Abstract
Prostaglandin E2 (PGE2), the most abundant COX-2–derived prostaglandin found in colorectal cancer, promotes tumor cell proliferation and survival via multiple signaling pathways. However, the role of PGE2 in tumor hypoxia is not well understood. Here, we show a synergistic effect of PGE2 and hypoxia on enhancing ANGPTL4 expression and that elevation of ANGPTL4 promotes colorectal cancer growth. PGE2 induces ANGPTL4 expression at both the mRNA and protein levels under hypoxic conditions. Moreover, hypoxia induces one of the PGE2 receptors, namely EP1. Activation of EP1 enhances ANGPTL4 expression, whereas blockage of EP1 by its antagonist inhibits PGE2 induction of ANGPTL4 under hypoxic conditions. Importantly, over-expression of ANGPTL4 promotes cell proliferation and tumor growth in vitro and in vivo. In addition, treatment with ANGPTL4 recombinant protein increases colorectal carcinoma cell proliferation through effects on STAT1 signaling. The MAPK and Src pathways mediate ANGPTL4-induced STAT1 expression and activation. These results are relevant to human disease since we found that the expression of ANGPTL4 and STAT1 are elevated in 50% of human colorectal cancers tested and there is a positive correlation between COX-2 and ANGPTL4 as well STAT1 expression in colorectal carcinomas. Collectively, these findings suggest that PGE2 plays an important role in promoting cancer cell proliferation via ANGPTL4 under hypoxic conditions.
Keywords: PGE2, hypoxia, ANGPTL4, colorectal cancer, proliferation
Introduction
Recent evidence confirms a reduction in risk for colorectal and other cancers in individuals who regularly take aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs). These drugs are potent inhibitors of cyclooxygenase-1 and -2 (COX-1 and COX-2). Elevation of COX-2 was found in approximately 50% of colorectal adenomas and 85% of adenocarcinomas (1, 2) and is associated with worse survival among colorectal cancer (CRC) patients (3). COX-2 converts free arachidonic acid into prostanoids, including prostaglandins (PGs) and thromboxanes (TXs). Prostaglandin E2 (PGE2) is the most abundant prostaglandin found in CRC (4). An increasingly large body of evidence has demonstrated that PGE2 mediates the tumor-promoting effects of COX-2 in CRC (5). PGE2 treatment dramatically increased both small and large intestinal adenoma burden in ApcMin/+ mice and significantly enhanced AOM-induced colon tumor incidence and multiplicity (6, 7). PGE2 promotes tumor growth by stimulating EP receptor signaling with subsequent enhancement of cellular proliferation, promotion of angiogenesis, inhibition of apoptosis, and stimulation of invasion/motility (8).
Hypoxia is one of the most common and critical factors identified thus far in the regulation of cancer progression and metastasis (9, 10). Adaptation of tumor cells to hypoxia is achieved largely by undergoing genetic changes that enable them to survive and become more malignant or invasive (11). Hypoxia induces COX-2 expression and PGE2 levels via HIF-1, a major transcription factor to regulate hypoxic response, in colorectal carcinoma cells (12, 13). Increased levels of PGE2 during hypoxia, in turn, potentiates HIF-1 transcriptional activity and promotes colorectal carcinoma cell growth and survival (12), suggesting that cross talk exists between PGE2 and hypoxia. However, the mechanism of how PGE2 promotes CRC progression in the hypoxic tumor microenvironment is unclear. We postulated that PGE2 might mediate a pivotal tumor cellular adaptive response to hypoxia with the implication for connecting tumor hypoxia to cancer progression.
Angiopoietin-like protein 4 (ANGPTL4) is a secretary protein and cleaved into an N-terminal coiled-coil fragment (N-ANGPTL4) and a C-terminal fibrinogen-like domain (C-ANGPTL4) that modulates the disposition of circulating triglycerides (14). Although ANGPTL4’s role in lipid metabolism has been well characterized, its role in cancer progression is poorly understood. Although recent studies suggest that ANGPTL4 is involved in cancer progression, the precise role of ANGPTL4 in angiogenesis and cancer progression is still debated. For example, ANGPTL4 has been reported to have pro-angiogenic effects and anti-angiogenic effects in different models (15-17). Moreover, ANGPTL4 mediates TGFβ-induced lung metastasis of breast cancer (18) but inhibits metastasis of melanoma cells (19). These conflicting data support the need of additional research to address role of ANGPTL4 in cancer progression.
In this study, ANGPTL4 is identified as a novel focal point of the crosstalk between PGE2 and hypoxia signaling pathways in CRC cells. Our results showed that PGE2 and hypoxia synergistically induce ANGPTL4 expression via EP1. Importantly, we demonstrate that the elevation of ANGPTL4 promotes tumor growth in vitro and in vivo. Moreover, we identified STAT1 as a new target of ANGPTL4 and found that inhibition of STAT1 attenuated ANGPTL4 induction of CRC cell proliferation. We further elucidated the ANGPTL4 signaling pathways in response to STAT1 expression and activation. The maximal induction of ANGPTL4 required both hypoxia and PGE2, indicating the complex nature of interactions within the tumor microenvironment that affect cancer signaling pathways.
Material and Methods
Reagents
PGE2, 17-phenyl-trinor-PGE2 and SC-19220 were purchased from Cayman Chemical Company (Ann Arbor, MI). U73122, BAPTA-AM, PD98059, SU6656, and diphenylene iodonium were purchased from EMD Chemicals (Gibbstown, NJ). ONO-AE3-208 kindly was provided by Ono Pharmaceutical Co. (Osaka, Japan). Full length and C-terminal ANGPTL4 recombinant proteins were obtained from R&D Systems.
Cell culture and treatment
LS-174T, HT-29 and HCT-116 were purchased from the ATCC (Manassas, VA). All cells were routinely maintained in McCoy’s 5A medium containing 10% fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin in a 5% CO2 humidified incubator at 37°C. Cells were exposed to hypoxia by placing them in a mixed-gas incubator that was infused with an atmosphere consisting of 94% N2, 5% CO2, and 1% O2. In addition, PGE2 was treated at same time.
Real time-quantitative PCR (RT-qPCR)
Total RNA was isolated by using TRIzol (Invitrogen). cDNA was synthesized from 2 μg of total RNA by using High-Capacity cDNA Reverse Transcription Kits (Applied Biosystems, Foster City, CA) and mixed with TaqMan® Gene Expression Assay Mix, sterile water and TaqMan® Fast Universal PCR Master Mix (Applied Biosystems). Real-time PCR was carried out using 7900 HT Fast (Applied Biosystems) system and expression of target genes mRNA relative to 18s rRNA was calculated.
Measurement of ANGPTL4 secretion
Cells were exposed to PGE2 or hypoxic conditions, conditioned medium was collected and then the amount of ANGPTL4 secreted into medium was measured using Human ANGPTL4 DuoSet ELISA kit (R&D Systems, Inc., Minneapolis, MN).
DNA construction
ANGPTL4 cDNA (Origene) was amplified with pfuUltra Hotstart DNA polymerase (Stratagene). The PCR products were digested with XhoI and NotI and were cloned into pBMN-I-GFP (addgen).
