilin and tetracycline while Plasmocin™ (InvivoGen) is composed of a macrolide and a quinolone. On the other hand, MRA and Ciprofloxacin are members of the quinolones family inhibiting bacterial DNA gyrase and replication of DNA (Helgason and Miller 2005; Uphoff and Drexler 2004, 2011).
Plasmocin™ and BM-Cyclin were efficient in removing mollicutes and curing 65 and 66.25% of our cell lines, respectively. While, Ciprofloxacin and MRA were considerably less efficient as they cured only 20 and 31.25% of our cell lines, respectively. The high efficiency of Plasmocin and BM-Cyclin were not surprising as they are composed of two types of bactericidal agents (www.plasmocin.com, Uphoff et al. 2002). Zakharova et al. (2010) showed that Plasmocin can effectively remove mycoplasma contamination in treatment of chronic mycoplasma infections.
Notably, Plasmocin™ and BM-Cyclin were highly efficient especially in curing of M. orale, M. hyorhinis and M. arginini infected cell lines which were resistant to other antibiotics used in this study. Fleckenstein and Drexler (1996) also reported these strains as resistant strains. For instance, we observed high level of ciprofloxacin as well as MRA resistance/regrowth after treating cell lines infected with above mentioned mollicutes.
As for regrowth, Plasmocin™ showed the lowest level (10%) in our experiment, while regrowth was observed in 16.25% of cell lines after treating with BM-Cyclin. On the other hand, ciprofloxacin and MRA showed striking levels of regrowth (80 and 58.75% respectively) in treated cells. It has been shown by other researchers that ciprofloxacin may increase the mycoplasma resistance to antibiotic treatment (Momynaliev et al. 2002). Interestingly, for each cell lines (except for J774 in which regrowth was observed after treating with all the studied antibiotics) for which regrowth was observed after BM-Cyclin treatment, Plasmocin™ may solve the problem and vice versa.
Surprisingly, Plasmocin™ showed the highest percentage (25%) of culture death, although plasmocin™ targets, the prokaryotic DNA replication and protein synthesis machineries, which are totally different from those of eukaryotic cells (www.plasmocin.com). However, Because of these two combined mechanisms, the cells are more affected by the antibiotic activity and the environment is more toxic for cells. Similarly, Singh et al. (2008) lost their culture during plasmocin™ treatment. BM-Cyclin showed slightly lower cytotoxic effects on the studied cell lines. Therefore, it might reduce the risk of culture loss, especially in the case of expensive or hard-to-obtain cell lines to treat with BM-Cyclin first before treating with Plasmocin.
Since the higher amount of culture death was observed during treatment with Plasmocin™ or BM-cyclin, treatment with other antibiotics such as MRA or ciprofloxacin must be considered spontaneously in the case of valuable cell lines.
On the other hand, ciprofloxacin showed no cell cytotoxicity at all, while MRA caused 10% cell death during or after treatment. Although cytotoxic effect of ciprofloxacin was not detected in this study, such observation has been reported before (Fleckenstein and Drexler 1996; Kloskowski et al. 2010; Sousa and Poiares-da-Silva 2001). Although the latter two antibiotics, showed lower percentage of cell cytotoxicity, they are not recommendable as they exhibited poor results in curing and regrowth experiments.
It is well known that several parameters are changed during infection of cultured cells by mycoplasma including reduction in the number of chromosomes in the cells or difficulty in the interpretation of the results of enzymatic activity (Souza et al. 2007). On the other hand different antibiotic doses administered during antibiotic treatment may cause different effects on cultured cells. Therefore it is difficult to detect and assess the effect of antibiotic treatment on the cell cycle stages during treatment of mycoplasmal infected cells. There are few studies on the antibiotic administration and its effect on different stages of the cell cycle. It has been reported that antibiotics may inhibit the cell proliferation and cause cell cycle arrest at the G2/M phase (Tsai et al. 2008; Koziel et al. 2010). For this reason the antibiotic concentration should be determined precisely while apoptosis induction or mitosis inhibition should be considered.
The combination of two or more antibiotics with different action mechanisms for treatment of a single cell culture makes the interpretation of the results more complicated. Based on our experiment this might increase the risk of culture death or antibiotic resistance of bacteria. For this reason we suggest that using two or more antibiotics in an alternating procedure may be suitable for a successful eradication. For example BM cyclin (inhibits the protein synthesis) and Ciprofoxacin (inhibits the DNA gyrase activity) with different action mechanisms can be used alternately during treatment period with specified intervals. It should be noted that the outcomes of cocktail effects are under investigation and will be discussed in more detail in the future publications.