Establishment of stable cell line
pBMN-I-GFP and pBMN-I-GFP-ANGPTL4 retroviral vectors were transfected into Phoenix cells in 60-mm dishes using Lipofectamine reagent (Invitrogen) according to the manufacturer’s protocol. Culture medium containing virus particles was collected 48 h later and was added to LS-174T cells. Cells infected were sorted by green fluorescent protein (GFP) positivity to eliminate uninfected cells.
Western blotting
Whole cell lysates were prepared for Western blot analyses using lysis buffer containing 20 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 1% TX-100, 1mmol/L EDTA, pH 8.0, and 1 mmol/L PMSF. Samples were denatured in a SDS sample buffer. Total proteins were separated by loading 20μg of total cell lysate on a denaturing 10% SDS-polyacrylamide gel and transferred to a nitrocellulose membrane. Membranes were blocked with 5% non-fat dry milk in phosphate-buffered saline containing 0.1% Triton X-100 and incubated with primary antibodies that recognize STAT1, Src, phospho-Src (Cell Signaling Technology, Denvers, MA), ANGPTL4 (R&D Systems), ERk, phospho-ERk (Santa Cruz Biotechnology) and Actin (Sigma-Aldrich, St. Louis, MO). Secondary antibody conjugated to horseradish peroxidase (Vector Laboratories Inc, Burlingame, CA) was used at 1:2,000 to detect primary antibodies and enzymatic signals were visualized by chemiluminescence.
Cell viability assay
Ninety-six-well plates were seeded with 5,000 cells per well and cells were treated without or with ANGPTL4 in serum-free medium for 3 d. Cell viability was determined using Cell Proliferation Reagent WST-1 (Roche Applied Science).
Immunocytochemistry
Cells were treated with ANGPTL4, washed with phosphate-buffered saline, and fixed with 4% paraformaldehyde for 30 min at room temperature, and subsequent blocking with phosphate-buffered saline containing 1% BSA and 0.1% TX-100 for 30 min at room temperature. They were incubated with STAT1 antibody (Cell Signaling Technology) for 2 h at room temperature, followed by biotinylated secondary antibody (Vector Laboratories Inc.) for 1 h, then fluorescein streptavidin (Vector Laboratories Inc.) for 30 min, and then DAPI (Invitrogen) for 5 min to visualize nuclei. Cells were examined under a fluorescence microscope (Nikon ECLIPSE TE300) to determine localization of STAT1.
Immunohystochemistry
Paraffin-embedded specimens were treated with xylene and ethanol to remove the paraffin. The slides were immersed in Borg decloaker solution (Biocare Medical, Inc.) and boiled in a pressure cooker at 125°C for 5 min for antigen retrieval. Endogenous peroxidase activity was blocked by incubating in 3% H2O2 containing PBS solution for 10 min. The slides were blocked with 5% normal goat serum and incubated with anti-STAT1 (Cell Signaling), HIF-1α (BD Biosciences), and ANGPTL4 (Adipobioscience, Santa Clara, CA) at 4°C overnight. After washing with PBS, the slides were incubated with Goat anti-Rabbit HRP (Vector Laboratories). After washing, the slides were developed with DAB reagent (Vector Laboratories) followed by counterstaining with Hematoxylin.
RNA interference
siGENOME SMARTpool siRNAs targeting STAT1 (M-003543-01-000) was purchased from Dharmacon, Inc. (Chicago, IL). LS174T cells were transfected with 20 nmol/L of STAT1 siRNA or non-targeting siRNA using Lipofectamine™ RNAiMAX (Invitrogen) according to the manufacturer’s specifications. The efficacy of knock-down was confirmed by Western blot analysis.
Xenograft study
All mice were housed and treated in accordance with protocols approved by the Institutional Animal Care and Use Committee at The University of Texas M.D. Anderson Cancer Center. LS-174T cells (5 × 105) selected for the stable expression of ANGPTL4 (ANGPTL4/LS-174T) or control GFP (GFP/LS-174T) were injected s.c. into the flanks of nude mice. The tumor size was measured starting from 13 to 28 days after injection. After the mice were euthanized using CO2 asphyxiation, necropsies were done to remove tumors and measurements were taken of tumor weight and size.
Gene expression data of colon cancer patients
Human colorectal carcinoma specimens were obtained from Tissue Procurement and Banking Facility (TPBF) at The University of Texas MD Anderson Cancer Center. Equal amounts of mRNA were analyzed by RT-qPCR for COX-2, ANGPTL4, and STAT1. Microarray data from Moffit Cancer Center (Moffit cohort, GSE17536, n = 177) were downloaded from GEO (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE17536). Kaplan-Meier plots and log-rank test were used to estimate patient prognosis.
Statistical analysis
Each experiment was done at least three times, and data are presented as the mean ± SE. Statistical significance was determined using Student’s t test, one-factor ANOVA, or two-factor ANOVA, where applicable. P < 0.05 was considered statistically significant.
Results
PGE2 enhances ANGPTL4 expression in colorectal carcinoma cells under hypoxic conditions
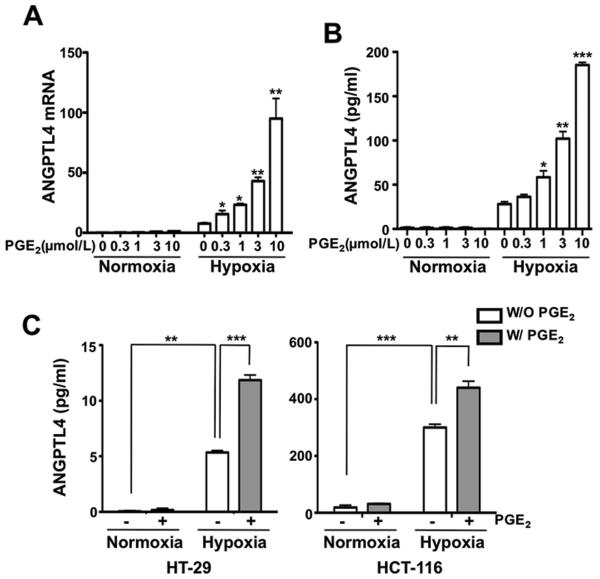
To help mimic the conditions present in the tumor microenvironment, we examined the role of PGE2 under normoxic and hypoxic conditions. We performed transcriptome analysis with RNA isolated from LS-174T cells exposed to PGE2 or/and hypoxia. LS-174T cells normally have low COX-2 levels and do not produce PGE2 under both normoxic and hypoxic conditions (data not shown). Analysis of our microarray data revealed that ANGPTL4 was one of PGE2 downstream targets in cells exposed to hypoxia. Multiple lines of evidence showed that hypoxia induces ANGPTL4 expression in several cell lines including monocytes, and adipocytes (20-22). As expected, hypoxia increased ANGPTL4 expression at both mRNA and protein levels in LS-174T cells (Fig. 1A and B). Strikingly, PGE2 treatment dramatically enhanced ANGPTL4 expression under hypoxic conditions at both mRNA and protein levels in dose-dependent manners in LS-174T cells, whereas PGE2 had no effect on ANGPTL4 expression under normoxic conditions (Fig. 1A and B). The synergistic effect of PGE2 and hypoxia on ANGPTL4 protein expression was also observed in HCT-116 and HT-29 colorectal carcinoma cells (Fig. 1C). These results demonstrate that PGE2 and hypoxia synergistically induce ANGPTL4 expression in CRC cells.
Figure 1. PGE2 enhances ANGPTL4 expression under hypoxic conditions.
A and B, the indicated concentrations of PGE2 were treated in LS-174T cells exposed to normoxia/hypoxia for 12 h (mRNA) or 48 h (protein) to determine the expression of ANGPTL4. ANGPTL4 mRNA (A) and protein (B) levels were determined by RT-qPCR and ELISA analysis, respectively. C, ELISA analysis was performed in HT-29 (left) and HCT-116 (right) cells exposed to normoxia/hypoxia or DMSO/ 1 μmol/L PGE2 for 48 h.
EP1 mediates PGE2-stimulated ANGPTL4 expression during hypoxia
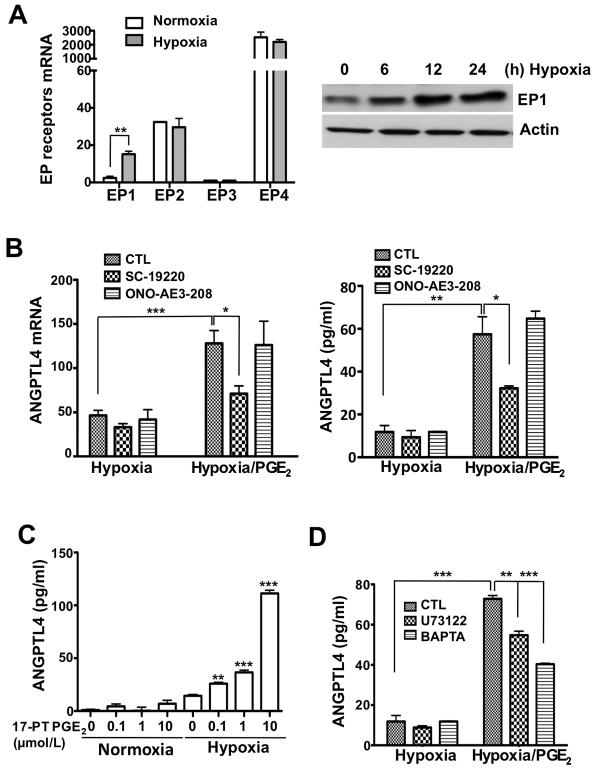
To explore why PGE2 induces ANGPTL4 expression only under hypoxic conditions but not under normoxic conditions, we first examined the effects of hypoxia on PGE2 receptor levels. There are four known PGE2 receptors (EP1, EP2, EP3, and EP4) that are typical G protein-coupled receptors. Since recent reports showed that hypoxia induced EP1 expression in osteoblastic cells (23), we examined the effect of hypoxia on EP1 expression in CRC cells. Indeed, hypoxia specifically increased only EP1 expression and not other PGE2 receptors in LS-174T cells (Fig. 2A). Since LS-174T cells express high levels of EP4, we examined which receptor mediates the effects of PGE2 on upregulation of ANGPTL4 expression. Treatment of LS-174T cells with an EP1 antagonist, SC19220, blocked PGE2-induced ANGPTL4 induction under hypoxic conditions, whereas an EP4 antagonist, ONO-AE3-208, had no effect on ANGPTL4 expression (Fig 2B). Consistent with these results, a selective EP1 agonist (17-PT PGE2) has the same effects as PGE2 on ANGPTL4 expression (Fig. 2C). Since EP1 is coupled to the Gq/PLC/Ca2+ pathway (24), we tested whether inhibition of this pathway attenuates PGE2 induction of ANGPTL4. Treatment of LS-174T cells with a PLC inhibitor (U73122) and a Ca2+ chelating agent (BAPTA) partially suppressed PGE2-induced ANGPTL4 expression under hypoxic conditions, respectively (Fig. 2D). These results reveal that EP1 is induced by hypoxia and mediates PGE2 induction of ANGPTL4.
Figure 2. EP1 mediates PGE2-induced ANGPTL4 expression during hypoxia.
A, LS-174T cells were exposed to hypoxia for 12 h and RT-qPCR was done to determine the expression of EP1, EP2, EP3, and EP4 receptors (left). LS-174T cells were exposed to hypoxia, and EP1 protein levels were analyzed by Western blotting over times as indicated (right). Actin was used as a protein loading control. B, EP1 (10 μmol/L SC-19220) and EP4 (10 μmol/L ONO-AE3-208) antagonists were pretreated for 1 h and then PGE2 were treated in LS-174T cells under normoxic or hypoxic conditions, following RT-qPCR and ELISA to detect ANGPTL4 mRNA and protein. C, ANGPTL4 levels were determined in the growth medium using a commercial ELISA after treatment with the indicated concentration of EP1 agonist (17-PT-PGE2) under normoxic or hypoxic conditions. D, Cells were pretreated with PLC inhibitor (10 μmol/L U73122) or Ca2+ chelator (1 μmol/L BAPTA-AM) for 1 h and then treated with PGE2 under normoxia/hypoxia for 48 h. ANGPTL4 levels were analyzed by ELISA.
ANGPTL4 promotes tumor growth in vitro and in vivo
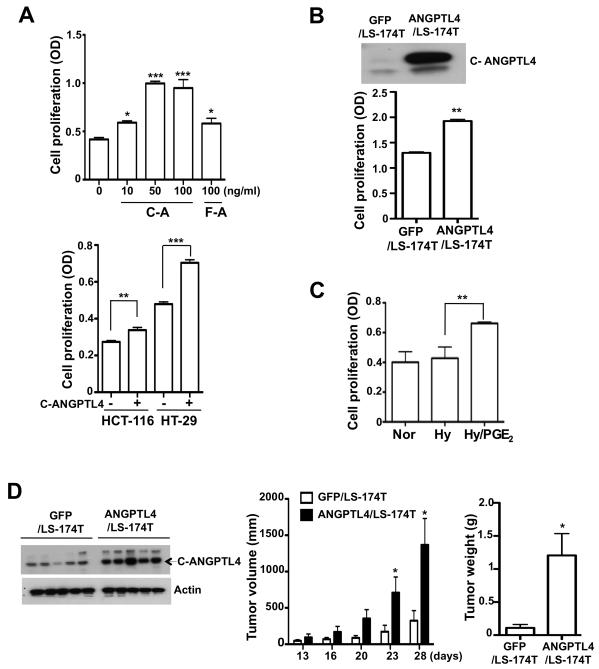
To determine the biologic role of ANGPTL4 in regulating cancer cell proliferation, LS-174T cells were treated with full-length recombinant ANGPTL4 (F-ANGPTL4) and C-ANGPTL4, respectively. Our results showed that C-ANGPTL4 more dramatically promoted LS-174T cell proliferation than F-ANGPTL4 (Fig. 3A, top). In addition, C-ANGPTL4 exhibited the similar effects on regulating HCT-116 and HT-29 cell proliferation (Fig. 3A, bottom). In contrast, both F-ANGPTL4 and C-ANGPTL4 have no effects on regulating LS-174T cell apoptosis (data not shown). To confirm the proliferative effect of ANGPTL4, we established LS-174T cells that overexpress ANGPTL4 (ANGPTL4/LS-174T) and control cells (GFP/LS-174T). C-ANGPTL4 (37 kDa) was easily detected in the conditioned medium of ANGPTL4/LS-174T cells (Fig. 3B, top). Because ANGPTL4 is proteolytically cleaved upon secretion from cells, F-ANGPTL4 was strongly detected in the cell lysate but not the conditioned medium (data not shown). Consistent with above results, over-expression of ANGPTL4 enhanced cell proliferation (Fig. 3B, bottom). These results are consistent with our observation that PGE2 increases cell proliferation under hypoxic conditions (Fig. 3C).