In conclusion, results obtained from Plasmocin™ and BM-cyclin in treatment efficacy were almost similar. They exhibited efficient eradication ability with minimum level of regrowth. However, relatively high percentage of cell cytotoxicity for these antibiotics introduces the risk of losing cell lines during the treatment which should be considered especially when dealing with expensive or hard-to-obtain cell lines. Finally it can be concluded that, ciprofloxacin and MRA did not efficiently eradicate mollicutes from the studied cell lines. BM cycline has been chosen as the best antibiotic with the highest amount of cured cells after treatment. Although Plasmocin showed the highest amount of cell death, it can be considered as a selective antibiotic, because of the lowest rate of mycoplasma regrowth compared to the other antibiotics.
Acknowledgment
The authors would like to express their appreciation to Prof. Ehsan Mostafavi (head of department of epidemiology) and Ali Jahanian Najafabadi for their technical assistance. The authors also would like to thank Pasteur Institute for their financial assistance.
References
- Bronckaers A, Balzarini J, Liekens S. The cytostatic activity of pyrimidine nucleosides is strongly modulated by Mycoplasma hyorhinis infection: implications for cancer therapy. Biochem Pharmacol. 2008;76:188–197. doi: 10.1016/j.bcp.2008.04.019. [DOI] [PubMed] [Google Scholar]
- Drexler HG, Dirks WG, MacLeod RAF, Quentmeier H, Steube KG, Uphoff CC (2001) DSMZ catalogue of human and animal cell lines, 8th edn. DSMZ, Braunschweig
- Drexler HG, Uphoff CC. Mycoplasma contamination of cell cultures: incidence, sources, effects, detection, elimination, prevention. Cytotechnology. 2002;39:75–90. doi: 10.1023/A:1022913015916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler HG, Gignac SM, Hu ZB, Hopert A, Fleckenstein E, Voges M, Uphoff CC (1994) Treatment of mycoplasma contamination in a large panel of cell cultures. In Vitro Cell Dev Biol Anim 30:344–347 [DOI] [PubMed]
- Drexler HG, Uphoff CC, Dirks W, MacLeod RAF. Mix-ups and mycoplasma: the enemies within. Leuk Res. 2002;26:329–333. doi: 10.1016/S0145-2126(01)00136-9. [DOI] [PubMed] [Google Scholar]
- Fleckenstein E, Drexler HG. Elimination of mycoplasma contamination in cell cultures. Biochemica. 1996;1:48–51. [Google Scholar]
- Helgason CD, Miller CL. Basic cell culture protocols. 3. New Jersey: Humana Press Inc; 2005. p. 27. [Google Scholar]
- Kloskowski T, Olkowska J, Nazlica A, Drewa T. The influence of ciprofloxacin on hamster ovarian cancer cell line CHO AA8. Acta Pol Pharm. 2010;67:345–349. [PubMed] [Google Scholar]
- Koziel R, Szczepanowska J, Magalska A, Piwocka K, Duszynski J, Zablocki K. Ciprofloxacin inhibits proliferation and promotes generation of aneuploidy in Jurkat cells. J Physiol Pharmacol. 2010;61:233–239. [PubMed] [Google Scholar]
- Mariotti E, Mirabelli P, Di Noto R, Fortunato G, Salvatore F. Rapid detection of mycoplasma in continuous cell lines using a selective biochemical test. Leuk Res. 2008;32:323–326. doi: 10.1016/j.leukres.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Mnif W, Duchesne MJ, Balaguer P, Nicolas JC, Cavaillès V, Bartegi A, Badia E. Efficient treatment of Mycoplasma hyorhinis contamination in MCF-7 breast cancer cells with doxycyclin. J Immuno Biol. 2007;22:107–110. [Google Scholar]