Figure 3. ANGPTL4 increases tumor growth in vitro and in vivo.
A, Full length (F-A) or C-terminal fragment (C-A) of recombinant ANGPTL4 protein was treated in LS-174T cells for 3 days to determine cell proliferation (top). HCT-116 and HT-29 cells were exposed to 100 ng/ ml C-ANGPTL4 for 3 days and cell proliferation was analyzed (bottom). B, LS-174T cells were stably transfected with GFP or ANGPTL4 constructs. C-ANGPTL4 expression was determined by Western blotting after the collection of conditioned medium (top). These cells were incubated in serum free medium for 3 days and then cell proliferation was tested (bottom). C, 1μmol/L of PGE2 was treated in LS-174T cells exposed to normoxia/hypoxia for 3 days and cell proliferation assay was done. D. LS-174T cell stably transfected with GFP or ANGPTL4 constructs were injected into the flanks of nude mice. The tumor size (middle) was measured from 13 to 26 days after injection and tumor weight (right) was also measured and C-ANGPTL4 level (left) were detected after mice were euthanized.
To validate our in vitro results in vivo, ANGPTL4/LS-174T or GFP/LS-174T cells were injected into the flanks of nude mice. The tumor size was measured starting from 13 to 28 days after injection. Tumor weight and C-ANGPTL4 levels were also measured after mice were euthanized. As expected, C-ANGPTL4 levels were higher in the tumor tissue of mice injected with cells expressing ANGPTL4 than controls (Fig. 3D, left). Moreover, cells expressing ANGPTL4 formed larger tumors than the control cells (Fig. 3D, middle and right), suggesting that elevation of ANGPTL4 promotes CRC growth. Taken together, these results suggest that ANGPTL4 accelerates CRC growth via enhancing cell proliferation.
STAT1 is important for ANGPTL4-mediated cell proliferation
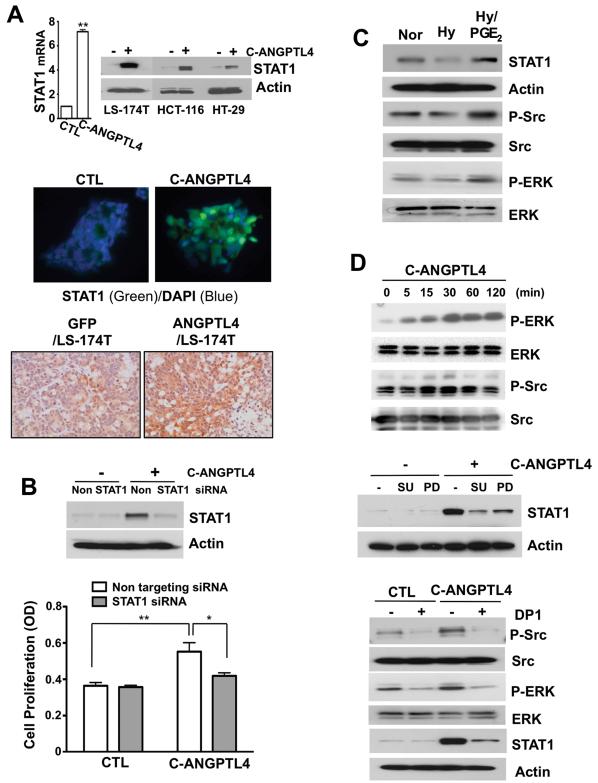
Although several recent reports have shown ANGPTL4’s role in cancer progression (15, 25, 26), little is known about how ANGPTL4 regulates cell proliferation and what downstream targets of ANGPTL4 are involved in regulating cell proliferation. The microarray analysis revealed that C-ANGPTL4 treatment increased interferon-related gene expression, such as STAT1, IFI35, IFI9, and IRF1. These genes were validated by RT-qPCR (data not shown). We further demonstrated that C-ANGPTL4 treatment induced STAT1 expression at both the mRNA and protein levels as well as its nuclear localization (Fig. 4A, top and middle). Immunohistochemical staining further revealed that STAT1 expression was elevated in tumor tissue derived from mice implanted with ANGPTL4/LS-174 cells as compared with the GFP control (Fig. 4A, bottom). Since STAT1 is over-expressed in head and neck and lung cancer and has been reported to have a proliferative effect (27, 28), we examined the contribution of STAT1 o n C-ANGPTL4-induced cell proliferation. Knockdown of STAT1 significantly suppressed C-ANGPTL4-induced cell proliferation (Fig. 4B). Since ERK and Src are known to be the critical signaling molecules for cell proliferation or survival (29), we determined whether these pathways are involved in C-ANGPTL4 induction of STAT1 expression. As shown in Fig. 4C, PGE2 was able to enhance the phosphorylation of Src and ERK as well as STAT1 expression under hypoxic conditions. Moreover, C-ANGPTL4 treatment induced phosphorylation of Src and ERK in time-dependent manners (Fig. 4D, top), whereas pharmacological inhibitors of Src (SU6656) and ERK (PD98059) blocked STAT1 expression stimulated by C-ANGPTL4, respectively (Fig. 4D, middle). Recently, ANGPTL4 has been reported to stimulate NADPH-oxidase-dependent production of O2−, which in turn triggers the Src and ERK pathway (30). Therefore, we examined whether ANGPTL4 regulated Src and ERK activation and STAT1 expression via up-regulating O2− production. Indeed, inhibition of O2− production by a NADPH oxidase inhibitor (diphenylene iodonium, DP1) suppressed ANGPTL4-stimulated the phosphorylation of Src and ERK and the expression of STAT1 (Fig. 4D, bottom). Collectively, these results indicate that STAT1 is a downstream target of C-ANGPTL4 for regulating cancer cell proliferation and the STAT1 induction is dependent on NADPH-oxidase-mediated production of O2−, Src, and MAPK pathways.
Figure 4. ANGPTL4 enhances cancer cell growth through STAT1.
A, LS-174T cells were exposed to C-ANGPTL4 for 12 h (mRNA) and 24 h (protein) and RT-qPCR and Western blot was done for STAT1 mRNA and protein levels, respectively (top). Cells treated with vehicle (CTL) or C-ANGPTL4 were stained with antibody against STAT1 (green) and counterstained with DAPI (blue). Immunofluorescence was detected by a fluorescence microscope (Nikon ECLIPSE TE300) (x 400) (middle). Immunohistochemistry was performed to detected STAT1 expression in tumor tissue generating by injection of ANGPTL4/LS-174 cells into the flanks of nude mice compared with the GFP control (bottom). B, Cells were transfected with non-targeting and STAT1 siRNA, respectively. After 24 h, transfecting cells were treated with C-ANGPTL4 for 3 days and subjected to Western blotting for STAT1 (top) and cell proliferation analysis (bottom). C, Western blot analysis was performed in LS-174T cells exposed to normoxia/hypoxia or DMSO/1 μmol/L PGE2 for 24 h to determine the expression of STAT1 and the phosphorylation of ERK and Src. D, Western blot analysis was carried out to examine the phosphorylation of ERK and Src in LS-174T cells in the presence or absence of C-ANGPTL4 (top). 1 μmol/L SU6656 (SU) or 10 μmol/L PD98059 (PD) were pretreated for 1 h and then STAT1 levels were determined in cells exposed to C-ANGPTL4 (middle). 2 μmol/L of diphenylene iodonium (DP1) was pretreated for 1 h and then the phosphorylation of ERK and Src (0.5 h) as well as the expression of STAT1 (24 h) were determined in cells exposed to C-ANGPTL4 (bottom).
ANGPTL4 and STAT1 are elevated in human CRC specimens
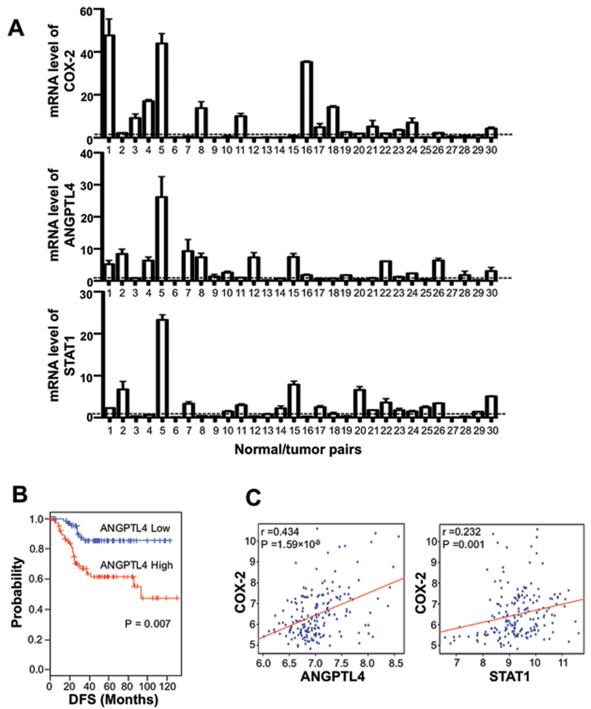
Since increased levels of COX-2 and a downstream product, PGE2, has been reported in most human colorectal cancers (1) and hypoxia is one of key elements in the tumor microenvironment that promotes cancer development and progression (9, 10), we examined ANGPTL4 levels in human CRC and the correlation of ANGPTL4 with COX-2 and STAT1 in human CRC. ANGPTL4 mRNA levels were elevated in 16 of 30 cancer specimens as compared with those in adjacent normal mucosa (Fig. 5A). Moreover, ANGPTL4 expression in human CRC specimens correlates with COX-2 expression (r=0.2433, p=0.0056). In addition, high STAT1 expression was found in 17 of 30 tumor tissues and is also correlated with ANGPTL4 expression (r=0.6861, p=<0.0001). To support these findings, we used a public microarray database to retrieve gene expression data of colorectal cancer patients. Patients in the Moffitt cohort (n = 177) were dichotomized according to expression level of ANGPTL4. Overall survival rate of patients with higher expression of ANGPTL4 were significantly worse than that of those with lower expression of ANGPTL4 (Fig. 5B). In addition, the expression of COX-2 positively correlated with the expression of ANGPTL4 (r=0.434, p=<0.0001) and STAT1 (r=0.232, P=0.001) in the Moffitt cohort (n = 177) (Fig. 5C). Therefore, these results suggest that elevation of ANGPTL4 could be relevant to human CRC progression.
Figure 5. COX-2, ANGPTL4 and STAT1 expressions in colorectal cancers.
A. Total RNA was isolated from 30 individual human colorectal cancer tissues and corresponding normal mucosa. Equal amounts of mRNA were analyzed by RT-qPCR for COX-2, ANGPTL4, and STAT1. The cross line indicates a ratio equal to 1. B, Colorectal cancer patients in the Moffitt cohort (n = 177) were dichotomized by expression of ANGPTL4 and patient with relative high expression or relative low expression of ANGPTL4 were considered for plotting. DFS, disease-free survival. C, Correlation of COX-2 and STAT1 or ANGPTL4expression in Moffitt Colorectal cancer patient cohort. Scatter plots between COX-2 and STAT1 or ANGPTL4 in Moffitt cohort (n = 177).
Discussion
Hypoxia in the tumor microenvironment contributes to cancer progression and metastasis by activating adaptive programs that promote cancer cell proliferation, survival, and motility as well as tumor-associated angiogenesis. It has been reported that there is a cross talk between the COX-2 pathway and signaling pathways affected by hypoxia. For example, hypoxia induces COX-2 expression in CRC cells and vascular endothelial cells (12, 31). Reciprocally, COX-2-derived PGE2 regulates HIF-1 expression and transcriptional activity in those cells (12, 32). Although a connection between COX-2 and hypoxia has been established, the precise mechanisms by which PGE2 and hypoxia synergistically promote cancer progression are largely unclear. In this study, we identify ANGPTL4 as a novel PGE2 downstream target under hypoxic conditions in CRC cells. We also found that hypoxia–induced EP1 mediates the effects of PGE2 on regulation of ANGPTL4. Elevation of ANGPTL4 promotes tumor growth in vitro and in vivo, suggesting that ANGPTL4 can act as a downstream effector of PGE2 for adaptation of cancer cells to hypoxia. Our results suggest that the COX-2-PGE2 pathway plays an important role in adapting cancer cell to the tumor microenvironment such as hypoxia for cancer progression.