- Molla Kazemiha V, Shokrgozar MA, Arabestani MR, Shojaei Moghadam M, Azari SH, Maleki S, Amanzadeh A, Jeddi Tehrani M, Shokri F. PCR-based detection and eradication of mycoplasmal infections from various mammalian cell lines: a local experience. Cytotechnology. 2009;61:117–124. doi: 10.1007/s10616-010-9252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momynaliev KT, Smirnova OV, Ladygina VG, Orlova VPh, Akopian TA, Govorun VM (2002) Development of flouroquinolone (ciprofloxacin) resistance in mycoplasma hominis in the presence of HeLa cells. Russ J Genet 38:771–776 [PubMed]
- Mowles JM. The use of ciprofloxacin for the elimination of mycoplasma from naturally infected cell lines. Cytotechnology. 1988;1:355–358. doi: 10.1007/BF00365081. [DOI] [PubMed] [Google Scholar]
- Nakai N, Kawaguchi C, Nawa K, Kobayashi S, Katsuta Y, Watanabe M. Detection and elimination of contaminating microorganisms in transplantable tumors and cell lines. Exp Anim. 2000;49:309–313. doi: 10.1538/expanim.49.309. [DOI] [PubMed] [Google Scholar]
- Ridgway GL, Mumtaz G, Gabriel FG, Oriel JD. The activity of ciprofloxacin and other 4-quinolones against Chlamydia trachomatis and Mycoplasmas in vitro. Eur J Clin Microbiol Infect Dis. 1984;3:344–346. doi: 10.1007/BF01977491. [DOI] [PubMed] [Google Scholar]
- Shin JH, Joo HS, Lee WH, Seok HB, Calsamig M, Pijoan C, Molitor TW. Identification and characterization of cytopathogenic Mycoplasma hyorhinis from swine farms with a history of abortions. J Vet Med Sci. 2003;65:501–509. doi: 10.1292/jvms.65.501. [DOI] [PubMed] [Google Scholar]
- Singh S, Puri SK, Srivastava K. Treatment and control of mycoplasma contamination in Plasmodium falciparum culture. Parasitol Res. 2008;104:181–184. doi: 10.1007/s00436-008-1181-3. [DOI] [PubMed] [Google Scholar]
- Sousa MC, Poiares-da-Silva J. The cytotoxic effects of ciprofloxacin in Giardia lamblia trophozoite. Toxicol In Vitro. 2001;15:297–301. doi: 10.1016/S0887-2333(01)00026-1. [DOI] [PubMed] [Google Scholar]
- Souza FT, Sostruznik LS, Scolari RC, Castro KJ, Giugliani R, Coelho JC. Comparison of the measurement of lysosomal hydrolase activity in mycoplasma-contaminated and non-contaminated human fibroblast cultures treated with mycoplasma removal agent. Clin Biochem. 2007;40:521–525. doi: 10.1016/j.clinbiochem.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Tsai WC, Hsu CC, Tang FT, Wong AM, Chen YC, Pang JH. Ciprofloxacin-mediated cell proliferation inhibition and G2/M cell cycle arrest in rat tendon cells. Arthritis Rheum. 2008;58:1657–1663. doi: 10.1002/art.23518. [DOI] [PubMed] [Google Scholar]
- Uphoff CC, Drexler HG. Elimination of Mycoplasma from infected cell lines using antibiotics. Cancer Cell Cult Method Mol Med. 2004;88:327–334. doi: 10.1385/1-59259-406-9:327. [DOI] [PubMed] [Google Scholar]
- Uphoff CC, Drexler HG. Eradication of Mycoplasma contamination. Basic Cell Cult Protoc Method Mol Biol. 2005;290:25–34. doi: 10.1385/1-59259-838-2:025. [DOI] [PubMed] [Google Scholar]
- Uphoff CC, Drexler HG. Elimination of Mycoplasmas from infected cell lines using antibiotics. Cancer Cell Cult Method Mol Biol. 2011;731:105–114. doi: 10.1007/978-1-61779-080-5_9. [DOI] [PubMed] [Google Scholar]
- Uphoff CC, Meyer C, Drexler HG. Elimination of mycoplasma from leukemia-lymphoma cell lines using antibiotics. Leukemia. 2002;16:284–288. doi: 10.1038/sj.leu.2402364. [DOI] [PubMed] [Google Scholar]
- Zakharova E, Grandhi J, Wewer MD, Gavrilin MA. Mycoplasma suppression of THP-1 cell TLR responses is corrected with antibiotics. PLoS One. 2010;5:e9900. doi: 10.1371/journal.pone.0009900. [DOI] [PMC free article] [PubMed] [Google Scholar]