PGE2 exerts its biological effects in an autocrine or paracrine manner by binding to its cognate cell surface receptors, EP1, EP2, EP3, and EP4. All four EP receptors have been implicated in regulating cancer progression in certain contexts. These receptors are categorized into three groups. EP1 couples to Gq to increase intracellular calcium, EP2 and EP4 couple to Gαs to induce intracellular cyclic AMP, and EP3 couples to Gαi to decrease cyclic AMP. EP receptors have distinct functions because each receptor has a specific tissue distribution and induces a specific signaling pathways (some redundancy may exit) (33). Here, we found that hypoxia specifically enhanced EP1 expression in colorectal carcinoma cells without significant effects on EP2, EP3 or EP4 (Fig. 2A). EP1 has been reported to promote cell survival and proliferation in different types of cancer cells (34, 35). A role for EP1 in colorectal tumorigenesis has been further confirmed in animal models of CRC. For example, inhibition of EP1 by genetic deletion or treatment of an EP1-selective antagonist reduced intestinal tumor burden in ApcMin/+ and carcinogen-treated EP1−/− mice (36), demonstrating that EP1 contributes to cancer development. Our result is supported by the observation that hypoxia specifically increases EP1 expression in bone cells. Hypoxia-induced EP1 may play a role in bone remodeling and repair in regions of compromised or damaged bone, where O2 tension is low (23). Our data demonstrated that only hypoxia-induced EP1 mediated PGE2 induction of ANGPTL4 although EP4 expression is high in both normoxic and hypoxic conditions (Fig, 2). This result explains why PGE2 only induced ANGPTL4 under hypoxic conditions but not under normoxic conditions. Moreover, our results from immunochemistry studies showed that ANGPTL4 is expressed in hypoxic regions of the tumors (Supplemental Fig. S1) although HIF-1α, the indicator of hypoxia, and ANGPTL4 did not exactly co-localize in same cells but overlap in same area because HIF-1α is a transcription factor and ANGPTL4 is a ligand to be secreted and diffused in the tumors. Collectively, these findings imply that tumor hypoxia enhances PGE2 signaling through up-regulating the EP1 receptor expression and the PGE2 signaling may allow adaptation of cancer cells to the tumor microenvironment.
The synergistic effects of hypoxia and other factors such as insulin, PDGF, and TNF-α targeting genes has been reported in multiple types of cells, including cancer cells (37, 38). In contrast to PGE2, these factors also induce the expression of target genes under normoxic conditions. Our results showed that PGE2 induced ANGPTL4 expression only under hypoxic conditions. Several studies revealed that ANGPTL4 is transcriptionally regulated in response to intracellular or extracellular signals (18, 39). Since hypoxic-inducible factor (HIF) mediates hypoxia up-regulation of ANGPTL4 in different types of cells and PGE2 enhances HIF-1 transcriptional activity (12, 13, 20-22), it is possible that HIF-1 mediates the effect of PGE2 on induction of ANGPTL4. However, our results showed that inhibition of HIF-1 did not affect PGE2 induction of ANGPTL4 (data not shown). Based on the observations that ANGPTL4 is one of peroxisome proliferator-activated receptors (PPARs) target genes (40) with several putative PPRE consensus sequences on its promoter (41) and PGE2 transactivates PPARδ activity (7), we postulate that PPARδ is a potential transcription factor that could regulate PGE2-mediated ANGPTL4 expression under hypoxic conditions. Further investigation is needed to test this hypothesis.
ANGPTL4’s functions have been well established in regulating lipid metabolism through the inhibition of lipoprotein lipase (LPL). N-ANGPTL4, which assembles into multimeric structures, is essential to inhibit the activity of LPL (39). However, C-ANGPTL4 exists as monomer and its functional role in monomeric form is still unclear. In addition to ANGPTL4’s role in lipid metabolism, several recent studies have linked F-ANGPTL4 to angiogenesis and cancer development (15, 25, 26). However, few studies have delineated the biological function of C- or N-ANGPTL4 in cancer progression. Here, we show a similar function of C-ANGPTL4 in inducing CRC cell proliferation that is more effective than F-ANGPTL4 (Fig. 3A). In addition, our results demonstrate for the first time that C-ANGPTL4 induced CRC proliferation through up-regulation of STAT1. STAT1 has been reported to have proliferative effects and is over-expressed in head and neck and lung cancer (27, 28). In addition, activation of STAT1 promotes tumor cell survival in Wilms’ tumor (27). Moreover, radiation induces STAT1 expression through activation epidermal growth factor receptor (EGFR) in breast cancer cells (42). STAT1 has been reported to regulate the oncogenic genes Fos and Egr1 in the absence of STAT3 (43). However, little information regarding the mechanism by which STAT1 regulates cell proliferation and oncogenesis is available. Since our microarray date showed that C-ANGPTL4 increased several oncogenic genes including Fos and Egr1 (data not shown), C-ANGPTL4 affect on these oncogenic genes via STAT1 may contribute to cancer cell proliferation. Moreover, we identified novel C-ANGPTL4 downstream targets, Src and ERK, which are critical for C-ANGPTL4 induction of STAT1 expression (Fig. 4). It has been well established previously that the Src and ERK are responsible to cell proliferation. Therefore, the role of ANGPTL4 in cancer development is likely conferred b y C-ANGPTL4 through activating the signaling pathways which regulate cell proliferation. Although C- and F-ANGPTL4 has fibrinogen-like domain of angiopoietin that binds to angiopoietin receptor Tie1 and Tie2, C- and F-ANGPTL4 do not have any significant affinity for those receptors (44). A recent report shows that C-ANGPTL4 interacts with integrin β1 and β5 and produces O2− to regulate anoikis resistance of cancer cells (30). Therefore, confirmation of the C-ANGPTL4 receptor such as integrins will add to our understanding of how C-ANGPTL4 regulates cell proliferation as well as angiogenesis and metastasis.
It is generally accepted that hypoxia in tumor microenvironment promotes chemoresistance (45). It has been reported that COX-2 overexpressing cancer cells are more resistant to doxorubicin and gefitinib (46, 47). Moreover, STAT1 has been shown to play a role in chemo- and radio-resistance. A recent study has shown that the interferon-related gene signature including STAT1 and its target genes are associated with resistance to DNA damage across multiple cancer cell lines and can affect resistance to chemotherapy (48). In addition, radioresistant tumor cells expressed higher STAT1 levels than control cells (28). In accordance with these published results, we found for the first time that STAT1 was upregulated by ANGPTL4 and our preliminary data showed that ANGPTL4 treatment suppressed oxaliplatin-induced cell death in LS-147T cells (data not shown). Furthermore, the expression of COX-2, ANGPTL4 and STAT1 in human CRC specimens appears to be correlated (Fig. 5). Based on these observations, it is conceivable that ANGPTL4 may contribute to the hypoxia or COX2-induced drug resistance and may offer a therapeutic option for CRC.
In conclusion, our studies reveal the molecular mechanism by which PGE2 signaling and hypoxia coordinately promotes tumor growth. We found that PGE2 enhanced ANGPTL4 expression via EP1 receptor signaling that was enhanced by hypoxia. Moreover, we provide in vitro and in vivo evidence showing that elevation of ANGPTL4 promotes tumor growth. These results indicate that PGE2 plays an important role in promoting cancer cellular adaptive response to hypoxia and cancer progression via ANGPTL4. The knowledge of PGE2’s role in tumor hypoxia may help to better understand how cancer cells adapt to hypoxia for their growth. It has not escaped our attention that other genes are also more dramatically induced under hypoxia conditions following PGE2 treatment. This group of genes may reveal other important connections between inflammatory mediators and cancer progression in the tumor microenvironment.
Supplementary Material
Acknowledgement
We thank Marivonne Rodriguez, Sung-Nam Cho and Hong Wu for technical assistance.
Financial supports: This work is supported by NIH Grants RO1DK 62112 (RND), P01-CA-77839 (RND), and R37-DK47297 (RND).
Reference
- 1.Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer. 2001;1:11–21. doi: 10.1038/35094017. [DOI] [PubMed] [Google Scholar]
- 2.Marnett LJ, DuBois RN. COX-2: a target for colon cancer prevention. Annu Rev Pharmacol Toxicol. 2002;42:55–80. doi: 10.1146/annurev.pharmtox.42.082301.164620. [DOI] [PubMed] [Google Scholar]
- 3.Ogino S, Kirkner GJ, Nosho K, Irahara N, Kure S, Shima K, et al. Cyclooxygenase-2 expression is an independent predictor of poor prognosis in colon cancer. Clin Cancer Res. 2008;14:8221–7. doi: 10.1158/1078-0432.CCR-08-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D, Dubois RN. Cyclooxygenase-2: a potential target in breast cancer. Semin Oncol. 2004;31:64–73. doi: 10.1053/j.seminoncol.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Wang D, Buchanan FG, Wang H, Dey SK, DuBois RN. Prostaglandin E2 enhances intestinal adenoma growth via activation of the Ras-mitogen-activated protein kinase cascade. Cancer Res. 2005;65:1822–9. doi: 10.1158/0008-5472.CAN-04-3671. [DOI] [PubMed] [Google Scholar]
- 6.Kawamori T, Uchiya N, Sugimura T, Wakabayashi K. Enhancement of colon carcinogenesis by prostaglandin E2 administration. Carcinogenesis. 2003;24:985–90. doi: 10.1093/carcin/bgg033. [DOI] [PubMed] [Google Scholar]
- 7.Wang D, Wang H, Shi Q, Katkuri S, Walhi W, Desvergne B, et al. Prostaglandin E(2) promotes colorectal adenoma growth via transactivation of the nuclear peroxisome proliferator-activated receptor delta. Cancer Cell. 2004;6:285–95. doi: 10.1016/j.ccr.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 10:181–93. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giatromanolaki A, Harris AL. Tumour hypoxia, hypoxia signaling pathways and hypoxia inducible factor expression in human cancer. Anticancer Res. 2001;21:4317–24. [PubMed] [Google Scholar]
- 10.Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 11.Lendahl U, Lee KL, Yang H, Poellinger L. Generating specificity and diversity in the transcriptional response to hypoxia. Nat Rev Genet. 2009;10:821–32. doi: 10.1038/nrg2665. [DOI] [PubMed] [Google Scholar]
- 12.Kaidi A, Qualtrough D, Williams AC, Paraskeva C. Direct transcriptional up-regulation of cyclooxygenase-2 by hypoxia-inducible factor (HIF)-1 promotes colorectal tumor cell survival and enhances HIF-1 transcriptional activity during hypoxia. Cancer Res. 2006;66:6683–91. doi: 10.1158/0008-5472.CAN-06-0425. [DOI] [PubMed] [Google Scholar]
- 13.Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, et al. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30:377–86. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- 14.Lee EC, Desai U, Gololobov G, Hong S, Feng X, Yu XC, et al. Identification of a new functional domain in angiopoietin-like 3 (ANGPTL3) and angiopoietin-like 4 (ANGPTL4) involved in binding and inhibition of lipoprotein lipase (LPL) J Biol Chem. 2009;284:13735–45. doi: 10.1074/jbc.M807899200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma T, Jham BC, Hu J, Friedman ER, Basile JR, Molinolo A, et al. Viral G protein-coupled receptor up-regulates Angiopoietin-like 4 promoting angiogenesis and vascular permeability in Kaposi’s sarcoma. Proc Natl Acad Sci U S A. 107:14363–8. doi: 10.1073/pnas.1001065107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Jan S, Amy C, Cazes A, Monnot C, Lamande N, Favier J, et al. Angiopoietin-like 4 is a proangiogenic factor produced during ischemia and in conventional renal cell carcinoma. Am J Pathol. 2003;162:1521–8. doi: 10.1016/S0002-9440(10)64285-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito Y, Oike Y, Yasunaga K, Hamada K, Miyata K, Matsumoto S, et al. Inhibition of angiogenesis and vascular leakiness by angiopoietin-related protein 4. Cancer Res. 2003;63:6651–7. [PubMed] [Google Scholar]
- 18.Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR, et al. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133:66–77. doi: 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galaup A, Cazes A, Le Jan S, Philippe J, Connault E, Le Coz E, et al. Angiopoietin-like 4 prevents metastasis through inhibition of vascular permeability and tumor cell motility and invasiveness. Proc Natl Acad Sci U S A. 2006;103:18721–6. doi: 10.1073/pnas.0609025103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knowles HJ, Cleton-Jansen AM, Korsching E, Athanasou NA. Hypoxia-inducible factor regulates osteoclast-mediated bone resorption: role of angiopoietin-like 4. FASEB J. 24:4648–59. doi: 10.1096/fj.10-162230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murata M, Yudo K, Nakamura H, Chiba J, Okamoto K, Suematsu N, et al. Hypoxia upregulates the expression of angiopoietin-like-4 in human articular chondrocytes: role of angiopoietin-like-4 in the expression of matrix metalloproteinases and cartilage degradation. J Orthop Res. 2009;27:50–7. doi: 10.1002/jor.20703. [DOI] [PubMed] [Google Scholar]
- 22.Wang B, Wood IS, Trayhurn P. Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch. 2007;455:479–92. doi: 10.1007/s00424-007-0301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Genetos DC, Lee CM, Wong A, Yellowley CE. HIF-1alpha regulates hypoxia-induced EP1 expression in osteoblastic cells. J Cell Biochem. 2009;107:233–9. doi: 10.1002/jcb.22119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimura M, Osumi S, Ogihara M. Prostaglandin E(2) (EP(1)) receptor agonist-induced DNA synthesis and proliferation in primary cultures of adult rat hepatocytes: the involvement of TGF-alpha. Endocrinology. 2001;142:4428–40. doi: 10.1210/endo.142.10.8450. [DOI] [PubMed] [Google Scholar]
- 25.Nakayama T, Hirakawa H, Shibata K, Abe K, Nagayasu T, Taguchi T. Expression of angiopoietin-like 4 in human gastric cancer: ANGPTL4 promotes venous invasion. Oncol Rep. 24:599–606. doi: 10.3892/or_00000897. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, Han B, Zhang Z, Pan J, Xia H. Expression of angiopoietin-like 4 and tenascin C but not cathepsin C mRNA predicts prognosis of oral tongue squamous cell carcinoma. Biomarkers. 15:39–46. doi: 10.3109/13547500903261362. [DOI] [PubMed] [Google Scholar]
- 27.Timofeeva OA, Plisov S, Evseev AA, Peng S, Jose-Kampfner M, Lovvorn HN, et al. Serine-phosphorylated STAT1 is a prosurvival factor in Wilms’ tumor pathogenesis. Oncogene. 2006;25:7555–64. doi: 10.1038/sj.onc.1209742. [DOI] [PubMed] [Google Scholar]
- 28.Khodarev NN, Beckett M, Labay E, Darga T, Roizman B, Weichselbaum RR. STAT1 is overexpressed in tumors selected for radioresistance and confers protection from radiation in transduced sensitive cells. Proc Natl Acad Sci U S A. 2004;101:1714–9. doi: 10.1073/pnas.0308102100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roskoski R., Jr. RAF protein-serine/threonine kinases: structure and regulation. Biochem Biophys Res Commun. 399:313–7. doi: 10.1016/j.bbrc.2010.07.092. [DOI] [PubMed] [Google Scholar]
- 30.Zhu P, Tan MJ, Huang RL, Tan CK, Chong HC, Pal M, et al. Angiopoietin-like 4 protein elevates the prosurvival intracellular O2(−):H2O2 ratio and confers anoikis resistance to tumors. Cancer Cell. 19:401–15. doi: 10.1016/j.ccr.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 31.Ji YS, Xu Q, Schmedtje JF., Jr. Hypoxia induces high-mobility-group protein I(Y) and transcription of the cyclooxygenase-2 gene in human vascular endothelium. Circ Res. 1998;83:295–304. doi: 10.1161/01.res.83.3.295. [DOI] [PubMed] [Google Scholar]
- 32.Ji R, Chou CL, Xu W, Chen XB, Woodward DF, Regan JW. EP1 prostanoid receptor coupling to G i/o up-regulates the expression of hypoxia-inducible factor-1 alpha through activation of a phosphoinositide-3 kinase signaling pathway. Mol Pharmacol. 77:1025–36. doi: 10.1124/mol.110.063933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cha YI, DuBois RN. NSAIDs and cancer prevention: targets downstream of COX-2. Annu Rev Med. 2007;58:239–52. doi: 10.1146/annurev.med.57.121304.131253. [DOI] [PubMed] [Google Scholar]
- 34.Zhang L, Jiang L, Sun Q, Peng T, Lou K, Liu N, et al. Prostaglandin E2 enhances mitogen-activated protein kinase/Erk pathway in human cholangiocarcinoma cells: involvement of EP1 receptor, calcium and EGF receptors signaling. Mol Cell Biochem. 2007;305:19–26. doi: 10.1007/s11010-007-9523-5. [DOI] [PubMed] [Google Scholar]
- 35.Makita H, Mutoh M, Maruyama T, Yonemoto K, Kobayashi A, Fujitsuka H, et al. A prostaglandin E2 receptor subtype EP1-selective antagonist, ONO-8711, suppresses 4-nitroquinoline 1-oxide-induced rat tongue carcinogenesis. Carcinogenesis. 2007;28:677–84. doi: 10.1093/carcin/bgl178. [DOI] [PubMed] [Google Scholar]
- 36.Kawamori T, Kitamura T, Watanabe K, Uchiya N, Maruyama T, Narumiya S, et al. Prostaglandin E receptor subtype EP(1) deficiency inhibits colon cancer development. Carcinogenesis. 2005;26:353–7. doi: 10.1093/carcin/bgh322. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Kimura H, Hirota K, Kasuno K, Torii K, Okada T, et al. Synergistic effect of hypoxia and TNF-alpha on production of PAI-1 in human proximal renal tubular cells. Kidney Int. 2005;68:569–83. doi: 10.1111/j.1523-1755.2005.00435.x. [DOI] [PubMed] [Google Scholar]
- 38.Meissner U, Ostreicher I, Allabauer I, Rascher W, Dotsch J. Synergistic effects of hypoxia and insulin are regulated by different transcriptional elements of the human leptin promoter. Biochem Biophys Res Commun. 2003;303:707–12. doi: 10.1016/s0006-291x(03)00401-7. [DOI] [PubMed] [Google Scholar]
- 39.Koliwad SK, Kuo T, Shipp LE, Gray NE, Backhed F, So AY, et al. Angiopoietin-like 4 (ANGPTL4, fasting-induced adipose factor) is a direct glucocorticoid receptor target and participates in glucocorticoid-regulated triglyceride metabolism. J Biol Chem. 2009;284:25593–601. doi: 10.1074/jbc.M109.025452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rieck M, Meissner W, Ries S, Muller-Brusselbach S, Muller R. Ligand-mediated regulation of peroxisome proliferator-activated receptor (PPAR) beta/delta: a comparative analysis of PPAR-selective agonists and all-trans retinoic acid. Mol Pharmacol. 2008;74:1269–77. doi: 10.1124/mol.108.050625. [DOI] [PubMed] [Google Scholar]
- 41.Kaddatz K, Adhikary T, Finkernagel F, Meissner W, Muller-Brusselbach S, Muller R. Transcriptional profiling identifies functional interactions of TGF beta and PPAR beta/delta signaling: synergistic induction of ANGPTL4 transcription. J Biol Chem. 285:29469–79. doi: 10.1074/jbc.M110.142018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amorino GP, Hamilton VM, Valerie K, Dent P, Lammering G, Schmidt-Ullrich RK. Epidermal growth factor receptor dependence of radiation-induced transcription factor activation in human breast carcinoma cells. Mol Biol Cell. 2002;13:2233–44. doi: 10.1091/mbc.01-12-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schiavone D, Avalle L, Dewilde S, Poli V. The immediate early genes Fos and Egr1 become STAT1 transcriptional targets in the absence of STAT3. FEBS Lett. doi: 10.1016/j.febslet.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 44.Oike Y, Akao M, Kubota Y, Suda T. Angiopoietin-like proteins: potential new targets for metabolic syndrome therapy. Trends Mol Med. 2005;11:473–9. doi: 10.1016/j.molmed.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Cosse JP, Michiels C. Tumour hypoxia affects the responsiveness of cancer cells to chemotherapy and promotes cancer progression. Anticancer Agents Med Chem. 2008;8:790–7. doi: 10.2174/187152008785914798. [DOI] [PubMed] [Google Scholar]
- 46.Singh B, Cook KR, Vincent L, Hall CS, Berry JA, Multani AS, et al. Cyclooxygenase-2 induces genomic instability, BCL2 expression, doxorubicin resistance, and altered cancer-initiating cell phenotype in MCF7 breast cancer cells. J Surg Res. 2008;147:240–6. doi: 10.1016/j.jss.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 47.Kim YM, Park SY, Pyo H. Cyclooxygenase-2 (COX-2) negatively regulates expression of epidermal growth factor receptor and causes resistance to gefitinib in COX-2-overexpressing cancer cells. Mol Cancer Res. 2009;7:1367–77. doi: 10.1158/1541-7786.MCR-09-0004. [DOI] [PubMed] [Google Scholar]
- 48.Weichselbaum RR, Ishwaran H, Yoon T, Nuyten DS, Baker SW, Khodarev N, et al. An interferon-related gene signature for DNA damage resistance is a predictive marker for chemotherapy and radiation for breast cancer. Proc Natl Acad Sci U S A. 2008;105:18490–5. doi: 10.1073/pnas.0809242105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.